Abstract
Background and Purpose
Intravenous thrombolysis (IVT) is underutilized in ethnic minorities and women. In order to disentangle individual and system-based factors determining disparities in IVT use, we investigated race/sex differences in IVT utilization among hospitals serving varying proportions of minority patients.
Methods
Ischemic stroke admissions were identified from the Nationwide Inpatient Sample between 2007 and 2011. Hospitals were categorized based on the percentage of minority patients admitted with stroke (<25% minority patients [“white hospitals”], 25–50% minority patients [“mixed hospitals”], or >50% minority patients [“minority hospitals”]). Logistic regression was used to evaluate the association between race/sex and IVT use within and between the different hospital strata.
Results
Among 337,201 stroke admissions, white men had the highest odds of IVT among all race/sex groups in any hospital strata, and the odds of IVT for white men did not differ by hospital strata. For white women and minority men the odds of IVT were significantly lower in minority hospitals compared to white hospitals (OR 0.83, 95% CI 0.71–0.97, for white women; and OR 0.82, 95% CI 0.69–0.99, for minority men). Race disparities in IVT use among women were observed in white hospitals (OR 0.88, 95% CI 0.78–0.99, in minority compared to white women), but not in minority hospitals (OR 0.94, 95% CI 0.82–1.09). Sex disparities in IVT use were observed among whites, but not minorities.
Conclusion
Minority men and white women have significantly lower odds of IVT in minority hospitals compared to white hospitals. IVT use in white men does not differ by hospital strata.
Keywords: thrombolysis, disparities, sex differences, minority race
Introduction
Ischemic stroke is a leading cause of disability and mortality in the United States1,2. Minorities, particularly black and Hispanic individuals, have higher age-standardized stroke prevalence rates compared to their white counterparts, and ischemic stroke occurs at an earlier age in blacks compared to whites3–5. Similarly, life-time stroke incidence is higher among women compared to men, mainly because of increased longevity among women in the setting of higher stroke rates in older age groups3,6.
Clinical outcomes after ischemic stroke are consistently worse in minorities and women, in part due to differences in quality of care between different race and sex groups during stroke hospitalizations4,7–9. Intravenous thrombolysis (IVT), the cornerstone of acute stroke therapy associated with improved mortality and functional outcomes, is underutilized in minorities, even when only considering eligible patients presenting within 3 hours of onset of symptoms10–14. Similarly, analyses of data from single-center studies, registries, and administrative databases have found lower odds of IVT in women compared to men9,15–18, although considerable between-study variation exits19,20. The underlying reasons for these observed race and sex disparities are not known, but may include individual (patient and provider) factors, as well as system (hospital) factors.
Institutional and health system-related factors contributing to the observed race and sex differences in IVT utilization have been insufficiently explored. Some hospitals, especially those located in urban neighborhoods, serve a substantially higher proportion of minority patients, and may differ from hospitals serving predominantly white populations in their organizational structure, availability of equipment and specialists, and funding21. Access to and outcome after medical and surgical interventions differs between patients admitted to a hospital predominantly serving white patients and those predominantly serving minorities, i.e. in patients admitted for myocardial infarction or carotid stenosis22–24; however, it is unclear if there are differences in IVT utilization by race or sex within and between predominantly white versus predominantly minority hospitals.
In order to disentangle individual and system-based factors contributing to disparities in IVT use in the United States, we sought to determine whether IVT utilization differs by race and sex among hospitals serving varying proportions of minority patients. Identifying individual and hospital factors contributing to underutilization of IVT may aid in developing strategies to mitigate present race and sex disparities.
Methods
Data source
Data were obtained from the Nationwide Inpatient Sample (NIS), part of the Healthcare Cost and Utilization Project (HCUP), sponsored by the Agency for Healthcare Research and Quality25. The NIS is the largest all-payer inpatient database in the US, representing a 20% stratified sample of all admissions to non-federal US hospitals. NIS captures information regarding demographics, hospital characteristics, primary and secondary diagnoses and procedures, comorbidities, and case severity measures on several million hospital discharges each year. All diagnoses and procedures are recorded using International Classification of Diseases version 9 Clinical Modification (ICD9-CM) codes. Detailed information on the design and contents of the NIS is available at http://www.hcup-us.ahrq.gov. Because NIS data are publicly available and contains no personal identifying information, this study was exempt from institutional review board approval.
Case selection
For this retrospective cross-sectional analysis of discharge data from the NIS, we identified adult cases with primary diagnosis of ischemic stroke by using ICD9-CM codes 433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.01, 434.11, 434.91, and 436 between 2007 and 201126,27. We excluded elective admissions and patients enrolled in a clinical trial (ICD9-CM code V70.7). The unit of observation in NIS is discharge after hospitalization. Cases transferred from another hospital were excluded because IVT is typically administered at the hospital of initial presentation and to prevent double counting of the same patient. This algorithm has been shown to identify acute ischemic stroke with high sensitivity and specificity28–30. In addition, cases with missing information on race/ethnicity and sex, the primary exposures of interest, were excluded.
Primary exposures, hospital strata, and outcome of interest
The primary exposures of interest were self-reported ethnic minority race, sex, and race-sex interaction. Minority race included all patients self-identifying as black, Hispanic, Asian, or Other, by self-reporting. Among ischemic stroke admissions, we compared differences in IVT administration between white men, white women, minority men, and minority women within strata of hospital serving predominantly white or predominantly minority patients. Hospitals were stratified into 3 groups based on the proportion of minority patients: predominantly white hospitals (<25% minority stroke patients), mixed hospitals (25–25% minority stroke patients), and predominantly minority hospitals (>50% minority stroke patients). The primary outcome of interest was administration of IV thrombolysis as identified by ICD9-CM procedure code 99.10.
