Abstract
Context:
Pheochromocytoma (PHEO) occurs in 50% of patients with multiple endocrine neoplasia type 2 (MEN2). It is unknown if the presence of PHEO is associated with more aggressive medullary thyroid cancer (MTC).
Objective:
To present our experience with MEN2 PHEO and evaluate whether PHEO impacts MTC overall survival in patients with RET codon 634 mutations.
Design:
We performed a retrospective chart review of MEN2 patients at MD Anderson Cancer Center from 1960 through 2012.
Patients:
The study group comprised 85 patients (group 1) with MEN2-associated PHEO. Of these, 59 patients (subgroup 1) with RET codon 634 mutations were compared to 48 patients (group 2) with RET codon 634 mutations, but without MEN2-associated PHEO.
Main Outcome Measures:
Of 85 patients with MEN2 and PHEO, 70 had MEN2A and 15 had MEN2B. Median age at PHEO diagnosis was 32 years. The initial manifestation of MEN2 was MTC in 60% of patients, synchronous MTC and PHEO in 34%, and PHEO in 6% of patients. Of patients, 72% had bilateral PHEO, and most tumors were synchronous (82%). Subgroup analysis of MEN2 patients with and without PHEO, who were carriers of RET codon 634, the most common mutation with PHEO, showed no significant differences in the stage of MTC at initial diagnosis. The median follow-up time for patients with PHEO was 249 months and without PHEO was 67 months (P < .01). Survival analyses among RET 634 carriers did not show shorter survival for patients with PHEO. The median survival time for patients with PHEO was 499 months and without PHEO was 444 months (P < .05).
Conclusions:
PHEO in MEN2 patients are usually bilateral and unlikely to be metastatic. Subgroup analysis of patients with RET 634 mutations with and without PHEO showed that PHEO was not associated with a more advanced stage of MTC at diagnosis or a shorter survival.
Mmultiple endocrine neoplasia type 2 (MEN2) is an autosomal-dominant inherited syndrome characterized by medullary thyroid carcinoma (MTC) and pheochromocytoma (PHEO) and caused by germline missense mutations in the RET proto-oncogene. The continued discovery of RET mutations has led to specific genotype-phenotype correlations (1–3) and the formation of the MEN consensus management guidelines in 2001 (4).
PHEO are mainly associated with codon 634 and 918 mutations of the RET proto-oncogene (5, 6) and could be a clinical marker for a genomic background predisposing patients to a more aggressive MTC. The aims of our study were to characterize the clinical expression of MEN2 PHEO, to determine if PHEO in patients with RET 634 is associated with an advanced stage of MTC at initial diagnosis, and to compare survival among patients with germline RET 634 mutations who present with and without PHEO.
Patients and Methods
After obtaining approval from the institutional review board, we identified 319 MEN2 patients between 1960 and 2012. A database was created with 160 demographic, clinical, laboratory, radiology, and pathological variables for each patient. The primary tumor size and location were determined by pathology, surgery, or radiology reports. In patients with MTC, pathology reports from initial surgery and baseline radiographic studies were determined by staging.
We classified PHEO or MTC based on screening or clinical presentation. Screening was defined when tumors were identified biochemically in asymptomatic patients or through radiological studies in probands and family members.
In patients with multiple PHEO, tumors were considered synchronous when diagnosed within 6 months of initial diagnosis and metachronous when discovered 6 months or more after the primary PHEO diagnosis (7). The primary tumor size was measured using the largest tumor dimension. Whenever PHEO and MTC were diagnosed within 1 year, the tumors were considered concomitant.
We defined prophylactic surgery for MTC as total thyroidectomy in a patient with germline RET 634 mutations before the age of 5 (5). Because many patients had cortex-sparing surgeries for PHEO and because of the limited descriptions of surgical resection margins on many pathology reports, it was difficult to determine if the appearance of a second PHEO in the ipsilateral adrenal gland suggested recurrence rather than a second primary tumor. However, in all of our patients with metachronous PHEO, the second PHEO presented in the contralateral adrenal gland was assumed to be a second primary tumor.
Inclusion and exclusion criteria
We included patients with MEN2 with and without PHEO. Patients whose diagnoses could not be confirmed by biochemical, radiographic, or histopathologic criteria were excluded. Patients who had prophylactic surgery for MTC were excluded from survival analysis.
