Abstract
The daily production of billions of platelets must be regulated to avoid spontaneous bleeding or arterial occlusion and organ damage. Complex mechanisms control platelet production and clearance in physiological and pathological conditions. This review will focus on the mechanisms of platelet senescence with specific emphasis on the role of post-translational modifications in platelet life-span and thrombopoietin production downstream of the hepatic Ashwell-Morrell receptor.
Keywords: Platelet clearance, glycans, Ashwell Morell Receptor, Thrombopoietin, JAK2, STAT3
Platelet clearance
Multiple mechanisms mediate platelet clearance. One mechanism appears to function via aging (senescence) induced signals, i.e. via glycan degradation and apoptotic mechanisms. Platelets are also removed by immune responses, i.e. via antibody or T-cell dependent mechanisms. This review will focus on senescence related platelet clearance mechanisms via the hepatic Ashwell-Morell Receptor (AMR) leading to regulation of thrombopoietin (TPO), the primary regulator of thrombopoiesis [1], in hepatocytes.
Senescence-induced platelet clearance by the Ashwell-Morrell receptor
Changes in the glycan composition are one mechanism for inducing platelet clearance [2, 3]. Sialic acid (SA) depletion is one determinant for the removal of senescent circulating platelets [4]. SA loss is likely mediated by upregulation of sialidases, Neu1 and Neu3, which platelets express in granular compartments [5]. The sialidase inhibitor oseltamivir phosphate (Tamiflu®), which is clinically used to treat influenza, has also been shown to increase platelet counts in 2 patients with ITP, as well as in a large cohort of patients from the Erasmus Medical Center, Rotterdam, independently of influenza diagnosis [6–8]. These studies indicate that platelets may regulate their SA content and survival using intrinsic sialidase activity. Our unpublished data show that injections of sialidase inhibitors into wild-type mice increase platelet life-span, supporting the notion that endogenous sialidase activity mediates loss of SA by platelets leading to clearance.
The highly conserved hepatic Ashwell-Morell receptor (AMR), originally termed asialoglycoprotein receptor (ASGPR), is a transmembrane heteroligomeric glycoprotein complex composed of two ASGPR1 and one ASGPR2 subunits. Its expression is restricted to hepatocytes. The AMR is one of the multiple lectins of the C-type family involved in recognition, binding, and clearance of asialoglycoproteins. It was the first cellular receptor to be identified and isolated, and the first lectin to be detected in mammals. It has largely been regarded as an endocytic receptor responsible for the removal of circulating plasma glycoproteins or glycolipids lacking SA [9]. Surprisingly, mice lacking a functional AMR due to genetic deletion of either ASGPR1 or ASGPR2 subunits, showed no increase in circulating glycolipids or glycoproteins [9]. The physiological role of the AMR has therefore remained unclear since its discovery for four decades until recent studies demonstrated that the AMR is responsible for the clearance of platelets with reduced α2,3-linked SA, such as during sepsis, after cold storage (in vitro aging), or in mice lacking the sialyltransferase ST3GalIV [10–13].
These findings led subsequently to the discovery that clearance of senescent, SA-deprived platelets is mediated by the hepatic AMR. Furthermore, this removal drives mRNA expression of TPO in hepatocytes in vivo and in HepG2 cells in vitro to increase the numbers of platelet precursors, bone marrow megakaryocytes (MKs), and de novo platelet production [4]. TPO mRNA expression is regulated via phosphorylation and activation of the tyrosine kinase JAK2 and the acute phase response signal transducer and activator of transcription STAT3 downstream of the AMR.
The notion that loss of SA determines platelet life span is not novel [10, 11, 13–16], however, the recent study elucidates that aged, desialylated platelets regulate hepatic TPO mRNA production in vivo via the AMR. This feedback mechanism presents the AMR-desialylated platelet pair as an important control point for TPO homeostasis and shows that TPO expression in hepatocytes is regulated and not constitutive, as previously believed (Figure 1). The detailed mechanisms will be discussed below.
