ABSTRACT
Population density and associated behavioral adjustments are potentially important in regulating physiological performance in many animals. In r-selected species like the fruit fly (Drosophila), where population density rapidly shifts in unpredictable and unstable environments, density-dependent physiological adjustments may aid survival of individuals living in a social environment. Yet, how population density (and associated social behaviors) affects physiological functions like metabolism is poorly understood in insects. Additionally, insects often show marked sexual dimorphism (larger females). Thus, in this study on D. melanogaster, we characterized the effects of fly density and sex on both mass-specific routine oxygen consumption (V̇O2) and hypoxia tolerance (PCrit). Females had significantly lower routine V̇O2 (∼4 µl O2 mg−1 h−1) than males (∼6 µl O2 mg−1 h−1) at an average fly density of 28 flies·respirometer chamber−1. However, V̇O2 was inversely related to fly density in males, with V̇O2 ranging from 4 to 11 µl O2 mg−1 h−1 at a density of 10 and 40 flies·chamber−1, respectively (r2=0.58, P<0.001). Female flies showed a similar but less pronounced effect, with a V̇O2 of 4 and 7 µl O2 mg−1 h−1 at a density of 10 and 40 flies·chamber−1, respectively (r2=0.43, P<0.001). PCrit (∼5.5 to 7.5 kPa) varied significantly with density in male (r2=0.50, P<0.01) but not female (r2=0.02, P>0.5) flies, with higher fly densities having a lower PCrit. An extensive survey of the literature on metabolism in fruit flies indicates that not all studies control for, or even report on, fly density and gender, both of which may affect metabolic measurements.
KEY WORDS: Drosophila, Oxygen consumption, Sex, Social interaction, Respirometry techniques
Summary: Technical advances allowing oxygen consumption measurement in individual fruit flies actually take them out of their normal highly social context, resulting in higher oxygen consumption rates than in natural groups.
INTRODUCTION
The metabolic rate of the fruit fly Drosophila has been measured in numerous studies, with numerous intentions. Investigators have long used the fruit fly as a model for investigations of life span, and accordingly have measured metabolic rate in an often unsuccessful attempt to correlate metabolism to longevity (Arking et al., 1988; Baldal et al., 2006; Hulbert et al., 2004; Khazaeli et al., 2005; Melvin et al., 2007; Miquel et al., 1982; Partridge et al., 2005; Promislow and Haselkorn, 2002; Van Voorhies et al., 2003, 2004b). Metabolic rate in Drosophila has also been investigated in the context of specific genotypes (Hoekstra and Montooth, 2013; Jensen et al., 2014; Jumbo-Lucioni et al., 2010; Montooth et al., 2003; Stone et al., 2013), to reveal how genotype underpins specific metabolic phenotypes. Metabolic rate has also been measured for insight into how specific metabolic pathways affect energy metabolism (Barros et al., 1991; Isobe et al., 2013). The study of function and limitations of gas exchange by a tracheal system have also involved metabolic rate measurements (Klok et al., 2010; Merkey et al., 2011; Mölich et al., 2012). Finally, but not exhausting the list of reasons for measuring metabolic rate in Drosophila, a driver for such studies has been comparative physiological aspects including the effects of temperature or oxygen as stressors (Berrigan and Partridge, 1997; Folguera et al., 2010; Isobe et al., 2013; Lighton, 2007; Orr, 1925; Skandalis et al., 2011; Van Voorhies, 2009; Williams et al., 2004).
As varied as the rationale for metabolic rate measurements in Drosophila are the methodologies that have been employed. Most common has been the measurement of carbon dioxide emission or the consumption of oxygen, detected by respiratory gas sensors using open respirometry, closed respirometry and intermittent respirometry (for review see Van Voorhies et al., 2008). However, heat production/microcalorimetry (Hulbert et al., 2004; Piper et al., 2014; Van Voorhies et al., 2008) and even doubly labeled water techniques (Piper et al., 2014) have been employed. Until the last decade or so, most measurements were on small groups of flies, necessary to assemble sufficient biomass for accurate measurement of metabolic rate. In recent years, however, sensitivity of instrumentation has grown to the point that metabolic rate measurement on single flies is routine.
Not surprisingly, given the variety of approaches to measuring metabolic rate and the various protocols used, a nearly 200-fold variation exists in estimates of routine metabolic rate in Drosophila. The reasons for this variation have typically been attributed to differences in techniques (Van Voorhies et al., 2008). Yet, a myriad of biotic reasons can account for variability in comparative physiological studies, including sex, prandial state, time of day, and history including epigenetic influences (Burggren, 2014).
One factor of potentially great importance in metabolic rate in Drosophila is behavior, and especially social interaction between individuals. Drosophila is an insect with fairly stereotypic and well-studied social interactions, including numerous sex-specific behaviors (Mowrey and Portman, 2012; Portman, 2007; Schneider et al., 2016; Villella and Hall, 2008; Yamamoto et al., 2014). Given the nature of the behavioral interactions between individuals, one might anticipate that social interactions and the associated stereotypic behaviors in Drosophila might also directly or indirectly influence their metabolic rate. However, the technology-enabled trend to single fly analyses has, for better or worse, eliminated social interaction as a variable. Indeed, as Piper et al. (2014) so aptly commented, “(metabolic chamber) measurements require separating individuals from any social context, and may only poorly reflect the environment in which the animals normally live”. Yet, few previous studies have addressed the potentially complex relationships between social interaction and metabolic rate specifically in Drosophila.
Another poorly controlled variable in the measurement of metabolic rate in Drosophila is sex. There is considerable sexual dimorphism in Drosophila, with females being as much as 40-50% heavier than males (Piper et al., 2014). Yet, the majority of studies have tended to ignore sex in their experimental groups or, alternatively, have used either all females or all males in their metabolic measurements. These approaches either obscure the effects of social interactions when multiple flies are assessed, or eliminate them when single flies are subject to experimentation.
Given the key importance of Drosophila as an animal model, and the prominence of metabolic rate measurements in current studies, there is a compelling need to understand biotic sources of variation in measurements of metabolic rate. Consequently, we have measured oxygen consumption in D. melanogaster independently in males and females, and as a function of density (number of flies per respirometer chamber). Specifically, we hypothesized that oxygen consumption in D. melanogaster would be influenced by both social interactions and by sex. We additionally measured Pcrit, the partial pressure of oxygen at which oxygen consumption begins to decline, as this variable is an indicator of hypoxic tolerance.
RESULTS
Body mass
Wet body mass was significantly higher (P<0.02) in adult female flies (0.693±0.050 mg) compared with adult male flies (0.505±0.047 mg) (Fig. 1). Similarly, dry body mass was significantly higher (P<0.009) in adult female flies (0.211±0.012 mg) compared with adult male flies (0.161±0.008 mg).
Fig. 1.
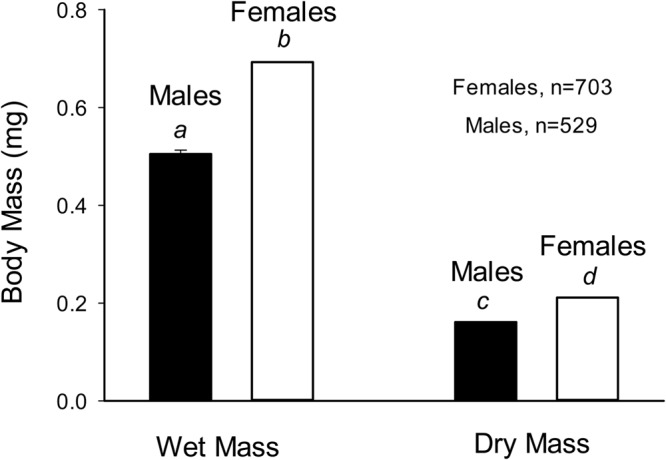
Wet and dry body mass in female and male D. melanogaster. Mean±s.e. are plotted, but standard errors are too small to be visible (see text). N=703 for females, 529 for males. Different lower case italic letters indicate significance differences between groups at the P<0.01 level (t-test).
Mass-specific routine oxygen consumption
Mass-specific V̇O2, based on either wet or dry mass, are shown for male and female adult D. melanogaster in Fig. 2. V̇O2 expressed for wet mass in males and females was ∼6.0 and 4.5 µl O2 mg−1 h−1, respectively. There was no significant difference (P>0.05) between V̇O2 in males and females, based on either wet or dry mass, when pooling all respirometry data irrespective of density of flies in the respirometer.
Fig. 2.