Comorbidity and severity adjustment
We calculated the modified Charlson comorbidity index, a weighted score of 17 different comorbidities validated for outcome adjustment for analyses of administrative data sets using ICD9-CM codes31,32, for each patient. This index has been validated for comorbidity adjustment via ICD-9 coding in ischemic stroke (Goldstein, Stroke, 2004). Case severity was determined using the All Patient Refined-Diagnosis Related Groups (APR-DRGs), a 4-point ordinal scale (minor, moderate, major, and extreme risk of mortality) derived from age, primary and secondary diagnoses, and procedures33. The APR-DRG algorithm is a validated and reliable indicator of mortality, and is commonly used as a severity indicator in studies relating to stroke34.
Statistical analysis
Clinical and hospital-level characteristics in the different hospital strata were compared using Chi2 for categorical variables and Kruskal-Wallis tests for continuous variables. Multivariable logistic regression was performed to determine the association of IVT with the four race-sex exposure groups of interest, and statistical interaction between race and sex was explored. Models were adjusted for age, hospital characteristics (teaching status, bed size, location, region, and annual volume of stroke cases), discharge quarter, weekend admission, modified Charlson Comorbidity Index, APR-DRG severity subclass, insurance status, median household income per patient’s ZIP code, hypertension, diabetes mellitus, dyslipidemia, coronary artery disease, peripheral vascular disease, atrial fibrillation, valvular disease, thrombocytopenia, alcohol abuse, drug abuse, and chronic kidney disease. Multicollinearity was assessed using variance inflation factors (VIFs), with values >5 suggestive of multicollinearity. Since the variable race had substantial missingness, sensitivity analysis including imputed values for race via multiple imputation via chained equations (MICE) was performed. We used a Generalized Estimation Equations (GEE) approach to account for clustering of patients within hospitals. Statistical analysis was performed using STATA version 13 (Stata Statistical Software: Release 13. College Station, TX). A p-value of <0.05 was considered statistically significant. 95% confidence intervals are reported.
Results
Patient characteristics
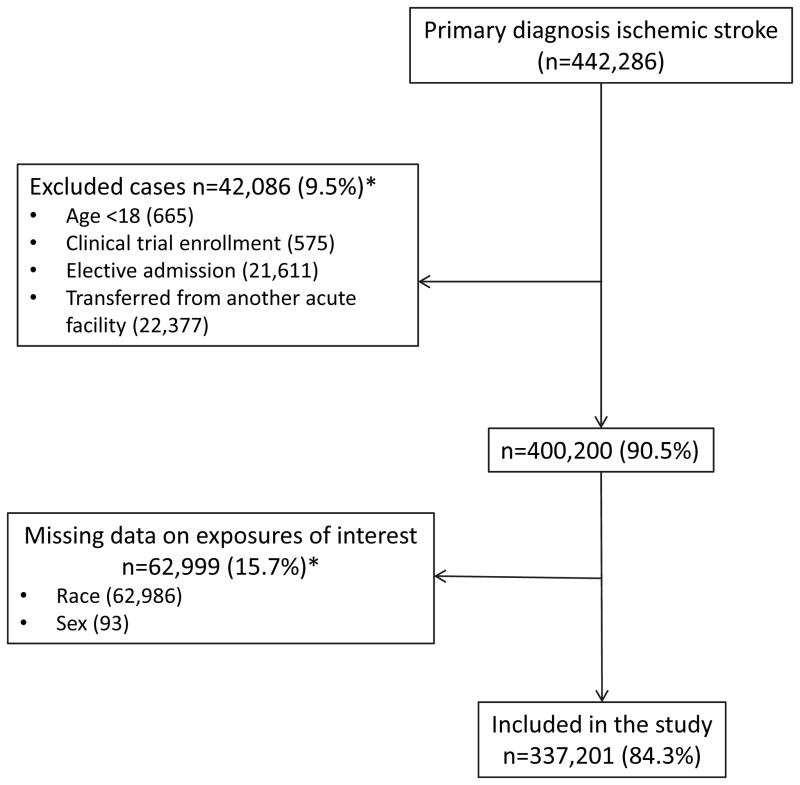
Among the 337,201 cases that met inclusion criteria (Figure 1), 176,389 (52.3%) were treated at predominantly white hospitals, 93,297 (27.7%) were treated at mixed hospitals, and 67,515 (20.0%) received care at hospitals serving predominantly minority stroke patients. Patient and hospital characteristics of subjects treated in hospitals with varying proportion of minority patients are summarized in Table 1. Baseline characteristics of patients by IVT use can be found in Supplemental Table I.
Figure 1.
Flow diagram indicating selection of the study population. *Not mutually exclusive.
Table 1.