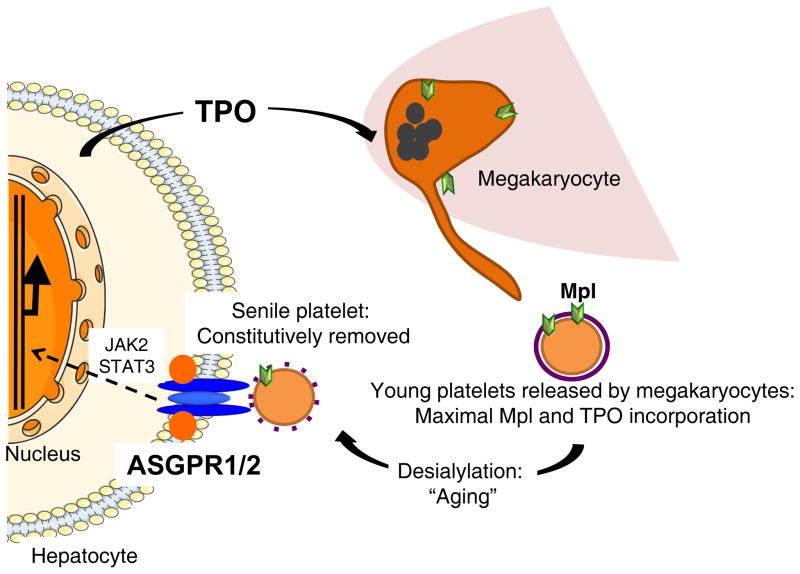
Figure 1. Scheme of hepatic TPO production via JAK2-STAT3 signaling after desialylated platelet uptake by the AMR.
Bone marrow megakaryocytes produce and release young sialic acid (purple ring)-containing platelets into the blood stream. Young platelets maximally internalize TPO via Mpl-mediated endocytosis. Circulating platelets become desialylated by active blood-borne sialidases as they age (dashed purple ring) and are recognized by the hepatic AMR. Desialylated platelet ingestion signaling positively stimulates hepatic TPO mRNA expression via JAK2-STAT3 activation, releasing TPO into plasma, thereby regulating bone marrow homeostasis and thrombopoiesis.
TPO regulation
TPO is the primary regulator of platelet production, supporting the survival, proliferation and differentiation of the bone marrow MKs [1, 17, 18]. TPO, acting through its receptor, Mpl, is required for hematopoietic stem cell maintenance and megakaryopoiesis. Since the discovery of TPO many molecular mechanisms of thrombopoiesis have been identified, including the development of polyploidy and proplatelet formation, the final fragmentation of the MK cytoplasm to yield blood platelets, and the regulation of this process [17, 19–22].
One unanswered question is the regulation of TPO production under steady state and under pathologic conditions. Multiple organs display TPO mRNA transcripts, with hepatocytes having the highest levels and being the primary cells responsible for the production and secretion of TPO into the bloodstream (Figure 1). TPO production has long been thought to be constitutive, with TPO plasma levels maintained solely by its uptake and metabolism by platelets and MKs [23–27]. TPO is taken up by platelets through Mpl-mediated endocytosis [20, 28]. Circulating TPO levels are elevated in patients with Congenital Amegakaryocytic Thrombocytopenia (CAMT), which is caused by germline Mpl mutations [29, 30], Thrombocytopenia-Absent Radius (TAR) syndrome [31],or acquired aplastic anemia [32, 33]. In these cases, circulating TPO levels are inversely correlated to platelet counts. Thus, the removal and destruction of TPO released into the bloodstream depends on platelet and MK mass and Mpl surface expression (Figure 1).
This model is supported by mouse models recently generated to specifically lack Mpl (Mplfl/fl Pf4-Cre mice) or Mpl-regulatory proteins in MKs and platelets, i.e. the Mpl-associated tyrosine kinase JAK2 (Jak2fl/fl Pf4-Cre mice) and the large endocytic GTPase dynamin 2 (Dnm2fl/fl Pf4-Cre mice), which plays a critical role in Mpl endocytosis and JAK2 signaling [20, 34, 35]. Mplfl/fl Pf4-Cre mice and Jak2fl/fl Pf4-Cre mice display profound megakaryocytosis and thrombocytosis with a remarkable expansion of MK-committed and multipotential progenitor cells, which normally express Mpl and JAK2 respectively. Multipotential progenitor cells display biological responses and a gene expression signature indicative of chronic TPO overstimulation as the underlying causative mechanism [34, 35]. This is an intriguing finding as Mplfl/fl Pf4-Cre mice and Jak2fl/fl Pf4-Cre mice are obviously able to “bypass” the respective lack of Mpl and JAK2 in the MK lineage. The studies conclude that TPO signaling in MKs is dispensable for platelet production, and that the key role of TPO signaling is in controlling platelet numbers via generation and stimulation of the bipotential MK precursors. On the other hand, Mpl expression in MKs and platelets is essential to prevent megakaryocytosis and myeloproliferation by restricting the amount of TPO available to stimulate the production of MKs from the progenitor cell pool. Even more surprising was the normal circulating TPO levels in these mice, presenting more evidence that circulating TPO levels are regulated in a complicated manner.
