Abstract
Context:
The polycystic ovary syndrome (PCOS) is a common and complex disease without a clear pattern of inheritance. Anti-Müllerian hormone (AMH) has an inhibitory effect on FSH-stimulated follicle growth. Serum AMH levels are higher in women with PCOS than in normo-ovulatory women. The elevated AMH levels may reflect abnormalities in AMH signaling.
Objective:
The purpose of this study was to evaluate the association of the anti-Müllerian hormone receptor 2 (AMHR2) −482 A>G polymorphism (rs2002555) with the pathophysiology of PCOS.
Design:
AMHR2 −482 A>G polymorphism genotyping were performed in a large cohort of women with PCOS and in a healthy control group.
Setting/Subjects:
A total of 858 Caucasian Greek women with PCOS and 309 healthy control women were studied.
Interventions:
Genotyping and hormonal measurements were preformed.
Main Outcome Measures:
Hormone levels in women with PCOS were analyzed.
Results:
The AMHR2 polymorphism was more common in women with PCOS than in control women (P = .026). Homozygous AMHR2 −482 A>G gene polymorphisms (GG) were associated with decreased levels of LH (P = .003) and lower LH to FSH ratios (P = .01) in women with PCOS, as well as with lower prolactin levels (P = .004). No other associations related to AMHR2 −482 A>G polymorphisms were observed in women with PCOS or control women.
Conclusion:
In this study, the role of the AMHR2 −482 A>G gene polymorphism in the pathogenesis of PCOS was suggested by the association of the variant with PCOS risk. Thus, further research is needed to elucidate a possible association of the AMHR2 −482 A>G gene polymorphism with AMH signaling and impaired ovarian function and its clinical significance in women with PCOS.
The polycystic ovary syndrome (PCOS) is a heterogeneous endocrine disorder affecting 6% to 10% of women of reproductive age (1). PCOS is characterized by androgen excess, oligo/anovulation, and polycystic ovarian morphology on ultrasound examination (2). There is evidence that PCOS is associated with both genetic and environmental factors (3, 4). The efforts to clarify the genetic component of PCOS pathogenesis have been focused on identifying genetic variants in loci directly or indirectly involved in various aspects of the syndrome.
The expression patterns of anti-Müllerian hormone (AMH) and its type II receptor (AMHR2) in the postnatal ovary indicated the importance of AMH signaling in ovarian folliculogenesis (5). In vivo studies in AMH knockout mice revealed that AMH had an inhibitory effect on FSH-stimulated follicle growth (6). It has also been shown that serum AMH levels are higher in women with PCOS than in normo-ovulatory women (7) and that AMH levels are higher in individual follicles from women with PCOS (8). The elevated AMH levels might inhibit FSH action in women with PCOS, providing an explanation for the disturbed folliculogenesis or may reflect abnormalities at the AMH receptor.
As far as we know, no correlation between AMHR2 polymorphisms and PCOS pathogenesis has ever been demonstrated (9, 10). In this study, we examined the AMHR2 −482 A>G polymorphism (rs2002555) in Caucasian Greek women with PCOS. The large number of the participants and their ethnic homogeneity act as enhancers in this genetic study to unmask a potential role of the AMHR2-482 A>G polymorphism in PCOS.
Patients and Methods
Patients
The study included 858 Caucasian Greek women with PCOS and 309 women used as control population. No woman reported use of any medication that could interfere with the normal function of the hypothalamic-pituitary-gonadal axis during the past 6 months.
The diagnosis of PCOS was based on the Rotterdam criteria (2). Exclusion criteria were type 2 diabetes, congenital adrenal hyperplasia, androgen-secreting tumors, and Cushing syndrome. All members of the control population had regular ovulation, serum progesterone levels >10 ng/mL in the luteal phase of the menstrual cycle, and no evidence of clinical or biochemical hyperandrogenism.
