Abstract
We used hepatic balance and tracer ([3H]glucose) techniques to examine the impact of “breakfast” on hepatic glucose metabolism later in the same day. From 0–240 min, 2 groups of conscious dogs (n = 9 dogs/group) received a duodenal infusion of glucose (GLC) or saline (SAL), then were fasted from 240–360 min. Three dogs from each group were euthanized and tissue collected at 360 min. From 360–600 min, the remaining dogs underwent a hyperinsulinemic (4× basal) hyperglycemic clamp (arterial blood glucose 146 ± 2 mg/dL) with portal GLC infusion. The total GLC infusion rate was 14% greater in dogs infused with GLC than in those receiving SAL (AUC360–600min 2,979 ± 296 vs. 2,597 ± 277 mg/kg, respectively). The rates of hepatic glucose uptake (5.8 ± 0.8 vs. 3.2 ± 0.3 mg ⋅ kg−1 ⋅ min−1) and glycogen storage (4.7 ± 0.6 vs. 2.9 ± 0.3 mg ⋅ kg−1 ⋅ min−1) during the clamp were markedly greater in dogs receiving GLC compared with those receiving SAL. Hepatic glycogen content was ∼50% greater, glycogen synthase activity was ∼50% greater, glycogen phosphorylase activity was ∼50% lower, and the amount of phosphorylated glycogen synthase was 34% lower, indicating activation of the enzyme, in dogs receiving GLC compared with those receiving SAL. Thus, morning GLC primed the liver to extract and store more glucose in the presence of hyperinsulinemic hyperglycemia later in the same day, indicating that breakfast enhances the liver’s role in glucose disposal in subsequent same-day meals.
Introduction
A number of studies, but not all, have suggested a metabolic advantage to consuming versus skipping breakfast (1–7). In particular, insulin insensitivity and the risk of overweight/obesity have been reported to be lower among those who regularly eat breakfast versus those who skip breakfast (8–11). In a study in which the same meal was given to normal individuals three times throughout the day, the excursions of glucose and insulin were twice as great in the morning as at midday or in the evening (12). Moreover, consumption of the largest meal of the day in the morning, rather than the evening, was associated with lower glycemia over 24 h in a small group of lean, healthy volunteers (13). These findings suggest a possible “priming” effect of breakfast on glucose metabolism throughout the remainder of the day.
The liver is an especially important organ in glucose metabolism, by virtue of its ability to both take up and release glucose as appropriate to maintain glucose homeostasis. The liver is responsible for the uptake of as much as one-third of a moderate-sized oral or duodenal glucose load, and its role in glucose homeostasis can be seen to be even greater when its full response (i.e., including suppression of net hepatic glucose output) is considered (14,15). The extent to which glucose metabolism by the liver later in the same day is affected by breakfast consumption remains unknown. Assessment of hepatic glucose balance in humans is not practical, given the difficulty in accessing the hepatic portal vein. Hepatic vascular access is also a problem in rodent models because of the small size of the vessels. A model using conscious, chronically catheterized dogs, on the other hand, allows for a robust assessment of both net and absolute hepatic glucose balance and whole-body glucose disposition (16).
We hypothesized that breakfast intake has a priming effect on the liver as a result of the excursions in glycemia and/or insulin it brings about, initiating molecular changes that facilitate net hepatic glucose uptake (NHGU) and storage during subsequent meals in the same day. As a first step in examining this hypothesis, we delivered saline or glucose via duodenal infusion in the morning and then assessed hepatic glucose metabolism later in the day. We used a canine model to precisely quantify NHGU and glycogen synthesis, and to examine molecular markers associated with glucose metabolism in the liver.
Research Design and Methods
Animal Care and Surgical Procedures
Adult mongrel dogs (10 male and 8 female; 20.6 ± 0.6 kg) acquired from a U.S. Department of Agriculture–licensed vendor were studied. The protocol was approved by the Vanderbilt University Institutional Animal Care and Use Committee, and the animals were housed and cared for according to AAALAC International guidelines. The dogs were fed once daily a chow-and-meat diet in an amount calculated to maintain weight (17), and they were maintained on a 12-h light/12-h dark cycle.
Approximately 16 days before the study, each dog underwent surgery under general anesthesia to insert sampling catheters in the femoral artery, hepatic portal vein, and left common hepatic vein; blood flow probes around the portal vein and hepatic artery; a splenic vein and a jejunal vein catheter to allow infusion into the portal circulation; and an infusion catheter in the inferior vena cava. In addition, a duodenal infusion catheter was inserted. All procedures were previously described (15,17).
On the day before the study, all dogs were fed their regular diet at noon, and any remaining food was removed within an hour. All dogs ate at least 75% of the daily ration the day before study.
Experimental Design
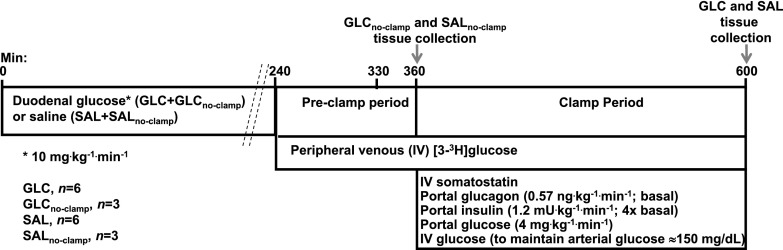
Figure 1 provides an outline of the study design. On the morning of the study, at the onset of the light cycle, the duodenal and arterial catheters were removed from their subcutaneous pockets under local anesthesia, and a continuous 4-h (0–240 min) infusion of 20% glucose at 10 mg ⋅ kg−1 ⋅ min−1 was initiated via the duodenal catheter in nine dogs to mimic a morning meal (GLC group). Another nine animals received an intraduodenal infusion of normal saline at a rate identical (in mL ⋅ kg−1 ⋅ min−1) to the infusion received by the group receiving glucose (SAL group). Arterial glucose and insulin concentrations were monitored in the groups during the 4-h duodenal infusion. The duodenal infusions ended at 240 min. At that point, six dogs in each group were prepared for a subsequent clamp period. The flow probes and the remainder of the infusion and sampling catheters were exteriorized and peripheral venous access was established (18). A primed (38 µCi), continuous (0.38 μCi/min) infusion of [3H]glucose was initiated via a peripheral vein; after a 90-min tracer equilibration period (240–330 min), postinfusion fasting blood samples were collected from the sampling catheters from 330–360 min (“preclamp” period). Three dogs that had undergone duodenal glucose infusion and three that had received duodenal saline infusion did not receive [3H]glucose but were euthanized at 360 min to collect hepatic tissues; these are referred to as the GLCno-clamp and SALno-clamp groups.