Dnm2fl/fl Pf4-Cre mice have impaired Mpl-mediated endocytosis, resulting in elevated plasma TPO levels and constitutive phosphorylation of JAK2, although JAK2 expression is reduced in Dnm2fl/fl Pf4-Cre platelets [20]. Dnm2fl/fl Pf4-Cre mice develop MK hyperplasia, myelofibrosis, extramedullary hematopoiesis, and severe and rapid (<3 weeks) splenomegaly. However, Dnm2fl/fl Pf4-Cre mice develop macrothrombocytopenia, not thrombocytosis, as DNM2 plays an additional critical role in the formation of the MK demarcation membrane system required for platelet formation. The low blood platelet numbers of Dnm2fl/fl Pf4-Cre mice and their inability to clear circulating TPO likely exacerbate their severe myelofibrosis phenotype.
A growing body of evidence lends credence to the assertion that platelet TPO metabolism is not the sole determinant of plasma TPO levels in humans. In contrast to the “autoregulation” model of blood TPO levels described above, serum TPO levels are lower than expected in patients with Immune Thrombocytopenia (ITP) [36, 37], and high in patients with Essential Thrombocythemia (ET) [38, 39]. In patients with thrombocytopenia little of the hepatocyte produced TPO is presumed to be removed by platelets and TPO blood levels rise. In contrast, thrombocytosis should be accompanied by low steady-state levels of blood TPO, because platelet-mediated TPO destruction surpasses its production [23–27]. However, Mpl expression levels on the membrane surface of platelets are strongly decreased in patients with ET presenting the somatic JAK2 mutation V617F [40, 41], which can explain decreased TPO uptake and high circulation TPO levels.
The notion that TPO production is regulated, rather than autonomous, is further supported by data showing that marrow stromal cells produce TPO in response to thrombocytopenia both in mice and in humans [42, 43]. Selective liver irradiation in mice stimulates hepatic TPO production [44]. Further, in addition to marrow stromal cell TPO production, a number of inflammatory states, e.g. ulcerative colitis, rheumatoid arthritis, ovarian cancer, are associated with increased blood TPO levels and thrombocytosis [38, 42, 45–52]. This inflammation-induced increase in TPO expression is mediated by interleukin 6 (IL-6), which stimulates hepatic TPO mRNA expression and production both in hepatocytes in vivo and in HepG2 and Hep3B cells in vitro [49–51, 53, 54]. If hepatic TPO regulation by IL-6 is now well characterized, the ligand-receptor pair regulating hepatic TPO production at steady-state has remained elusive. The new model detailed below furthers our understanding of the regulation of blood TPO levels and thrombopoiesis: desialylated, senescent platelet clearance via the hepatic AMR enhances hepatic TPO production (Figure 1).
The AMR-IL-6R connection
Interestingly, the AMR signaling cascade shares similarities with that of IL-6, as it involves JAK2 and STAT3 tyrosine phosphorylation and STAT3 translocation to the nucleus (Figure 2) [4, 55]. Binding of IL-6 to its hepatic receptor, IL-6R/gp80, engages the signal transducing subunit gp130, leading to STAT3 tyrosine phosphorylation and activation by gp130-associated JAK1, and to a lesser extent JAK2. Thus, both desialylated platelets and IL-6 lead to STAT3-mediated hepatic TPO mRNA expression downstream of the AMR-JAK2 and IL-6R-JAK1 signaling cascades, respectively.
