Abstract
Objectives
We previously reported high rates of urinary incontinence among gynecologic cancer survivors and aimed to evaluate the effectiveness of a simple intervention for treatment of urinary incontinence in this population.
Methods
We recruited 40 gynecologic cancer survivors who reported urinary incontinence on a validated questionnaire. Women were randomized to either pelvic floor muscle training/behavioral therapy (treatment group) or usual care (control group). The primary outcome measure, assessed at 12 weeks post intervention, was a 40% difference in the validated Patient Global Impression of Improvement (PGI-I) score. Fisher’s exact test was used to identify differences between groups for frequency data; two sample t-test was conducted for continuous measurements.
Results
Mean age of this cohort was 57 (range: 37–79). The majority of the survivors had uterine cancer (60%), 18% had received radiation therapy, 95% had received surgical therapy, and 35% had received chemotherapy. At three months, 80% of the treatment and 40% of the control group reported that their urinary incontinence was “much better” or “very much better” as evaluated by the Patient Global Impression of Improvement scale (p = 0.02). Brink’s scores were significantly improved in the treatment group as compared to those of the controls (p < 0.0001). Treatment group adherence was high; the treatment group performed exercises with an average of 22 days/month.
Conclusions
Urinary incontinence negatively affects quality of life, and despite a high prevalence among gynecologic cancer survivors, it is often under-assessed and undertreated. We found a simple intervention that included pelvic floor muscle training and behavioral therapy, which significantly improved cancer survivor’s urinary incontinence.
Keywords: Cancer survivorship, Quality of life, Urinary incontinence
Introduction
There are over one million gynecologic cancer survivors in the United States as of 2009. Each year, approximately 80,000 women are diagnosed with a gynecologic cancer including uterine, cervical, and ovarian and vulvar malignancies [1]. Cancer therapy has improved to the state where many people have curable disease or their cancer is considered a more chronic illness. With these advances have come the challenges of treating long term effects of cancer treatment. Treatment of gynecologic cancer often involves a multimodality approach with radical surgery, pelvic radiation, and/or systemic chemotherapy. All of these therapies cause direct or indirect injury to the pelvic organ anatomy and physiology and can impact pelvic floor function. Our group conducted a cohort study to define the prevalence of pelvic floor disorders in our gynecologic cancer survivors. Of 200 gynecologic cancer survivors that were disease and treatment free for >1 year, 67% of women reported moderate to severe urinary incontinence [2]. In contrast, in the general female population, the prevalence of urinary incontinence is estimated to range between 10 and 40% [3].
Treatment for urinary incontinence includes behavioral, medical or surgical interventions. The most common nonsurgical treatment for incontinence is pelvic floor muscle training (PFMT) or “Kegel’s” exercises, named after the first physician to describe pelvic floor exercises as well as behavioral interventions regarding fluid intake, control and avoidance of constipation [4,5]. In 1948, Kegel reported a success rate of 84% in treating various types of incontinence with pelvic floor muscle training and behavior interventions. Despite proven effectiveness in the general female population, the effectiveness of PFMT and behavioral therapy, both being simple interventions, has not been evaluated in gynecologic cancer survivors. The effectiveness of these interventions may differ in cancer survivors since radiation, chemotherapy and radical pelvic surgery can result in significant anatomical functional changes in the pelvis and lower urinary tract, including damage of nerve fibers and compromise of vascular supply with resultant fibrosis. Data are lacking evaluating treatment options for gynecologic cancer patients who are incontinent. In this pilot study, we aimed to evaluate the effectiveness and feasibility of a simple intervention, pelvic floor exercise training and behavioral therapy, for the treatment of urinary incontinence among gynecologic cancer survivors. We hypothesized that cancer survivors randomized to a behavioral intervention would demonstrate improved continence and quality of life.