Baseline characteristics of the study population stratified by hospitals with varying proportions of minority patients (n=337,201).
| Characteristics | White hospitals (<25% minorities) (n=176,389) | Mixed hospitals (25–50% minorities) (n=93,297) | Minority hospitals (>50% minorities) (n=67,515) | p-value |
|---|---|---|---|---|
| Age – years: median (IQR) | 76 (64–84) | 72 (60–82) | 69 (57–80) | <0.001 |
| Female – n (%) | 94,886 (53.8) | 49,625 (53.2) | 35,153 (52.1) | <0.001 |
| Race – n (%) | <0.001 | |||
| White | 156,240 (88.6) | 59,256 (63.5) | 19,882 (29.5) | |
| Black | 11,763 (6.7) | 20,671 (22.2) | 25,101 (37.2) | |
| Hispanic | 4,071 (2.3) | 7,401 (7.9) | 13,380 (19.8) | |
| Asian or Pacific Islander | 1,774 (1.0) | 3,086 (3.3) | 4,261 (6.3) | |
| Other | 2,541 (1.4) | 2,883 (3.1) | 4,891 (7.2) | |
| Primary expected payer – n (%) | <0.001 | |||
| Private Insurance | 32,521 (18.4) | 19,576 (21.0) | 12,936 (19.2) | |
| Medicare | 125,911 (71.4) | 58,894 (63.1) | 38,363 (56.8) | |
| Medicaid | 7,644 (4.3) | 6,475 (6.9) | 8,606 (12.8) | |
| Self-pay | 6,354 (3.6) | 5,119 (5.5) | 4,994 (7.4) | |
| No charge/other | 3,700 (2.1) | 2,971 (3.2) | 2,476 (3.7) | |
| Missing information | 259 (0.2) | 262 (0.3) | 140 (0.2) | |
| Median household income for patient ZIP code: quartiles – n (%) | <0.001 | |||
| Quartile 1 | 42,712 (24.2) | 25,931 (27.8) | 26,980 (40.0) | |
| Quartile 2 | 48,129 (27.3) | 22,033 (23.6) | 13,235 (19.6) | |
| Quartile 3 | 42,466 (24.1) | 21,164 (22.7) | 14,507 (21.5) | |
| Quartile 4 | 39,741 (22.5) | 22,160 (23.8) | 10,943 (16.2) | |
| Missing information | 3,341 (1.9) | 2,009 (2.2) | 1,850 (2.7) | |
| Weekend admission | 45,893 (26.0) | 24,218 (26.0) | 17,453 (25.9) | 0.697 |
| Missing information* | <10 (0.0) | <10 (0.0) | <10 (0.0) | |
| Hospital region – n (%) | 0.001 | |||
| Northeast | 43,705 (24.8) | 11,970 (12.8) | 15,072 (22.3) | |
| Midwest | 38,804 (22.0) | 6,962 (7.5) | 7,529 (11.2) | |
| South | 62,402 (35.4) | 52,383 (56.2) | 29,496 (43.7) | |
| West | 31,478 (17.8) | 21,982 (23.5) | 15,418 (22.8) | |
| Hospital location – n (%) | <0.001 | |||
| Rural | 29,974 (17.0) | 5,989 (6.4) | 4,368 (6.5) | |
| Urban | 144,998 (82.2) | 86,725 (93.0) | 61,936 (91.7) | |
| Missing information | 1,417 (0.8) | 583 (0.6) | 1,211 (1.8) | |
| Teaching Hospital – n (%) | 58,553 (33.2) | 44,560 (47.8) | 40,430 (59.9) | <0.001 |
| Missing information | 1,417 (0.8) | 583 (0.6) | 1,211 (1.8) | |
| Hospital bed size – n (%) | <0.001 | |||
| Small | 24,785 (14.1) | 8,391 (9.0) | 5,460 (8.1) | |
| Medium | 43,703 (24.8) | 21,590 (23.1) | 16,706 (24.7) | |
| Large | 106,484 (60.4) | 62,733 (67.2) | 44,138 (65.4) | |
| Missing information | 1,417 (0.8) | 583 (0.6) | 1,211 (1.8) | |
| Hospital stroke case volume (cases/yr) | <0.001 | |||
| Quartile 1 | 54,296 (30.8) | 16,825 (18.0) | 13,549 (20.1) | |
| Quartile 2 | 43,983 (24.9) | 22,185 (23.8) | 19,283 (28.6) | |
| Quartile 3 | 41,052 (23.3) | 26,881 (28.8) | 15,336 (22.7) | |
| Quartile 4 | 37,058 (21.0) | 27,406 (29.4) | 19,347 (28.7) | |
| Charlson comorbidity index | <0.001 | |||
| 1 | 50,569 (28.7) | 25,537 (27.4) | 17,333 (25.7) | |
| 2 | 40,060 (22.7) | 21,422 (23.0) | 15,693 (23.2) | |
| 3 | 37,355 (21.2) | 19,820 (21.2) | 13,875 (20.6) | |
| ≥4 | 48,405 (27.4) | 26,518 (28.4) | 20,614 (30.5) | |
| Hypertension – n (%) | 138,984 (78.8) | 75,156 (80.6) | 56,114 (83.1) | <0.001 |
| Diabetes Mellitus – n (%) | 55,157 (31.3) | 32,345 (34.7) | 26,927 (39.9) | <0.001 |
| Dyslipidemia – n (%) | 85,878 (48.7) | 45,381 (48.6) | 30,155 (44.7) | <0.001 |
| Coronary artery disease – n (%) | 28,680 (16.3) | 14,157 (15.2) | 9,726 (14.4) | <0.001 |
| Atrial fibrillation – n (%) | 43,422 (24.6) | 20,339 (21.8) | 11,892 (17.6) | <0.001 |
| Valvular disease – n (%) | 19,906 (11.3) | 9,302 (10.0) | 5,255 (7.8) | <0.001 |
| Peripheral vascular disease – n (%) | 16,809 (9.5) | 8,084 (8.7) | 5,134 (7.6) | <0.001 |
| Chronic kidney disease – n (%) | 21,382 (12.1) | 12,113 (13.0) | 9,298 (13.8) | <0.001 |
| Thrombocytopenia – n (%) | 3,195 (1.8) | 1,816 (2.0) | 1,220 (1.8) | 0.035 |
| Alcohol abuse – n (%) | 6,214 (3.5) | 3,700 (4.0) | 3,084 (4.6) | <0.001 |
| Drug abuse – n (%) | 2,254 (1.3) | 2,169 (2.3) | 2,645 (3.9) | <0.001 |
| APR-DRG: loss of function | <0.001 | |||
| Minor | 16,572 (9.4) | 8,789 (9.4) | 6,678 (9.9) | |
| Moderate | 86,985 (49.3) | 44,568 (47.8) | 32,619 (48.3) | |
| Major | 59,510 (33.7) | 31,254 (33.5) | 21,235 (31.5) | |
| Extreme | 13,313 (7.6) | 8,677 (9.3) | 6,980 (10.3) | |
| Missing information* | <10 (0.0) | <10 (0.0) | <10 (0.0) |
APR-DRG: all patient refined diagnosis-related group.