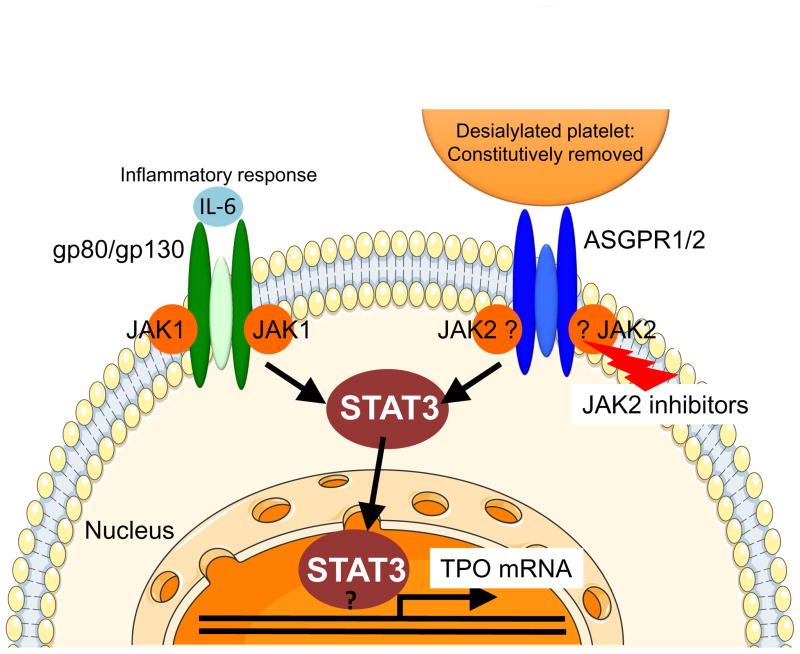
Figure 2. Comparison between the AMR and IL-6R signaling pathways to hepatic TPO mRNA expression.
Binding of desialylated platelets to the hepatic AMR composed of one ASGPR2 and two ASGPR1 subunits activates JAK2. IL-6 binding to its hepatic receptor composed of one gp80 and two gp130 subunits activates gp130-associated JAK1, and to a lesser extent JAK2. Both JAK1 and JAK2 phosphorylate STAT3, resulting in its translocation to the nucleus where it stimulates mRNA expression of TPO and acute phase response proteins. It is unclear whether JAK2 directly associates with ASGPR1 and whether STAT3 directly binds to the TPO promoter.
Importantly, disruption of AMR-desialylated platelet signaling by the JAK1/2 inhibitors adversely affects hepatic TPO mRNA expression and secretion in mouse hepatocytes in vivo and in human HepG2 cells in vitro [4]. Thrombocytopenia is a common adverse event of JAK1/2 inhibitor treatment, which is clinically used in myeloproliferative neoplasms (MPNs) [56, 57]. JAK1/2 inhibitors target hematopoietic stem and precursor cell mutant JAK2-V617F as well as wild-type JAK2, activation of which is essential for red blood cell and platelet production [58, 59]. This new study indicates that inhibition of TPO production downstream of the hepatic AMR-JAK2 signaling cascade could additionally contribute to the thrombocytopenia associated with JAK1/2 treatment. Clinical studies are necessary to investigate this notion, particularly to determine whether MPN patients treated with JAK1/2 inhibitors have low circulating TPO levels.
Hepatic STAT3 controls the transcription of mRNA for acute phase plasma proteins [60]. It is therefore tempting to speculate that acute phase proteins are produced in response to AMR ligation, which would establish clearance of desialylated platelets as a component of the acute phase response. Consistent with this hypothesis, the AMR-mediated removal of desialylated platelets improves the probability of host survival during sepsis [9]. Separate studies have shown that liver regeneration following injury is promoted by platelets [61] and requires AMR and hepatic STAT3 function [62]. Thus, the platelet-AMR-JAK2-STAT3 signaling cascade may connect desialylated platelet to inflammatory responses.
Conclusion
Recent evidence shows that the hepatic AMR recognizes senescent, desialylated platelets under steady-state conditions. Desialylated platelets and the AMR are the physiological ligand-receptor pair regulating hepatic TPO mRNA production, as AMR-mediated removal of desialylated platelets regulates hepatic TPO synthesis by recruiting JAK2 and STAT3 to increase thrombopoiesis. Further studies will continue to elucidate the mechanisms regulating billions of platelets that are produced and cleared daily.
Acknowledgments
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants HL089224 and HL107146 (to K.M.H.); and bridge grants from the Brigham Research Institute and the American Society of Hematology (to H.F.).