Figure 1.
Study design. The dogs were randomly assigned to duodenal infusion of either glucose or saline. After 4 h of duodenal infusion, there was a 2-h period before the clamp, followed by a 4-h hyperinsulinemic-hyperglycemic clamp with portal glucose infusion. The dogs receiving no clamp were euthanized and tissue collected at 360 min. IV, intravenous.
From 360–600 min, all dogs in the GLC and SAL groups (n = 6 dogs/group) underwent a hyperinsulinemic-hyperglycemic clamp with portal vein glucose infusion to mimic the conditions during absorption of a later meal. During the clamp, somatostatin (0.8 µU ⋅ kg−1 ⋅ min−1) was infused via the peripheral vein to suppress endocrine pancreatic secretion, and regular insulin (four times basal values; 1.2 mU ⋅ kg−1 ⋅ min−1) and glucagon (basal; 0.57 ng ⋅ kg−1 ⋅ min−1) were replaced via intraportal infusion. Glucose (20%) was infused continuously at 4 mg ⋅ kg−1 ⋅ min−1 into the portal circulation. This provided a constant, readily quantifiable load of glucose to the liver. In conjunction with this, a primed, continuous infusion of 50% glucose was administered via a peripheral vein to create and maintain hyperglycemia; the infusion rate was adjusted as required, on the basis of 0.2-mL samples taken every 5 min, to clamp arterial blood glucose at 150 mg/dL. We previously demonstrated that this dual infusion method is effective in clamping glucose delivery to both the liver and the peripheral tissues at very constant values (see, e.g, ref. 19). Larger samples were taken every 15–30 min from the artery, portal vein, and hepatic vein catheters to allow measurement of hormones and substrates. At the end of the study, each animal was deeply anesthetized with sodium pentobarbital while all infusions continued, and tissues from three liver lobes were rapidly (within 3 min) freeze-clamped in situ and stored at −80°C for later analysis. The dogs were then euthanized.
Analyses
Real-time PCR and Western Blotting: Enzyme Activities
RNA isolation, cDNA synthesis, quantitative PCR primers and analysis, and Western blotting procedures were performed as described previously (20). Antibodies were purchased from Cell Signaling Technology, with the exception of the protein targeting to glycogen (PTG) antibody (PPP1R3C), which was obtained from Santa Cruz Biotechnology. ImageJ software (http://rsb.info.nih.gov/ij/) was used for quantification. Quantities of specific mRNAs and proteins of interest were normalized to that of RPL32 and β-actin, respectively (Sigma-Aldrich). Glucokinase (GK), glycogen synthase (GS), and glycogen phosphorylase (GP) activities were determined as previously described (21).
Biochemical Analysis
Plasma glucose, [3H]glucose, glucagon, insulin, and nonesterified fatty acid (NEFA) levels, and blood lactate and glycerol concentrations, were measured using standard methods, as described previously (19). The method described by Keppler and Decker (22) was used to determine hepatic glycogen concentrations.
Lipids were extracted from hepatic tissue as described by Folch et al. (23). Lipids were recovered from the filtered extracts in the chloroform phase. Individual lipid classes were separated by thin-layer chromatography and then scraped from the plates and methylated (24). The methylated fatty acids were extracted and analyzed using gas chromatography. Inclusion of lipid standards with odd-chain fatty acids permitted quantitation of the amount of lipid in the sample. Dipentadecanoyl phosphatidylcholine (C15:0), diheptadecanoin (C17:0), trieicosenoin (C20:1), and cholesteryl eicosenoate (C20:1) were used as standards.
Calculations
Unidirectional hepatic glucose uptake (HGU) and non-HGU were calculated with [3H]glucose, as described previously (19). Net hepatic substrate balances, net hepatic carbon retention, and non-HGU were also calculated with cold glucose concentrations using the arteriovenous difference method (19). An indirect method was used to calculate NHGU in order to reduce any error introduced by streaming infusate in the portal vein (19). Hepatic sinusoidal plasma insulin and glucagon concentrations were calculated as previously described (19). Glycogen synthesis via the direct pathway was calculated by dividing hepatic [3H]-labeled glycogen by the average inflowing plasma [3H]glucose-specific radioactivity (19). Net hepatic carbon retention, previously shown to be a reliable index of glycogen synthesis, was calculated as described previously (19).
Statistical Analyses
Data are expressed as means ± SEMs. Two-way ANOVA with or without a repeated measures design was used (SigmaStat; Systat, Richmond, CA), and post hoc analysis was performed using the Student-Newman-Keuls multiple comparisons test. A P value <0.05 was considered significant.
Results
Morning Infusion Period
Arterial insulin and glucose concentrations before the morning infusion period did not differ between the SAL and SALno-clamp versus the GLC and GLCno-clamp groups (Table 1). In the SAL and SALno-clamp groups, blood glucose concentrations drifted down slightly but significantly over the course of the infusion, whereas insulin concentrations did not change significantly. In the GLC and GLCno-clamp groups, concentrations of both glucose and insulin were highest early in the duodenal glucose infusion period, with glucose levels at 60 min nearly 1.5-fold basal values and insulin concentrations nearly seven times basal values. By 180 min, the glucose and insulin concentrations had fallen to only 130% and 157% of basal values, respectively. The blood glucose concentrations remained stable from 180 to 240 min, whereas at 240 min the insulin concentrations were approximately threefold the basal values. Blood glucose and plasma insulin concentrations were significantly greater during duodenal infusion of glucose compared with saline, as anticipated (Table 1). Glucagon concentrations remained unchanged from basal values throughout the 240-min period in all dogs (data not shown). In addition, cortisol concentrations did not change over time during the morning period within the groups and did not differ between treatments (mean for the period 2.7 ± 0.2 vs. 2.3 ± 0.2 µg/dL in SAL + SALno-clamp vs. GLC + GLCno-clamp groups, respectively; P = 0.11).
