Abstract
Context:
Dehydroepiandrosterone (DHEA) and T hormones are advertised as antiaging, antiobesity products. However, the evidence that these hormones have beneficial effects on adipose tissue metabolism is limited.
Objective:
The objective of the study was to determine the effect of DHEA and T supplementation on systemic lipolysis during a mixed-meal tolerance test (MMTT) and an iv glucose tolerance test (IVGTT).
Design:
This was a 2-year randomized, double-blind, placebo-controlled trial.
Setting:
The study was conducted at a general clinical research center.
Participants:
Sixty elderly women with low DHEA concentrations and 92 elderly men with low DHEA and bioavailable T concentrations participated in the study.
Interventions:
Elderly women received 50 mg DHEA (n = 30) or placebo (n = 30). Elderly men received 75 mg DHEA (n = 30), 5 mg T (n = 30), or placebo (n = 32).
Main Outcome Measures:
In vivo measures of systemic lipolysis (palmitate rate of appearance) during a MMTT or IVGTT.
Results:
At baseline there was no difference in insulin suppression of lipolysis measured during MMTT and IVGTT between the treatment groups and placebo. For both sexes, a univariate analysis showed no difference in changes in systemic lipolysis during the MMTT or IVGTT in the DHEA group and T group when compared with placebo. There was no change in the results after adjusting for the resting energy expenditure, except for a small, but significant (P = .03) lowering of MMTT nadir palmitate rate of appearance in women who received DHEA.
Conclusion:
In elderly individuals with concentrations of DHEA (men and women) or T (men) below the normal range for young adults, supplementation of these hormones has no effect on insulin suppression of systemic lipolysis.
We evaluated the effects of supplementation with DHEA (in elderly men and women) and testosterone (in elderly men) on postprandial or iv insulin suppression of lipolysis. We found no effect of these hormones on systemic lipolysis.
The production of the androgens dehydroepiandrosterone (DHEA) and T declines with aging (1–3). Whether this decline has detrimental consequences to overall health is unknown, but DHEA and T are being used to improve muscle mass/strength and reduce body fat. There are well-documented, sex-specific associations between androgen levels and body fat distribution; however, association does not mean causality. Hence, replacement of androgens in elderly men and women who have plasma concentrations of DHEA and T below the normal range for young adults may not improve fat distribution or adipose function.
The studies that tested the effects of androgens on body composition, adipose metabolism, adipocyte differentiation, and lipolysis have given inconsistent results (4–12). Several human studies report that DHEA and T stimulate lipolysis (8, 12, 13).
We conducted a randomized, double-blind, placebo-controlled trial to evaluate the effects on body composition, quality of life, glucose, and fat metabolism of DHEA replacement in elderly men and women and T replacement in elderly men, with low concentrations of these hormones. We found no significant, clinically relevant effects on body composition, quality of life, and insulin sensitivity after 24 months of supplementation with DHEA or T (14, 15). The group that received T had an increase in meal fatty acid storage in the sc abdominal adipose tissue (AT) compared with femoral sc AT (16). Although we could not detect an effect of DHEA or T on postabsorptive lipolysis (16), we cannot infer the same for postprandial lipolysis. Furthermore, the differences in meal fatty acid storage previously reported (16) could be offset by androgen-induced changes in insulin suppression of lipolysis in the postprandial state. As part of this 2-year study of DHEA and T replacement in elderly men and women, we collected data to test the hypotheses that T or DHEA supplementation had an effect on systemic lipolysis during a mixed-meal tolerance test (MMTT) or an insulin-modified iv glucose tolerance test (IVGTT).
Materials and Methods
Herein we report pre- and postintervention postprandial and insulin-suppressed free fatty acid (FFA) data from 135 elderly adults (>60 y) who participated in a published trial (14) assessing the effect of 2 years of placebo vs supplementation with DHEA or T on body composition, glucose metabolism, and bone density. This study was approved by the Mayo Institutional Review Board. Participants providing informed consent included 60 elderly women with sulfated DHEA levels less than 0.95 μg/mL and 92 elderly men with bioavailable T levels less than 103 ng/dL and sulfated DHEA levels less than 1.57 μg/mL. Bioavailable T levels of less than 103 ng/dL correspond to levels less than 2 SD of the mean, normal concentration for young men, and DHEA levels less than 1.57 μg/mL, corresponding to levels less than 1.5 SD of the mean, normal concentration for young men (20–30 y of age).
Women were randomly assigned to receive DHEA (n = 30) or placebo (n = 30), and men were randomly assigned to receive DHEA (n = 30), T (n = 30), or placebo (n = 32) (14). T was given as a 5-mg patch applied daily and thus resulted in continuous delivery of T to the men. DHEA was taken as tablets each morning, 50 mg for women and 75 mg for men. The FFA flux study procedures have been previously described in detail (16, 17). Briefly, participants underwent outpatient and inpatient visits at baseline and 24 months after replacement with placebo, DHEA, or T. The outpatient visits included dual-energy x-ray absorptiometry and single-slice abdominal computed tomography for body composition. The participants underwent two inpatient visits to measure the suppression of lipolysis in response to a meal and an iv insulin profile. They first underwent an insulin-modified IVGTT followed by a second inpatient visit for MMTT. We used a continuous infusion of [9,10-3H]palmitate (0.3 μCi/min) during both IVGTT and MMTT to assess palmitate kinetics. The details of the protocol used for IVGTT and MMTT have been published (17). The medications (T patch, DHEA, and placebo) were continued during the inpatient visits and were given each morning. Of the initially randomized volunteers, eight elderly women and nine elderly men did not complete the studies of palmitate kinetics.