Footnotes
Conflict-of-interest disclosure
The authors have no conflicts of interest to declare.
References
- 1.Kaushansky K. The molecular mechanisms that control thrombopoiesis. The Journal of clinical investigation. 2005;115:3339–47. doi: 10.1172/JCI26674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rumjantseva V, Hoffmeister KM. Novel and unexpected clearance mechanisms for cold platelets. Transfus Apher Sci. 2010;42:63–70. doi: 10.1016/j.transci.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmeister KM. The role of lectins and glycans in platelet clearance. J Thromb Haemost. 2011;9(Suppl 1):35–43. doi: 10.1111/j.1538-7836.2011.04276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grozovsky R, Begonja AJ, Liu K, Visner G, Hartwig JH, Falet H, et al. The Ashwell-Morell receptor regulates hepatic thrombopoietin production via JAK2-STAT3 signaling. Nature medicine. 2015;21:47–54. doi: 10.1038/nm.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jansen AJ, Josefsson EC, Rumjantseva V, Liu QP, Falet H, Bergmeier W, et al. Desialylation accelerates platelet clearance after refrigeration and initiates GPIbalpha metalloproteinase-mediated cleavage in mice. Blood. 2012;119:1263–73. doi: 10.1182/blood-2011-05-355628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alioglu B, Tasar A, Ozen C, Selver B, Dallar Y. An experience of oseltamivir phosphate (tamiflu) in a pediatric patient with chronic idiopathic thrombocytopenic purpura: a case report. Pathophysiol Haemost Thromb. 2010;37:55–8. doi: 10.1159/000321379. [DOI] [PubMed] [Google Scholar]
- 7.Shao L, Wu Y, Zhou H, Qin P, Ni H, Peng J, et al. Successful treatment with oseltamivir phosphate in a patient with chronic immune thrombocytopenia positive for anti-GPIb/IX autoantibody. Platelets. 2014:1–3. doi: 10.3109/09537104.2014.948838. [DOI] [PubMed] [Google Scholar]
- 8.Jansen AJ, Peng J, Zhao HG, Hou M, Ni H. Sialidase inhibition to increase platelet counts: A new treatment option for thrombocytopenia. Am J Hematol. 2015;90:E94–5. doi: 10.1002/ajh.23953. [DOI] [PubMed] [Google Scholar]
- 9.Grewal PK. The Ashwell-Morell receptor. Methods Enzymol. 2010;479:223–41. doi: 10.1016/S0076-6879(10)79013-3. [DOI] [PubMed] [Google Scholar]
- 10.Rumjantseva V, Grewal PK, Wandall HH, Josefsson EC, Sorensen AL, Larson G, et al. Dual roles for hepatic lectin receptors in the clearance of chilled platelets. Nature medicine. 2009;15:1273–80. doi: 10.1038/nm.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sorensen AL, Rumjantseva V, Nayeb-Hashemi S, Clausen H, Hartwig JH, Wandall HH, et al. Role of sialic acid for platelet life span: exposure of beta-galactose results in the rapid clearance of platelets from the circulation by asialoglycoprotein receptor-expressing liver macrophages and hepatocytes. Blood. 2009;114:1645–54. doi: 10.1182/blood-2009-01-199414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grewal PK, Aziz PV, Uchiyama S, Rubio GR, Lardone RD, Le D, et al. Inducing host protection in pneumococcal sepsis by preactivation of the Ashwell-Morell receptor. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1313905110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grewal PK, Uchiyama S, Ditto D, Varki N, Le DT, Nizet V, et al. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat Med. 2008;14:648–55. doi: 10.1038/nm1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crook M. Platelet sialic Acid and its significance to platelet life-spans. Platelets. 1990;1:167. doi: 10.3109/09537109009005484. [DOI] [PubMed] [Google Scholar]
- 15.Crook M. Sialic Acid: its importance to platelet function in health and disease. Platelets. 1991;2:1–10. doi: 10.3109/09537109109005496. [DOI] [PubMed] [Google Scholar]