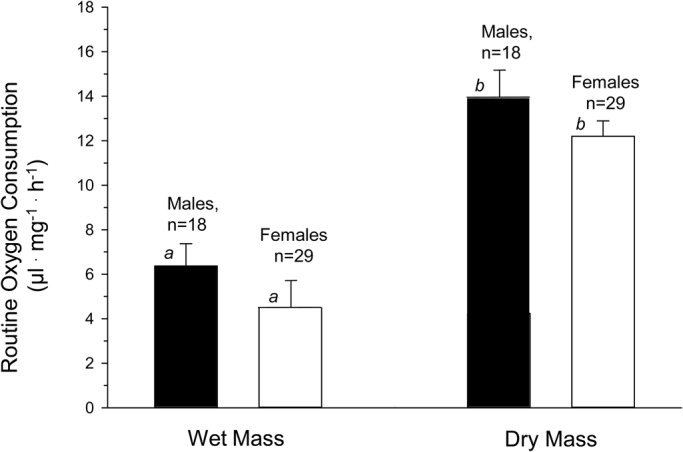
Wet and dry mass-specific routine oxygen consumption (V̇O2) in male and female D. melanogaster. Mean values±s.e. are presented. Also indicated are n values, where each value contributing to n is a separate trial comprising multiple flies in each respirometer. The average number of flies in the respirometer (density) for n runs, was 18±3 for males and 29±3 for females. Different lower case italic letters indicate significance differences between groups at the P<0.01 level (t-test).
This general approach of pooling all data obfuscates sex differences that emerge when also controlling for fly density. Fig. 3 shows the relationship between mass-specific V̇O2 as a function of fly density within the respirometer. Both male and female flies exhibited greatly decreased V̇O2 as fly density increased. V̇O2 decreased to just 15% and 40% in males and females, respectively, as fly density increased from 10 to 50 flies·respirometer chamber−1. Assuming a linear relationship, extrapolation of the relationship between density and V̇O2 back to density of one fly per respirometer indicated a profound sex-based difference in V̇O2, with a hypothetical single male having a V̇O2 of ∼14 µl O2 mg−1 h−1 but only ∼8 µl O2 mg−1 h−1 in a single female. The sex-dependent difference in the V̇O2 relationship is underscored by the fact that the regressions relating V̇O2 to density in males and females had significantly different slopes (−0.27 males, −0.12 females; P=0.017) and intercepts (13.87 males, 7.84 females; P=0.001). Importantly, these decreases in metabolic rate are not a function of decreased PO2 within the respirometers related to crowding – all data reported in Fig. 3 were recorded in a ‘normoxic’ PO2 above 16 kPa.
Fig. 3.
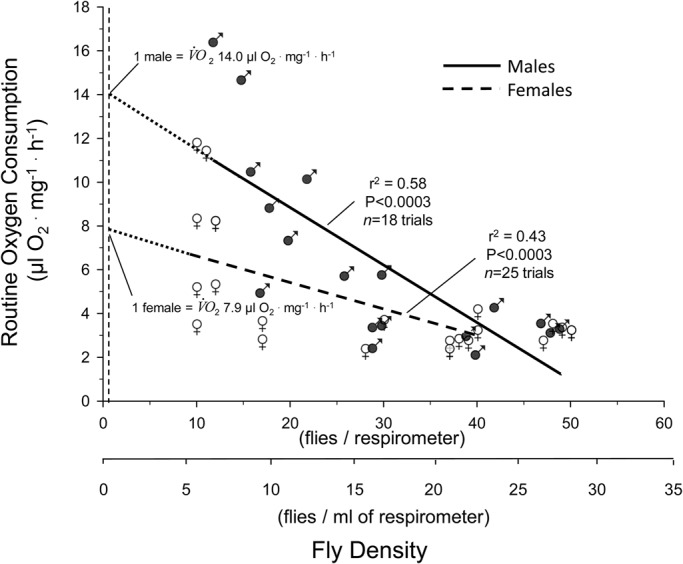
Mass-specific routine V̇O2 in normoxia as a function of density in male and female D. melanogaster. Density is shown on both a per respirometer and per ml of respirometer basis. Separate linear regressions for male (n=18) and female (n=25) flies are provided, along with the correlation coefficient and P value for each. Dashed lines represent the extrapolation of each relationship back to the value for a single fly. The slope of the two lines was significantly different (P<0.02, least squares method).
Noteworthy is that extrapolation of these V̇O2 values back to the origin to predict values for single flies is based on a highly significant (P<0.003) linear regression. Use of a quadratic equation to describe the existing data produces a similarly high level of significance (P<0.001), but does not render realistic values when extrapolated back beyond the existing data towards the origin for determination of values for individual flies.
Critical partial pressure (PCrit)
Critical partial pressure (PCrit) was determined in 14 separate trials involving 211 flies of both sexes. Fig. 4A shows a scatterplot of the acquired data, indicating that most flies ceased to consume oxygen at around 2 kPa. Fig. 4B shows a representative trial of 17 female flies, yielding a PCrit of 5.9 kPa for this respirometry trial.
Fig. 4.
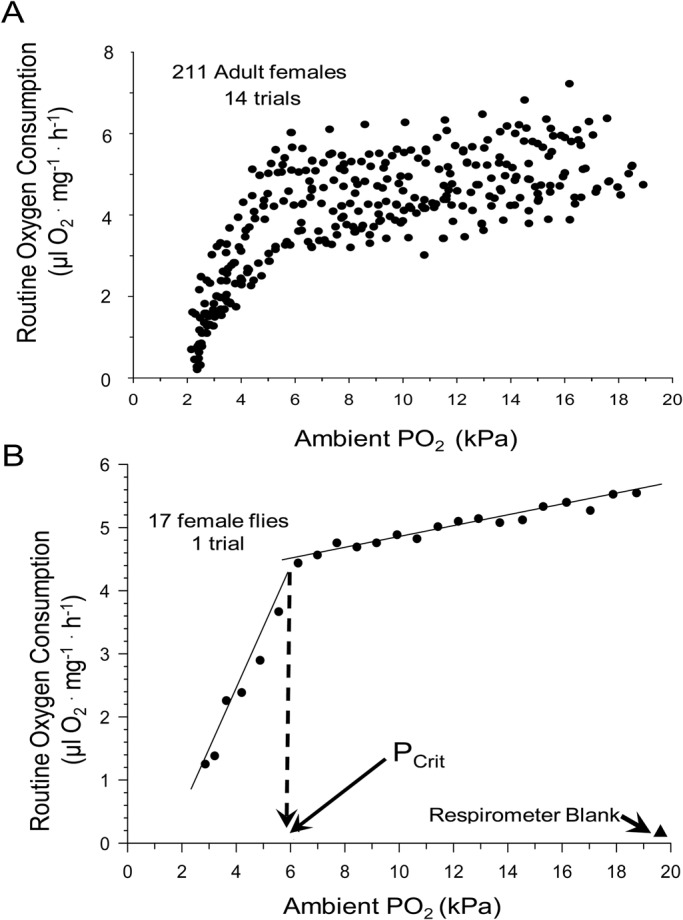
PCrit determination in adult D. melanogaster. (A) A scatter plot of 11 PCrit trials (211 females in total). (B) Representative routine V̇O2 data derived from a single experimental trial of a respirometer containing 17 female flies. Also shown are the values for a respirometer blank. The intersection of the two linear regressions indicates the PCrit value, identified on the X-axis by the vertical dashed arrow (see text for details of PCrit calculation and statistics).
PCrit as a function of fly density in the respirometry chambers is shown in Fig. 5. PCrit in female flies was ∼5-6 kPa, with the extrapolated value for a single fly being 5.6 kPa. For females, density had no significant effect on PCrit (P>0.05); however, PCrit was highly dependent upon fly density for males (P<0.005), with values ranging from ∼5.5 kPa at 50 flies chamber−1 up to an extrapolated value of 8.5 kPa for a single fly per chamber.
Fig. 5.