Materials and methods
This study was performed at the University of New Mexico through the Department of Obstetrics and Gynecology. Institutional review board approval was obtained and all women gave written informed consent. Participants were women who attended the gynecologic oncology clinics for routine surveillance visits who were ≥ 30 years old and had a history of uterine, cervical, ovarian, or vulvar cancer. All participants had been disease- and treatment-free for at least one year and currently had no evidence of cancer. Eligible patients based on cancer history and treatment free interval were then screened for urinary incontinence using the Incontinence Severity Index (ISI), a validated symptom severity scale, to determine the presence of urinary incontinence [6]. The ISI Scale categorizes mild incontinence for scores 1–2, moderate incontinence for scores 3–4, and severe incontinence for scores 6–8. If women had any degree of urinary incontinence, defined as a score >0, they were offered enrollment in the study. All study participants completed the Questionnaire for Urinary Incontinence Diagnosis (QUID) at enrollment. The QUID is a validated 6-item questionnaire for female urinary incontinence type diagnosis [7]. Participants also completed the Urinary Distress Inventory (UDI-6) and the Incontinence Impact Questionnaire (IIQ-7), which measure the bother from incontinence as well as its impact on quality of life, respectively. Patient demographics, cancer history, cancer treatment, surgical history, and previous incontinence treatments were recorded by the provider at the initial visit. Participants were then randomized to the treatment group or the usual or control group. Randomization assignment was by a research staff not involved in the clinical care of the patients. All randomization assignments were kept in sealed opaque envelopes, which were opened when women met inclusion criteria and gave written consent. The randomization assignments were generated from a random number table using the random allocation rule. If randomized to the treatment group, the participant began the training program on the first visit day. Women were given a handout and instruction describing behavioral management tips for urinary incontinence. This included information and suggestions about optimal volume fluid intake, constipation management, measures to reduce urinary urgency by decreasing fluid intake, and avoiding caffeine and other bladder irritants that have proved effective in other intervention trials [8]. The provider then conducted a training session during the clinic visit designed to teach the participant to contract her pelvic floor muscles correctly. The training session required approximately 15 min. The provider confirmed appropriate contraction of the pelvic floor by palpation of the levator ani during a contraction and rated the strength of the contraction using the Brink’s scale. The Brink’s scale rates pelvic floor contractions from 3 to 12 and has been validated for the evaluation of pelvic floor strength. Appropriate feedback was given to avoid contraction of abdominal, gluteal, or adductor muscles. The provider performing the training attended two pelvic floor physical therapy sessions with experienced pelvic floor physical therapists. The pelvic floor muscle training program was explained to the participant verbally and in written form. The training program consisted of the participant performing 10 pelvic floor muscle contractions with a goal of holding the contraction for 5 s; women were asked to perform 3 sets daily for the twelve week study period. To promote adherence to the training program, the participants in the training group received a reminder phone call approximately four weeks after the first study visit. The phone call reviewed the training instructions and addressed any concerns or questions the participant had. If randomized to the control group, the participant did not have the above training program and did not undertake exercises. This is representative of usual care in our gynecologic oncology clinics. The control participants completed the same questionnaires as the treatment group participants both at enrollment and at 12 weeks and underwent assessment of pelvic floor muscle strength using the Brink’s scale. Because incontinent women may be interested in treatment, we did offer the training program to the women in the control group after they completed the study.
Twelve weeks after randomization, the participants returned for the second study visit. At this visit, participants completed questions regarding treatment compliance such as how many exercises they performed per day, and how well they complied with the exercise program. Participants also completed the validated Patient Global Impression of Improvement scale. In addition, they also completed the ISI, QUID, UDI-6, and IIQ-7 questionnaires. The trainer also completed a Brink’s scale at both visits to evaluate the strength of the participant’s contractions.
The control group did not undergo the above training program or receive the behavioral therapy handouts.
The primary outcome, assessed at 12 weeks, was improvement in Patient Global Impression of Improvement (PGI-I) rating. The PGI-I is a validated single item that asks the participant to rate improvement of her continence status using a seven-point Likert scale. The PGI-I global index is capable of reflecting a woman’s overall appraisal of her condition and response to treatment. The PGI-I tool when developed correlated significantly with incontinence episode frequency, stress pad tests and disease specific quality of life questionnaires [9] Participants were considered “successfully” treated if they report that they are “very much better” or “much better”. All other response options were defined as treatment failures. Secondary outcome measures included the change in the ISI, UDI, and IIQ scores which measure the impact of urinary incontinence on quality of life [10]. The Brink’s scale was used by trainers for the treatment group visits to evaluate the strength of the contractions in a qualitative manner [11].
Statistical tests
Power analysis was performed. We anticipated a dropout rate of 10%. Previous studies suggest that approximately 23% of the usual care subjects will report substantial improvement. If this holds, then a two- sided t-test at the 5% level would have 60% power to detect a 40% improvement in the treatment group (i.e. an increase from 25% to 65%), and 80% power to detect a 48% increase in improvement with group sizes of 18. Baseline demographic and clinical characteristics were compared between the intervention and control groups with the use of the two sample t-test and the Mann–Whitney test for continuous measurements with and without normal distribution, respectively. The Fisher’s exact test was used to identify difference between groups for frequency data. Two-sided p values b 0.05 were considered to indicate statistical significance. All statistical analyses were performed using SPSS version 17.0. The trial was registered with Clinicaltrials.gov NCT01806350.