During these studies palmitate concentration and specific activity were measured at baseline (before the meal or iv insulin) and every 30 minutes for 3 hours during the MMTT and at 20, 31, 45, 90, and 120 minutes during the IVGTT. Suppression of lipolysis during either test is reported as the nadir palmitate rate of appearance (Ra) or the area under the curve (AUC) for the palmitate Ra. The AUC for palmitate concentration and AUC for Ra was the AUC for these parameters estimated throughout the entire duration of the MMTT or IVGTT.
The techniques used for each of the hormone concentration, substrate, and palmitate assays have been previously reported (16, 17). Nonsteady-state formulas were used to calculate palmitate Ra for the postprandial and IVGTT studies (18).
The methods used to measure hormone levels are described below.
Total T was measured using a competitive chemiluminescent immunoassay on the DxI 800 automated immunoassay system (Beckman Instruments). Intraassay coefficients of variation (CVs) are 6.5% at 69 ng/dL and 3.3% at 862 ng/dL. Interassay CVs are 7.4% at 116 ng/dL, 8.6% at 407 ng/dL, and 4.0% at 761 ng/dL.
Bioavailable T was measured by the differential precipitation of SHBG by ammonium sulfate after the equilibration of the serum sample with tracer amounts of tritium-labeled T. The results are expressed as the percentage of T free or albumin bound (not precipitated with SHBG) compared with an albumin standard. The product of this percentage and the total T measurement is the total bioavailable T. Intraassay CV was 5.4 at 14.1% of bioavailable T. Interassay CVs are 7.5 at 28.4%, 4.9 at 41.9%, and 4.9 at 50.8% of bioavailable T.
SHBG was measured by a solid phase, two-site chemiluminescent assay on the Siemens Immulite 2000 automated immunoassay system (Siemens Healthcare Diagnostics). Intraassay CVs are 2.7% and 3.1% at 5.5 and 95.9 nmol/L, respectively. Interassay CVs are 4.0% at 5.4 and 5.9% at 74 nmol/L.
DHEA sulfate was measured by a solid-phase, competitive chemiluminescent enzyme immunoassay (EIA) on the Siemens Immulite 2000 automated immunoassay system (Siemens Healthcare Diagnostics). The intraassay CVs are 5.7%, 5.7%, 5.7%, and 5.1% at 26.4, 77, 203, and 493 μg/dL. The interassay CVs are 5.2%, 3.6%, and 5.4% at 80.1, 211, and 515 μg/dL, respectively.
Statistical analysis
Baseline characteristics and descriptive data are presented as median (interquartile range) for continuous variables and proportion for categorical variables. Differences in baseline characteristics between the treatment groups was determined by an ANOVA. The Pearson coefficient was used for the correlation of continuous variables when normally distributed, and the Spearman coefficient was used when the data were not normally distributed. The Kruskal-Wallis test was used to analyze the differences in the palmitate concentration or palmitate Ra (expressed as either nadir levels or AUC) between the treatments groups by sex. A multiple linear regression analysis was used to adjust for potential confounders for the relationship between the suppression of lipolysis and treatment received. Two-sided statistical tests and an alpha <.05 was used to determine statistical significance for all analyses. All analyses were performed using JMP software (version 10.0.0; SAS Institute, Cary, NC, USA).
Sample size
Because the effect on T and DHEA on the suppression of lipolysis was not the primary outcome related to fatty acid metabolism of this study, we did not perform power calculations for this analysis a priori. To estimate the statistical power, we assessed the minimal statistically significant difference we could detect between the groups based on our sample size. We used data from previous studies to develop post hoc power calculations. Under conditions comparable with these experiments, we reported that the intrasubject variability of overnight postabsorptive palmitate Ra was 14% ± 8% (19); when insulin is given, it is 8% ± 4% (20). In previous animal and in vitro studies, the change of lipolysis after replacement with androgens varies from 35% to 80% (8, 13, 21). With a sample size of 26 per group, a power of 80%, and a significance level of 0.05, we could have detected a difference in nadir palmitate Ra of 10 μmol/min. Based upon the literature, differences less than 10 μmol/min are unlikely to be clinically relevant, considerably larger differences exist between insulin-sensitive and insulin-resistant states (22, 23).
Palmitate Ra was expressed in micromoles per minute and adjusted for resting energy expenditure (REE) for statistical analysis. Some investigators express free fatty acid flux per kilogram of fat-free mass; however, we have previously demonstrated that fat-free mass does not correlate with palmitate Ra in adult men and women (19), whereas REE is the best predictor and positively correlates with free fatty acid flux (16, 19, 24).