Table 1.
Venous blood glucose and plasma insulin concentrations before (0 min) and during (60–240 min) the morning infusion period
| Parameter and groups | 0 min | 60 min | 120 min | 180 min | 240 min |
|---|---|---|---|---|---|
| Blood glucose (mg/dL) | |||||
| SAL + SALno-clamp | 87 ± 1 | 87 ± 2 | 86 ± 2 | 83 ± 1† | 84 ± 2 |
| GLC + GLCno-clamp | 87 ± 3 | 122 ± 5*† | 111 ± 6*† | 108 ± 6*† | 107 ± 11*† |
| Plasma insulin (µU/mL) | |||||
| SAL + SALno-clamp | 9 ± 1 | 7 ± 1 | 9 ± 2 | 7 ± 1 | 8 ± 1 |
| GLC + GLCno-clamp | 7 ± 2 | 47 ± 24*† | 26 ± 1*† | 11 ± 1*† | 21 ± 3*† |
Data are mean ± SEM. SAL + SALno-clamp includes six dogs that underwent the clamp (SAL) and three dogs from which tissue was harvested before the clamp (SALno-clamp). GLC + GLCno-clamp includes six dogs that underwent the clamp (GLC) and three dogs from which tissue was harvested before the clamp (GLCno-clamp).
*P < 0.05 vs. SAL + SALno-clamp;
†P < 0.05- vs. 0-min sample in the same group.
Data Before and During the Clamp Period
Hormone and Hepatic Blood Flow Data
The plasma insulin concentrations during the preclamp and afternoon clamp periods did not differ significantly between the SAL and GLC groups (Table 2). The clamp period concentrations of insulin were approximately fourfold higher than the preclamp values in both groups, mimicking postprandial conditions. The arterial plasma glucagon and cortisol concentrations were not different during the preclamp and clamp periods, and they did not differ between the two groups at any time (Table 2). In addition, hepatic blood flow did not differ between the SAL and GLC groups at any time (Table 2).
Table 2.
Arterial plasma insulin, glucagon, and cortisol concentrations and hepatic blood flow during the preclamp and clamp periods
| Parameter and group | Before the clamp (330–360 min) | Clamp period (360–600 min) |
|---|---|---|
| Arterial insulin (µU/mL) | ||
| SAL | 5 ± 1 | 19 ± 2 |
| GLC | 6 ± 1 | 23 ± 2 |
| Arterial glucagon (ng/L) | ||
| SAL | 30 ± 4 | 31 ± 5 |
| GLC | 40 ± 4 | 39 ± 4 |
| Arterial cortisol (µg/dL) | ||
| SAL | 2.7 ± 0.6 | 3.4 ± 0.8 |
| GLC | 1.7 ± 0.5 | 2.8 ± 0.7 |
| Total hepatic blood flow (mL ⋅ kg−1 ⋅ min−1) | ||
| SAL | 34 ± 1 | 31 ± 2 |
| GLC | 30 ± 3 | 30 ± 4 |
Data are mean ± SEM (n = 6 dogs/group) for all time points during the indicated period. There were no significant differences between groups.
Glucose Data
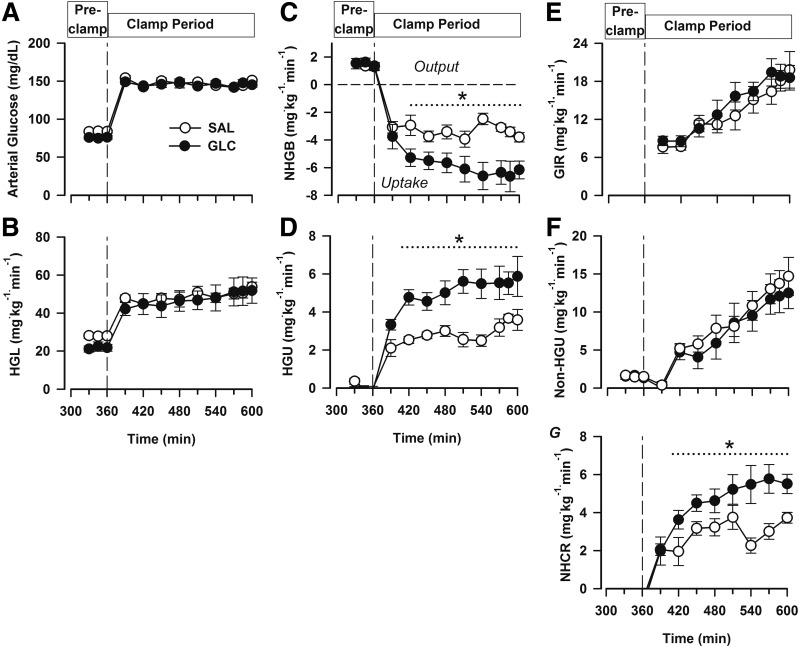
Blood glucose concentrations were no different between the SAL and GLC groups during the preclamp or the clamp periods. In keeping with this, the hepatic glucose loads were virtually identical in the two groups (Fig. 2A and B). During the preclamp period, all animals were in a state of net hepatic glucose output (1.4 ± 0.1 and 1.6 ± 0.2 mg ⋅ kg−1 ⋅ min−1 in the SAL and GLC groups, respectively; P = 0.72) (Fig. 2C); with the onset of the hyperinsulinemic-hyperglycemic clamp in the presence of portal glucose infusion, they shifted to NHGU, with the mean rate in the GLC group ∼2.4 mg ⋅ kg−1 ⋅ min−1 greater than that in the SAL group (P < 0.05). Unidirectional (tracer-determined) HGU was near zero in both groups during the basal period (Fig. 2D). HGU increased markedly in both groups with the onset of the clamp, with mean rates of 5.0 ± 0.5 vs. 2.9 ± 0.2 mg ⋅ kg−1 ⋅ min−1 in the GLC and SAL groups, respectively (Fig. 2D).
Figure 2.