Results
Ninety-eight women were screened for enrollment into the study. Fifty-eight women were excluded secondary to ineligibility because they did not report urinary incontinence. The remaining 23 women refused to participate for various reasons; the most common reason for not participating was that they did not feel their incontinence warranted treatment. Forty women who underwent initial evaluation were randomly allocated into two groups: 20 participants were assigned to the treatment and 20 participants to the control group (Fig. 1).
Fig. 1.
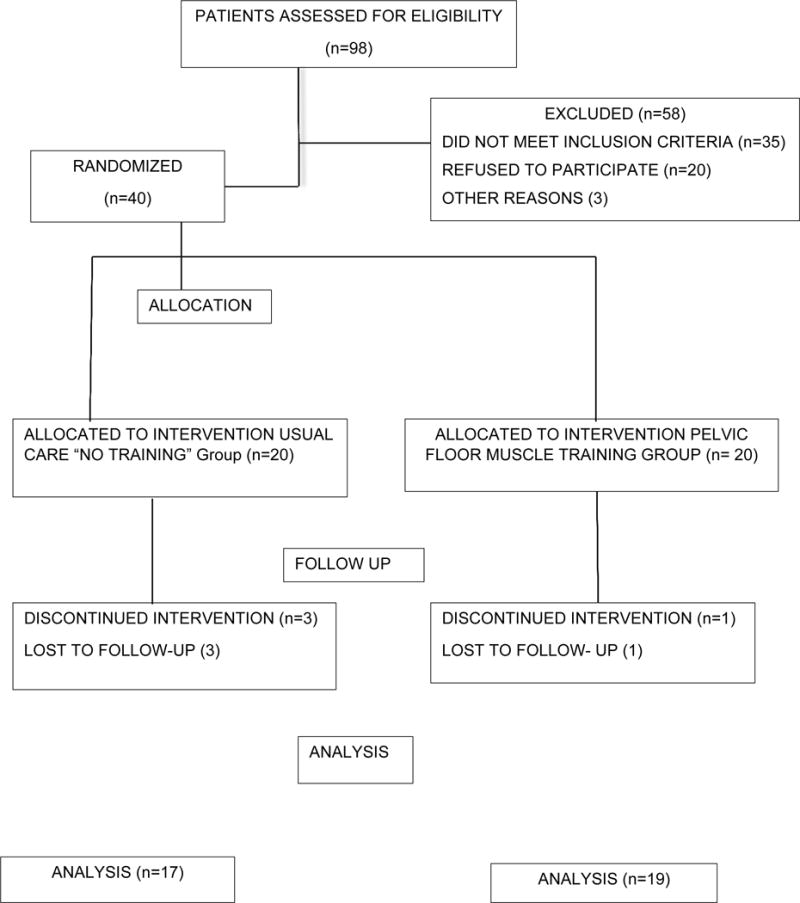
Consort diagram
The baseline characteristics are summarized in Table 1. There were no differences in the two groups in any of the baseline descriptive characteristics. The mean age for the entire cohort was 57 ± 7.2 years (range: 37–79). Obesity was common in both groups, with a mean BMI in the control group of 31 ± 8.7 and in the PFMT group of 35 ± 9.7, p = 0.19. The most prevalent cancer was endometrial (24 participants, 60%), followed by ovarian (9 participants, 23%) and cervical (5 participants, 13%). Eighteen percent had received radiation therapy as part of their cancer therapy. The median interval between cancer treatment and enrollment was 2.5 years (range 1–5). Stress urinary incontinence was the most common type of incontinence on the QUID questionnaire representing 70% of the study population and mixed incontinence symptoms were seen in 25% of participants.
Table 1.