Results
Baseline characteristics of the participants who completed both pre- and posttreatment FFA flux studies are presented in Table 1. There were no significant differences in weight, age, body composition, baseline levels of sulfated DHEA (DHEAS), total T, or bioavailable T between elderly men in the three treatment groups or elderly women in the two groups. The baseline median DHEA levels were 0.6 and 0.3 μg/mL for men and women, respectively, and increased to an average of approximately 4 μg/mL in both groups assigned to DHEA (Table 2). The baseline median total T levels in men assigned to T was 362 ng/dL and increased to 494 ng/dL 24 months after therapy (14) (Table 2). There was no statistically significant difference in T or DHEA concentrations measured throughout the study in the groups assigned to placebo. After 2 years of hormone supplementation, there was a small increase in fat-free mass for elderly men on T (14) but no other significant changes in body weight or body composition between the groups.
Table 1.
Baseline Characteristics of the 135 Elderly Men and Women Who Participated in the Lipolysis Studies
| Characteristic | Elderly Women |
Elderly Men |
|||
|---|---|---|---|---|---|
| Placebo (n = 27) | DHEA (n = 25) | Placebo (n = 29) | DHEA (n = 28) | T (n = 26) | |
| Age, y | 69 (65–75) | 68 (66–69) | 67 (64–73) | 69 (67–72) | 66 (62–72) |
| Weight, kg | 74 (65–80) | 70 (59–79) | 86 (79–98) | 84 (74–92) | 86 (78–92) |
| BMI, kg/m2 | 28.3 (26–30.4) | 26.6 (24.7–29.5) | 27.4 (26–29.8) | 27 (24.4–29.1) | 28.2 (25.7–30.2) |
| Body fat, % | 46 (41–48) | 44 (40–49) | 30 (25–34) | 27 (23–31) | 28 (25–33) |
| Fat-free mass, kg | 37.9 (35.5–40.1) | 36 (34–39.4) | 58.2 (53.6–61.8) | 56.2 (52.1–60.4) | 56.1 (53.8–60.6) |
| REE, kcal/d | 1318 (1241–1442) | 1301 (1206–1401) | 1662 (1589–1811) | 1635 (1524–1719) | 1645 (1527–1802) |
| Serum triglycerides, mg/dL | 123 (99–145) | 121 (68–144) | 112 (78–150) | 103 (74–138) | 127 (87–154) |
Abbreviation: BMI, body mass index. Data are expressed as median (interquartile range).
Table 2.
DHEA, SHBG, and T Values at Baseline and After 2 Years of Supplementation With DHEA or T
| Younga |
Elderly Females |
Elderly Males |
|||||
|---|---|---|---|---|---|---|---|
| Hormone Values | F | M | Placebo | DHEA | Placebo | DHEA | T |
| SHBG, nmol/L | |||||||
| Baseline | 72.2 (45.6, 144.5)b | 23.4 (19, 29)c | 50 (36.6, 73.8) | 52.4 (34.9, 71.4) | 41.7 (35.1, 50.8) | 33.1 (28.5, 36.8) | 36.8 (30.2, 40.8) |
| Year 2 | 54.2 (37, 67.7) | 37.8 (24.9, 45.9) | 41.2 (36.6, 49.4) | 31.2 (26.4, 36.6) | 36.6 (31.3, 44.4) | ||
| Changed | −1 (−8.5, 5.4) | −13.6 (−27.2, −4.5)e | 0.4 (−5, 6) | −2.4 (−5, 2.6)f | −0.2 (−5.1, 3.4)f | ||
| DHEAS, μg/mL | |||||||
| Baseline | 1.4 (1, 2)b | 2.75 (1.9, 3.7)c | 0.3 (0.3, 0.4) | 0.3 (0.3, 0.5) | 0.62 (0.42, 1) | 0.65 (0.4, 0.9) | 0.6 (0.3, 0.9) |
| Year 2 | 0.3 (0.3, 0.4) | 3.66 (2.7, 6) | 0.55 (0.3, 0.8) | 3.96 (3.1, 6.3) | 0.5 (0.3, 0.7) | ||
| Changed | 0 (−0.04, 0) | 3.9 (2.9, 5)g | −0.1 (−0.2, 0.02) | 3.9 (2.8, 5.3)e | −0.07 (−0.17, 0) | ||
| Total T, ng/dL | |||||||
| Baseline | 543 (470, 675)c | 387 (294, 460) | 373.5 (254, 440) | 362 (295, 459) | |||
| Year 2 | 383 (331, 527) | 348 (276, 451) | 494 (421, 637) | ||||
| Changed | 24 (−14.5, 67.5) | 1.5 (-23, 32.8) | 111 (39, 252)e | ||||
| Bioavailable T, ng/dL | |||||||
| Baseline | 174.4 (125, 238)c | 52.7 (43.6, 63.6) | 63.5 (51.6, 76.5) | 57.1 (45.7, 65.7) | |||
| Year 2 | 52.9 (38.6, 65.1) | 65.3 (53.4, 88.1) | 82.8 (65, 115.1) | ||||
| Changed | −0.78 (−7.7, 11.6) | 3.5 (−5.7, 19) | 30.5 (−0.9, 62.5)h | ||||
Abbreviations: F, female; M, male. Data are expressed as median (interquartile range).