Written consent was given by all participants according to guidelines of the Institutional Review Council (institutional review board) of the School of Medicine, University of Patras and the Aristotle University of Thessaloniki. The study complied with the principles of the Declaration of Helsinki, and all participants gave their consent.
Materials and Methods
Blood samples were taken between the third and fourth day of the cycle in healthy control women or between the third and fourth day after spontaneous bleeding in women with PCOS, after overnight fasting. For those women with an interval between menstruation cycles greater than 90 days, 100 mg of micronized progesterone was given for 10 days to achieve an induced menstruation (less than 5% of the total population of women with PCOS included in the study).
LH, FSH, testosterone, free testosterone, and insulin were measured by chemiluminescence (Elecsys 2010; Roche Diagnostics). Androstenedione, dehydroepiandrosterone sulfate (DHEAS), sex hormone–binding globulin (SHBG), and 17-hydroxyprogesterone were measured by RIA (BioSource). Serum lipids were measured with an automatic biochemical analyzer (Olympus; Medicon System Reagent Hellas). AMH concentrations were measured with an enzymatically amplified 2-sided immunoassay (DSL-10–14400 active Müllerian-inhibiting substance/AMH ELISA kit; DSL Laboratories). The theoretical sensitivity of the method is 0.006 ng/mL, the intra-assay coefficient of variation for high values is 3.3%, and the interassay coefficient of variation for high values is 6.7%.
Genotyping
Genomic DNA was extracted from whole peripheral blood by the standard method with phenol-chloroform.
The genotyping was performed by primer extension of multiplex products with detection by matrix-assisted laser desorption ionization/time of flight mass spectroscopy using a Sequenom platform. The single nucleotide polymorphism (SNP) did not deviate from Hardy-Weinberg equilibrium in case patients or control subjects (P > 10−3).
Statistical analysis
Values are expressed as means ± SD. The genotype frequency distributions between groups and control women were compared using the χ2 test. All variables (clinical and hormonal) were tested for normal distribution using the Kolmogorov-Smirnov test.
The comparison between women with PCOS and control women (total values and not between subgroups) was adjusted to the effect of age via the general linear model. Variables that did not follow normal distribution were log-transformed so that the general linear model could be applied. The distribution comparison across subgroups (genotypes within PCOS or control women) was done using parametric methods (ANOVA) for variables after normal distribution and nonparametric methods (Kruskal-Wallis) for variables not following normal distribution. The Bonferroni adjustment for multiple comparisons was applied in the post hoc analysis (pairwise comparison). Values were considered to be statistically significant at P < .05. Statistical analysis was done using PASW 19 for Windows (IBM SPSS Statistics; IBM software).
Results
Women with PCOS were younger than control women. After adjustment for the comparison to the effect of age, AMH, FSH, LH, DHEAS, testosterone, androstenedione, and 17-hydroxyprogesterone levels and the free androgen index were higher in women with PCOS than in control women, whereas SHBG levels were lower (Table 1).
Table 1.