Patients characteristics.
| Demographic detail | PFMT group N = 20 | Control group N = 20 | P value |
|---|---|---|---|
| Age (yrs) mean ± SD | 57.9 ± 6.6 | 57.5 ± 7.5 | 0.9010 |
| Race/Ethnicity (%) | Native American 3 (15%) African American 1 (5%) Caucasian 11 (55%) Hispanic 5 (25%) |
Native American 1 (5%) African American 0 (0%) Caucasian 14 (70%) Hispanic 5 (25%) |
0.6333 |
| Smoking (%) | Yes 3 (15%) No 17 (85%) |
Yes 3 (15%) No 17 (85%) |
1.0000 |
| Parity (mean ± SD) | 1.8 ± 0.9 | 2.0 ± 1.1 | 0.6780 |
| BMI (mean ± SD) | 31.2 ± 2.0 | 35.1 ± 1.9 | 0.1900 |
| % Using HRT | 3 (15%) | 2 (10%) | 0.6614 |
| % Hysterectomy/BSO | 18 (90%) | 18 (90%) | 1.0000 |
| Type of cancer | |||
| - Uterine % | 14 (70%) | 10 (50%) | 0.6996 |
| - Ovarian % | 3 (15%) | 6 (30%) | |
| - Cervical % | 2 (10%) | 3 (15%) | |
| - Other % | 1 (5%) | 1 (5%) | |
| Time since treatment (yrs) (mean ± SD) | 2.3 ± 1.5 | 2.8 ± 1.9 | 0.2869 |
| % prior pelvic surgery treatment | 19 (95%) | 19 (95%) | 1.0000 |
| % radiation treatment | 3 (15%) | 4 (20%) | 1.0000 |
| % chemotherapy | 7 (35%) | 7 (35%) | 1.0000 |
| % prior incontinence treatment | 2 (10%) | 4 (20%) | 0.6614 |
Women in the treatment group judged their compliance with the exercise therapy to be ‘excellent’ or ‘good’ in 75% of cases, ‘poor’ in 20%, while in the remaining 5% the exercises were not performed at all. There were no significant differences in demographic characteristics between compliant vs. noncompliant participants. For analyses women remained in their assigned treatment groups for an intent-to-treat analysis.
The results of the subjective assessment after three months are shown in Table 2. The majority of women in the treatment group reported improvement in their urinary incontinence. At three months, 80% of the PFMT group and 40% of the control group reported that their urinary incontinence was “much better” or “very much better” as evaluated by the Patient Global Impression of Improvement scale (p = 0.02).
Table 2.
Patient Global Impression of Improvement scale (PGI-I).
| Outcome measure | PFMT group N = 20 | Control group N = 20 | p value |
|---|---|---|---|
| PGI-I % successful score | |||
| “Much better” or “Very much better” | 16 (80%) | 8 (40%) | |
| “No change” of “worse” | 4 (20%) | 12 (60%) | 0.025 |
Most study participants had stress predominant urinary incontinence based on the QUID responses, 80% in the PFMT group and 85% in the control group. At three months, 70% of the PFMT group and 50% of the control group reported lack of bother from their urinary incontinence, as assessed by UDI-6, p = 0.62, not different. The condition specific quality of life scores (IIQ-7) were not different between the two groups as compared pre and post intervention.
The median ISI score for the treatment group was 3 (± 0.8) compared with the control group median ISI score which was 4 (± 0.6), both scores indicate moderate to severe urinary incontinence [6]. The treatment group did demonstrate improvement in the ISI score after three months. Prior to treatment 7 women reported mild urinary incontinence and 13 women reported moderate/severe incontinence and after the three month PFMT program 8 women reported moderate/severe and 12 reported mild urinary incontinence (Fig. 2).
Fig. 2.
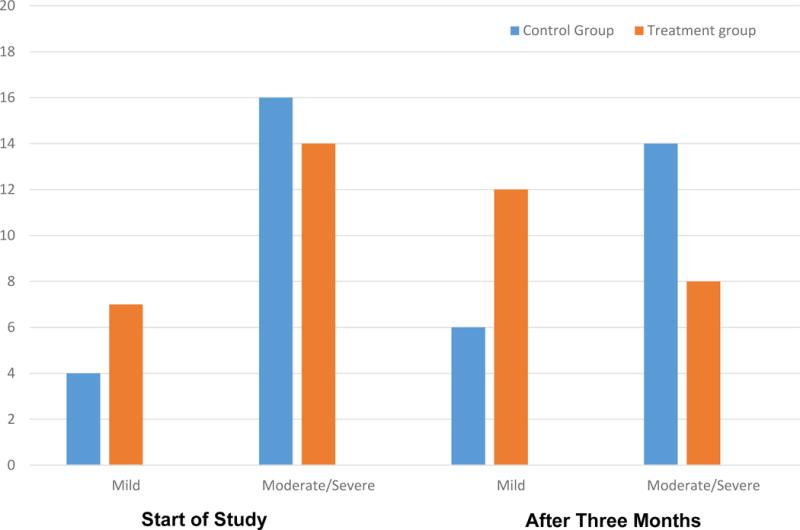
Change in the Incontinence Severity Index (ISI) score in control group and treatment group before and after intervention
The treatment group demonstrated significant improvement in the measure of pelvic floor muscle function as measured by the Brink’s score (p = 0.0001). The average Brink’s score was 3 points higher in the treatment group at the three month evaluation compared with the study enrollment score.