- 16.Reimers HJ, Greenberg J, Cazenave JP, Packham MA, Mustard JF. Experimental modification of platelet survival. Adv Exp Med Biol. 1977;82:231–3. doi: 10.1007/978-1-4613-4220-5_48. [DOI] [PubMed] [Google Scholar]
- 17.Kaushansky K. Determinants of platelet number and regulation of thrombopoiesis. Hematology Am Soc Hematol Educ Program. 2009:147–52. doi: 10.1182/asheducation-2009.1.147. [DOI] [PubMed] [Google Scholar]
- 18.Kuter DJ. Thrombopoietin and thrombopoietin mimetics in the treatment of thrombocytopenia. Annu Rev Med. 2009;60:193–206. doi: 10.1146/annurev.med.60.042307.181154. [DOI] [PubMed] [Google Scholar]
- 19.Machlus KR, Thon JN, Italiano JE., Jr Interpreting the developmental dance of the megakaryocyte: a review of the cellular and molecular processes mediating platelet formation. Br J Haematol. 2014;165:227–36. doi: 10.1111/bjh.12758. [DOI] [PubMed] [Google Scholar]
- 20.Bender M, Giannini S, Grozovsky R, Jonsson T, Christensen H, Pluthero FG, et al. Dynamin 2-dependent endocytosis is required for normal megakaryocyte development. Blood. 2015;125:1014–24. doi: 10.1182/blood-2014-07-587857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Orban M, Lorenz M, Barocke V, Braun D, Urtz N, et al. A novel role of sphingosine 1-phosphate receptor S1pr1 in mouse thrombopoiesis. J Exp Med. 2012;209:2165–81. doi: 10.1084/jem.20121090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaushansky K. Thrombopoiesis. Semin Hematol. 2015;52:4–11. doi: 10.1053/j.seminhematol.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Kuter DJ, Rosenberg RD. The reciprocal relationship of thrombopoietin (c-Mpl ligand) to changes in the platelet mass during busulfan-induced thrombocytopenia in the rabbit. Blood. 1995;85:2720–30. [PubMed] [Google Scholar]
- 24.Cohen-Solal K, Villeval J, Titeux M, Lok S, Vainchenker W, Wendling F. Constitutive expression of Mpl ligand transcripts during thrombocytopenia or thrombocytosis. Blood. 1996;88:2578–28. [PubMed] [Google Scholar]
- 25.Fielder PJ, Gurney AL, Stefanich E, Marian M, Moore MW, Carver-Moore K, et al. Regulation of thrombopoietin levels by c-mpl-mediated binding to platelets. Blood. 1996;87:2154–61. [PubMed] [Google Scholar]
- 26.Shinjo K, Takeshita A, Nakamura S, Naitoh K, Yanagi M, Tobita T, et al. Serum thrombopoietin levels in patients correlate inversely with platelet counts during chemotherapy-induced thrombocytopenia. Leukemia. 1998;12:295–300. doi: 10.1038/sj.leu.2400946. [DOI] [PubMed] [Google Scholar]
- 27.Engel C, Loeffler M, Franke H, Schmitz S. Endogenous thrombopoietin serum levels during multicycle chemotherapy. Br J Haematol. 1999;105:832–8. doi: 10.1046/j.1365-2141.1999.01459.x. [DOI] [PubMed] [Google Scholar]
- 28.Hitchcock IS, Chen MM, King JR, Kaushansky K. YRRL motifs in the cytoplasmic domain of the thrombopoietin receptor regulate receptor internalization and degradation. Blood. 2008;112:2222–31. doi: 10.1182/blood-2008-01-134049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ihara K, Ishii E, Eguchi M, Takada H, Suminoe A, Good RA, et al. Identification of mutations in the c-mpl gene in congenital amegakaryocytic thrombocytopenia. Proc Natl Acad Sci U S A. 1999;96:3132–6. doi: 10.1073/pnas.96.6.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballmaier M, Germeshausen M, Schulze H, Cherkaoui K, Lang S, Gaudig A, et al. c-mpl mutations are the cause of congenital amegakaryocytic thrombocytopenia. Blood. 2001;97:139–46. doi: 10.1182/blood.v97.1.139. [DOI] [PubMed] [Google Scholar]
- 31.Ballmaier M, Schulze H, Strauss G, Cherkaoui K, Wittner N, Lynen S, et al. Thrombopoietin in patients with congenital thrombocytopenia and absent radii: elevated serum levels, normal receptor expression, but defective reactivity to thrombopoietin. Blood. 1997;90:612–9. [PubMed] [Google Scholar]