Survival
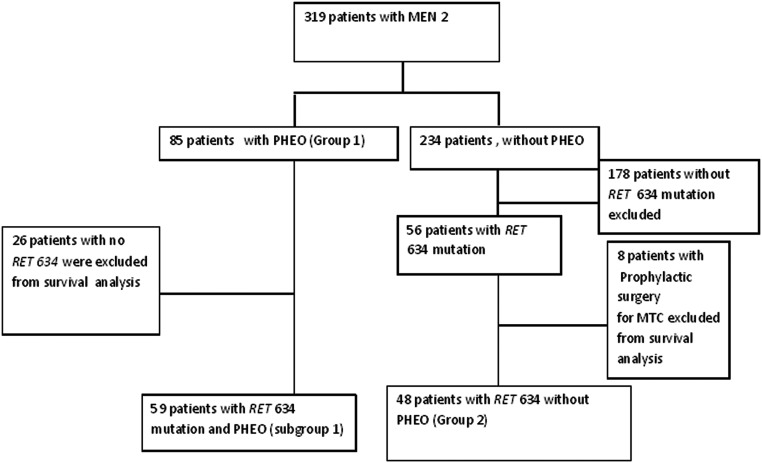
Overall survival was calculated from the date of MTC diagnosis to the date of death or last follow-up. To determine the effect of PHEO on stage of MTC at initial diagnosis, we compared 59 patients with PHEO (subgroup1) to 48 patients without PHEO (group 2) in patients with germline RET 634 mutations (Figure 1). Death information was retrieved from medical records and the United States Social Security Database.
Figure 1.
Flow diagram of study selection process.
Statistical analysis
Analyses were performed using SAS and SPSS (version 17.0). Descriptive statistics were used to summarize the data. Medians and ranges were computed for continuous variables, given the unequally distributed population in our cohort. For categorical variables, frequencies and percentages were calculated. The Wilcoxon rank sum test and other semiparametric tests were used to evaluate differences between patient subgroups. χ2 analyses were used to compare categorical variables. The Kaplan-Meier method was used to determine overall survival and the log-rank test was used to make comparisons among survival times. Cox proportional hazards regression models were used to determine the specific clinical factors associated with survival. All tests were two-sided, and a P value less than .05 was considered statistically significant.
Results
Demographics
The study group (group 1) consisted of 85 patients with MEN2-associated PHEO. Of these, 70 had MEN2A and 15 had MEN2B. There were 54 women and 31 men: 81% of patients were Caucasian, 68% had a family history of MTC, and 67% had a family history of PHEO. Sixty-six patients were diagnosed with PHEO through screening (Table 1).
Table 1.
Baseline Characteristics of 85 Patients (Group 1) With MEN2 Associated PHEO With Subclassification of Patients With MEN2A and MEN2B
| Patient Demographics | MEN2A (n = 70) | MEN2B (n = 15) |
|---|---|---|
| Sex | ||
| Male | 26 (37%) | 5 (33%) |
| Female | 44 (63%) | 10 (67%) |
| Race | ||
| Caucasian | 59 (84%) | 10 (67%) |
| Other | 11 (16%) | 5 (33%) |
| Age at PHEO diagnosis, ya | 34 (17–60) | 25 (18–40) |
| Age at MTC diagnosis, ya | 26 (10–60) | 22 (8–39) |
| MTC tumor size, cma | 1.5 (0.5–6) | 1.8 (0.9–4.5) |
| PHEO tumor size, cma | 3.8 (1–14) | 2.5 (1–6.4) |
| Family history of MTC | 55 (79%) | 3 (20%) |
| Family history of PHEO | 45 (64%) | 2 (13%) |
| PHEO diagnosis secondary to screening | 48 (69%) | 9 (60%) |
| Median follow-up time for PHEO, mo | 119 (0–527) | 57 (1–251) |
| Bilateral PHEO | 51 (73%) | 10 (67%) |
| Synchronous | 43 (84%) | 7 (70%) |
| Metachronous | 8 (16%) | 3 (30%) |
Data represent median (minimum-maximum).