Comparison of Clinical and Hormonal Characteristics of Women With PCOS and Healthy Control Women With Different AMHR2 −482 A>G Polymorphism Genotypes
| PCOS |
Control |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 858) | AA (n = 585; 68.2%) | AG (n = 248; 28.9%) | GG (n = 25; 2.9%) | P Value | Total (n = 309) | AA (n = 234; 75%) | AG (n = 71; 23.7%) | GG (n = 4; 1.3%) | P Value | |
| Age, y | 24.02 ± 5.82 | 24.25 ± 5.73 | 23.53 ± 5.86 | 23.34 ± 7.32 | .172 | 30.85 ± 5.76 | 30.93 ± 5.94 | 30.57 ± 5.32 | 31 | .970 |
| Body mass index, kg/m2 | 26.55 ± 6.98 | 26.40 ± 6.70 | 26.88 ± 7.48 | 27.38 ± 8.78 | .875 | 27.23 ± 6.89 | 26.76 ± 6.63 | 28.72 ± 7.82 | 30.84 | .455 |
| Waist to hip ratio | 0.79 ± 0.25 | 0.78 ± 0.07 | 0.81 ± 0.46 | 0.80 ± 0.08 | .668 | 0.78 ± 0.07 | 0.78 ± 0.07 | 0.77 ± 0.07 | 0.79 | .825 |
| AMH, ng/mL | 5.88 ± 6.03c | 5.18 ± 2.61 | 5.82 ± 1.84 | 4.11 ± 2.38 | .688 | 3.12 ± 1.36 | 3.2 ± 1.39 | 2.85 ± 1.36 | ||
| FSH, mIU/mL | 5.99 ± 1.80c | 5.99 ± 1.84 | 6.05 ± 1.69 | 5.27 ± 1.62 | .197 | 6.93 ± 2.39 | 7.19 ± 2.55 | 6.13 ± 1.46 | 4.53 | .118 |
| LH, mIU/mL | 7.69 ± 5.35c | 7.78 ± 5.18 | 7.77 ± 5.88 | 4.43 ± 2.43a,b | .003 | 5.61 ± 2.71 | 5.88 ± 2.82 | 4.52 ± 2.04 | 7.96 | .085 |
| LH to FSH ratio | 1.34 ± 0.93c | 1.36 ± 0.91 | 1.34 ± 0.98 | 0.83 ± 0.41a,b | .010 | 0.85 ± 0.40 | 0.87 ± 0.42 | 0.74 ± 0.27 | 1.76 | .118 |
| DHEAS, μg/dL | 3013 ± 1315c | 3044 ± 1310 | 2986 ± 1293 | 2475 ± 1596 | .152 | 1967 ± 802 | 1955 ± 806 | 2042 ± 813 | 1323 | .660 |
| Prolactin, ng/mL | 14.20 ± 7.17 | 14.64 ± 7.32 | 13.48 ± 6.81 | 10.07 ± 4.54a | .004 | 13.49 ± 6.19 | 13.40 ± 6.42 | 13.43 ± 5.32 | 21.50 | .434 |
| Testosterone, ng/dL | 74.69 ± 29.58c | 75.36 ± 30.19 | 74.11 ± 28.53 | 63.33 ± 22.37 | .146 | 39.99 ± 11.13 | 39.78 ± 11.24 | 41.05 ± 11.09 | 33 | .741 |
| Androstenedione, ng/mL | 2.77 ± 1.07c | 2.78 ± 1.07 | 2.81 ± 1.10 | 2.33 ± 0.79 | .194 | 1.71 ± 0.47 | 1.72 ± 0.46 | 1.68 ± 0.52 | 1.41 | .775 |
| 17-Hydroxyprogesterone, ng/mL | 1.13 ± 0.56c | 1.12 ± 0.55 | 1.16 ± 0.60 | 1.06 ± 0.49 | .856 | 0.79 ± 0.39 | 0.81 ± 0.38 | 0.71 ± 0.42 | 0.52 | .448 |
| SHBG, nmol/L | 43.39 ± 26.82c | 44.15 ± 27.94 | 42.07 ± 24.48 | 37.34 ± 18.77 | .477 | 60.44 ± 32.86 | 59.65 ± 30.91 | 62.79 ± 40.42 | 70.81 | .748 |
| Free androgen index | 8.37 ± 6.83c | 8.35 ± 6.86 | 8.58 ± 7.04 | 8.37 ± 6.83 | .812 | 2.91 ± 1.65 | 2.90 ± 1.63 | 2.99 ± 1.77 | 1.62 | .523 |
Values are means ± SD. P values reflect comparisons within each group separately (women with PCOS and control women):
P < .05 vs AA;
P < .05 vs GA;
P < .001 vs control, adjusted for the effect of age.