The HCUP data user agreement precludes reporting individual cell counts ≤10.
Minority race and female sex are associated with decreased odds of IVT
IVT rates overall were lower among minorities compared to whites (4.25% vs. 4.89%, p<0.001) and among women compared to men (4.44% vs. 5.00%, p<0.001). In multivariable models adjusted for other demographics, comorbidities and hospital characteristics, the odds of IVT was lower in minorities compared to whites, and women compared to men (Table 2). White men had the highest rate of IVT (5.30%), compared to 4.53% in white women, 4.31% in minority men, and 4.20% in minority women. In adjusted models, the difference in odds of IVT between minority and white patients was more pronounced among men compared to women (OR 0.74, 95% CI 0.68–0.80, in minority men compared to their white counterparts vs. OR 0.85, 95% CI 0.79–0.93, in minority women compared to white women, p-value for interaction 0.001; Table 2). Sex disparities in IVT administration were present among whites (OR 0.89, 95% CI 0.85–0.92, in white women compared to white men), but not in minorities (OR 1.02, 95% CI 0.95–1.09, in minority women compared to minority men; Table 2). Sensitivity analysis after multiple imputation of missing values for race yielded similar results as complete-case analysis with regard to effect size, direction, and statistical significance (Supplemental Table II).
Table 2.
Multivariable analysis of IVT use after stroke for race, sex, and race-sex interaction. Models were adjusted for sociodemographic factors (age, insurance status, median household income per ZIP code), hospital characteristics (teaching status, bed size, location, region, and annual volume of stroke cases), discharge quarter, weekend admission status, medical comorbidities and disease severity measures (hypertension, diabetes mellitus, dyslipidemia, coronary artery disease, peripheral vascular disease, atrial fibrillation, valvular disease, chronic kidney disease, thrombocytopenia, alcohol abuse, drug abuse, modified Charlson Comorbidity Index, and APR-DRG).
| Variable | OR | 95% CI | p-value | p for interaction |
|---|---|---|---|---|
| Race | ||||
| White | 1 (ref) | |||
| All Minorities | 0.80 | 0.74–0.85 | <0.001 | |
| Sex | ||||
| Men | 1 (ref) | |||
| Women | 0.92 | 0.89–0.96 | <0.001 | |
| Race/Sex | 0.001 | |||
| White Men | 1 (ref) | |||
| Minority Men | 0.74 | 0.68–0.80 | <0.001 | |
| White Women | 0.89 | 0.85–0.92 | <0.001 | |
| Minority Women | 0.76 | 0.70–0.82 | <0.001 |
IVT utilization by sex and race within strata of hospitals with varying proportion of minority patients
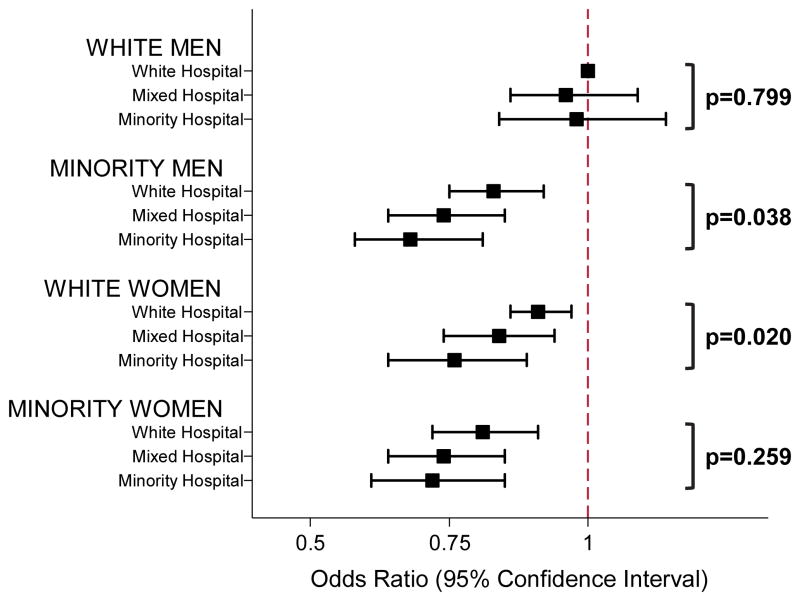
White men had the highest odds of IVT in all hospital strata (Table 3), and the odds of IVT among white men did not differ significantly by hospital strata (Table 4 and Figure 2). Compared to white men, the odds of IVT in white women was significantly lower in minority hospitals compared to white hospitals (Table 3). White women presenting to minority hospitals had 17% lower odds of IVT than those treated in white hospitals (95% CI 0.71–0.97, p=0.020; Table 4 and Figure 2). In hospitals caring predominantly for minorities, the odds of IVT were similarly lower for white women (OR 0.75, 95% CI 0.67–0.85), minority men (OR 0.72, 95% CI 0.62–0.83), and minority women (OR 0.74, 95% CI 0.64–0.86), when compared to white men (Table 3). There were no sex disparities among minorities in either hospital strata, but minority men and women had the lowest odds of IVT in any of the strata (Table 3). Among minority men the odds of IVT was significantly lower in minority hospitals compared to white hospitals (OR 0.69, 95% CI 0.59–0.81, vs. OR 0.84, 95% CI 0.75–0.93, p=0.038; Table 4 and Figure 2). In minority women, the odds of IVT did not significantly differ by hospital strata (Table 4; Figure 2). Compared to their white counterparts, minority women had significantly lower odds of IVT in white hospitals (OR 0.88, 95% CI 0.78–0.99; Table 4) and mixed hospitals (OR 0.88, 95% CI 0.79–0.97), but not minority hospitals (OR 0.94, 95% CI 0.82–1.09). Sensitivity analysis after multiple imputation of missing values for race revealed similar results (Supplemental Table III).