Thirty-eight healthy young women and 37 healthy young men baseline hormonal values obtained compared with values in the elderly groups.
P < .001 young females vs elderly females at baseline.
P < .001 young males vs elderly males at baseline.
Change in hormone levels (year 2 − baseline).
P < .001 vs placebo.
No significant change compared with placebo.
P < .0001 vs placebo.
P < .01 vs placebo.
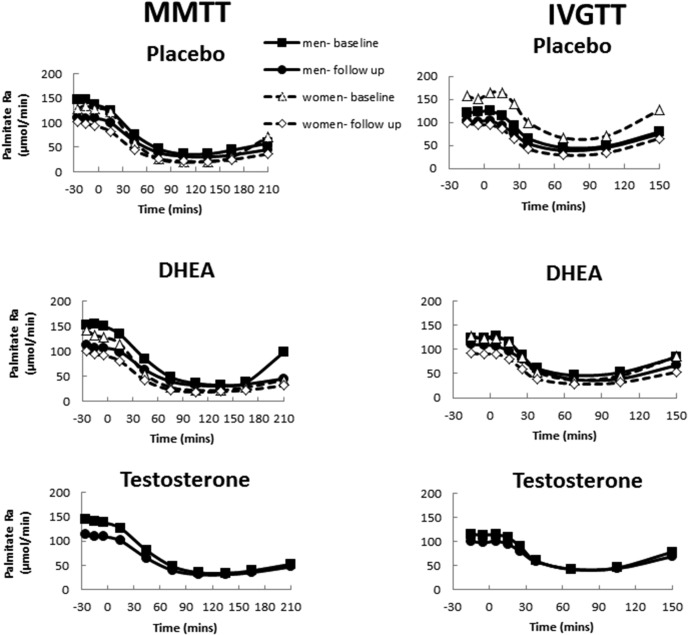
The pre- and posttreatment palmitate Ra responses to the MMTT and the IVGTT for the placebo groups are shown in the top panels of Figure 1. The palmitate Ra responses to DHEA (men and women) and T (men) before and after treatment are depicted in the middle and bottom panels of Figure 1, respectively. We found an overall trend for decrease in the nadir palmitate Ra at 24 months during the MMTT and IVGTT in men and women in all groups (Table 3). There was a median decrease in MMTT nadir palmitate Ra of 4 μmol/min (−2, 10 μmol/min) and 1 μmol/min (−3, 6 μmol/min) for men and women, respectively. The IVGTT nadir palmitate rate of appearance decreased by a median of 5 μmol/min (−9, 17) for men and 7 μmol/min (−3, −14) for women. Although most of these changes were statistically significant (except for women after a mixed meal), the absolute changes were quite small of unknown physiological relevance.
Figure 1. Palmitate Ra at baseline and after 2 years of hormone replacement (follow-up) according to treatment group during the MMTT (left panel) and during the IVGTT (right panel).
Table 3.
Change in Systemic Lipolysis at 24 Months in Men and Women According to Treatment Group
| Men |
Women |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Change in Palmitate Ra (Baseline to 24 mo) | Placebo | DHEA | T | P Valuea | P Valueb | Placebo | DHEA | P Valuea | P Valueb |
| MMTT | |||||||||
| Nadir Ra, μmol/min | 4 (−2, 8) | 3 (−7, 11) | 3 (−2, 12) | .82 | .51 D .65 T |
1 (−4, 4) | 3 (−2, 10) | .16 | .03 |
| AUC Ra, μmol/min | 1924 (93, −3824) | 1341 (419, 4165) | 1722 (−400, 3704) | .92 | .92 D .79 T |
509 (−329, 1713) | 780 (−518, 3040) | .46 | .09 |
| IVGTT | |||||||||
| Nadir Ra, μmol/min | 4 (−7, 16) | 8 (−8, 18) | 4 (−11, 17) | .74 | .59 D .57 T |
8 (−3, 14) | 5 (−3, 14) | 1.00 | .71 |
| AUC Ra, μmol/min | 794 (−1048, 2622) | 1470 (−901, 2239) | 1029 (−1395, 2357) | .88 | .3 D .62 T |
1443 (−139, 3618) | 1536 (−301, 3186) | .63 | .65 |
Abbreviations: D, P value of the DHEA group compared with placebo; T, P value of the T group compared with placebo; Nadir Ra, nadir palmitate rate of appearance; AUC Ra, area under the curve of palmitate rate of appearance. Data are expressed as median (interquartile range).
Unadjusted P value for any difference between the three groups in men and two groups in women.
Adjusted P value for change in REE.