Germline RET mutations associated with PHEO
Germline RET testing results were identified for 62 patients with MEN2 and PHEO. Mutations associated with PHEO were found in codons 634, 918, 618, 666, and 883. Sixty-nine percent of patients had RET 634 codon mutations and 8% had RET 918 mutations. If a family member carried a RET mutation, other family members with the MEN2 phenotype were assumed to carry the same mutation and were included in the study. This led to an additional nine patients who were identified with MEN2 due to genetic testing of family members. There were 14 patients who met clinical criteria for MEN2 who did not have RET testing, as most of these patients were diagnosed with MTC before the availability of RET testing.
PHEO and MTC median age at diagnosis
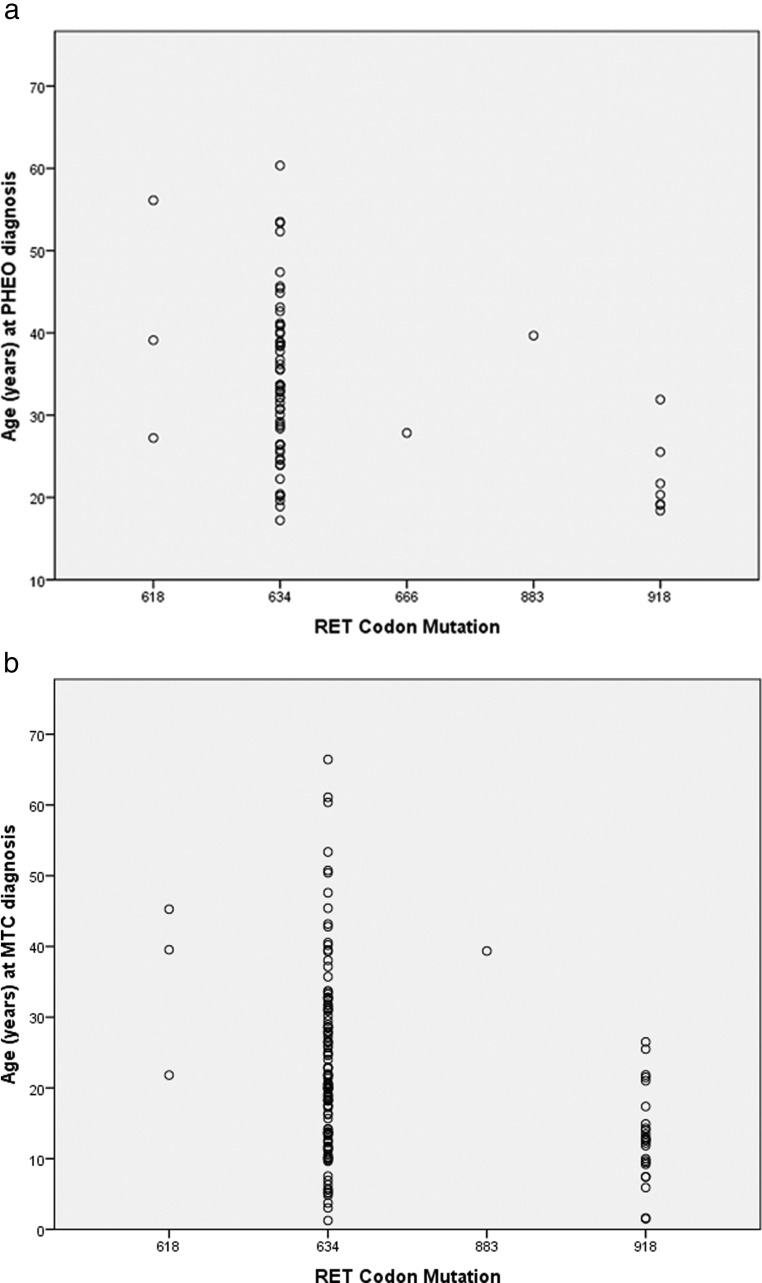
The median patient age at diagnosis of PHEO was 32 years (range: 17–60) (Figure 2A). Subgroup analysis showed the median ages at PHEO diagnosis for patients with MEN2A and MEN2B were 34 years (range: 17–60) and 25 years (range: 18–40), respectively (P < .05). Most patients, regardless of RET mutation type, were diagnosed with PHEO by the age of 40. The median age at diagnosis of MTC was 24 years (range: 8–60) across various RET codon mutation subtypes (Figure 2B). Subgroup analysis showed that the median age at MTC diagnosis was 26 years (range: 10–60) in MEN2A and 22 years (range: 8–39) in MEN2B patients (P < .01).