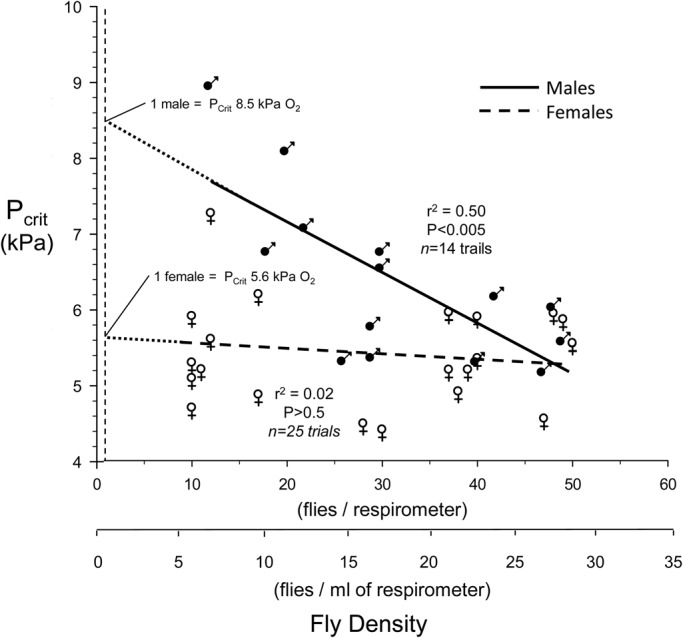
PCrit as a function of respirometer fly density in male and female D. melanogaster. Density is shown on both a per respirometer and per ml of respirometer basis. Separate linear regressions for male (n=14) and female (n=25) flies are provided, along with the correlation coefficient and P value for each (least squares method). Dashed lines represent the extrapolation of each relationship back to the value for a single fly. The slope of the line for female flies was not significant (P>0.05), indicating that PCrit in females as lines was unaffected by fly density.
Reflecting the same sex-related patterns for PCrit and fly density, the relationship between PCrit and V̇O2 was not significant in females (P>0.05) but highly significant in males (P<0.001). Thus, in males with a higher routine V̇O2, PCrit was correspondingly higher (Fig. 6).
Fig. 6.
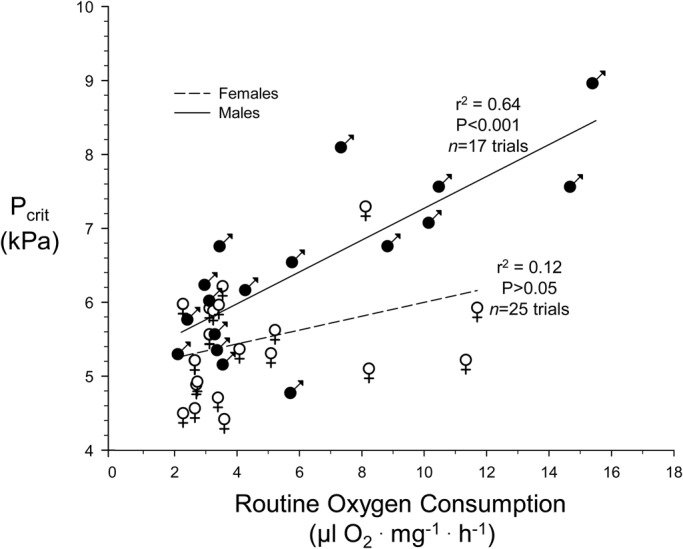
Relationship between PCrit and routine V̇O2 in male and female D. melanogaster. N=17 trials for males and 25 trials for females (least squares method). Average fly density of all trials was 29±2 flies respirometer−1.
Prandial effects on routine V̇O2 and PCrit
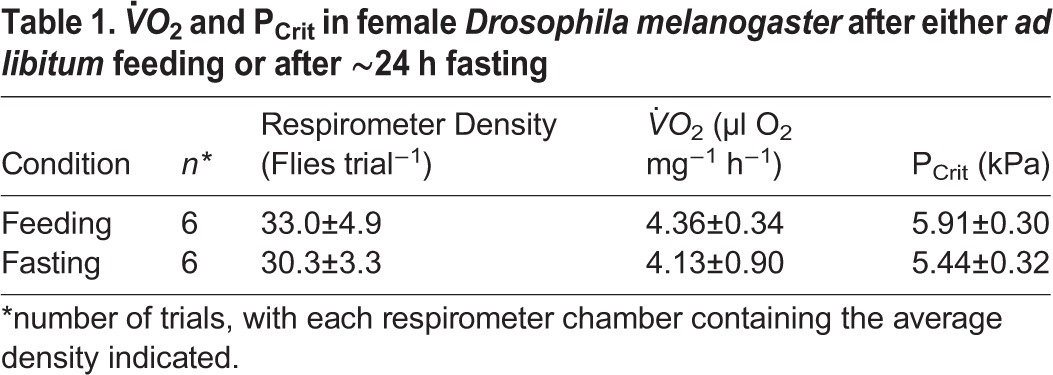
The effects of 24 h of fasting on V̇O2 and PCrit values in D. melanogaster fed ad libitum and fasted for 24 h are provided in Table 1. A t-test indicated that fasting to this extent had no significant effect on either variable.
Table 1.
V̇O2 and PCrit in female Drosophila melanogaster after either ad libitum feeding or after ∼24 h fasting
DISCUSSION
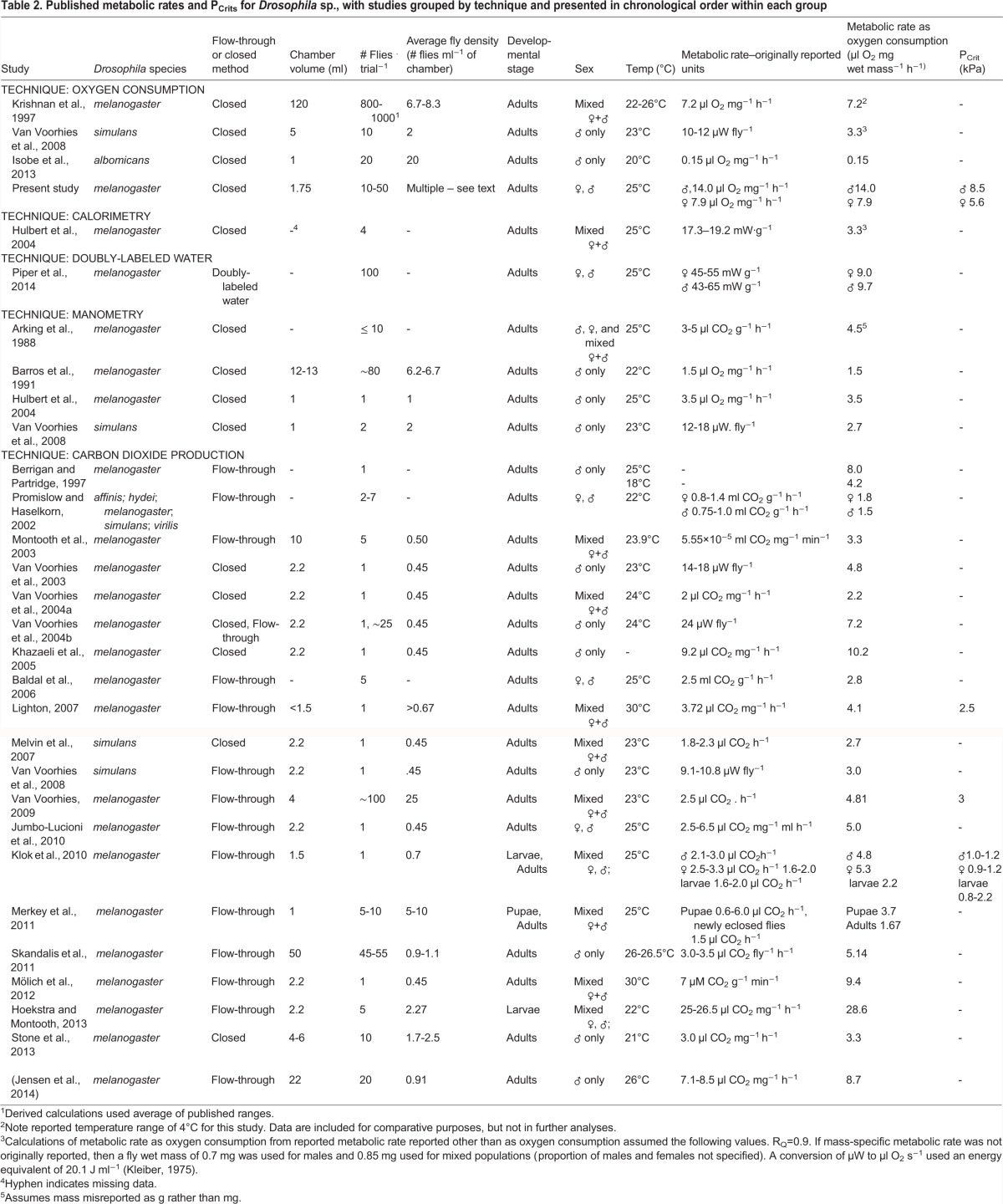
Interpretation of the experimental data from the present study is best done against the backdrop of the extensive, yet highly variable and contradictory, literature on the metabolic rate of Drosophila spp. Consequently, we have assembled published data for this species (Table 2). Noteworthy is that not all listed studies reported temperature, gender or sufficient data to calculate fly density in the respirometers during oxygen consumption measurement, preventing a robust meta-analysis.
Table 2.