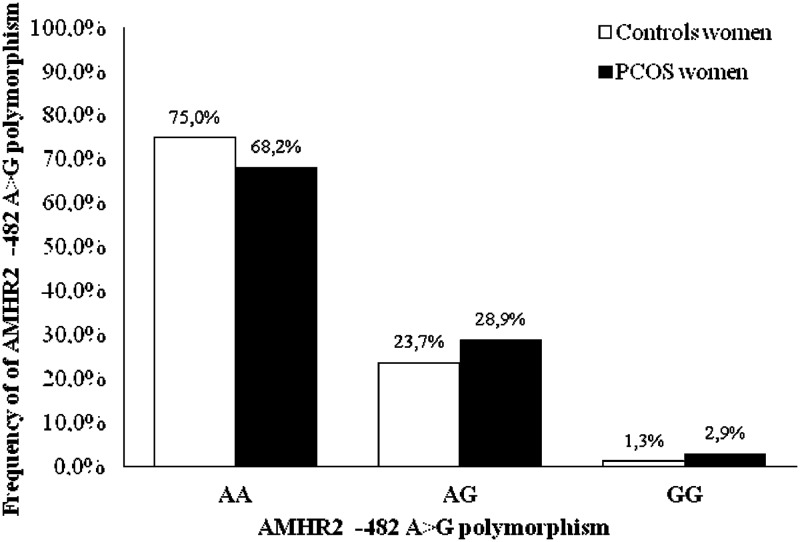
The AMHR2 −482 A>G gene polymorphism (rs2002555) was more common in women with PCOS than in control women (P = .026) (odds ratio, 1.64 ± 0.15; P = .001) (Figure 1). The relationship between AMHR2 −482 A>G (rs2002555) and PCOS remained when only subjects meeting the National Institutes of Health criteria for PCOS were examined (odds ratio, 1.66 ± 0.15; P < .001).
Figure 1.
Schematic representation of the AMHR2 −482 A>G polymorphism genotype frequency among women with PCOS and healthy control women. The frequency of the AMHR2 −482 A>G polymorphism in the PCOS groups compared with that in the control group (χ2 test) varies and was statistically significant for women with PCOS (P = .026).
The homozygosis of the AMHR2 −482 A>G gene polymorphism (GG) was associated with decreased levels of LH (P = .003) and a lower LH to FSH ratio in women with PCOS, but not in control women. It was also associated with lower prolactin levels (P = .004) in the PCOS group (Table 1). No other association related to the AMHR2 −482 A>G polymorphism was observed in women with PCOS or control women.
Discussion
The results of the present study demonstrate a significant difference in the frequency of genotypes of the AMHR2 −482 A>G polymorphism (rs2002555) in women with PCOS compared with the control ovulatory population. The data suggest that the AMHR2 polymorphism results in increased susceptibility to PCOS. The polymorphism is also associated with lower levels of LH as well as with a lower LH to FSH ratio in women with PCOS.
Our data add to previous studies that examined the AMHR2 −482 A>G polymorphism in women with PCOS. Sproul et al (10) found no differences in the frequency of the AMHR2 −482 A>G between women with PCOS and women in the general population. This study involved a sufficient number of women with PCOS (n = 335), and the diagnosis of the syndrome was based on the strict National Institutes of Health criteria. The failure of the study to demonstrate a relationship with the variant may be due to the heterogeneity of the ethnic origin in that study, whereas our cohort of women consists of a homogeneous Caucasian Greek population. Kevenaar et al (9) also failed to demonstrate an association of the AMHR2 −482 A>G variant with PCOS or PCOS phenotypic features. A possible explanation for the difference between our results and their findings could be the small control group in that previous study (n = 32).
Serum AMH levels are higher in women with PCOS (7) who also have disturbed folliculogenesis. A recent in vitro study showed that the production of AMH is increased up to 75% in women with PCOS compared with that in control women (8). The current study supports the possibility that a change in AMH signaling through the receptor affects endogenous follicular dysfunction. It does not appear that the receptor changes resulted in higher AMH levels in serum, although the numbers were small and the hypothesis needs further investigation. Thus, the role of this SNP on the receptor's functionality or the signaling activity needs further investigation.