Figure 2.
Age at PHEO diagnosis based on RET codon mutation subtype (A) and at MTC diagnosis based on RET codon mutation type (B).
PHEO and MTC size
The median tumor size for PHEO in the entire cohort was 3.5 cm (range: 1–14). Comparison of MEN2A and MEN2B patients revealed median tumor sizes of 3.8 cm (range: 1–14) and 2.5 cm (range: 1.5–6.4), respectively (P < .01). Median PHEO sizes were also categorized by the decade of diagnosis, which showed a decrease in tumor size at identification since the 1970s, possibly due to earlier screening for PHEO. Conversely, the median age at diagnosis of PHEO was unchanged during the study period. The primary MTC tumor size for the entire cohort was 1.5 cm (range: 0.5–6), with a median of 1.5 cm (range: 0.5–6) in patients with MEN2A and a median of 1.8 cm (range: 0.9–4.5) in patients with MEN2B (P > .05). Median MTC sizes when categorized by decades showed a decrease in tumor size at identification when comparing patients identified before and after 1970. However, over the last 40 years, there has not been a significant decrease in median tumor size at diagnosis. Comparison of the median age at MTC diagnosis through the decades showed identification of MTC after age 20 before 1980 and before age 20 after 2000.
Chronology of PHEO and MTC in MEN2 patients
Sixty percent of patients presented with MTC as the initial presentation of MEN2, whereas 34% were simultaneously diagnosed with both MTC and PHEO. Only five patients, all carriers of RET 634, presented with PHEO first, and of these, only one patient had apparently sporadic PHEO, with no previous family history of PHEO or MTC and lack of bilateral disease on presentation. The remaining patients had a family history suggestive of MEN2, although this was not noted by their physicians. Of note, it is possible in these patients that MTC may have been present at the time of PHEO diagnosis but was not detected due to lack of knowledge of the association at that time. The median time between MTC and PHEO diagnoses in our cohort was 5.4 years with patients diagnosed with PHEO up to 32 years after their initial diagnosis of MTC and MTC diagnosis 6, 7, 8, 12, and 21 years after PHEO diagnosis. None of the patients had metastatic PHEO.
Bilateral PHEO and biochemical profile
Seventy-two percent of patients had bilateral PHEO, with 82% of these patients having synchronous presentation. In metachronous presentations, the median time between diagnosis of the first PHEO and second PHEO was 9.4 years (range: 1.85–20.76). Biochemical analysis showed that metanephrines and normetanephrines were on average 2.5 and 3.8 times higher than the upper limits for each assay.
Deaths
Twenty deaths (24%) had occurred in our series of 85 patients with PHEO. The cause of death was linked to PHEO in 2 patients (10%), MTC in 17 patients (85%), and unknown in 1 patient. The median age of death was 38 years (range: 25–73). Deaths occurred secondary to hypertensive crises during anesthesia and during contrast administration in the two patients in whom PHEO was identified as cause of death. Both patients were identified with their PHEO on autopsy in the 1960s; no deaths attributable to PHEO have since been recorded in our database.
Subgroup analysis of MEN2 patients with germline RET 634 mutations
A subgroup analysis was performed on the 115 patients with germline RET 634 mutations to evaluate the overall effect of PHEO on stage at MTC diagnosis and overall survival in these patients. After removal of eight patients who had prophylactic thyroidectomy for MTC, the remaining patients were divided into those with PHEO (subgroup 1) and those without PHEO (group 2). The baseline characteristics are summarized in Table 2.
Table 2.