Published metabolic rates and PCrits for Drosophila sp., with studies grouped by technique and presented in chronological order within each group
Sex and oxygen consumption
The specific influence of sex on metabolic rate is not clear from our survey of the literature (Table 2). A relatively early study of the responses of D. melanogaster to anoxia concluded that there were no physiological differences between sexes (Krishnan et al., 1997). In contrast, while statistics were not explicitly given, the metabolic rate (measured as CO2 production) of isolated individual female flies appeared to be anywhere from 30-40% higher (Van Voorhies et al., 2004b) to 200-300% higher (Van Voorhies et al., 2004a) than males under the same conditions. Another study indicates that there is no significance difference in V̇O2 between male and female flies measured at a density of 1 fly ml−1 of respirometer (Klok et al., 2010). The present study suggests that at higher respirometer densities there is little or no difference between oxygen consumption in males and females, but there is a significant effect of density such that as fly density declines, males begin to exhibit higher metabolic rates than females (Fig. 3). The discrepancy among studies may be due to the interaction of sex and factors such as rearing condition (mixed sex, density, food source), fly age, or female condition (virgin versus non-virgin). In aggregate, only about one half of the studies identified in our survey (Table 2) actually control for sex, with many studies using unsorted mixtures of male and female flies. Clearly, future studies that control for and investigate sex of the flies are called for.
Critical PO2 for oxygen consumption
The critical PO2 for oxygen consumption, PCrit, provides an indication of the ability of the animal to maintain its V̇O2 as ambient PO2 declines, and is often viewed as an indicator of tolerance to acute hypoxia. PCrit is much less frequently measured than metabolic rate itself because of the necessity of a more involved experimental protocol. Indeed, our survey revealed that PCrit values were measured in only four studies of Drosophila (including the present study), averaging 3.4±1.3 kPa. PCrit values for insects vary widely, but generally fall in the range of 2-8 kPa (Chown and Nicolson, 2004; Greenlee and Harrison, 2004; Harrison et al., 2006; Harrison and Roberts, 2000; Hoback and Stanley, 2001; Lease et al., 2012; Owings et al., 2014). In the current study, PCrit for single flies, determined from extrapolation, was ∼5.6 for females and 8.5 for males. These values, while in line with published values for other insects, are higher than mean value of ∼3.4 kPa derived from the few values revealed in our survey and much higher than the very low estimates of PCrit of less than 1 for adult D. melanogaster measured by Klok and his collaborators (Klok et al., 2010) (Table 2).
One critique of the current methodology of the current for measuring Pcrit was that it required several hours in a closed respirometer for PCrit determination. It is possible that during that measurement period the beginning of desiccation and/or starvation could have begun influencing oxygen consumption or other physiological variables, since these are potentially profound stressors in Drosophila (Gibbs, 2002; Hardy et al., 2015; Masek et al., 2014; Rajpurohit et al., 2016). Consequently, we wish to emphasize not the overall PCrit values for adult D. melanogaster determined in our study, but rather the fact that the values in the present study were dependent on fly density, which is a novel observation. Additionally, the current study is among the first on Drosophila to examine sex-dependence of PCrit in insects. Here we report a large difference in PCrit estimated for individual flies in males (8.5 kPa) compared to females (5.6 kPa). Males not only had a higher PCrit but also showed a sharply decreasing PCrit as fly density in the respirometer increased (Fig. 5). The mechanism underlying these pronounced sex-based differences is not clear. The females of Drosophila are generally larger than males (Fig. 1), which might be thought to lead to slighter larger diffusion distances for O2 within the tracheal system; however, this would lead to higher, rather than lower, PCrit in females. In any event, a systematic examination of the literature has indicated that PCrit in insects is independent of body mass (Harrison et al., 2014; Lease et al., 2012). Another possibility would arise if males have intrinsically higher levels of V̇O2 which are correlated with a higher PCrit (Fig. 6). Yet, in aggregate, there is no significant difference in V̇O2 between males and females (Fig. 2). Clearly, further experimentation is required to verify these findings and determine the basis for sex-based differences in PCrit in Drosophila.
Density-dependence of oxygen consumption V̇O2 and PCrit
Density of flies in respirometers, like sex of the flies examined, varies widely in the literature, with densities ranging from 1-100 flies per respirometer (Table 2). New methodologies with greater sensitivities for measuring metabolic rate, especially using CO2 production, are now enabling metabolic rate measurements in individual flies, which has become the new norm in the last decade (Table 2). This protocol very closely mirrors that used for decades (truly, for centuries) for larger animals, which are typically measured individually, in part because this protocol streamlines the calculations of mass-specific V̇O2. Indeed, the ability to measure metabolic rate in individual flies has been promoted as an advancement “which greatly increases the sample size and statistical power of experimental studies and allows the effects of individual differences in body size to be taken into account” (Van Voorhies et al., 2004b). Indeed, most studies are now done on individual flies (Table 2).
What may be the consequences of social isolation to the measurement of metabolic rate in Drosophila? Clearly, measuring metabolic rate in an individual fruit fly (or ant or other small social insect) is a technical tour de force. Yet at least in the present study a protocol that varies fly density in the respirometer profoundly affects both metabolic rate and PCrit. Although females are less sensitive to the effects of fly density than males, in both sexes the estimated routine mass-specific V̇O2 of an individual, as estimated from extrapolation from multiple fly groups, is much higher than groups of flies. A single previous study that we could identify has investigated the ‘group effect’, investigating the difference between metabolic rate measured in individual flies and in groups of 20 individuals, and reported no difference as a function of density (Van Voorhies et al., 2004b). That study also reported, perhaps not surprisingly, that actual physical confinement (entrapment with cotton balls) elevated metabolic rate by as much as 50%. However, even at the highest densities of ∼50 flies per respirometer in the current study, there was no forced physical contact between flies, nor any physical confinement within the respirometer itself. Importantly, the densities used in the present experiments designed to measure routine oxygen consumption are unlikely to have resulted in any depression of metabolism from the development of hypoxia, because all respirometer runs for routine oxygen consumption were completed after a decline in PO2 of just a few kPa and well before PO2 levels fell below PCrit. Another factor to be considered in contemplating density effects on routine oxygen consumption involves a possible effect of enhanced male aggression, which might lead to elevated rates of oxygen consumption. Yet, one could reasonably expect such aggression to increase as fly density in the respirometers increased, but our findings show quite the opposite effect where increased density leads to reduced routine oxygen consumption.
What, then, could be the mechanism for these high metabolic rates in individual flies or flies in smaller groups from the present study? One explanation is that the high metabolic rates we extrapolate for individual flies represent flies experiencing stress as a result of their social isolation. This begs the question of what is a ‘routine’ metabolic rate for a social insect, that of an individual out of its social context, or that while interacting normally with other individuals? Adult fruit flies are involved in a multitude of social behaviors that often surround courtship and aggression (Eban-Rothschild and Bloch, 2012; Herrero, 2012; Kravitz and Fernandez Mde, 2015; Pavlou and Goodwin, 2013; Villella and Hall, 2008; Yamamoto et al., 2014). While we may think of courtship and aggression as social behaviors that elevate metabolic rate, social isolation of individuals is not normal for fruit flies and may lead to stress (and thus an elevated metabolic rate) equal to or greater than that resulting from normal social behaviors between flies. Indeed, there is a long-established literature, including that oriented towards ‘animal well-being’, e.g. Hirata et al. (2016) and Lyons et al. (1993), that suggests that social isolation can directly elevates physiological rates. This is a broadly based finding for schooling fishes (Nadler et al., 2016; Parker, 1973; Schleuter et al., 2007), birds (Khan et al., 2015; Soleimani et al., 2012) and mammals (Krause and Ruxton, 2002; Martin et al., 1980; Rushen et al., 1999). Generally, the hypothalamic-pituitary-adrenocortical (HPA) system is implicated in these responses (Hennessy et al., 2009; Hernandez et al., 2010; Herskin et al., 2007). Stress-related phenomena associated with social isolation are also being explored in insects, including Drosophila (Kohlmeier et al., 2016; McNeil et al., 2015; Soleimani et al., 2012; Ueda and Wu, 2009), where both short- and long-term influences of isolation on aggressive behavior and basic physiological processes (e.g. neuromuscular excitability, altered cellular ROS regulation) are being revealed. Flies are presumably carrying out interactive behaviors in any study with multiple individuals per respirometer. Olfactory cues are important to the behavior of Drosophila (Eban-Rothschild and Bloch, 2012), with flies likely responding to pheromones or other environmental cues in a respirometer. It is unclear whether this ‘pheromone hypothesis’ would be affected by measurements that use either closed or flow-through (‘open’) respirometry. Given the potency of insect pheromones, including those of Drosophila (Kohl et al., 2015), and the very low flow rates of most flow-through respirometry, it is likely that multiple flies in a respirometer are exposed to pheromones with either technique. Ironically then, the increasing trend to measure metabolic rate in individual fruit flies, in part because we now can, has artificially removed these animals from their normal social context involving a multitude of behavioral interactions largely driven by the release, reception and perception of pheromones and visual and tactile clues (Dweck et al., 2015; Piper et al., 2014; Schneider et al., 2016; Vijayan et al., 2014).