- 32.Schrezenmeier H, Griesshammer M, Hornkohl A, Nichol JL, Hecht T, Heimpel H, et al. Thrombopoietin serum levels in patients with aplastic anaemia: correlation with platelet count and persistent elevation in remission. Br J Haematol. 1998;100:571–6. doi: 10.1046/j.1365-2141.1998.00590.x. [DOI] [PubMed] [Google Scholar]
- 33.Gu J, Lu L, Xu R, Chen X. Plasma thrombopoietin levels in patients with aplastic anemia and idiopathic thrombocytopenic purpura. Chinese medical journal. 2002;115:983–6. [PubMed] [Google Scholar]
- 34.Ng AP, Kauppi M, Metcalf D, Hyland CD, Josefsson EC, Lebois M, et al. Mpl expression on megakaryocytes and platelets is dispensable for thrombopoiesis but essential to prevent myeloproliferation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:5884–9. doi: 10.1073/pnas.1404354111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer SC, Keller MD, Woods BA, LaFave LM, Bastian L, Kleppe M, et al. Genetic studies reveal an unexpected negative regulatory role for Jak2 in thrombopoiesis. Blood. 2014;124:2280–4. doi: 10.1182/blood-2014-03-560441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kosugi S, Kurata Y, Tomiyama Y, Tahara T, Kato T, Tadokoro S, et al. Circulating thrombopoietin level in chronic immune thrombocytopenic purpura. Br J Haematol. 1996;93:704–6. doi: 10.1046/j.1365-2141.1996.d01-1702.x. [DOI] [PubMed] [Google Scholar]
- 37.Ichikawa N, Ishida F, Shimodaira S, Tahara T, Kato T, Kitano K. Regulation of serum thrombopoietin levels by platelets and megakaryocytes in patients with aplastic anaemia and idiopathic thrombocytopenic purpura. Thromb Haemost. 1996;76:156–60. [PubMed] [Google Scholar]
- 38.Wang JC, Chen C, Novetsky AD, Lichter SM, Ahmed F, Friedberg NM. Blood thrombopoietin levels in clonal thrombocytosis and reactive thrombocytosis. Am J Med. 1998;104:451–5. doi: 10.1016/s0002-9343(98)00090-4. [DOI] [PubMed] [Google Scholar]
- 39.Griesshammer M, Hornkohl A, Nichol JL, Hecht T, Raghavachar A, Heimpel H, et al. High levels of thrombopoietin in sera of patients with essential thrombocythemia: cause or consequence of abnormal platelet production? Ann Hematol. 1998;77:211–5. doi: 10.1007/s002770050445. [DOI] [PubMed] [Google Scholar]
- 40.Besancenot R, Chaligne R, Tonetti C, Pasquier F, Marty C, Lecluse Y, et al. A senescence-like cell-cycle arrest occurs during megakaryocytic maturation: implications for physiological and pathological megakaryocytic proliferation. PLoS biology. 2010:8. doi: 10.1371/journal.pbio.1000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pecquet C, Diaconu CC, Staerk J, Girardot M, Marty C, Royer Y, et al. Thrombopoietin receptor down-modulation by JAK2 V617F: restoration of receptor levels by inhibitors of pathologic JAK2 signaling and of proteasomes. Blood. 2012;119:4625–35. doi: 10.1182/blood-2011-08-372524. [DOI] [PubMed] [Google Scholar]
- 42.McCarty J, Sprugel K, Fox N, Sabath D, Kaushansky K. Murine thrombopoietin mRNA levels are modulated by platelet count. Blood. 1995;86:3668–875. [PubMed] [Google Scholar]
- 43.Sungaran R, Chisholm OT, Markovic B, Khachigian LM, Tanaka Y, Chong BH. The role of platelet alpha-granular proteins in the regulation of thrombopoietin messenger RNA expression in human bone marrow stromal cells. Blood. 2000;95:3094–101. [PubMed] [Google Scholar]
- 44.Mouthon M, Vandamme M, Gourmelon P, Vainchenker W, Wendling F. Preferential liver irradiation enhances hematopoiesis through a thrombopoietin-independent mechanism. Radiat Res. 1999;152:390–7. [PubMed] [Google Scholar]
- 45.Sungaran R, Markovic B, Chong B. Localization and regulation of thrombopoietin mRNa expression in human kidney, liver, bone marrow, and spleen using in situ hybridization. Blood. 1997;89:101–7. [PubMed] [Google Scholar]