Baseline Characteristics of Patients With Germline RET 634 Mutations Excluding Patients With Prophylactic Thyroidectomy for MTC
| Patient Demographics | Subgroup 1 | Group 2 | P Value |
|---|---|---|---|
| (n = 59) | (n = 48) | ||
| Sex | .81 | ||
| Male | 22 (37%) | 19 (40%) | |
| Female | 37 (63%) | 29 (60%) | |
| Race | <.0001 | ||
| Caucasian | 51 (86%) | 23 (48%) | |
| Other | 8 (14%) | 25 (52%) | |
| Age at PHEO diagnosis, ya | 34 (17–60) | n/a | n/a |
| Age at MTC diagnosis, ya | 24 (10–61) | 20 (6–66) | .05 |
| MTC tumor size, cma | 1.5 (0.5–6) | 0.95 (0.15–8) | .06 |
| PHEO tumor size, cma | 3.5 (1–14) | n/a | n/a |
| Family history of MTC | 48 (81%) | 39 (81%) | .87 |
| Family history of PHEO | 39 (66%) | 19 (40%) | .006 |
| PHEO diagnosis secondary to screening | 43 (73%) | n/a | n/a |
| MTC diagnosis secondary to screening | 45 (76%) | 32 (66%) | .46 |
| Median follow-up time for MTC, mo | 249 | 67 | <.01 |
| Median age at last follow-up, y | 46 (22–75) | 28 (6–72) | <.01 |
Data represent median (minimum-maximum).
MTC stage at diagnosis and overall survival
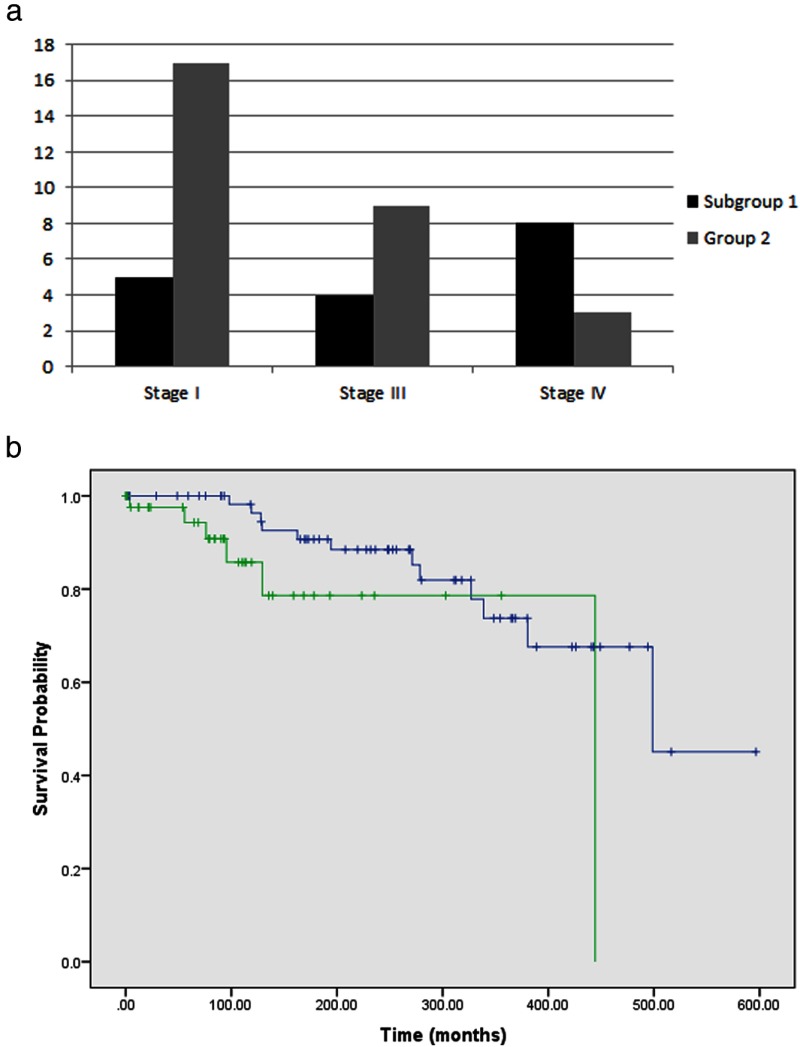
Of 107 patients with germline RET 634 mutations, all of whom had MTC, 59 had PHEO, which is consistent with the reported penetrance (8). Due to limited availability of pathology reports, the stage at initial surgery for MTC (5) was identified in only 46 patients. From this group, in patients with PHEO, the distribution of MTC stage at initial diagnosis was as follows: stage I in 5 patients, stage III in 4 patients, and stage IV in 8 patients. For patients without PHEO, the MTC stage at initial diagnosis was stage I in 17 patients, stage III in 9 patients, and stage IV in 3 patients (Figure 3A).