The AMHR2 −482 A>G polymorphism was found to be associated with higher follicular phase estradiol levels in normo-ovulatory women (11), suggesting a role for AMH in the regulation of FSH sensitivity in the ovary through its receptor. In a prospective study (12), this polymorphism was also found to be correlated with natural age at menopause, suggesting a role for AMH signaling in the usage of the primordial follicle pool in women. In accordance with these findings (13), a more recent study confirms that the variation in the AMHR2 gene modifies the relation between parity and age at natural menopause. There is a discrepancy only with the study by Kevenaar et al (12), regarding nulliparous women with the rs2002555 G/G genotype who had a 2.6-year earlier menopause that nulliparous women with the A/A genotype. The authors considered the possibility that genes involved in primary follicle recruitment influence the timing of menopause. In agreement with these studies are the observations on AMH knockout mice (5, 14), which although fertile presented more growing follicles resulting in depletion of the primordial pool and early cessation of ovulation (5). The data from these studies might suggest a negative influence on AMH signaling because of the presence of the AMHR2 −482 A>G polymorphism.
Rigon et al (15) showed that the genetic variants of the AMHR2 gene seem to be associated with unexplained infertility, suggesting a role in the pathophysiology of normoestrogenic and normo-ovulatory infertility. A hypothesis that might provide an explanation for the involvement of the AMHR2 SNP in pathological procedures, without clear justification, is that the AMHR2 −482 A>G polymorphism could have a role in ovarian dysfunction even when it is not evident in the hormonal profile.
The data provided by previous studies suggest diminished G allele AMHR2 signaling in women with regular menstrual cycles, whereas there is no evidence about the implications of this variant in women with PCOS. It has been reported that the 482 A>G polymorphism is located at a potential c-Myb and c-Myc transcription factor–binding site (12) and therefore may modify promoter activity. In a recent study it was shown that LH reduces AMHR2 expression by lutein GCs from normo-ovulatory women but had no effect on AMHR2 mRNA levels in oligo-ovulatory or anovulatory women with PCOS (16). We can hypothesize that the altered hormonal profile in PCOS might have a stronger effect on transcription or bioactivity of G allele AMHR2 than on the A allele, suggesting a favorable or enhancing role of the G allele in PCOS pathogenesis.
In women with PCOS, the serum AMH level is related to increased serum LH levels (7). Although the AMHR2 −482 A>G polymorphism was not correlated with serum AMH levels, its presence, by decreasing AMH signaling, might be associated with lower LH levels and a lower LH to FSH ratio, by an as-yet unexplained mechanism. Hyperprolactinemia is a common finding among patients with PCOS, but the underlying cause of the link between these 2 disorders has not been clearly established (17). The association of the AMHR2 −482 A>G polymorphism with lower prolactin serum levels is more difficult to explain because one might expect higher levels with the possible increased estradiol associated with the polymorphism in previous studies (11).
A limitation of the present study is the lack of solid data regarding serum estradiol levels in our women with PCOS. Indeed, serum estradiol levels would have been of real value in the interpretation of the contribution of AMH to the regulation of FSH sensitivity in the ovary through its AMHR2 −482 A>G polymorphic receptor.
In conclusion, in this study the role of the AMHR2 −482 A>G gene polymorphism in the pathogenesis of PCOS has been suggested by the association of the variant with PCOS risk. Thus, further research is needed to elucidate a possible association of the AMHR2 −482 A>G gene polymorphism with AMH signaling and impaired ovarian function and its clinical significance in women with PCOS.
Acknowledgments
We thank George Sakellaropoulos, Assistant Professor of Medical Physics, University of Patras Medical School (Patras, Greece), for his aid in the statistical evaluation of the present study.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AMH
- anti-Müllerian hormone
- AMHR2
- anti-Müllerian hormone receptor 2
- DHEAS
- dehydroepiandrosterone sulfate
- PCOS
- polycystic ovary syndrome
- SHBG
- sex hormone–binding globulin
- SNP
- single nucleotide polymorphism.