Discussion
This pilot study demonstrated that a three-month pelvic floor muscle training program in combination with behavioral therapy resulted in significant improvement in urinary incontinence symptoms for gynecologic cancer survivors; 80% of survivors felt their condition had improved or was cured versus a 40% improvement in the control group who received usual care. PGI-I, ISI and Brink’s scores all demonstrated significant improvement for the treatment group in our study. We did not see improvement in condition specific quality of life or bother from incontinence, although it is not uncommon for quality of life measure changes to lag behind symptom improvement. This is one of the few studies evaluating the treatment of urinary incontinence in gynecologic cancer survivors, although these interventions have already proved effective in the general population. In this pilot study, we set out to evaluate the feasibility and effectiveness of introducing urinary incontinence screening and treatment into a gynecologic oncology practice. We chose to evaluate a simple intervention, pelvic floor exercise training and behavioral therapy, so that our results and evaluation could be implemented into routine gynecologic oncology care. We have found that this multimodal behavioral therapy was effective in this population and was feasible to perform. We are not able to separate out which aspect of the intervention was more effective but combined therapy is most often instituted in clinical practice. Symptoms of urinary incontinence are often ignored during routine cancer surveillance visits; appropriately oncologists are typically more focused on cancer prevention and cure rather than on changes in quality of life which may significantly affect survivors. We feel that our results highlight the importance of recognizing this prevalent problem in our patients and the simplicity of introducing a simple teaching session of PFMT can have an impact on patient symptoms.
Some studies have found that a third of women are not able to contract the pelvic floor musculature correctly [4, 5]. In this pilot study, we conducted a brief teaching session including tips on correct contraction techniques and we used vaginal palpation at the first evaluation to teach and confirm the ability to perform a correct contraction. This feedback was performed during the pelvic exam for the patient’s cancer assessment.
Urinary incontinence affects quality of life in a multitude of ways and has been associated with social isolation, poor self-rated health, decreased psychological well-being, and impaired sexual relations [12, 13]. Gynecologic cancer survivors have a significant risk of developing urinary dysfunction and incontinence following treatment for their cancer. This is supported not only by our own results but also by other reports. One descriptive study including 70 endometrial cancer patients evaluated the prevalence of urinary symptoms and the effect incontinence had on post treatment quality of life. The survey found that over 80% of women after treatment for endometrial cancer reported urinary incontinence. The use of adjuvant radiation therapy was associated with more severe incontinence symptoms and greater impact on overall QOL when compared to women who did not require radiation therapy [14].
This study has limitations. First, the study was conducted at a single center with a small sample size. Second, the duration and intensity of the treatment program was short to accommodate study feasibility and may have been inadequate to verify the effects of exercise. Third, the evaluator at the twelve week visit was not blinded to the treatment arm for assessing pelvic floor muscle strength. Finally, our sample size is too small to evaluate the effects of type of prior treatments, such as radiation and chemotherapy on continence and response to the intervention. The study strengths include random assignment to treatment groups, use of validated outcome measures, use of a simple intervention, and focused on cancer survivors. Cancer survivors were eager to participate in a study aimed at improving their quality of life.
Pelvic floor muscle training has demonstrated effectiveness in women with all types of incontinence [4]. The intervention is simple to teach and is cost effective. A recent systematic review reported that women treated with PFMT were more likely to report cure, improvement, or better quality of life than controls [15]. From our data, we conclude that pelvic floor muscle training and behavioral therapy is an effective intervention for incontinent gynecologic cancer survivors. Studies with longer duration of intervention and follow-up and larger sample size are needed to evaluate the effectiveness and generalizability of this intervention in gynecologic cancer survivors. These studies are needed to help improve cancer survivor’s quality of life after cure.
HIGHLIGHTS.