Figure 3.
A, Distribution of MTC Stage at initial diagnosis in RET codon 634 mutation carriers (n = 46) and B, survival comparison between patients with PHEO (Subgroup 1) and without PHEO (Group 2).
For patients with PHEO, 100%, 98%, and 90% of patients were alive at 5, 10, and 15 years after MEN2 diagnosis, respectively. For patients without PHEO, excluding patients who had prophylactic surgeries, 98%, 85%, and 78% of patients were alive at 5, 10, and 15 years, respectively. The median survival time for patients with PHEO was 499 months, compared to 444 months for patients with MTC only (P < .05) (Figure 3B). The median follow-up time for patients with PHEO was 249 months and without PHEO was 67 months (P < .01).
Hazard ratios (HRs) for death: Cox proportional hazards regression model
In the univariate analysis, the age at which MTC was diagnosed was significant, with each year delay in diagnosis leading to a 6% increase in death (HR = 1.062; 95% confidence interval [CI]: 1.020–1.106, P < .01). In addition, for each 1-cm increase in PHEO size at diagnosis, survival decreased by 58% (HR = 1.575; 95% CI: 1.186–2.092, P < .0017). Patients who were diagnosed with MTC without screening had twice the risk of death of patients with screening (HR = 2.360; 95% CI: 0.829–6.712, P > .05). Compared with patients with PHEO, patients without PHEO had three times the risk of death during the study period (HR = 2.9; 95% CI: 0.97–8.886, P > .05). When these variables were analyzed in a stepwise fashion in the multivariate model, however, none remained significant.
Discussion
Over the last decades, the MEN2 literature has focused primarily on MTC. This study is unique because in addition to describing MEN2-associated PHEO in a single institution database, we discuss the more recently discovered RET mutations associated with PHEO, explore the interaction between PHEO and MTC, and consider the change in the clinical presentations for MEN2 at the beginning of the 21st century.
Our study describes the natural history of MEN2 PHEO with confirmation that approximately 50% of patients with RET 634 mutations develop PHEO, with age of presentation in the third and fourth decades of life (9). Approximately two-thirds present with MTC first, while one-third present with MTC and PHEO concomitantly. In <5% of patients, PHEO is the presenting feature, with <1% of PHEO developing as an as apparently sporadic tumor. These patients did not have data on calcitonin or thyroid imaging. It is possible that some may have had concomitant MTC. PHEOs secrete both adrenaline and noradrenaline. Our study confirms the association of PHEO with RET mutations in codons 634, 918, 618, 666, and 883, with codon 634 as the most common mutation encountered. Although RET codon mutation 620 has been described with PHEO (10), this mutation is rarely associated with PHEO.
Genetic testing is recommended to every young patient with an apparently sporadic PHEO (11–13). The type of gene testing depends on the age at presentation, primary tumor location, malignancy, biochemical phenotype, and incidence (14–16). For patients older than 50 years, testing for RET mutations is rarely indicated as there is a low probability for MEN2 (17).
No metastatic PHEO were found despite having patients with primary tumor sizes >5 cm, a clinical predictor of malignancy (7, 18). In addition, MEN2 patients did not develop paragangliomas. Because metastases are very rare, computed tomography and magnetic resonance imaging are probably sufficient to localize MEN2 PHEO.
MEN2 PHEO are frequently bilateral and synchronous. Surgical treatment should be carefully evaluated, giving preference to cortical-sparing procedures to decrease the risk of adrenal insufficiency (19, 20). When PHEO present metachronously, they can appear up to 15 years later in patients followed with annual biochemical screening; thus patients with PHEO need long-term annual screening. Rarely, the diagnosis of PHEO occurs in patients older than 60 years of age, and therefore, the screening interval with plasma metanephrines could be extended to every 2 or 3 years. In addition, a recent study noted differences in the incidence of PHEO based on ATA mutation subclass and carrier age, recommending a change in the frequency of screening based on the expected peak incidence rather than applying standard recommendations to all RET mutation carriers (21). Only 18% of our patients had unilateral PHEO; most of these patients would however develop another PHEO with continued screening. Interestingly, most patients with PHEO were Caucasian as compared to patients without PHEO, suggesting that there may be unrecognized biologic or genetic factors accounting for these differences. Also, this may reflect limited access to medical care among non-Caucasian populations.