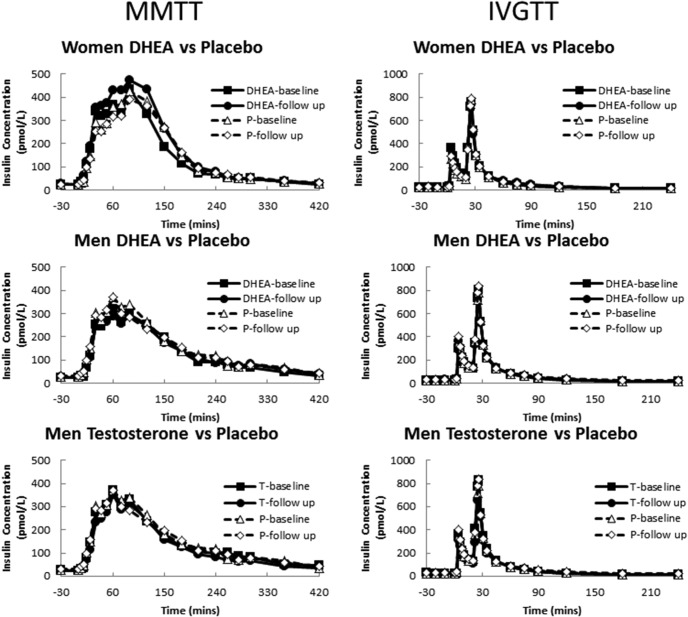
During the pretreatment MMTT, there were no differences in the nadir palmitate concentrations for women or men. For women, the median (interquartile range) nadir palmitate concentrations were 9 μmol/L (8–12) and 9 μmol/L (8–11) for the DHEA and placebo groups, respectively (P = .98). For men, the median nadir palmitate concentrations were 13 (10–19), 12 (9–17), and 13 (10–18) μmol/L in the T, DHEA, and placebo groups, respectively (P = .57). There were similar patterns during the IVGTT (data not shown). There was no statistically significant difference in the basal and peak insulin concentrations achieved in the pretreatment and posttreatment studies between the groups (Figure 2).
Figure 2. Insulin concentrations in the participants in these studies.
P, placebo.
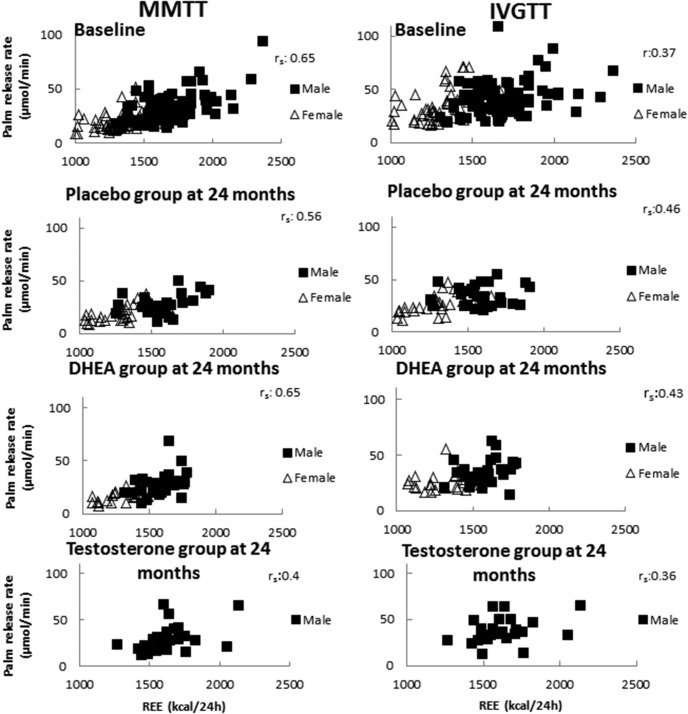
We analyzed the data from the pretreatment studies and found a significant, positive correlation between the REE and the MMTT nadir of systemic lipolysis as measured by palmitate Ra (Figure 3). This association was present in the 24-month data in all groups (Figure 3, left panel). We also found a positive correlation between REE and nadir IVGTT palmitate Ra at baseline in all groups. However, at 24 months the correlations between the nadir palmitate Ra and REE for men on placebo and women on DHEA were no longer statistically significant (Figure 3, right panel).
Figure 3. Correlations between REE and palmitate release rate during a MMTT and IVGTT.
r, Pearson's correlation coefficient; rs, Spearman's correlation coefficient.
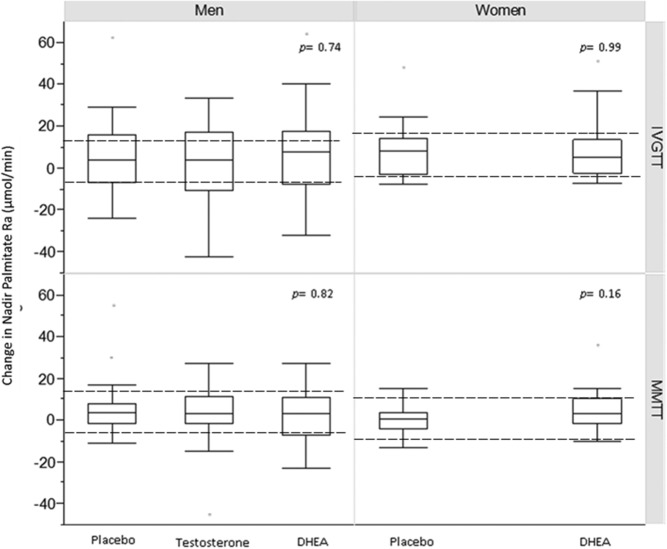
Univariate analysis of data from the MMTT and IVGTT did not detect statistically significant differences between changes in systemic lipolysis in women that received DHEA or in men that received DHEA or T compared with placebo (Figure 4). The results were similar whether lipolysis was expressed as the nadir palmitate Ra or AUC of the palmitate Ra. The lack of statistical significance persisted after adjusting for the changes in either the fat-free mass or REE, except for a small but statistically significant difference in nadir palmitate Ra in women receiving DHEA compared with placebo during the MMTT when the REE was included in the multivariate model (P = .03) (Table 3).