Data from the fasting period following the morning infusion (before the clamp [Pre-clamp]; 330–360 min) and during a subsequent hyperinsulinemic-hyperglycemic clamp with intraportal glucose infusion (clamp period). Arterial blood glucose (A), hepatic glucose load (HGL; B), net hepatic glucose balance (NHGB; C), unidirectional (tracer-determined) HGU (D), glucose infusion rate (GIR; E), non-HGU (F), and net hepatic carbon retention (NHCR, in milligrams glucose equivalents per kilogram per minute) (G). From 0–240 min (data not shown), the groups (n = 6 dogs/group) received a duodenal infusion of saline (white circles) or glucose (black circles). *P < 0.05 between groups.
The total glucose infusion rate (the fixed-rate portal vein infusion plus the variable peripheral glucose infusion required to maintain equivalent hyperglycemia) was slightly but not significantly greater in the GLC group versus the SAL group (mean clamp period rates: 14.4 ± 1.4 vs. 13.3 ± 1.5 mg ⋅ kg−1 ⋅ min−1, respectively; P = 0.28) (Fig. 2E). Non-HGU increased throughout the clamp period in both groups, with no significant difference in the rates between groups (mean 8.6 ± 1.8 and 9.9 ± 1.5 mg ⋅ kg−1 ⋅ min−1 in the GLC and SAL groups, respectively) (Fig. 2F). In addition, net hepatic carbon retention, an index of glycogen synthesis, was increased >50% in the GLC group versus the SAL group (Fig. 2G).
Lactate, Glycerol, and NEFA
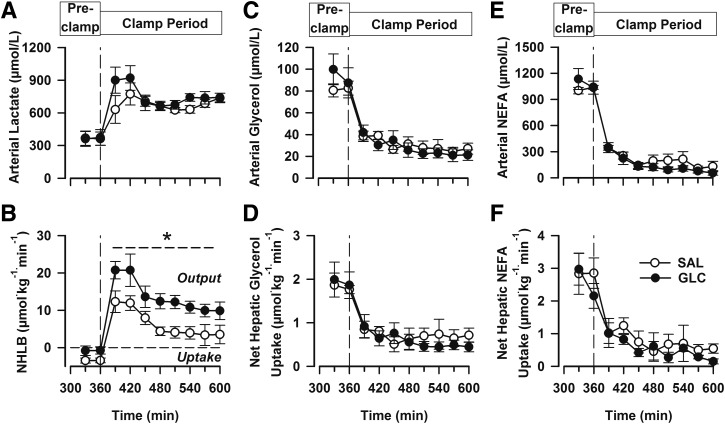
Arterial blood lactate concentrations increased in both groups with the onset of the clamp period and did not differ between groups (Fig. 3A). Both groups exhibited net hepatic lactate uptake during the late-morning fasting period and rapidly switched to net hepatic lactate output (NHLO) after the start of the clamp (Fig. 3B). The rates of NHLO were higher in the GLC group than in the SAL group throughout the clamp period (P < 0.05), such that the area under the curve (AUC) of the change in NHLO from the fasting period after breakfast was 46% greater in the GLC than the SAL group (P < 0.05).
Figure 3.
Data from the fasting period following the morning infusion (before the clamp [Pre-clamp]; 330–360 min) and during a subsequent hyperinsulinemic-hyperglycemic clamp with intraportal glucose infusion (clamp period). Arterial blood lactate (A), net hepatic lactate balance (NHLB; B), arterial blood glycerol (C), net hepatic glycerol uptake (D), arterial plasma NEFA uptake (E), and net hepatic NEFA uptake (F). From 0–240 min (data not shown), the groups (n = 6 dogs/group) received a duodenal infusion of saline (white circles) or glucose (black circles). *P < 0.05 between groups.
The arterial concentrations of glycerol and NEFA declined quickly with the onset of the clamp in both groups and remained low throughout the clamp, indicating no difference in lipolytic suppression. In addition, no between-group differences in glycerol or NEFA uptake by the liver occurred at any time (Fig. 3C–F).
Liver Tissue Analyses
Glycogen, Triglyceride, and mRNA Data
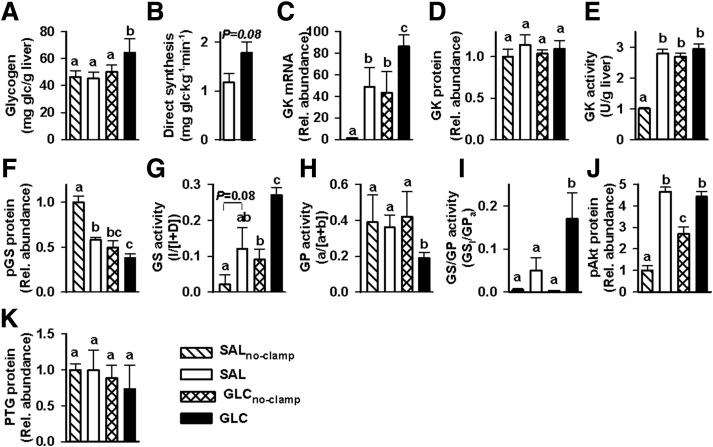
Hepatic glycogen concentrations at the end of study did not differ among the SAL, SALno-clamp, and GLCno-clamp groups (Fig. 4A). By contrast, the concentrations in the GLC group were ∼40% greater than in the SAL and SALno-clamp groups and 28% greater than in the GLCno-clamp group (P < 0.05 for the GLC group vs. all other groups). Glycogen synthesis via the direct pathway was ∼50% greater in the GLC versus the SAL group (P = 0.08) (Fig. 4B).
Figure 4.
Hepatic tissue analyses: glycogen concentrations (A), glycogen synthesis via the direct pathway (B), GK mRNA (C), relative (Rel.) GK protein (D), GK activity (E), pGS protein (F), GS activity (active/total) (G), GP activity (active/total) (H), GS activity relative to that of GP (I), pAKT protein (J), and relative PTG protein (K) in the SALno-clamp (hatched bars), SAL (white bars), GLCno-clamp (cross-hatched bars), and GLC (black bars) groups. Samples were taken at the end of study (360 min for the no-clamp groups and 600 min for the SAL and GLC groups). RNA and protein levels in the SAL, GLCno-clamp, and GLC groups are expressed relative to those in the SALno-clamp group. Direct glycogen synthesis is not shown for the no-clamp groups because they received no [3H]glucose. Data marked with different lowercase letters differed significantly from one another (P < 0.05), whereas those marked with the same letter were not significantly different from one another.