MTC is almost universal in MEN2 patients, but it is still unclear why certain patients develop PHEO and others do not. Some studies suggest that, for patients with germline RET 634 mutations, different amino acid substitutions can account for the variable expression rates of PHEO (8, 22). We speculated that the specific molecular changes leading to PHEO formation may create an environment contributing to more aggressive MTC. We found no survival difference among patients with germline RET 634 mutations with and without PHEO. Conversely, patients with PHEO had a significant increased survival compared to patients without PHEO. The interpretation of this finding should be done cautiously as the patients without PHEO had a shorter follow-up time. Furthermore, the patients without PHEO were younger and it is possible that some could later develop PHEO.
We found that in patients with RET 634 mutations, older age at initial MTC diagnosis and larger MTC primary tumor size were inversely associated with decreased overall survival. The MTC and PHEO found at the beginning of the 21st century seem to be smaller than those identified in the previous century, suggesting that the incorporation of genetic testing and presymptomatic screening may have impacted morbidity and survival. In fact, over the last decade, none of our MEN2 patients with PHEO have experienced a catecholamine crisis or death. However, we were not able to find any significance in the multivariate model, possibly because of our limited sample size.
Our study has limitations, including referral bias, small sample size, and a large number of patients without pathology reports from their thyroidectomies preventing adequate comparison of stage of MTC in patients with and without PHEO.
Overall, this study characterizes the nature of MEN2 PHEO. For the first time, we are presenting objective data describing how the epidemiology of PHEO and MTC in the setting of MEN2 has changed over time, which is perhaps a reflection of the historical impact of RET testing and the MEN guidelines. Furthermore, this study uniquely explores the interaction between PHEO and MTC, raising the question of whether patients with PHEO represent a different spectrum of disease as compared to patients without PHEO. Larger prospective studies done over an extended period of time are needed to confirm our findings.
Acknowledgments
We thank Rena Sellin, MD for review of this manuscript and Zachary Bohannan from the Department of Scientific Publications for grammatical review.
This work was supported by The University of Texas MD Anderson Cancer Center Support Grant CA016672 and by Mr Clarence Cazalot and Mrs Margaret Cazalot and Mr William Granek and Mrs Marle Granek.
Disclosure Summary: The authors have no conflicts of interest to disclose.
Funding Statement
This work was supported by The University of Texas MD Anderson Cancer Center Support Grant CA016672 and by Mr Clarence Cazalot and Mrs Margaret Cazalot and Mr William Granek and Mrs Marle Granek.
Footnotes
- CI
- confidence interval
- HR
- hazard ratio
- MEN2
- multiple endocrine neoplasia type 2
- MTC
- medullary thyroid cancer
- PHEO
- pheochromocytoma.
References
- 1. Toledo SP, dos Santos MA, Toledo Rde A, Lourenco DM Jr. Impact of RET proto-oncogene analysis on the clinical management of multiple endocrine neoplasia type 2. Clinics (Sao Paulo). 2006;61(1):59–70. [DOI] [PubMed] [Google Scholar]
- 2. Eng C, Clayton D, Schuffenecker I, et al. The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA. 1996;276(19):1575–1579. [PubMed] [Google Scholar]
- 3. Mulligan LM, Eng C, Healey CS, et al. Specific mutations of the RET proto-oncogene are related to disease phenotype in MEN 2A and FMTC. Nat Genet. 1994;6(1):70–74. [DOI] [PubMed] [Google Scholar]
- 4. Brandi ML, Gagel RF, Angeli A, et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86(12):5658–5671. [DOI] [PubMed] [Google Scholar]