Although beyond the scope of the present experiments, worth mentioning is that consideration of fly density is typically in the context of the individual. Yet, there may be implications of density variation and metabolic rate at the level of the colony in social insects. For example, behavioral organization in colonies of the seed-harvester Pogonomyrmex californicus affects metabolic rate and alterations in growth patterns at the colony-level (Waters et al., 2010). How density within the colony plays into the allometric scaling, activity and growth of whole colonies of social insects will be a highly interesting focus of future studies.
Finally, fly density goes beyond effects on metabolism. Experiments raising larval Drosophila under various group densities discovered an inverse exponential relationship between group density and nervous system morphology, including synaptic bouton numbers, as well as the number and length of axonal branches (Stewart and McLean, 2004). Additionally, there was a direct density-dependence of concentration of Fasciclin-II, a cell membrane glycoprotein important in the process of axonal fasciculation.
Further experimentation is warranted to determine the full extent of group density on the biology of Drosophila, and especially to determine metabolic rate in the presence and absence of cues associated with social behaviors. Additional future experiments on density effects should also establish whether the relationship between respirometer fly density and V̇O2 is in fact a U- or V-shaped curve, with higher densities than used in the current study leading to stress-related increases in V̇O2.
Oxygen consumption and prandial state
The present study discovered no significant difference between adult Drosophila feeding ad libitum and flies starved for 24 h. Similarly, a fasting period of 4 h had no effect on metabolic rate, although the RQ value was decreased by fasting (Van Voorhies et al., 2004b). Collectively, these data suggest that there is little or no specific dynamic action (SDA) in either the short- or long-term. In contrast to these findings, Baldal et al. reported lowered metabolic rate as a result of starvation but, in the long-term across generations, elevated metabolic rates associated with starvation resistance (Baldal et al., 2006). Again, variation exists in the literature, with another long-term study reporting no change in metabolic rate produced by chronic diet restriction (Hulbert et al., 2004). Interestingly, starvation has no effect on the respiration of mitochondria isolated from fasting adults (Partridge et al., 2005). Clearly, more experiments are required to understand the full interactions between metabolic rate and prandial state in Drosophila.
A complex set of interactions occurs between various activities, desiccation, starvation/feeding and numerous physiological processes (Hardy et al., 2015; Masek et al., 2014; Rajpurohit et al., 2016; Slocumb et al., 2015). How fly density may interact with these variables is currently not well understood. Future studies to both tease apart these factors as well as explore synergies are highly warranted.
Conclusions
This study is the first to systematically report on the effect of density (and thus social interaction) on the metabolic rate and critical O2 partial pressure of Drosophila. Highly significant effects of respirometer fly density are evident for both physiological parameters. Values of V̇O2 determined in the present study are within the range of those previously published for Drosophila. Our findings, plus examination of previously published data (Table 2), suggests that greater attention be paid to controlling for (and reporting) not only respirometer density, but also sex, temperature and genetic strain.
MATERIALS AND METHODS
Maintenance and identification
Fruit flies D. melanogaster (Meigen, 1830) (wild-type, Oregon R strain, Carolina Biological Supply Company, Burlington, NC, USA) were grown and maintained at 25°C in a 14 h light:10 h dark photoperiod. Flies were kept in standard densities of ∼150 flies per 250 ml volume plastic vial, with ∼30 ml of standard Drosophila medium plus Baker's yeast (Formula 4-24 Instant Drosophila Medium, Carolina Biological Supply Company) in the bottom of the rearing bottle. Drosophila stock was maintained by transferring flies to new culture vials every 10-14 days. For experiments, ∼30 mixed-sex adult flies from stock culture were placed into a fresh vial on day 0. On day 2, the adult flies were removed from the vials, and eggs laid in the vials were allowed to develop into adult flies. On day 12, adult flies were transferred without anesthesia to fresh vials. Thereafter, adult flies were transferred every sixth day to fresh vials until assayed.
Metabolic rate of 5-day-old adult flies is significantly higher than that at 16, 29 or 47 days of age, with these three older stages not being significantly different from each other (Van Voorhies et al., 2003). Consequently, we restricted our measurements to adult flies 10-20 days old. Flies were anesthetized with 2-3 min of exposure to FlyNap® (Carolina Biological Supply Company) prior to sorting for sex determination. Males were identified based on the presence of sex combs. Males and females were kept separated and allowed to recover from anesthesia with free access to food for at least one day prior to oxygen consumption measurement. However, one group was fasted for 24 h prior to metabolic measurements (see below).
Body mass
Immediately following measurement of oxygen consumption (see below), flies were anesthetized with FlyNap® and wet mass (mg) determined to the nearest 0.01 mg. Flies were then placed in a 60°C oven to dry for 24 h prior to measuring dry mass (mg).
Measurement of routine oxygen consumption
Routine oxygen consumption (V̇O2) was measured at 25°C on individual groups comprising a known number of flies, ranging from 10 to 50 individuals. Each group of flies was placed in a polypropylene centrifuge tube with the following specifications: (8.2 mm diameter, 35 mm length, 0.9 mm wall thickness) sealed with a polypropylene cap of identical thickness. Net respirometer chamber gas volume was 1.751±0.002 ml (n=15), as measured by weighing the water-filled respirometer at 25°C and using water density to determine volume. Given the very small variation in respirometer gas volume from assembly to assembly, a respirometer gas volume of 1.751 ml was used in all V̇O2 calculations. Each chamber contained 50 mg of soda lime pellets for CO2 absorption, which were kept separate from the flies by a small wad of cotton batting (soda lime also absorbs water vapor, so flies were likely subjected to some small degree of desiccation especially during the longer V̇O2 trials, which was not controlled for). The volume of the cotton and soda lime was determined for each run, and subtracted from the respirometer volume for each measurement. All respirometers were submerged in a water bath maintained at 25°C±0.2°C during the V̇O2 measurements.
Flies were allowed a 30 min acclimation period in the chamber, which was gently flushed with fresh air during this period. The respirometer chamber was then sealed, and a fiberoptic O2 detection probe (see below) was gently inserted through a 1 mm gas-tight orifice in the center of the respirometer cap, and advanced to the center of the respirometer. A blank was created by an identically treated respirometer chamber without flies. Respirometer chambers were then gently placed into a 25°C covered water bath, where they remained for the duration of the V̇O2 measurement period. Experiments were carried out in the darkened respirometers.
PO2 in the respirometer chamber was recorded in real time using a FOXY 40 Hz O2 probe attached to a MultiFrequency Phase Fluorometer (MFPF-100) system made by OceanOptics, Inc. (Dunedin, FL, USA). The output of the system was attached to a computer running Tau Theta software (OceanOptics Inc.). Response time of the probe was <1 s. The probe was subjected to a three point calibration with dried gases at a PO2 of 1, 10 and 20 kPa. Preliminary experiments revealed that the movements of the flies themselves (body movements, wing movements) created sufficient gas convection currents within the respirometer to keep the gas within sufficiently mixed, so no potentially disturbing additional gas mixing within the chambers was provided by the researchers.
Given the small oxygen consumptions of the flies, any significant inward diffusion of oxygen across the respirometer walls, or leakage of water through the respirometer lid, could affect the accuracy of the measurements. Thus, to verify the suitability of the respirometers for these experiments, permeability measurements were made on six empty respirometers to determine the rate of inward O2 diffusion from the surrounding water. The respirometers were filled with 100% N2 gas and then submerged in the water bath. The subsequent increase in PO2 within each respirometer was then measured at the end of 2.5 h of submergence. No water was detected leaking into any of the respirometers. The rate of PO2 increase in the submerged respirometers over the submersion period was only 0.0021±0.007 kPa h−1 kPa−1 of inward PO2 pressure gradient driving diffusion (mean±standard error, n=6), even with an inward partial pressure gradient across the wall of ∼20 kPa. As described below, 5 h was the longest time period that any flies spent in the respirometers and that the lowest PO2s at the end of that 5 h period were ∼2 kPa. Thus, the respirometer testing was under conditions as lengthy and with an inward diffusion pressure gradient larger than would exist under even the most extreme O2 depletion occurring during actual respirometer runs. Consequently, we concluded that the respirometers were functionally both O2 impermeable and leak-proof, and no corrections were necessary for the subsequent V̇O2 calculations.