Figure 4. Unadjusted change in nadir palmitate Ra at 24 months (baseline follow-up) according to treatment group.
The dashed lines indicate the change in palmitate Ra of ±10 μmol/min (from post hoc power calculation) in the treatment groups compared with placebo, necessary to detect a statistically significant difference.
Discussion
In this randomized, placebo-controlled trial of supplementation of DHEA in elderly men and women and T in elderly men, we evaluated the effects of these hormones on the suppression of lipolysis during an IVGTT and a mixed-meal tolerance test. We have previously reported that neither DHEA nor T alters meal fatty acid metabolism or postabsorptive lipolysis (15, 16), but we were unable to include the lipolysis suppression data in that already lengthy report. Subsequent analysis revealed that postprandial suppression of lipolysis is a better predictor of some aspects of metabolic dysregulation than postabsorptive lipolysis (17). We now report that neither DHEA nor T replacement for 24 months has a clinically significant effect on postprandial or iv insulin suppression of lipolysis.
Previous studies indicate that DHEA replacement decreases body fat by 6%–30% (4, 6). It has been postulated that this effect is mediated by the stimulation of lipolysis (8) via activation of hormone sensitive lipase (25) and adipose triglyceride lipase (21). In contrast, we did not find an effect of DHEA on body composition or on systemic lipolysis. We found a small, statistically significant difference in change in the nadir palmitate Ra for women on DHEA compared with placebo during MMTT. However, we did not find a difference when lipolysis was measured as an AUC palmitate Ra or during IVGTT, which suggests that the former difference is probably not clinically relevant; furthermore, we cannot exclude a type 1 statistical error due to multiple comparison testing. There are other differences between the aforementioned studies and ours; the studies that found differences in body composition were short-term studies of 6 months' duration or less and the effect on lipolysis was predominantly evaluated in animal (25) or in vitro studies (21), which may not reflect the effects of DHEA in AT in vivo.
In the case of T, there are conflicting results reported of the effects on AT lipolysis. In cultured human adipocytes and preadipocytes from young healthy men and women, exposure to T had either no effect on lipolysis (11) or suppression of catecholamine-stimulated lipolysis (26). However, in another study, lipolysis was stimulated in preadipocytes cultured with DHT during differentiation (12). Increased in vivo lipolysis was also seen after 6 weeks of treatment with T in middle-aged men (13). The methodological differences between these studies and ours make it difficult to directly compare the results. We believe our data provide strong evidence that T administration to elderly men with mildly reduced levels at baseline does not alter systemic lipolysis in the absence of substantial losses of body fat.
The study design we used controls for potential selection bias or confounders for the association between the interventions and systemic lipolysis. The longer duration of therapy with T and DHEA avoids flawed results caused by regression toward the mean. However, this phenomenon may explain why some of the short-term studies with DHEA on body composition (4, 6, 7) or T on lipolysis (13) showed significant differences between the treatment groups. However, we should note that insulin suppression of lipolysis was not the primary outcome of this randomized trial, and, however unlikely, it is possible that our negative findings are the result of a type 2 statistical error. That said, the absolute differences in the changes of palmitate Ra we identified between the treatment groups were below the levels that might be physiologically relevant. Different body fat depots may contribute disproportionately to systemic lipolysis under basal (27, 28) and insulin-suppressed (22, 29) conditions. We have previously shown that upper body sc fat is the main source of excess FFA, and the splanchnic bed (visceral fat) accounts for 15%–30% of systemic FFA (27). It is possible that DHEA or T altered the relative contributions of FFA from the various fat depots. For example, these hormones could alter FFA release from visceral depot and this would not be reflected in our measures of systemic lipolysis. However, changes in FFA delivery to the liver would be expected to alter hepatic insulin sensitivity with regard to glucose and/or triglyceride metabolism; we found no effects of these hormones on insulin-regulated glucose production (15, 30) or plasma triglyceride concentrations (14). Regardless of hypothetical different effects these hormones might have on specific fat depots, the lack of change in systemic lipolysis is consistent with the finding that supplementing these hormones in the elderly also does not change peripheral insulin sensitivity (15, 30).
Abnormalities of AT lipolysis causes excess FFA, which can result in hepatic and muscle insulin resistance, increased very low-density lipoprotein-triglyceride-level production, abnormal vascular constriction, and excess insulin secretion. Our study was a mechanistic study with the goal of identifying whether DHEA or T have effects on adipose tissue metabolism by measuring changes in systemic lipolysis. The lack of effect of these hormones on systemic lipolysis suggests that these hormones may not have significant effects on AT metabolism as it relates to the above-outlined metabolic variables. We cannot say that these hormones have no effect on AT metabolism because other measures in adipose tissue were not performed, such as AT inflammation, adipokine production, etc. However, we think our study adds more relevant information to help us understand the role of these hormones in AT metabolism.