Table 3.
IVT utilization by race and sex, stratified by hospital.
| Variable | Adjusted OR (95% CI) of IVT | p-value* |
|---|---|---|
| White hospitals (<25% minorities) | ||
| White Men | 1 (ref) | |
| White Women | 0.92 (0.87–0.97) | |
| Minority Men | 0.83 (0.75–0.93) | 0.001 |
| Minority Women | 0.81 (0.72–0.92) | 0.046 |
| Mixed hospitals (25–50% minorities) | ||
| White Men | 1 (ref) | |
| White Women | 0.87 (0.81–0.94) | |
| Minority Men | 0.74 (0.66–0.83) | <0.001 |
| Minority Women | 0.75 (0.68–0.83) | <0.001 |
| Minority hospitals (>50% minorities) | ||
| White Men | 1 (ref) | |
| White Women | 0.75 (0.67–0.85) | |
| Minority Men | 0.72 (0.62–0.83) | <0.001 |
| Minority Women | 0.74 (0.64–0.86) | 0.840 |
P-value for race differences within sex strata, i.e. for comparison of minority men to white men, and minority women to white women within hospital strata.
Models were adjusted for sociodemographic factors (age, insurance status, median household income per ZIP code), hospital characteristics (teaching status, bed size, location, region, and annual volume of stroke cases), discharge quarter, weekend admission status, medical comorbidities and disease severity measures (hypertension, diabetes mellitus, dyslipidemia, coronary artery disease, peripheral vascular disease, atrial fibrillation, valvular disease, chronic kidney disease, thrombocytopenia, alcohol abuse, drug abuse, modified Charlson Comorbidity Index, and APR-DRG).
Table 4.
Odds of IVT administration within hospital categories for men, women, and men and women combined. Models were adjusted for sociodemographic factors (age, insurance status, median household income per ZIP code), hospital characteristics (teaching status, bed size, location, region, and annual volume of stroke cases), discharge quarter, weekend admission status, medical comorbidities and disease severity measures (hypertension, diabetes mellitus, dyslipidemia, coronary artery disease, peripheral vascular disease, atrial fibrillation, valvular disease, chronic kidney disease, thrombocytopenia, alcohol abuse, drug abuse, modified Charlson Comorbidity Index, and APR-DRG).
| Variable | OR (95% CI) in White hospitals (<25% minorities) | OR (95% CI) in Mixed hospitals (25–50% minorities) | OR (95% CI) in Minority hospitals (>50% minorities) |
|---|---|---|---|
| All men and women | |||
| White | 1 (ref) | 0.94 (0.84–1.05) | 0.90 (0.78–1.04) |
| Minority | 0.86 (0.79–0.93) | 0.77 (0.68–0.87) | 0.74 (0.63–0.86) |
| Men only | |||
| White | 1 (ref) | 0.96 (0.85–1.08) | 0.98 (0.84–1.14) |
| Minority | 0.84 (0.75–0.93) | 0.74 (0.64–0.85) | 0.69 (0.59–0.81) |
| Women only | |||
| White | 1 (ref) | 0.92 (0.82–1.04) | 0.83 (0.71–0.97) |
| Minority | 0.88 (0.78–0.99) | 0.81 (0.70–0.92) | 0.78 (0.66–0.93) |
Figure 2.
Graphic representation of odds ratios of IVT in hospitals with varying proportions of minority patients, stratified by race/sex. White hospital refers to hospitals with <25% minority stroke patients, mixed hospital refers to hospitals with 25–50% minority stroke patients, and minority hospital refers to hospitals with >50% minority stoke patients. White men in white hospitals are the reference. P-values compare odds of IVT in minority hospitals to white hospitals within each of the four race/sex strata.