Experimental protocols for routine V̇O2 and critical partial pressure
The first series of respirometry runs (trials) were designed to measure routine V̇O2. PO2 was allowed to fall from atmospheric (∼20 kPa) to no lower than ∼16 kPa during these runs, generally requiring 1-2 h, depending on fly density in the respirometers (greater density required less time for O2 depletion). This level of O2 was well above the PO2 at which oxygen consumption began to be affected by ambient PO2.
The second series of runs determined critical oxygen partial pressure, PCrit (expressed in kPa), essentially the ambient PO2 below which an animal's V̇O2 begins to decline, that is, below which V̇O2 cannot be maintained. For these experiments, flies were kept in the respirometer until PO2 had decreased to severely hypoxic levels, typically to a point at which no further changes in PO2 were detected, a process typically taking 3-5 h in total (the 1-2 h to reach the PCrit plus the additional 2-4 h to move to a PO2 well below Pcrit). Again, the time for depletion varied greatly, as PCrit was determined over a fly density range of 10 to 50 flies per respirometers.
Prandial state and routine oxygen consumption
In an experiment designed to consider the effect of prandial state on V̇O2 and hypoxia tolerance, female flies were separated into two groups, one with ad libitum access to food prior to placement in the respirometer, while the others were starved, with free access to water via moistened filter paper, for 18 h prior to V̇O2 measurement. Changes in lipid and other metabolic pathways occur in as little as 4-6 h of fasting in Drosophila (Chatterjee et al., 2014; Choi et al., 2015), with 24 h of fasting being sufficient to cause major biochemical disturbances (Menger et al., 2015; Park et al., 2014). Thus, 24 h of fasting was viewed as a strong stressor without creating a morbid metabolic physiology that accompanies >30-40 h starvation at 25°C. Flies from feeding and fasting groups were then placed in the plastic respirometers and their V̇O2 and PCrit determined as indicated above.
Routine oxygen consumption calculation
Mass-specific oxygen routine consumption, V̇O2, was calculated from the chamber volume (minus the volume of the soda lime pellets, cotton batting and estimated volume of the flies), the rate of decline of PO2 in the respirometer chamber, and the total mass of the flies in the respirometer, and was expressed as µl O2 mg−1 h−1. All V̇O2 values are expressed on a wet mass basis unless indicated otherwise.
Statistics
Differences in V̇O2 between males and females (independent of fly density) were tested with separate t-tests for wet and dry body mass calculations. Linear regressions were generated and tested for significance of routine oxygen consumption as a function of fly density and sex. PCrit was then determined for each respirometry run using a MATLAB program designed to analyze critical inflection points in the relationship between ambient PO2 and V̇O2 (Yeager and Ultsch, 1989). The slopes and intercepts of regression lines were compared for significant difference using Student's t-test. A significance level of 0.05 was adopted for all statistical tests, which were performed using SigmaPlot (San Jose, CA, USA) and Statistica (Dell Statistica, Tulsa, OK, USA) statistical software.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
D.H.H. and B.M.S. conducted the experiments, did the basic calculations of oxygen consumption and edited the manuscript. W.B. helped design the experiments, wrote the initial draft of the manuscript and performed the literature survey.
Funding
This work was supported by the National Science Foundation (IOS1543301 to W.B., and IOS-1025823 to W.B.).
References
- Arking R., Buck S., Wells R. A. and Pretzlaff R. (1988). Metabolic rates in genetically based long lived strains of Drosophila. Exp. Gerontol. 23, 59-76. 10.1016/0531-5565(88)90020-4 [DOI] [PubMed] [Google Scholar]
- Baldal E. A., Brakefield P. M. and Zwaan B. J. (2006). Multitrait evolution in lines of Drosophila melanogaster selected for increased starvation resistance: the role of metabolic rate and implications for the evolution of longevity. Evolution 60, 1435-1444. 10.1111/j.0014-3820.2006.tb01222.x [DOI] [PubMed] [Google Scholar]
- Barros A. R., Sierra L. M. and Comendador M. A. (1991). Decreased metabolic rate as an acrolein resistance mechanism in Drosophila melanogaster. Behav. Genet. 21, 445-451. 10.1007/BF01066723 [DOI] [PubMed] [Google Scholar]
- Berrigan D. and Partridge L. (1997). Influence of temperature and activity on the metabolic rate of adult Drosophila melanogaster. Comp. Biochem. Physiol. A Physiol. 118, 1301-1307. 10.1016/S0300-9629(97)00030-3 [DOI] [PubMed] [Google Scholar]
- Burggren W. W. (2014). Epigenetics as a source of variation in comparative animal physiology–or-Lamarck is lookin’ pretty good these days. J. Exp. Biol. 217, 682-689. 10.1242/jeb.086132 [DOI] [PubMed] [Google Scholar]
- Chatterjee D., Katewa S. D., Qi Y., Jackson S. A., Kapahi P. and Jasper H. (2014). Control of metabolic adaptation to fasting by dILP6-induced insulin signaling in Drosophila oenocytes. Proc. Natl. Acad. USA 111, 17959-17964. 10.1073/pnas.1409241111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S., Lim D.-S. and Chung J. (2015). Feeding and fasting signals converge on the LKB1-SIK3 pathway to regulate lipid metabolism in Drosophila. PLoS Genet. 11, e1005263 10.1371/journal.pgen.1005263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chown S. L. and Nicolson S. W. (2004). Insect Physiological Ecology: Mechanisms and Patterns. New York: Oxford University Press. [Google Scholar]
- Dweck H. K. M., Ebrahim S. A. M., Thoma M., Mohamed A. A. M., Keesey I. W., Trona F., Lavista-Llanos S., Svatoš A., Sachse S., Knaden M. et al. (2015). Pheromones mediating copulation and attraction in Drosophila. Proc. Natl. Acad. Sci. USA 112, E2829-E2835. 10.1073/pnas.1504527112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eban-Rothschild A. and Bloch G. (2012). Social influences on circadian rhythms and sleep in insects. Adv. Genet. 77, 1-32. 10.1016/b978-0-12-387687-4.00001-5 [DOI] [PubMed] [Google Scholar]
- Folguera G., Mensch J., Muñoz J. L., Ceballos S. G., Hasson E. and Bozinovic F. (2010). Ontogenetic stage-dependent effect of temperature on developmental and metabolic rates in a holometabolous insect. J. Insect Physiol. 56, 1679-1684. 10.1016/j.jinsphys.2010.06.015 [DOI] [PubMed] [Google Scholar]
- Gibbs A. G. (2002). Water balance in desert Drosophila: lessons from non-charismatic microfauna. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 133, 781-789. 10.1016/S1095-6433(02)00208-8 [DOI] [PubMed] [Google Scholar]
- Greenlee K. J. and Harrison J. F. (2004). Development of respiratory function in the American locust Schistocerca americana. I. Across-instar effects. J. Exp. Biol. 207, 497-508. 10.1242/jeb.00767 [DOI] [PubMed] [Google Scholar]
- Hardy C. M., Birse R. T., Wolf M. J., Yu L., Bodmer R. and Gibbs A. G. (2015). Obesity-associated cardiac dysfunction in starvation-selected Drosophila melanogaster. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R658-R667. 10.1152/ajpregu.00160.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J. F. and Roberts S. P. (2000). Flight respiration and energetics. Annu. Rev. Physiol. 62, 179-205. 10.1146/annurev.physiol.62.1.179 [DOI] [PubMed] [Google Scholar]
- Harrison J., Frazier M. R., Henry J. R., Kaiser A., Klok C. J. and Rascón B. (2006). Responses of terrestrial insects to hypoxia or hyperoxia. Respir. Physiol. Neurobiol. 154, 4-17. 10.1016/j.resp.2006.02.008 [DOI] [PubMed] [Google Scholar]
- Harrison J. F., Klok C. J. and Waters J. S. (2014). Critical PO2 is size-independent in insects: implications for the metabolic theory of ecology. Curr. Opin. Insect Sci. 4, 54-59. 10.1016/j.cois.2014.08.012 [DOI] [PubMed] [Google Scholar]