The total hepatic triglyceride content at the end of the clamp did not differ between the SAL and GLC groups, averaging 713 ± 27 µg/g tissue in both groups. In addition, the fatty acid composition of hepatic triglycerides did not differ significantly between groups, with one exception. None of the livers from the SAL group exhibited detectable levels of 18:3ω3 (α-linolenic acid [ALA]), whereas ALA was quantifiable in all livers in the GLC group, albeit at the lower limit of detection (Table 3).
Table 3.
Hepatic triglyceride composition
| Group | Fatty acid species (µg/g tissue) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 14:0 | 16:0 | 16:1 | 18:0 | 18:1ω9 | 18:1ω7 | 18:2 | 18:3ω6 | 18:3ω3 | 20:4 | |
| SAL | 12 ± 2 | 195 ± 37 | 24 ± 3 | 62 ± 10 | 214 ± 36 | 46 ± 20 | 111 ± 22 | 15 ± 9 | – | 34 ± 7 |
| GLC | 14 ± 1 | 186 ± 29 | 26 ± 2 | 69 ± 7 | 232 ± 18 | 24 ± 2 | 120 ± 16 | 4 ± 2 | 5 ± 1 | 33 ± 6 |
Samples (n = 6/group) were collected at the end of the clamp period. There were no significant differences between groups for any of the species detected in both groups. The species 18:3ω3 was not detected in the SAL group; in the GLC group, it appeared at the lower limit of detection. 14:0, tetradecanoic acid; 16:0, hexadecanoic acid; 16:1, 9-tetradecenoic acid; 18:0, octadecanoic acid; 18:1ω9, (Z)-octadec-9-enoic (oleic) acid; 18:1ω7, 11-octadecenoic acid; 18:2, 9,12-octadecadienoic (linoleic) acid; 18:3ω6, all-cis-6,9,12-octadecatrienoic acid; 18:3ω3, all-cis-9,12,15-octadecatrienoic (α-linolenic) acid; 20:4, all-cis-5,8,11,14-icosatetraenoic acid.
The SAL, GLC, and GLCno-clamp groups exhibited significant enhancement in GK mRNA compared with the SALno-clamp group; the increase in the GLC group was 77% greater than that in the SAL group and double that in the GLCno-clamp group (Fig. 4C). This suggests that the impact of the morning glucose infusion was to double the GK message evident during the afternoon clamp.
Western Blotting and Enzyme Activities
Despite the enhancement in GK mRNA, relative GK protein did not differ significantly among the four treatment groups (Fig. 4D). Nevertheless, the SALno-clamp group exhibited reduced GK activity (P < 0.05) in comparison with all three of the other groups, which did not differ significantly from one another (Fig. 4E). The amount of phosphorylated GS (pGS) protein was significantly reduced in the three test groups compared with the SALno-clamp group, and the GLC group had significantly lower (P < 0.05) relative pGS protein than the SAL group (Fig. 4F).
GS activity was increased by morning glucose infusion (GLCno-clamp group) and by the clamp (SAL group), and the combination of the two (GLC group) brought about a further significant increase in GS activity (Fig. 4G). While GP activity was not altered in the GLCno-clamp and SAL groups, the combination of morning glucose delivery and the afternoon clamp reduced it by ∼50% (Fig. 4H). Thus, the GS activity–to–GP activity ratio (GS-I [activity of glucose-6-phosphate–independent form]/GPa [active form]) increment was >3.5-fold greater in the GLC group, with the results of GLCno-clamp deducted from it, compared with the data from the SAL group, with the results from SALno-clamp deducted from it (P < 0.05) (Fig. 4I).
Relative phosphorylated Akt (pAkt) protein was enhanced in all other groups versus the SALno-clamp group (P < 0.05) (Fig. 4J). The enhancement in the GLCno-clamp group was significantly reduced in comparison with that in both the SAL and GLC groups, which did not differ from one another. Moreover, PTG expression did not differ significantly among any of the groups (Fig. 4K).
Discussion
In this study we used duodenal glucose versus saline infusions in the morning as surrogates for breakfast and no breakfast intake, respectively, and used hyperinsulinemic-hyperglycemic clamp conditions in the presence of the portal glucose signal as a surrogate for a later meal. This ensured that the GLC and SAL groups would have indistinguishable pancreatic hormone concentrations and hepatic glucose loads during the final study period, an important element of the study design since these factors are key regulators of HGU (16). HGU, NHGU, net hepatic carbon retention, and terminal hepatic glycogen concentrations were all at least 50% greater in the GLC group versus the SAL group, consistent with a priming effect of a morning carbohydrate load on the liver. The GLC group did not display a global increase in glucose disposal in comparison with the SAL group, even though the total glucose infusion rate was ∼1 mg ⋅ kg−1 ⋅ min−1 (∼9%) greater in the GLC group (P = 0.28). The enhancement of liver glucose uptake (∼2.4 mg ⋅ kg−1 ⋅ min−1) in the GLC group was offset by a tendency toward a reduction in the non-HGU rate in the same group (1.3 mg ⋅ kg−1 ⋅ min−1, or ∼15%; P = 0.28), which is probably the result of an increase in first-pass glucose extraction by the liver. This is consistent with our previous observation of a reduction in muscle glucose uptake in response to a glucose portal signal (25). These data suggest that the benefits of morning hyperglycemia and/or hyperinsulinemia were largely centered on hepatic metabolism and enhanced glycogen deposition. Indeed, the difference in hepatic glycogen deposition accounted for ∼86% of the difference in HGU between groups, with hepatic glycolysis—that is, net hepatic lactate production (Fig. 3)—accounting for an additional ∼13–14%. We demonstrated that hepatic glucose oxidation is remarkably constant (∼0.3 mg glucose ⋅ kg−1 ⋅ min−1) under a variety of experimental clamp conditions (26–28) and thus would account for a very small amount of HGU in both the SAL and GLC groups.