Discussion
The underlying causes for race and sex disparities in IVT utilization after stroke are poorly understood. In the United States, there remains significant residential segregation by race resulting in clustering of minority patients in a relatively small number of “minority-serving” hospitals21,35,36. In the present study, we aimed to determine whether individual (patient or provider) or hospital factors best explain disparities in IVT administration by race and sex in a large administrative dataset. We found that minority men and white women have significantly lower odds of IVT in hospitals serving predominantly ethnic minority patients compared to hospitals serving predominantly whites, while IVT use in white men does not differ between minority vs. white hospitals.
Differences in quality of care and utilization of standard of care treatment may depend on a number of system factors, such as supply of specialists, technological capabilities, or practice patterns21, e.g. hospitals with vascular neurology consultation capacities have higher IVT rates that those who do not37. In our study, structural differences such as a lack of 24/7 access to a vascular neurology specialist in minority hospitals may in part explain the observed lower IVT rates for minority men and white women in minority compared to white hospitals. However, IVT rates for white men did not significantly differ between white and minority hospitals, indicating that minority hospitals in principle are capable of providing comparable care with regard to IVT administration, at least for a distinct subgroup of patients. Therefore, technological capabilities and structural differences are less likely to explain differences by sex and race within any of the hospital strata, as these should not differ within a given hospital (regardless of whether the hospital serves predominantly white or predominantly minority patients). Thus, the presence of differences in IVT administration rates between minority and white hospitals for some groups (minority men and white women), but not others (white men), suggests that individual factors on the provider or patient side may be the main determinants of IVT administration. Of note, the IVT rate in minority women did not significantly decrease with increasing proportions of minorities treated per hospital, however, this was mainly due to the already low rate of IVT in white hospitals.
Minority hospitals more commonly serve predominantly poor and underinsured populations21,21,38, and emergency department arrival time may be linked to low income and other markers of socioeconomic status39. Differences in arrival time and potential contraindications to IVT were not systematically captured in NIS. Although we adjusted for income and insurance status as surrogate markers of socioeconomic status, other variables related to arrival time not captured in NIS, such as health literacy, marital status, availability of transportation, or access to telephone, may differ between white women/minority men living in the vicinity of a predominantly white hospital and white women/minority men who live in close proximity to a hospital serving predominantly minority patients. Similarly, since NIS lacks information on factors determining individual provider decision-making, we were unable to determine whether implicit bias among providers may in part explain the disparities observed in our study, as has been shown for thrombolysis decisions in clinical vignettes of patients with myocardial infarction40.
Despite accounting for hospital stroke case volume and other hospital characteristics, we acknowledge that other process of care measures not accounted for in our analysis, such as door-to-physician times or door-to-imaging times, may differ between predominantly minority versus white hospitals, and explain some of the observed differences41–43. In addition to the above mentioned limitations, our analysis is limited by the potential for miscoded and missing data in large administrative datasets reliant on ICD9-CM coding; however, it is unlikely that there is differential miscoding of diagnoses or procedures by race or sex. To address missingness for race, we performed a sensitivity analysis after multiple imputation for the race variable. While race is typically self-reported, it is possible that information captured in the race variable is not entirely accurate. We attempted to mitigate the absence of clinical and physiological stroke data in NIS by adjusting all regression models for the modified Charlson Comorbidity Index, a validated measure of patient comorbidities in ischemic stroke and ICH32,44. Other limitations include the lack of information on patient language, and provider attitudes or behaviors.
Despite these limitations, our data suggest that IVT is systematically underutilized in white women, and minority men and women, particularly in hospital settings predominantly serving minorities. Differences between hospital strata were only present for women and minorities but not for white men, suggesting that individual more than system and process of care determinants may explaining the observed disparities in IVT use. The identification of specific subpopulations in which IVT is underutilized provides a unique opportunity to implement interventions aimed at mitigating disparities. Strategies improving IVT utilization may best be directed specifically towards non-white/-male patients in hospitals serving minorities and their providers. Resources addressing access to care, health literacy, patient-provider communication, provider and health system cultural competency, implicit racial bias, and quality-of-care for acute stroke may reduce the observed disparities. Further investigation of factors determining systemic differences in delivery of stroke care between predominantly minority and predominantly white hospitals may allow targeted allocation of resources, and serve to develop effective approaches to mitigate and eventually eliminate disparities in IVT utilization across the US.
Supplementary Material
Acknowledgments
Sources of Funding
Dr Faigle is supported by an institutional KL2 grant from the Johns Hopkins Institute for Clinical and Translational Research (ICTR), which is funded in part by Grant Number KL2TR001077 from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Dr. Cooper is supported by a grant from the National Heart, Lung, and Blood Institute (K24HL083113).
Footnotes
Disclosures
Dr. Gottesman is an Associate Editor for Neurology. The authors report no other conflicts of interest.