References
- 1. Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. [DOI] [PubMed] [Google Scholar]
- 2. The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. 2004. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 19:41–47. [DOI] [PubMed] [Google Scholar]
- 3. Coviello AD, Zhuang WV, Lunetta KL, et al. . Circulating testosterone and SHBG concentrations are heritable in women: the Framingham Heart Study. J Clin Endocrinol Metab. 2011;96:E1491–E1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Diamanti-Kandarakis E, Piperi C. Genetics of polycystic ovary syndrome: searching for the way out of the labyrinth. Hum Reprod Update. 2005;11:631–643. [DOI] [PubMed] [Google Scholar]
- 5. Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Müllerian hormone. Reproduction. 2002;124:601–609. [DOI] [PubMed] [Google Scholar]
- 6. Durlinger AL, Gruijters MJ, Kramer P, et al. . Anti-Müllerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143:1076–1084. [DOI] [PubMed] [Google Scholar]
- 7. Piouka A, Farmakiotis D, Katsikis I, Macut D, Gerou S, Panidis D. Anti-Mullerian hormone levels reflect severity of PCOS but are negatively influenced by obesity: relationship with increased luteinizing hormone levels. Am J Physiol Endocrinol Metab. 2009;296:E238–E243. [DOI] [PubMed] [Google Scholar]
- 8. Pellatt L, Hanna L, Brincat M, et al. . Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92:240–245. [DOI] [PubMed] [Google Scholar]
- 9. Kevenaar ME, Laven JS, Fong SL, et al. . A functional anti-mullerian hormone gene polymorphism is associated with follicle number and androgen levels in polycystic ovary syndrome patients. J Clin Endocrinol Metab. 2008;93:1310–1316. [DOI] [PubMed] [Google Scholar]
- 10. Sproul K, Jones MR, Mathur R, Azziz R, Goodarzi MO. Association study of four key folliculogenesis genes in polycystic ovary syndrome. BJOG. 2010;117:756–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kevenaar ME, Themmen AP, Laven JS, et al. . Anti-Müllerian hormone and anti-Müllerian hormone type II receptor polymorphisms are associated with follicular phase estradiol levels in normo-ovulatory women. Hum Reprod. 2007;22:1547–1554. [DOI] [PubMed] [Google Scholar]
- 12. Kevenaar ME, Themmen AP, Rivadeneira F, et al. . A polymorphism in the AMH type II receptor gene is associated with age at menopause in interaction with parity. Hum Reprod. 2007;22:2382–2388. [DOI] [PubMed] [Google Scholar]
- 13. Voorhuis M, Broekmans FJ, Fauser BC, Onland-Moret NC, van der Schouw YT. Genes involved in initial follicle recruitment may be associated with age at menopause. J Clin Endocrinol Metab. 2011;96:E473–E479. [DOI] [PubMed] [Google Scholar]
- 14. Durlinger AL, Gruijters MJ, Kramer P, et al. . Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142:4891–4899. [DOI] [PubMed] [Google Scholar]
- 15. Rigon C, Andrisani A, Forzan M, et al. . Association study of AMH and AMHRII polymorphisms with unexplained infertility. Fertil Steril. 2010;94:1244–1248. [DOI] [PubMed] [Google Scholar]
- 16. Pierre A, Peigné M, Grynberg M, et al. . Loss of LH-induced down-regulation of anti-Mullerian hormone receptor expression may contribute to anovulation in women with polycystic ovary syndrome. Hum Reprod. 2013;28:762–769. [DOI] [PubMed] [Google Scholar]
- 17. Bracero N, Zacur HA. Polycystic ovary syndrome and hyperprolactinemia. Obstet Gynecol Clin North Am. 2001;28:77–84. [DOI] [PubMed] [Google Scholar]