- 5. Kloos RT, Eng C, Evans DB, et al. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid. 2009;19(6):565–612. [DOI] [PubMed] [Google Scholar]
- 6. Bryant J, Farmer J, Kessler LJ, Townsend RR, Nathanson KL. Pheochromocytoma: the expanding genetic differential diagnosis. J Natl Cancer Inst. 2003;95(16):1196–1204. [DOI] [PubMed] [Google Scholar]
- 7. Ayala-Ramirez M, Feng L, Johnson MM, et al. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab. 2011;96(3):717–725. [DOI] [PubMed] [Google Scholar]
- 8. Quayle FJ, Fialkowski EA, Benveniste R, Moley JF. Pheochromocytoma penetrance varies by RET mutation in MEN 2A. Surgery. 2007;142(6):800–805; discussion 805.e1. [DOI] [PubMed] [Google Scholar]
- 9. Nguyen L, Niccoli-Sire P, Caron P, et al. Pheochromocytoma in multiple endocrine neoplasia type 2: a prospective study. Eur J Endocrinol. 2001;144(1):37–44. [DOI] [PubMed] [Google Scholar]
- 10. Machens A, Brauckhoff M, Holzhausen HJ, Thanh PN, Lehnert H, Dralle H. Codon-specific development of pheochromocytoma in multiple endocrine neoplasia type 2. J Clin Endocrinol Metab. 2005;90(7):3999–4003. [DOI] [PubMed] [Google Scholar]
- 11. Erlic Z, Neumann HP. When should genetic testing be obtained in a patient with phaeochromocytoma or paraganglioma? Clin Endocrinol (Oxf),. 2009;70(3):354–347. [DOI] [PubMed] [Google Scholar]
- 12. Pomares FJ, Canas R, Rodriguez JM, Hernandez AM, Parrilla P, Tebar FJ. Differences between sporadic and multiple endocrine neoplasia type 2A phaeochromocytoma. Clin Endocrinol (Oxf). 1998;48(2):195–200. [DOI] [PubMed] [Google Scholar]
- 13. Waguespack SG, Rich T, Grubbs E, Ying AK, Perrier ND, Ayala-Ramirez M, Jimenez C. A current review of the etiology, diagnosis, and treatment of pediatric pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2010;95(5):2023–2037. [DOI] [PubMed] [Google Scholar]
- 14. Benn DE, Robinson BG. Genetic basis of phaeochromocytoma and paraganglioma. Best Pract Res Clin Endocrinol Metab. 2006;20(3):435–450. [DOI] [PubMed] [Google Scholar]
- 15. Machens A, Brauckhoff M, Gimm O, Dralle H. Risk-oriented approach to hereditary adrenal pheochromocytoma. Ann N Y Acad Sci. 2006;1073:417–428. [DOI] [PubMed] [Google Scholar]
- 16. Eisenhofer G, Lenders JW, Timmers H, et al. Measurements of plasma methoxytyramine, normetanephrine, and metanephrine as discriminators of different hereditary forms of pheochromocytoma. Clin Chem. 2011;57(3):411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jimenez C, Cote G, Arnold A, Gagel RF. Review: Should patients with apparently sporadic pheochromocytomas or paragangliomas be screened for hereditary syndromes? J Clin Endocrinol Metab. 2006;91(8):2851–2858. [DOI] [PubMed] [Google Scholar]
- 18. Raue F, Frank-Raue K, Grauer A. Multiple endocrine neoplasia type 2. Clinical features and screening. Endocrinol Metab Clin North Am. 1994;23(1):137–156. [PubMed] [Google Scholar]
- 19. Brunt LM, Lairmore TC, Doherty GM, Quasebarth MA, DeBenedetti M, Moley JF. Adrenalectomy for familial pheochromocytoma in the laparoscopic era. Ann Surg. 2002;235(5):713–720; discussion 720–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lairmore TC, Ball DW, Baylin SB, Wells SA Jr. Management of pheochromocytomas in patients with multiple endocrine neoplasia type 2 syndromes. Ann Surg. 1993;217(6):595–601; discussion 601–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Machens A, Lorenz K, Dralle H. Peak incidence of pheochromocytoma and primary hyperparathyroidism in multiple endocrine neoplasia 2: need for age-adjusted biochemical screening. J Clin Endocrinol Metab. 2013;98(2):E336–E345. [DOI] [PubMed] [Google Scholar]
- 22. Rodriguez JM, Balsalobre M, Ponce JL, Rios A, Torregrosa NM, Tebar J, Parrilla P. Pheochromocytoma in MEN 2A syndrome. study of 54 patients. World J Surg. 2008;32(11):2520–2526. [DOI] [PubMed] [Google Scholar]