Suppression of circulating glycerol and NEFA levels was equivalent in the SAL and GLC groups. Because lipolysis is highly sensitive to hyperinsulinemia in a healthy state (29), this was not unexpected. Nevertheless, these findings are consistent with the impact of morning hyperinsulinemia and hyperglycemia being focused on the liver. Although ALA was detectable in the livers of dogs in the GLC group, but not those in the SAL group, it is not clear whether this could have acutely affected HGU. Chronic consumption of a high-ALA diet in mice fed a high-fat diet was associated with reduced hepatic glucose production, in association with lower levels of steatosis (30). Also, oral and intravenous glucose tolerance were modestly improved in Zucker diabetic fatty rats fed a high-ALA diet for 8 weeks, although this was not associated with altered expression of any of the hepatic inflammatory markers examined (31). The acute effect of changes in hepatic ALA have not, to our knowledge, been examined.
Hepatic glycogen concentrations, net hepatic carbon retention, and glycogen synthesis via the direct pathway were all higher in the GLC group than in the SAL group. Given the metabolic importance of GK to the liver (32,33), the impact of insulin on GK transcription (34), and the long half-life of GK once it is elevated (35), we originally speculated that the insulin excursion associated with morning hyperglycemia might bring about an increase in GK transcription and a resulting increase in GK protein. Consistent with this, the increase in GK mRNA in the GLC group was approximately equal to the sum of the increases in the SAL and GLCno-clamp groups; thus, the mRNA response to morning glucose infusion seemed to persist to 360 min, with the afternoon clamp having an additive effect. In spite of the difference in mRNA, neither GK protein nor the activity of the enzyme differed between the SAL and GLC groups, indicating that an impact of the morning glucose infusion was not additive on either GK protein or GK activity. This suggests that GK protein and its activity were maximally stimulated by either the morning duodenal glucose infusion or the afternoon clamp, and if there was any preferential impact on GK in the GLC group, it lies in stimulating the translocation of the enzyme.
In contrast to our findings with GK, the activity of GS was significantly enhanced and that of GP was significantly reduced in the GLC group versus the others, resulting in significantly greater GS activity relative to GP activity in the GLC group compared with all other groups. Our previous work (36) and that of others (37) demonstrated the importance of suppressing GP activity relative to GS activity in the stimulation of HGU and hepatic glucose storage. We examined the hepatic expression of pAkt and PTG in an attempt to identify other regulatory proteins that might have affected glucose uptake and storage in the SAL and GLC groups, but we found no evidence for a differential effect.
The glucose and insulin excursions in response to standardized midday and evening meals are significantly smaller than those following breakfast in normal men (12). Moreover, when obese or overweight women were randomly assigned to diets causing isocaloric weight loss, with 500 kcal at lunch and either 700 kcal at breakfast and 200 kcal at dinner, or 200 kcal at breakfast and 700 at dinner, the group consuming the larger breakfast exhibited significantly reduced fasting glucose, insulin, and HOMA–insulin resistance in comparison with the group consuming the larger evening meal after 12 weeks of treatment (11). In addition, the insulin and glucose AUCs were significantly reduced at all three meals in the group eating a larger breakfast versus that eating the larger dinner (11). Given the importance of the liver in determining glucose and meal tolerance (38), the marked improvement in meal tolerance on the day when a larger breakfast was consumed is consistent with enhanced HGU on that day.
Most (39–44), but not all (12,45), data from healthy humans indicate that meal or glucose tolerance has a diurnal pattern, such that it decreases over the course of the day in subjects receiving three daily meals or glucose loads. This has been suggested to be related to a loss of insulin sensitivity and a decrease in the β-cell response to glucose from morning to evening (44). A decline in meal/glucose tolerance from morning to evening has been observed in subjects with impaired fasting glucose (46) but not in obese subjects with normal glucose tolerance (44), non–insulin-treated individuals with type 2 diabetes (47), or adults with type 1 diabetes (48). The current study differs from these previous studies in that it was not aimed at examining diurnal changes in either glucose tolerance or β-cell responsiveness, but instead was specifically designed to quantify the impact of morning hyperinsulinemia and hyperglycemia, brought about by a duodenal glucose infusion, on hepatic glucose metabolism in the same day. It remains to be determined whether hyperinsulinemia, hyperglycemia, or both are key elements in the enhancement of the HGU and hepatic glucose storage observed in the GLC group.
Individuals with diabetes have had lower postprandial levels of hepatic glycogen than normal controls in some (12,49,50), but not all (51–54), investigations. The degree of diabetes control seems to be a key determinant of postprandial hepatic glycogen content (54). Decreased hepatic glycogen might increase the vulnerability of people with type 1 diabetes to hypoglycemia, particularly during the nocturnal period. We recently showed that a liver-brain counterregulatory axis that is responsive to the mass of hepatic glycogen affects the counterregulatory response to hypoglycemia (28). Our data suggest that an increase in insulinemia and/or glycemia early in the day might improve hepatic glycogen stores and possibly reduce the risk of hypoglycemia during the night.
In conclusion, our data indicate that a morning glucose load exerts a priming effect on the liver, such that glucose uptake and storage are enhanced during a “meal” (i.e., a period of hyperinsulinemia and hyperglycemia) later in the day. Our findings also suggest that the quality and quantity of breakfast intake may be especially important in optimizing hepatic glycogen reserves, thus potentially reducing the risk of hypoglycemia in those with diabetes (28).
Article Information
Funding. This work was supported by National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01-DK-18243 (to A.D.C.). These studies used the Metabolic Physiology Shared Resource, the Hormone Assay & Analytical Services Core, and the Lipid Core, all supported by NIH/NIDDK grant DK020593. The Hormone Assay & Analytical Services Core also receives support from NIH/NIDDK grant DK059637. A.D.C. is the Jacquelyn A. Turner and Dr. Dorothy J. Turner Chair in Diabetes Research and thus receives support from this source.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.C.M. directed all experiments, collected and interpreted data, and drafted and revised the manuscript. M.C.M., P.E.W., and A.D.C. designed the experiments. M.S.S. performed the biochemical and tissue analyses. M.S.S., B.F., and G.K. performed the experiments. B.F. and P.E.W. were responsible for the surgical preparation and care of the animals. M.S. provided supervision and gave advice to M.S.S. during certain analytical processes. A.D.C. was involved in all aspects of the study, including review of the data. All authors provided input during the preparation of the manuscript. A.D.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this work were presented at the 75th Scientific Sessions of the American Diabetes Association, Boston, MA, 5–9 June 2015.