- Hennessy M. B., Kaiser S. and Sachser N. (2009). Social buffering of the stress response: diversity, mechanisms, and functions. Front. Neuroendocrinol. 30, 470-482. 10.1016/j.yfrne.2009.06.001 [DOI] [PubMed] [Google Scholar]
- Hernandez C. E., Matthews L. R., Oliver M. H., Bloomfield F. H. and Harding J. E. (2010). Effects of sex, litter size and periconceptional ewe nutrition on offspring behavioural and physiological response to isolation. Physiol. Behav. 101, 588-594. 10.1016/j.physbeh.2010.08.020 [DOI] [PubMed] [Google Scholar]
- Herrero P. (2012). Fruit fly behavior in response to chemosensory signals. Peptides 38, 228-237. 10.1016/j.peptides.2012.09.019 [DOI] [PubMed] [Google Scholar]
- Herskin M. S., Munksgaard L. and Andersen J. B. (2007). Effects of social isolation and restraint on adrenocortical responses and hypoalgesia in loose-housed dairy cows. J. Anim. Sci. 85, 240-247. 10.2527/jas.2005-346 [DOI] [PubMed] [Google Scholar]
- Hirata M., Kubo S., Taketomi I. and Matsumoto Y. (2016). Responsiveness of beef cattle (Bos taurus) to human approach, novelty, social isolation, restraint and trade-offs between feeding and social companionship. Anim. Sci. J. 87, 1443-1452. 10.1111/asj.12598 [DOI] [PubMed] [Google Scholar]
- Hoback W. W. and Stanley D. W. (2001). Insects in hypoxia. J. Insect Physiol. 47, 533-542. 10.1016/S0022-1910(00)00153-0 [DOI] [PubMed] [Google Scholar]
- Hoekstra L. A. and Montooth K. L. (2013). Inducing extra copies of the Hsp70 gene in Drosophila melanogaster increases energetic demand. BMC Evol. Biol. 13, 68 10.1186/1471-2148-13-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert A. J., Clancy D. J., Mair W., Braeckman B. P., Gems D. and Partridge L. (2004). Metabolic rate is not reduced by dietary-restriction or by lowered insulin/IGF-1 signalling and is not correlated with individual lifespan in Drosophila melanogaster. Exp. Gerontol. 39, 1137-1143. 10.1016/j.exger.2004.04.006 [DOI] [PubMed] [Google Scholar]
- Isobe K., Takahashi A. and Tamura K. (2013). Cold tolerance and metabolic rate increased by cold acclimation in Drosophila albomicans from natural populations. Genes Genet. Syst. 88, 289-300. 10.1266/ggs.88.289 [DOI] [PubMed] [Google Scholar]
- Jensen P., Overgaard J., Loeschcke V., Schou M. F., Malte H. and Kristensen T. N. (2014). Inbreeding effects on standard metabolic rate investigated at cold, benign and hot temperatures in Drosophila melanogaster. J. Insect Physiol. 62, 11-20. 10.1016/j.jinsphys.2014.01.003 [DOI] [PubMed] [Google Scholar]
- Jumbo-Lucioni P., Ayroles J. F., Chambers M. M., Jordan K. W., Leips J., Mackay T. F. C. and De Luca M. (2010). Systems genetics analysis of body weight and energy metabolism traits in Drosophila melanogaster. BMC Genomics 11, 297 10.1186/1471-2164-11-297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. S. I., Shigeoka C., Takahara Y., Matsuda S. and Tachibana T. (2015). Ontogeny of the corticotrophin-releasing hormone system in slow- and fast-growing chicks (Gallus gallus). Physiol. Behav. 151, 38-45. 10.1016/j.physbeh.2015.06.033 [DOI] [PubMed] [Google Scholar]
- Khazaeli A. A., Van Voorhies W. and Curtsinger J. W. (2005). Longevity and metabolism in Drosophila melanogaster: genetic correlations between life span and age-specific metabolic rate in populations artificially selected for long life. Genetics 169, 231-242. 10.1534/genetics.104.030403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiber M. (1975). Metabolic turnover rate: a physiological meaning of the metabolic rate per unit body weight. J. Theor. Biol. 53, 199-204. 10.1016/0022-5193(75)90110-1 [DOI] [PubMed] [Google Scholar]
- Klok C. J., Kaiser A., Lighton J. R. B. and Harrison J. F. (2010). Critical oxygen partial pressures and maximal tracheal conductances for Drosophila melanogaster reared for multiple generations in hypoxia or hyperoxia. J. Insect Physiol. 56, 461-469. 10.1016/j.jinsphys.2009.08.004 [DOI] [PubMed] [Google Scholar]
- Kohl J., Huoviala P. and Jefferis G. S. X. E. (2015). Pheromone processing in Drosophila. Curr. Opin. Neurobiol. 34, 149-157. 10.1016/j.conb.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmeier P., Hollander K. and Meunier J. (2016). Survival after pathogen exposure in group-living insects: don't forget the stress of social isolation! J. Evol. Biol. 29, 1867-1872. 10.1111/jeb.12916 [DOI] [PubMed] [Google Scholar]
- Krause J. and Ruxton G. (2002). Living in Groups. Oxford: Oxford University Press. [Google Scholar]
- Kravitz E. A. and Fernandez Mde L. (2015). Aggression in Drosophila. Behav. Neurosci. 129, 549-563. 10.1037/bne0000089 [DOI] [PubMed] [Google Scholar]
- Krishnan S. N., Sun Y.-A., Mohsenin A., Wyman R. J. and Haddad G. G. (1997). Behavioral and electrophysiological responses of Drosophila melanogaster to prolonged periods of anoxia. J. Insect Physiol. 43, 203-210. 10.1016/S0022-1910(96)00084-4 [DOI] [PubMed] [Google Scholar]
- Lease H. M., Klok C. J., Kaiser A. and Harrison J. F. (2012). Body size is not critical for critical PO(2) in scarabaeid and tenebrionid beetles. J. Exp. Biol. 215, 2524-2533. 10.1242/jeb.057141 [DOI] [PubMed] [Google Scholar]
- Lighton J. R. B. (2007). Hot hypoxic flies: Whole organism interactions between hypoxic and thermal stressors in Drosophila melanogaster. J. Therm. Biol. 32, 134-143. 10.1016/j.jtherbio.2007.01.009 [DOI] [Google Scholar]
- Lyons D. M., Price E. O. and Moberg G. P. (1993). Social grouping tendencies and separation-induced distress in juvenile sheep and goats. Dev. Psychobiol. 26, 251-259. 10.1002/dev.420260503 [DOI] [PubMed] [Google Scholar]
- Martin R. A., Fiorentini M. and Connors F. (1980). Social facilitation of reduced oxygen consumption in Mus musculus and Meriones unguiculatus. Comp. Biochem. Physiol. 65, 519-522. 10.1016/0300-9629(80)90072-9 [DOI] [Google Scholar]
- Masek P., Reynolds L. A., Bollinger W. L., Moody C., Mehta A., Murakami K., Yoshizawa M., Gibbs A. G. and Keene A. C. (2014). Altered regulation of sleep and feeding contributes to starvation resistance in Drosophila melanogaster. J. Exp. Biol. 217, 3122-3132. 10.1242/jeb.103309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil A. R., Jolley S. N., Akinleye A. A., Nurilov M., Rouzyi Z., Milunovich A. J., Chambers M. C. and Simon A. F. (2015). Conditions Affecting Social Space in Drosophila melanogaster. J. Vis. Exp. 105, e53242 10.3791/53242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvin R. G., Van Voorhies W. A. and Ballard J. W. O. (2007). Working harder to stay alive: metabolic rate increases with age in Drosophila simulans but does not correlate with life span. J. Insect Physiol. 53, 1300-1306. 10.1016/j.jinsphys.2007.07.006 [DOI] [PubMed] [Google Scholar]
- Menger K. E., James A. M., Cochemé H. M., Harbour M. E., Chouchani E. T., Ding S., Fearnley I. M., Partridge L. and Murphy M. P. (2015). Fasting, but not aging, dramatically alters the redox status of cysteine residues on proteins in Drosophila melanogaster. Cell Rep. 11, 1856-1865. 10.1016/j.celrep.2015.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkey A. B., Wong C. K., Hoshizaki D. K. and Gibbs A. G. (2011). Energetics of metamorphosis in Drosophila melanogaster. J. Insect Physiol. 57, 1437-1445. 10.1016/j.jinsphys.2011.07.013 [DOI] [PubMed] [Google Scholar]
- Miquel J., Fleming J. and Economos A. C. (1982). Antioxidants, metabolic rate and aging in Drosophila. Arch. Gerontol. Geriatr. 1, 159-165. 10.1016/0167-4943(82)90016-4 [DOI] [PubMed] [Google Scholar]