The definitions of low plasma DHEAS concentrations used for inclusion in our study (<1.57 μg/mL for men and <0.95 μg/mL for women) were based on concentrations found in young, healthy men and women (1.5 SD below the mean). However, the age-specific reference range for DHEAS in adult women above the age of 60 is less than 0.15–1.57 μg/mL and for elderly men 0.25–1.31 μg/mL. None of our participants had DHEAS concentrations below the lower end of their age-specific reference range. Our results indicate that DHEA supplements given to elderly adults with plasma DHEAS concentrations in the age-specific normal range have negligible effects on adipose tissue metabolism.
The assays for measuring T have changed during recent years. In this study we used a chemiluminescence immunoassay, whereas currently mass spectrometry is the preferred method. However, there is a good correlation between T levels by chemiluminescence immunoassay compared with mass spectrometry (31). The assay used in our study when compared with mass spectroscopy by liquid chromatography mass spectrometry were well correlated (r = 0.9 for total T levels and 0.95 for bioavailable T) (32).
We defined low T levels using bioavailable T criteria because it is considered to be the fraction readily available to cells. Our elderly male participants had moderately reduced bioavailable T concentrations, and some men had total and bioavailable T concentrations in the lower part of the normal range. The lower end of the reference range for total T in adults is 300 ng/dL and 40 ng/dL for bioavailable T for the assays used in our study; 24% and 11% of the male participants had concentrations below these levels for total T and bioavailable T, respectively. Therefore, the results of our study should be interpreted in the appropriated context and may not be generalizable to men with frank hypogonadism. Moreover, although the dose of T we used caused a significant increase in bioavailable T and fat-free mass as well as reductions in FSH and LH, the median increase did not reach the middle of the normal range. This is the goal recommended by Endocrine Society guidelines for patients with symptomatic hypogonadism. Nevertheless, the significant increment in T levels in our study was not associated with significant changes in systemic lipolysis.
In summary, DHEA or T supplementation has no significant effect on the suppression of systemic lipolysis measured by after either a mixed meal or an IVGTT in elderly women with low concentrations of DHEA or elderly men, with concentrations of DHEA and T below those observed in young men.
Acknowledgments
We thank the volunteers who participated in this study. We also thank Jean Feehan, Barbara Norby, and the members of the Mayo Clinic Clinical Research Unit nursing, dietary, and support laboratory staff for technical assistance in performing the study.
This study had a clinical trial registration number of NCT00254371.
This work was supported by Grants DK07352, PO1 AG14283, DK40484, and RR00585 from the US Public Health Service and the Mayo Foundation.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported by Grants DK07352, PO1 AG14283, DK40484, and RR00585 from the US Public Health Service and the Mayo Foundation.
Footnotes
- AT
- adipose tissue
- AUC
- area under the curve
- CV
- coefficient of variation
- DHEA
- dehydroepiandrosterone
- DHEAS
- sulfated dehydroepiandrosterone
- FFA
- free fatty acid
- IVGTT
- iv glucose tolerance test
- MMTT
- mixed-meal tolerance test
- Ra
- rate of appearance
- REE
- resting energy expenditure.
References
- 1. Nippoldt TB, Nair KS. Is there a case for DHEA replacement? Baillieres Clin Endocrinol Metab. 1998;12:507–520. [DOI] [PubMed] [Google Scholar]
- 2. Wu FC, Tajar A, Pye SR, et al. . Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93:2737–2745. [DOI] [PubMed] [Google Scholar]
- 3. Purifoy FE, Koopmans LH, Mayes DM. Age differences in serum androgen levels in normal adult males. Hum Biol. 1981;53:499–511. [PubMed] [Google Scholar]
- 4. Nestler JE, Barlascini CO, Clore JN, Blackard WG. Dehydroepiandrosterone reduces serum low density lipoprotein levels and body fat but does not alter insulin sensitivity in normal men. J Clin Endocrinol Metab. 1988;66:57–61. [DOI] [PubMed] [Google Scholar]
- 5. Morales AJ, Nolan JJ, Nelson JC, Yen SS. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab. 1994;78:1360–1367. [DOI] [PubMed] [Google Scholar]
- 6. Morales AJ, Haubrich RH, Hwang JY, Asakura H, Yen SSC. The effect of six months treatment with a 100 mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clin Endocrinol (Oxf). 1998;49:421–432. [DOI] [PubMed] [Google Scholar]
- 7. Villareal DT, Holloszy JO. Effect of DHEA on abdominal fat and insulin action in elderly women and men: a randomized controlled trial. JAMA. 2004;292:2243–2248. [DOI] [PubMed] [Google Scholar]
- 8. Hernández-Morante JJ, Pérez-de-Heredia F, Luján JA, Zamora S, Garaulet M. Role of DHEA-S on body fat distribution: Gender- and depot-specific stimulation of adipose tissue lipolysis. Steroids. 2008;73:209–215. [DOI] [PubMed] [Google Scholar]