References
- 1.Betts JA, Richardson JD, Chowdhury EA, Holman GD, Tsintzas K, Thompson D. The causal role of breakfast in energy balance and health: a randomized controlled trial in lean adults. Am J Clin Nutr 2014;100:539–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garaulet M, Gómez-Abellán P. Timing of food intake and obesity: a novel association. Physiol Behav 2014;134:44–50 [DOI] [PubMed] [Google Scholar]
- 3.Odegaard AO, Jacobs DR Jr, Steffen LM, Van Horn L, Ludwig DS, Pereira MA. Breakfast frequency and development of metabolic risk. Diabetes Care 2013;36:3100–3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song WO, Chun OK, Obayashi S, Cho S, Chung CE. Is consumption of breakfast associated with body mass index in US adults? J Am Diet Assoc 2005;105:1373–1382 [DOI] [PubMed] [Google Scholar]
- 5.Wennberg M, Gustafsson PE, Wennberg P, Hammarström A. Poor breakfast habits in adolescence predict the metabolic syndrome in adulthood. Public Health Nutr 2015;18:122–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donin AS, Nightingale CM, Owen CG, et al. . Regular breakfast consumption and type 2 diabetes risk markers in 9- to 10-year-old children in the Child Heart and Health Study in England (CHASE): a cross-sectional analysis. PLoS Med 2014;11:e1001703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jakubowicz D, Wainstein J, Ahren B, Landau Z, Bar-Dayan Y, Froy O. Fasting until noon triggers increased postprandial hyperglycemia and impaired insulin response after lunch and dinner in individuals with type 2 diabetes: a randomized clinical trial. Diabetes Care 2015;38:1820–1826 [DOI] [PubMed] [Google Scholar]
- 8.Deshmukh-Taskar PR, Nicklas TA, O’Neil CE, Keast DR, Radcliffe JD, Cho S. The relationship of breakfast skipping and type of breakfast consumption with nutrient intake and weight status in children and adolescents: the National Health and Nutrition Examination Survey 1999–2006. J Am Diet Assoc 2010;110:869–878 [DOI] [PubMed] [Google Scholar]
- 9.Farshchi HR, Taylor MA, Macdonald IA. Deleterious effects of omitting breakfast on insulin sensitivity and fasting lipid profiles in healthy lean women. Am J Clin Nutr 2005;81:388–396 [DOI] [PubMed] [Google Scholar]
- 10.Lazarou C, Matalas AL. Breakfast intake is associated with nutritional status, Mediterranean diet adherence, serum iron and fasting glucose: the CYFamilies study. Public Health Nutr 2015;18:1308–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring) 2013;21:2504–2512 [DOI] [PubMed] [Google Scholar]
- 12.Hwang JH, Perseghin G, Rothman DL, et al. . Impaired net hepatic glycogen synthesis in insulin-dependent diabetic subjects during mixed meal ingestion. A 13C nuclear magnetic resonance spectroscopy study. J Clin Invest 1995;95:783–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan LM, Shi JW, Hampton SM, Frost G. Effect of meal timing and glycaemic index on glucose control and insulin secretion in healthy volunteers. Br J Nutr 2012;108:1286–1291 [DOI] [PubMed] [Google Scholar]
- 14.Abumrad NN, Cherrington AD, Williams PE, Lacy WW, Rabin D. Absorption and disposition of a glucose load in the conscious dog. Am J Physiol 1982;242:E398–E406 [DOI] [PubMed] [Google Scholar]
- 15.Moore MC, Cherrington AD, Cline G, et al. . Sources of carbon for hepatic glycogen synthesis in the conscious dog. J Clin Invest 1991;88:578–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore MC, Coate KC, Winnick JJ, An Z, Cherrington AD. Regulation of hepatic glucose uptake and storage in vivo. Adv Nutr 2012;3:286–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coate KC, Kraft G, Lautz M, Smith M, Neal DW, Cherrington AD. A high-fat, high-fructose diet accelerates nutrient absorption and impairs net hepatic glucose uptake in response to a mixed meal in partially pancreatectomized dogs. J Nutr 2011;141:1643–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adkins-Marshall B, Pagliassotti MJ, Asher JR, et al. . Role of hepatic nerves in response of liver to intraportal glucose delivery in dogs. Am J Physiol 1992;262:E679–E686 [DOI] [PubMed] [Google Scholar]
- 19.Coate KC, Kraft G, Irimia JM, et al. . Portal vein glucose entry triggers a coordinated cellular response that potentiates hepatic glucose uptake and storage in normal but not high-fat/high-fructose-fed dogs. Diabetes 2013;62:392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramnanan CJ, Edgerton DS, Rivera N, et al. . Molecular characterization of insulin-mediated suppression of hepatic glucose production in vivo. Diabetes 2010;59:1302–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres TP, Fujimoto Y, Donahue EP, et al. . Defective glycogenesis contributes toward the inability to suppress hepatic glucose production in response to hyperglycemia and hyperinsulinemia in zucker diabetic fatty rats. Diabetes 2011;60:2225–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keppler D, Decker K. Glycogen: determination with amyloglycosidase. In Methods of Enzymatic Analysis. 2nd ed. Bergmeyer HU, Ed. New York, Academic Press, 1974, p. 1127–1131 [Google Scholar]
- 23.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509 [PubMed] [Google Scholar]
- 24.Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J Lipid Res 1964;5:600–608 [PubMed] [Google Scholar]
- 25.Moore MC, Hsieh PS, Neal DW, Cherrington AD. Nonhepatic response to portal glucose delivery in conscious dogs. Am J Physiol Endocrinol Metab 2000;279:E1271–E1277 [DOI] [PubMed] [Google Scholar]
- 26.Satake S, Moore MC, Igawa K, et al. . Direct and indirect effects of insulin on glucose uptake and storage by the liver. Diabetes 2002;51:1663–1671 [DOI] [PubMed] [Google Scholar]