References
- 1.Lawrence ES, Coshall C, Dundas R, Stewart J, Rudd AG, Howard R, et al. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke. 2001;32:1279–1284. doi: 10.1161/01.str.32.6.1279. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 4.Stansbury JP, Jia H, Williams LS, Vogel WB, Duncan PW. Ethnic disparities in stroke: epidemiology, acute care, and postacute outcomes. Stroke. 2005;36:374–386. doi: 10.1161/01.STR.0000153065.39325.fd. [DOI] [PubMed] [Google Scholar]
- 5.Morgenstern LB, Smith MA, Lisabeth LD, Risser JM, Uchino K, Garcia N, et al. Excess stroke in Mexican Americans compared with non-Hispanic Whites: the Brain Attack Surveillance in Corpus Christi Project. Am J Epidemiol. 2004;160:376–383. doi: 10.1093/aje/kwh225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, et al. The lifetime risk of stroke: estimates from the Framingham Study. Stroke. 2006;37:345–350. doi: 10.1161/01.STR.0000199613.38911.b2. [DOI] [PubMed] [Google Scholar]
- 7.Schwamm LH, Reeves MJ, Pan W, Smith EE, Frankel MR, Olson D, et al. Race/ethnicity, quality of care, and outcomes in ischemic stroke. Circulation. 2010;121:1492–1501. doi: 10.1161/CIRCULATIONAHA.109.881490. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs BS, Birbeck G, Mullard AJ, Hickenbottom S, Kothari R, Roberts S, et al. Quality of hospital care in African American and white patients with ischemic stroke and TIA. Neurology. 2006;66:809–814. doi: 10.1212/01.wnl.0000203335.45804.72. [DOI] [PubMed] [Google Scholar]
- 9.Reeves MJ, Fonarow GC, Zhao X, Smith EE, Schwamm LH Get With The Guidelines-Stroke Steering Committee & Investigators. Quality of care in women with ischemic stroke in the GWTG program. Stroke. 2009;40:1127–1133. doi: 10.1161/STROKEAHA.108.543157. [DOI] [PubMed] [Google Scholar]
- 10.Aparicio HJ, Carr BG, Kasner SE, Kallan MJ, Albright KC, Kleindorfer DO, et al. Racial Disparities in Intravenous Recombinant Tissue Plasminogen Activator Use Persist at Primary Stroke Centers. J Am Heart Assoc. 2015;4:e001877. doi: 10.1161/JAHA.115.001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimball MM, Neal D, Waters MF, Hoh BL. Race and income disparity in ischemic stroke care: nationwide inpatient sample database, 2002 to 2008. J Stroke Cerebrovasc Dis. 2014;23:17–24. doi: 10.1016/j.jstrokecerebrovasdis.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Kumar N, Khera R, Pandey A, Garg N. Racial Differences in Outcomes after Acute Ischemic Stroke Hospitalization in the United States. J Stroke Cerebrovasc Dis. 2016;25:1970–1977. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.049. [DOI] [PubMed] [Google Scholar]
- 13.Hsia AW, Edwards DF, Morgenstern LB, Wing JJ, Brown NC, Coles R, et al. Racial disparities in tissue plasminogen activator treatment rate for stroke: a population-based study. Stroke. 2011;42:2217–2221. doi: 10.1161/STROKEAHA.111.613828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messe SR, Khatri P, Reeves MJ, Smith EE, Saver JL, Bhatt DL, et al. Why are acute ischemic stroke patients not receiving IV tPA? Results from a national registry. Neurology. 2016 doi: 10.1212/WNL.0000000000003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng YZ, Reeves MJ, Jacobs BS, Birbeck GL, Kothari RU, Hickenbottom SL, et al. IV tissue plasminogen activator use in acute stroke: experience from a statewide registry. Neurology. 2006;66:306–312. doi: 10.1212/01.wnl.0000196478.77152.fc. [DOI] [PubMed] [Google Scholar]
- 16.Reid JM, Dai D, Gubitz GJ, Kapral MK, Christian C, Phillips SJ. Gender differences in stroke examined in a 10-year cohort of patients admitted to a Canadian teaching hospital. Stroke. 2008;39:1090–1095. doi: 10.1161/STROKEAHA.107.495143. [DOI] [PubMed] [Google Scholar]
- 17.Schumacher HC, Bateman BT, Boden-Albala B, Berman MF, Mohr JP, Sacco RL, et al. Use of thrombolysis in acute ischemic stroke: analysis of the Nationwide Inpatient Sample 1999 to 2004. Ann Emerg Med. 2007;50:99–107. doi: 10.1016/j.annemergmed.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Asdaghi N, Romano JG, Wang K, Ciliberti-Vargas MA, Koch S, Gardener H, et al. Sex Disparities in Ischemic Stroke Care: FL-PR CReSD Study (Florida-Puerto Rico Collaboration to Reduce Stroke Disparities) Stroke. 2016;47:2618–2626. doi: 10.1161/STROKEAHA.116.013059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reeves M, Bhatt A, Jajou P, Brown M, Lisabeth L. Sex differences in the use of intravenous rt-PA thrombolysis treatment for acute ischemic stroke: a meta-analysis. Stroke. 2009;40:1743–1749. doi: 10.1161/STROKEAHA.108.543181. [DOI] [PubMed] [Google Scholar]
- 20.Reeves MJ, Wilkins T, Lisabeth LD, Schwamm LH. Thrombolysis treatment for acute stroke: issues of efficacy and utilization in women. Womens Health (Lond) 2011;7:383–390. doi: 10.2217/whe.11.31. [DOI] [PubMed] [Google Scholar]
- 21.Baicker K, Chandra A, Skinner JS. Geographic variation in health care and the problem of measuring racial disparities. Perspect Biol Med. 2005;48:S42–53. [PubMed] [Google Scholar]