- Mölich A. B., Förster T. D. and Lighton J. R. B. (2012). Hyperthermic overdrive: oxygen delivery does not limit thermal tolerance in Drosophila melanogaster. J. Insect. Sci. 12, 109 10.1673/031.012.10901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montooth K. L., Marden J. H. and Clark A. G. (2003). Mapping determinants of variation in energy metabolism, respiration and flight in Drosophila. Genetics 165, 623-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowrey W. R. and Portman D. S. (2012). Sex differences in behavioral decision-making and the modulation of shared neural circuits. Biol. Sex Differ. 3, 8 10.1186/2042-6410-3-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler L. E., Killen S. S., McClure E. C., Munday P. L. and McCormick M. I. (2016). Shoaling reduces metabolic rate in a gregarious coral reef fish species. J. Exp. Biol. 219, 2802-2805. 10.1242/jeb.139493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr P. R. (1925). Critical thermal increments for oxygen consumption of an insect, Drosophila melanogaster. J. Gen. Physiol. 7, 731-734. 10.1085/jgp.7.6.731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owings A. A., Yocum G. D., Rinehart J. P., Kemp W. P. and Greenlee K. J. (2014). Changes in respiratory structure and function during post-diapause development in the alfalfa leafcutting bee, Megachile rotundata. J. Insect Physiol. 66, 20-27. 10.1016/j.jinsphys.2014.05.003 [DOI] [PubMed] [Google Scholar]
- Park S., Alfa R. W., Topper S. M., Kim G. E. S., Kockel L. and Kim S. K. (2014). A genetic strategy to measure circulating Drosophila insulin reveals genes regulating insulin production and secretion. PLoS Genet. 10, e1004555 10.1371/journal.pgen.1004555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker F. R. (1973). Reduced metabolic rates in fishes as a result of induced schooling. Trans. Am. Fish. Soc. 102, 125-131. [DOI] [Google Scholar]
- Partridge L., Piper M. D. W. and Mair W. (2005). Dietary restriction in Drosophila. Mech. Ageing Dev. 126, 938-950. 10.1016/j.mad.2005.03.023 [DOI] [PubMed] [Google Scholar]
- Pavlou H. J. and Goodwin S. F. (2013). Courtship behavior in Drosophila melanogaster: towards a ‘courtship connectome’. Curr. Opin. Neurobiol. 23, 76-83. 10.1016/j.conb.2012.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M. D. W., Selman C., Speakman J. R. and Partridge L. (2014). Using doubly-labeled water to measure energy expenditure in an important small ectotherm Drosophila melanogaster. J. Genet. Genomics 41, 505-512. 10.1016/j.jgg.2014.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portman D. S. (2007). Genetic control of sex differences in C. elegans neurobiology and behavior. Adv. Genet. 59, 1-37. 10.1016/S0065-2660(07)59001-2 [DOI] [PubMed] [Google Scholar]
- Promislow D. E. L. and Haselkorn T. S. (2002). Age-specific metabolic rates and mortality rates in the genus Drosophila. Aging Cell 1, 66-74. 10.1046/j.1474-9728.2002.00009.x [DOI] [PubMed] [Google Scholar]
- Rajpurohit S., Peterson L. M., Orr A. J., Marlon A. J. and Gibbs A. G. (2016). An experimental evolution test of the relationship between melanism and desiccation survival in insects. PLoS ONE 11, e0163414 10.1371/journal.pone.0163414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushen J., Boissy A., Terlouw E. M. and de Passillé A. M. (1999). Opioid peptides and behavioral and physiological responses of dairy cows to social isolation in unfamiliar surroundings. J. Anim. Sci. 77, 2918-2924. 10.2527/1999.77112918x [DOI] [PubMed] [Google Scholar]
- Schleuter D., Haertel-Borer S., Fischer P. and Eckmann R. (2007). Respiration rates of Eurasian perch Perca fluviatilis and ruffe: lower energy costs in groups. Trans. Am. Fish. Soc. 136, 43-55. 10.1577/T06-123.1 [DOI] [Google Scholar]
- Schneider J., Atallah J. and Levine J. D. (2016). Social structure and indirect genetic effects: genetics of social behaviour. Biol. Rev. Camb. Philos. Soc. Epub ahead of print, doi:10.1111/brv.12267 10.1111/brv.12267 [DOI] [PubMed] [Google Scholar]
- Skandalis D. A., Stuart J. A. and Tattersall G. J. (2011). Responses of Drosophila melanogaster to atypical oxygen atmospheres. J. Insect Physiol. 57, 444-451. 10.1016/j.jinsphys.2011.01.005 [DOI] [PubMed] [Google Scholar]
- Slocumb M. E., Regalado J. M., Yoshizawa M., Neely G. G., Masek P., Gibbs A. G. and Keene A. C. (2015). Enhanced sleep is an evolutionarily adaptive response to starvation stress in Drosophila. PLoS ONE 10, e0131275 10.1371/journal.pone.0131275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani A. F., Zulkifli I., Omar A. R. and Raha A. R. (2012). The relationship between adrenocortical function and Hsp70 expression in socially isolated Japanese quail. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 161, 140-144. 10.1016/j.cbpa.2011.10.003 [DOI] [PubMed] [Google Scholar]
- Stewart B. A. and McLean J. R. (2004). Population density regulates Drosophila synaptic morphology in a Fasciclin-II-dependent manner. J. Neurobiol. 61, 392-399. 10.1002/neu.20096 [DOI] [PubMed] [Google Scholar]
- Stone B., Evans L., Coleman J. and Kuebler D. (2013). Genetic and pharmacological manipulations that alter metabolism suppress seizure-like activity in Drosophila. Brain Res. 1496, 94-103. 10.1016/j.brainres.2012.12.007 [DOI] [PubMed] [Google Scholar]
- Ueda A. and Wu C.-F. (2009). Effects of social isolation on neuromuscular excitability and aggressive behaviors in Drosophila: altered responses by Hk and gsts1, two mutations implicated in redox regulation. J. Neurogenet. 23, 378-394. 10.3109/01677060903063026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhies W. A. (2009). Metabolic function in Drosophila melanogaster in response to hypoxia and pure oxygen. J. Exp. Biol. 212, 3132-3141. 10.1242/jeb.031179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhies W. A., Khazaeli A. A. and Curtsinger J. W. (2003). Selected contribution: long-lived Drosophila melanogaster lines exhibit normal metabolic rates. J. Appl. Physiol. 95, 2605-2613; discussion 2604 10.1152/japplphysiol.00448.2003 [DOI] [PubMed] [Google Scholar]
- Van Voorhies W. A., Khazaeli A. A. and Curtsinger J. W. (2004a). Lack of correlation between body mass and metabolic rate in Drosophila melanogaster. J. Insect Physiol. 50, 445-453. 10.1016/j.jinsphys.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Van Voorhies W. A., Khazaeli A. A. and Curtsinger J. W. (2004b). Testing the “rate of living” model: further evidence that longevity and metabolic rate are not inversely correlated in Drosophila melanogaster. J. Appl. Physiol. (1985) 97, 1915-1922. 10.1152/japplphysiol.00505.2004 [DOI] [PubMed] [Google Scholar]
- Van Voorhies W. A., Melvin R. G., Ballard J. W. O. and Williams J. B. (2008). Validation of manometric microrespirometers for measuring oxygen consumption in small arthropods. J. Insect Physiol. 54, 1132-1137. 10.1016/j.jinsphys.2008.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan V., Thistle R., Liu T., Starostina E. and Pikielny C. W. (2014). Drosophila pheromone-sensing neurons expressing the ppk25 ion channel subunit stimulate male courtship and female receptivity. PLoS Genet. 10, e1004238 10.1371/journal.pgen.1004238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villella A. and Hall J. C. (2008). Neurogenetics of courtship and mating in Drosophila. Adv. Genet. 62, 67-184. 10.1016/S0065-2660(08)00603-2 [DOI] [PubMed] [Google Scholar]
- Waters J. S., Holbrook C. T., Fewell J. H. and Harrison J. F. (2010). Allometric scaling of metabolism, growth, and activity in whole colonies of the seed-harvester ant Pogonomyrmex californicus. Am. Nat. 176, 501-510. 10.1086/656266 [DOI] [PubMed] [Google Scholar]
- Williams A. E., Rose M. R. and Bradley T. J. (2004). The respiratory pattern in Drosophila melanogaster selected for desiccation resistance is not associated with the observed evolution of decreased locomotory activity. Physiol. Biochem. Zool. 77, 10-17. 10.1086/381467 [DOI] [PubMed] [Google Scholar]
- Yamamoto D., Sato K. and Koganezawa M. (2014). Neuroethology of male courtship in Drosophila: from the gene to behavior. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 200, 251-264. 10.1007/s00359-014-0891-5 [DOI] [PubMed] [Google Scholar]
- Yeager D. P. and Ultsch G. R. (1989). Physiological regulation and conformation: a BASIC program for the determination of critical points. Physiol. Zool. 62, 888-907. 10.1086/physzool.62.4.30157935 [DOI] [Google Scholar]