- 46.Qian S, Fu F, Li W, Chen Q, de Sauvage F. Primary role of the liver in thrombopoietin production shown by tissue-specific knockout. Blood. 1998;92:2189–91. [PubMed] [Google Scholar]
- 47.Wolber EM, Ganschow R, Burdelski M, Jelkmann W. Hepatic thrombopoietin mRNA levels in acute and chronic liver failure of childhood. Hepatology. 1999;29:1739–42. doi: 10.1002/hep.510290627. [DOI] [PubMed] [Google Scholar]
- 48.McIntosh B, Kaushansky K. Transcriptional regulation of bone marrow thrombopoietin by platelet proteins. Exp Hematol. 2008;36:799–806. doi: 10.1016/j.exphem.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolber EM, Fandrey J, Frackowski U, Jelkmann W. Hepatic thrombopoietin mRNA is increased in acute inflammation. Thromb Haemost. 2001;86:1421–4. [PubMed] [Google Scholar]
- 50.Kaser A, Brandacher G, Steurer W, Kaser S, Offner FA, Zoller H, et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001;98:2720–5. doi: 10.1182/blood.v98.9.2720. [DOI] [PubMed] [Google Scholar]
- 51.Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366:610–8. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cerutti A, Custodi P, Duranti M, Noris P, Balduini CL. Thrombopoietin levels in patients with primary and reactive thrombocytosis. Br J Haematol. 1997;99:281–4. doi: 10.1046/j.1365-2141.1997.3823196.x. [DOI] [PubMed] [Google Scholar]
- 53.Wolber E, Jelkmann W. Interleukin-6 increases thrombopoietin production in human hepatoma cells HepG2 and Hep3B. J Interferon Cytokine Res. 2000;20:499–506. doi: 10.1089/10799900050023915. [DOI] [PubMed] [Google Scholar]
- 54.Burmester H, Wolber EM, Freitag P, Fandrey J, Jelkmann W. Thrombopoietin production in wild-type and interleukin-6 knockout mice with acute inflammation. J Interferon Cytokine Res. 2005;25:407–13. doi: 10.1089/jir.2005.25.407. [DOI] [PubMed] [Google Scholar]
- 55.Eulenfeld R, Dittrich A, Khouri C, Muller PJ, Mutze B, Wolf A, et al. Interleukin-6 signalling: more than Jaks and STATs. Eur J Cell Biol. 2012;91:486–95. doi: 10.1016/j.ejcb.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 56.Levine RL, Gilliland DG. Myeloproliferative disorders. Blood. 2008;112:2190–8. doi: 10.1182/blood-2008-03-077966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.LaFave LM, Levine RL. JAK2 the future: therapeutic strategies for JAK-dependent malignancies. Trends in pharmacological sciences. 2012;33:574–82. doi: 10.1016/j.tips.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Park SO, Wamsley HL, Bae K, Hu Z, Li X, Choe SW, et al. Conditional deletion of Jak2 reveals an essential role in hematopoiesis throughout mouse ontogeny: implications for Jak2 inhibition in humans. PLoS One. 2013;8:e59675. doi: 10.1371/journal.pone.0059675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grisouard J, Hao-Shen H, Dirnhofer S, Wagner KU, Skoda RC. Selective deletion of Jak2 in adult mouse hematopoietic cells leads to lethal anemia and thrombocytopenia. Haematologica. 2014;99:e52–4. doi: 10.3324/haematol.2013.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alonzi T, Maritano D, Gorgoni B, Rizzuto G, Libert C, Poli V. Essential role of STAT3 in the control of the acute-phase response as revealed by inducible gene inactivation [correction of activation] in the liver. Mol Cell Biol. 2001;21:1621–32. doi: 10.1128/MCB.21.5.1621-1632.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lesurtel M, Graf R, Aleil B, Walther D, Tian Y, Jochum W, et al. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104–7. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- 62.Dalton SR, Lee SM, King RN, Nanji AA, Kharbanda KK, Casey CA, et al. Carbon tetrachloride-induced liver damage in asialoglycoprotein receptor-deficient mice. Biochem Pharmacol. 2009;77:1283–90. doi: 10.1016/j.bcp.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]