Urinary incontinence is common among gynecologic cancer survivors
Evaluate pelvic floor muscle training (PFMT) for treatment of urinary incontinence
PFMT is an effective intervention for incontinent gynecologic cancer survivors.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Ries L, Harkins D, Krapcho M, Mariotto A, Miller B, Feuer E, et al. SEER Cancer Statistics Review, 1975–2004. Bethesda: National Cancer Institute; 2007. [cited 2007]; Available from: http://seer.cancer.gov/csr/1975_2004/ [Google Scholar]
- 2.Rutledge TL, Heckman SR, Qualls C, Muller CY, Rogers RG. Pelvic floor disorders and sexual function in gynecologic cancer survivors: a cohort study. Am J Obstet Gynecol. 2010;203(5):514. doi: 10.1016/j.ajog.2010.08.004. Epub 2010/09/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fantl JA, Newman D, Colling J, DeLancey J, Keeys C, Loughery R, et al. In: Urinary incontinence in adults: acute and chronic management. 2nd. Department of Health and Human Services PHS, editor. 1996. [Google Scholar]
- 4.Kegel AH. Progressive resistance exercise in the functional restoration of the perineal muscles. Am J Obstet Gynecol. 1948;56(2):238–48. doi: 10.1016/0002-9378(48)90266-x. Epub 1948/08/01. [DOI] [PubMed] [Google Scholar]
- 5.Kegel AH. Physiologic therapy for urinary stress incontinence. JAMA. 1951;146(10):915–7. doi: 10.1001/jama.1951.03670100035008. Epub 1951/07/07. [DOI] [PubMed] [Google Scholar]
- 6.Sandvik H, Hunskaar S, Seim A, Hermstad R, Vanvik A, Bratt H. Validation of a severity index in female urinary incontinence and its implementation in an epidemiological survey. J Epidemiol Community Health. 1993;47(6):497–9. doi: 10.1136/jech.47.6.497. Epub 1993/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley CS, Rovner ES, Morgan MA, Berlin M, Novi JM, Shea JA, et al. A new questionnaire for urinary incontinence diagnosis in women: development and testing. Am J Obstet Gynecol. 2005;192(1):66–73. doi: 10.1016/j.ajog.2004.07.037. Epub 2005/01/27. [DOI] [PubMed] [Google Scholar]
- 8.Burgio KL, Kraus SR, Menefee S, Borello-France D, Corton M, Johnson HW, et al. Behavioral therapy to enable women with urge incontinence to discontinue drug treatment: a randomized trial. Ann Intern Med. 2008;149(3):161–9. doi: 10.7326/0003-4819-149-3-200808050-00005. Epub 2008/08/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yalcin I, Bump RC. Validation of two global impression questionnaires for incontinence. Am J Obstet Gynecol. 2003;189(1):98–101. doi: 10.1067/mob.2003.379. Epub 2003/07/16. [DOI] [PubMed] [Google Scholar]
- 10.Barber MD, Walters MD, Bump RC. Short forms of two condition-specific quality-of- life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7) Am J Obstet Gynecol. 2005;193(1):103–13. doi: 10.1016/j.ajog.2004.12.025. Epub 2005/07/16. [DOI] [PubMed] [Google Scholar]
- 11.Brink CA, Wells TJ, Sampselle CM, Taillie ER, Mayer R. A digital test for pelvic muscle strength in women with urinary incontinence. Nurs Res. 1994;43(6):352–6. Epub 1994/11/01. [PubMed] [Google Scholar]
- 12.Hunskaar S, Vinsnes A. The quality of life in women with urinary incontinence as measured by the sickness impact profile. J Am Geriatr Soc. 1991;39(4):378–82. doi: 10.1111/j.1532-5415.1991.tb02903.x. Epub 1991/04/01. [DOI] [PubMed] [Google Scholar]
- 13.Johnson TM, 2nd, Kincade JE, Bernard SL, Busby-Whitehead J, Hertz-Picciotto I, DeFriese GH. The association of urinary incontinence with poor self-rated health. J Am Geriatr Soc. 1998;46(6):693–9. doi: 10.1111/j.1532-5415.1998.tb03802.x. Epub 1998/06/13. [DOI] [PubMed] [Google Scholar]
- 14.Erekson EA, Sung VW, DiSilvestro PA, Myers DL. Urinary symptoms and impact on quality of life in women after treatment for endometrial cancer. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(2):159–63. doi: 10.1007/s00192-008-0755-z. Epub 2008/11/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumoulin C, Hay-Smith J. Pelvic floor muscle training versus no treatment for urinary incontinence in women. A Cochrane systematic review. Eur J Phys Rehabil Med. 2008;44(1):47–63. Epub 2008/04/04. [PubMed] [Google Scholar]