- 27.Winnick JJ, An Z, Ramnanan CJ, et al. . Hepatic glycogen supercompensation activates AMP-activated protein kinase, impairs insulin signaling, and reduces glycogen deposition in the liver. Diabetes 2011;60:398–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winnick JJ, Kraft G, Gregory JM, et al. . Hepatic glycogen can regulate hypoglycemic counterregulation via a liver-brain axis. J Clin Invest 2016;126:2236–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaworski K, Sarkadi-Nagy E, Duncan RE, Ahmadian M, Sul HS. Regulation of triglyceride metabolism. IV. Hormonal regulation of lipolysis in adipose tissue. Am J Physiol Gastrointest Liver Physiol 2007;293:G1–G4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliveira V, Marinho R, Vitorino D, et al. . Diets containing α-linolenic (ω3) or oleic (ω9) fatty acids rescues obese mice from insulin resistance. Endocrinology 2015;156:4033–4046 [DOI] [PubMed] [Google Scholar]
- 31.Matravadia S, Zabielski P, Chabowski A, Mutch DM, Holloway GP. LA and ALA prevent glucose intolerance in obese male rats without reducing reactive lipid content, but cause tissue-specific changes in fatty acid composition. Am J Physiol Regul Integr Comp Physiol 2016;310:R619–R630 [DOI] [PubMed] [Google Scholar]
- 32.Agius L. Glucokinase and molecular aspects of liver glycogen metabolism. Biochem J 2008;414:1–18 [DOI] [PubMed] [Google Scholar]
- 33.Torres TP, Catlin RL, Chan R, et al. . Restoration of hepatic glucokinase expression corrects hepatic glucose flux and normalizes plasma glucose in zucker diabetic fatty rats. Diabetes 2009;58:78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramnanan CJ, Saraswathi V, Smith MS, et al. . Brain insulin action augments hepatic glycogen synthesis without suppressing glucose production or gluconeogenesis in dogs. J Clin Invest 2011;121:3713–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sibrowski W, Seitz HJ. Hepatic glucokinase turnover in intact and adrenalectomized rats in vivo. Eur J Biochem 1980;113:121–129 [DOI] [PubMed] [Google Scholar]
- 36.Coate KC, Smith MS, Shiota M, et al. . Hepatic glucose metabolism in late pregnancy: normal versus high-fat and -fructose diet. Diabetes 2013;62:753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagy L, Docsa T, Szántó M, et al. . Glycogen phosphorylase inhibitor N-(3,5-dimethyl-benzoyl)-N′-(β-D-glucopyranosyl)urea improves glucose tolerance under normoglycemic and diabetic conditions and rearranges hepatic metabolism. PLoS One 2013;8:e69420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Capaldo B, Gastaldelli A, Antoniello S, et al. . Splanchnic and leg substrate exchange after ingestion of a natural mixed meal in humans. Diabetes 1999;48:958–966 [DOI] [PubMed] [Google Scholar]
- 39.Saad A, Dalla Man C, Nandy DK, et al. . Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 2012;61:2691–2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshino J, Almeda-Valdes P, Patterson BW, et al. . Diurnal variation in insulin sensitivity of glucose metabolism is associated with diurnal variations in whole-body and cellular fatty acid metabolism in metabolically normal women. J Clin Endocrinol Metab 2014;99:E1666–E1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jarrett RJ, Baker IA, Keen H, Oakley NW. Diurnal variation in oral glucose tolerance: blood sugar and plasma insulin levels morning, afternoon, and evening. BMJ 1972;1:199–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Service FJ, Hall LD, Westland RE, et al. . Effects of size, time of day and sequence of meal ingestion on carbohydrate tolerance in normal subjects. Diabetologia 1983;25:316–321 [DOI] [PubMed] [Google Scholar]
- 43.Van Cauter E, Shapiro ET, Tillil H, Polonsky KS. Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol 1992;262:E467–E475 [DOI] [PubMed] [Google Scholar]
- 44.Lee A, Ader M, Bray GA, Bergman RN. Diurnal variation in glucose tolerance. Cyclic suppression of insulin action and insulin secretion in normal-weight, but not obese, subjects. Diabetes 1992;41:750–759 [DOI] [PubMed] [Google Scholar]
- 45.Malherbe C, De Gasparo M, De Hertogh R, Hoet JJ. Circadian variations of blood sugar and plasma insulin levels in man. Diabetologia 1969;5:397–404 [DOI] [PubMed] [Google Scholar]
- 46.Sonnier T, Rood J, Gimble JM, Peterson CM. Glycemic control is impaired in the evening in prediabetes through multiple diurnal rhythms. J Diabetes Complications 2014;28:836–843 [DOI] [PubMed] [Google Scholar]
- 47.Peter R, Dunseath G, Luzio SD, Chudleigh R, Roy Choudhury S, Owens DR. Daytime variability of postprandial glucose tolerance and pancreatic B-cell function using 12-h profiles in persons with type 2 diabetes. Diabet Med 2010;27:266–273 [DOI] [PubMed] [Google Scholar]
- 48.Hinshaw L, Dalla Man C, Nandy DK, et al. . Diurnal pattern of insulin action in type 1 diabetes: implications for a closed-loop system. Diabetes 2013;62:2223–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krssak M, Brehm A, Bernroider E, et al. . Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes 2004;53:3048–3056 [DOI] [PubMed] [Google Scholar]
- 50.Bischof MG, Krssak M, Krebs M, et al. . Effects of short-term improvement of insulin treatment and glycemia on hepatic glycogen metabolism in type 1 diabetes. Diabetes 2001;50:392–398 [DOI] [PubMed] [Google Scholar]
- 51.Bally L, Buehler T, Dokumaci AS, Boesch C, Stettler C. Hepatic and intramyocellular glycogen stores in adults with type 1 diabetes and healthy controls. Diabetes Res Clin Pract 2015;109:e1–e3 [DOI] [PubMed] [Google Scholar]
- 52.Flück CE, Slotboom J, Nuoffer JM, Kreis R, Boesch C, Mullis PE. Normal hepatic glycogen storage after fasting and feeding in children and adolescents with type 1 diabetes. Pediatr Diabetes 2003;4:70–76 [DOI] [PubMed] [Google Scholar]
- 53.Matyka K, Dixon RM, Mohn A, et al. . Daytime liver glycogen accumulation, measured by 13C magnetic resonance spectroscopy, in young children with type 1 diabetes mellitus. Diabet Med 2001;18:659–662 [DOI] [PubMed] [Google Scholar]
- 54.Bischof MG, Bernroider E, Krssak M, et al. . Hepatic glycogen metabolism in type 1 diabetes after long-term near normoglycemia. Diabetes 2002;51:49–54 [DOI] [PubMed] [Google Scholar]