- 9. Allan CA, Strauss BJG, Burger HG, Forbes EA, McLachlan RI. Testosterone therapy prevents gain in visceral adipose tissue and loss of skeletal muscle in nonobese aging men. J Clin Endocrinol Metab. 2008;93:139–146. [DOI] [PubMed] [Google Scholar]
- 10. Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol. 2001;56:M266–M272. [DOI] [PubMed] [Google Scholar]
- 11. Blouin K, Nadeau M, Perreault M, et al. . Effects of androgens on adipocyte differentiation and adipose tissue explant metabolism in men and women. Clin Endocrinol (Oxf). 2010;72:176–188. [DOI] [PubMed] [Google Scholar]
- 12. Gupta V, Bhasin S, Guo W, et al. . Effects of dihydrotestosterone on differentiation and proliferation of human mesenchymal stem cells and preadipocytes. Mol Cell Endocrinol. 2008;296:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rebuffe-Scrive M, Marin P, Bjorntorp P. Effect of testosterone on abdominal adipose tissue in men. Int J Obes. 1991;15:791–795. [PubMed] [Google Scholar]
- 14. Nair KS, Rizza RA, O'Brien P, et al. . DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355:1647–1659. [DOI] [PubMed] [Google Scholar]
- 15. Basu R, Dalla Man C, Campioni M, et al. . Two years of treatment with dehydroepiandrosterone does not improve insulin secretion, insulin action, or postprandial glucose turnover in elderly men or women. Diabetes. [Erratum (2007) 56(5):1486] 2007;56:753–766. [DOI] [PubMed] [Google Scholar]
- 16. Koutsari C, Ali AH, Nair KS, et al. . Fatty acid metabolism in the elderly: effects of dehydroepiandrosterone and testosterone replacement in hormonally deficient men and women. J Clin Endocrinol Metab. 2009;94:3414–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bush NC, Basu R, Rizza RA, Nair KS, Khosla S, Jensen MD. Insulin-mediated FFA suppression is associated with triglyceridemia and insulin sensitivity independent of adiposity. J Clin Endocrinol Metab. 2012;97:4130–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jensen MD, Heiling V, Miles JM. Measurement of non-steady-state free fatty acid turnover. Am J Physiol. 1990;258:E103–E108. [DOI] [PubMed] [Google Scholar]
- 19. Nielsen S, Guo Z, Albu JB, Klein S, O'Brien PC, Jensen MD. Energy expenditure, sex, and endogenous fuel availability in humans. J Clin Invest. 2003;111:981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jensen MD, Caruso M, Heiling V, Miles JM. Insulin regulation of lipolysis in nondiabetic and IDDM subjects. Diabetes. 1989;38:1595–1601. [DOI] [PubMed] [Google Scholar]
- 21. Karbowska J, Kochan Z. Fat-reducing effects of dehydroepiandrosterone involve upregulation of ATGL and HSL expression, and stimulation of lipolysis in adipose tissue. Steroids. 2012;77:1359–1365. [DOI] [PubMed] [Google Scholar]
- 22. Guo ZK, Hensrud DD, Johnson CM, Jensen MD. Regional postprandial fatty acid metabolism in different obesity phenotypes. Diabetes. 1999;48:1586–1592. [DOI] [PubMed] [Google Scholar]
- 23. Roust LR, Jensen MD. Postprandial free fatty acid kinetics are abnormal in upper body obesity. Diabetes. 1993;42:1567–1573. [DOI] [PubMed] [Google Scholar]
- 24. Shadid S, Koutsari C, Jensen MD. Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes. 2007;56:1369–1375. [DOI] [PubMed] [Google Scholar]
- 25. Mauriege P, Martel C, Langin D, et al. . Chronic effects of dehydroepiandrosterone on rat adipose tissue metabolism. Metabolism. 2003;52:264–272. [DOI] [PubMed] [Google Scholar]
- 26. Dicker A, Ryden M, Naslund E, et al. . Effect of testosterone on lipolysis in human pre-adipocytes from different fat depots. Diabetologia. 2004;47:420–428. [DOI] [PubMed] [Google Scholar]
- 27. Martin ML, Jensen MD. Effects of body fat distribution on regional lipolysis in obesity. J Clin Invest. 1991;88:609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nielsen S, Guo ZK, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meek S, Nair KS, Jensen MD. Insulin regulation of regional free fatty acid metabolism. Diabetes. 1999;48:10–14. [DOI] [PubMed] [Google Scholar]
- 30. Basu R, Dalla Man C, Campioni M, et al. . Effect of 2 years of testosterone replacement on insulin secretion, insulin action, glucose effectiveness, hepatic insulin clearance, and postprandial glucose turnover in elderly men. Diabetes Care. 2007;30:1972–1978. [DOI] [PubMed] [Google Scholar]
- 31. Haring R, Baumeister SE, Nauck M, et al. . Testosterone and cardiometabolic risk in the general population—the impact of measurement method on risk associations: a comparative study between immunoassay and mass spectrometry. Eur J Endocrinol. 2013;169:463–470. [DOI] [PubMed] [Google Scholar]
- 32. Khosla S, Amin S, Singh RJ, Atkinson EJ, Melton LJ 3rd, Riggs BL. Comparison of sex steroid measurements in men by immunoassay versus mass spectroscopy and relationships with cortical and trabecular volumetric bone mineral density. Osteoporos Int. 2008;19:1465–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]