- 22.Skinner J, Chandra A, Staiger D, Lee J, McClellan M. Mortality after acute myocardial infarction in hospitals that disproportionately treat black patients. Circulation. 2005;112:2634–2641. doi: 10.1161/CIRCULATIONAHA.105.543231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucas FL, Stukel TA, Morris AM, Siewers AE, Birkmeyer JD. Race and surgical mortality in the United States. Ann Surg. 2006;243:281–286. doi: 10.1097/01.sla.0000197560.92456.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joynt KE, Orav EJ, Jha AK. Thirty-day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305:675–681. doi: 10.1001/jama.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steiner C, Elixhauser A, Schnaier J. The healthcare cost and utilization project: an overview. Eff Clin Pract. 2002;5:143–151. [PubMed] [Google Scholar]
- 26.Goldstein LB. Accuracy of ICD-9-CM coding for the identification of patients with acute ischemic stroke: effect of modifier codes. Stroke. 1998;29:1602–1604. doi: 10.1161/01.str.29.8.1602. [DOI] [PubMed] [Google Scholar]
- 27.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke. 2005;36:1776–1781. doi: 10.1161/01.STR.0000174293.17959.a1. [DOI] [PubMed] [Google Scholar]
- 29.Tirschwell DL, Longstreth WT., Jr Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. doi: 10.1161/01.str.0000032240.28636.bd. [DOI] [PubMed] [Google Scholar]
- 30.Jones SA, Gottesman RF, Shahar E, Wruck L, Rosamond WD. Validity of hospital discharge diagnosis codes for stroke: the Atherosclerosis Risk in Communities Study. Stroke. 2014;45:3219–3225. doi: 10.1161/STROKEAHA.114.006316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 32.Bar B, Hemphill JC., 3rd Charlson comorbidity index adjustment in intracerebral hemorrhage. Stroke. 2011;42:2944–2946. doi: 10.1161/STROKEAHA.111.617639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards N, Honemann D, Burley D, Navarro M. Refinement of the Medicare diagnosis-related groups to incorporate a measure of severity. Health Care Financ Rev. 1994;16:45–64. [PMC free article] [PubMed] [Google Scholar]
- 34.Xian Y, Holloway RG, Pan W, Peterson ED. Challenges in assessing hospital-level stroke mortality as a quality measure: comparison of ischemic, intracerebral hemorrhage, and total stroke mortality rates. Stroke. 2012;43:1687–1690. doi: 10.1161/STROKEAHA.111.648600. [DOI] [PubMed] [Google Scholar]
- 35.Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL. Primary care physicians who treat blacks and whites. N Engl J Med. 2004;351:575–584. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- 36.Jha AK, Stone R, Lave J, Chen H, Klusaritz H, Volpp K. The concentration of hospital care for black veterans in Veterans Affairs hospitals: implications for clinical outcomes. J Healthc Qual. 2010;32:52–61. doi: 10.1111/j.1945-1474.2010.00085.x. [DOI] [PubMed] [Google Scholar]
- 37.Prabhakaran S, McNulty M, O’Neill K, Ouyang B. Intravenous thrombolysis for stroke increases over time at primary stroke centers. Stroke. 2012;43:875–877. doi: 10.1161/STROKEAHA.111.640060. [DOI] [PubMed] [Google Scholar]
- 38.Haider AH, Ong’uti S, Efron DT, Oyetunji TA, Crandall ML, Scott VK, et al. Association between hospitals caring for a disproportionately high percentage of minority trauma patients and increased mortality: a nationwide analysis of 434 hospitals. Arch Surg. 2012;147:63–70. doi: 10.1001/archsurg.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kleindorfer DO, Lindsell CJ, Broderick JP, Flaherty ML, Woo D, Ewing I, et al. Community socioeconomic status and prehospital times in acute stroke and transient ischemic attack: do poorer patients have longer delays from 911 call to the emergency department? Stroke. 2006;37:1508–1513. doi: 10.1161/01.STR.0000222933.94460.dd. [DOI] [PubMed] [Google Scholar]
- 40.Green AR, Carney DR, Pallin DJ, Ngo LH, Raymond KL, Iezzoni LI, et al. Implicit bias among physicians and its prediction of thrombolysis decisions for black and white patients. J Gen Intern Med. 2007;22:1231–1238. doi: 10.1007/s11606-007-0258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gargano JW, Wehner S, Reeves MJ. Do presenting symptoms explain sex differences in emergency department delays among patients with acute stroke? Stroke. 2009;40:1114–1120. doi: 10.1161/STROKEAHA.108.543116. [DOI] [PubMed] [Google Scholar]
- 42.Karve SJ, Balkrishnan R, Mohammad YM, Levine DA. Racial/ethnic disparities in emergency department waiting time for stroke patients in the United States. J Stroke Cerebrovasc Dis. 2011;20:30–40. doi: 10.1016/j.jstrokecerebrovasdis.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Lacy CR, Suh DC, Bueno M, Kostis JB. Delay in presentation and evaluation for acute stroke: Stroke Time Registry for Outcomes Knowledge and Epidemiology (S.T.R.O.K.E.) Stroke. 2001;32:63–69. doi: 10.1161/01.str.32.1.63. [DOI] [PubMed] [Google Scholar]
- 44.Goldstein LB, Samsa GP, Matchar DB, Horner RD. Charlson Index comorbidity adjustment for ischemic stroke outcome studies. Stroke. 2004;35:1941–1945. doi: 10.1161/01.STR.0000135225.80898.1c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.