Abstract
Among the therapeutic avenues being explored for replacement of the functional islet β-cell mass lost in type 1 diabetes (T1D), reprogramming of adult cell types into new β-cells has been actively pursued. Notably, mouse islet α-cells will transdifferentiate into β-cells under conditions of near β-cell loss, a condition similar to T1D. Moreover, human islet α-cells also appear to poised for reprogramming into insulin-positive cells. Here we have generated transgenic mice conditionally expressing the islet β-cell–enriched Mafa and/or Pdx1 transcription factors to examine their potential to transdifferentiate embryonic pan–islet cell Ngn3-positive progenitors and the later glucagon-positive α-cell population into β-cells. Mafa was found to both potentiate the ability of Pdx1 to induce β-cell formation from Ngn3-positive endocrine precursors and enable Pdx1 to produce β-cells from α-cells. These results provide valuable insight into the fundamental mechanisms influencing islet cell plasticity in vivo.
Introduction
While current therapies of insulin treatment afford glycemic control to patients with type 1 diabetes (T1D), these relatively static methods do not completely recapitulate the acute regulation of the endogenous islet β-cells destroyed by autoimmune destruction. Consequently, patients with T1D have a dramatically shortened life expectancy due to serious long-term diabetes complications, including coronary and renal disease. A variety of innovative approaches are being explored to produce β-cells from embryonic stem cells (1,2) and adult cell types (3–5). A supposition in these efforts involves producing conditions that correctly regulate the transcription factor networks required in programming pancreatic progenitor cells into β-cells and subsequently controlling mature islet cell function. These include transcription factors like Pdx1 (6–10), which is essential in the formation of early pancreatic epithelium, developing β-cells and adult islet β-cells, as well as neurogenin 3 (Ngn3) (11–13), which is required during embryogenesis for specification of all islet cell types (i.e., β-cells, glucagon hormone–producing α-cells, somatostatin δ-cells, pancreatic polypeptide (PP) cells, and ghrelin ε-cells). In addition, there are transcription factors like Mafa (14,15) that are influential later during postnatal β-cell maturation and adult cell function. Indeed, ectopic expression of Pdx1, Ngn3, and Mafa can reprogram pancreatic exocrine cells (3) and intestinal cells (4) into functional β-like cells in vivo.
T1D results from the specific loss of islet β-cells. Interestingly, functional β-like cells are produced from endogenous mouse islet α-cells (16) or δ-cells (17) after near total targeted destruction of this cell population, a model mimicking the disease state (16). Furthermore, epigenomic findings suggest that human α-cells are poised for reprogramming, with treatment to prevent histone 3 repressor site marking at lysine 27 leading to the appearance of insulin-positive–glucagon-positive bihormonal cells in human islets (18).
Here, we generated transgenic mice that allow conditionally and targeted expression of Mafa or Pdx1 to determine their contribution to β-cell generation from embryonic endocrine Ngn3-positive and committed glucagon-positive progenitors. Earlier studies had established that forced Pdx1 expression in this endocrine precursor population results in greater β-cell production at the expense of α-cells, with no effect on δ-cells or PP cells (19). We found that Mafa not only was found to potentiate the ability of Pdx1 to reprogram Ngn3-positive endocrine progenitor cells to insulin-positive cells but also empowered Pdx1 to transdifferentiate committed glucagon-positive α-cells to this cell fate. These results provide further support for the essential role of Mafa and Pdx1 in the production of therapeutic β-cells for treatment of patients with T1D.
Research Design and Methods
CAG-CAT-Pdx1flag, CAG-CAT-Mafamyc, Cre, and ROSA26 Mice
CAG-CAT-Mafamyc (20), CAG-CAT-Pdx1flag (21), Ngn3-Cre (12), Gcg-Cre (22), ROSA26-LacZ (23), and ROSA26-GFP (24) mice have previously been described. All animal procedures were approved by the Ethics Review Committee for Animal Experimentation of the Osaka University Graduate School of Medicine.
Immunohistochemistry and Cell Quantification
Pancreata were dissected and fixed in 4% paraformaldehyde in PBS at 4°C, washed in PBS, immersed in sucrose solution, embedded and frozen in Tissue-Tek (O.C.T. Compound; Sakura), or processed routinely for paraffin embedding. Frozen and paraffin blocks were sectioned at 6-μm thickness and immunostained. The following primary antibodies were used at the given dilutions: rabbit anti-MafA (1:500) (Bethyl Laboratories, Inc., Montgomery, TX); goat anti-MafA (25) (1:200); rabbit anti-Pdx1 (26) (1:1,000); rabbit anti-Nkx6.1 (1:200) (Sigma-Aldrich, St. Louis, MO); rabbit anti-MafB (1:200) (Bethyl Laboratories, Inc.); goat anti-Arx (1:200) (Santa Cruz Biotechnology, Inc., Dallas, TX); rabbit anti-myc (1:200) (Cell Signaling Technology, Inc., Danvers, MA); rabbit anti-flag (1:100) (Affinity BioReagents, Golden, CO); mouse anti-flag (1:500) (TransGenic Inc., Kobe, Japan); rabbit anti-Glut2 (1:200) (abcam, Cambridge, U.K.); guinea pig anti-insulin (1:2,000) (DAKO, Glostrup, Denmark); rabbit anti-glucagon (1:500) (DAKO); guinea pig anti-glucagon (1:200) (Millipore, St. Charles, MO); rabbit anti–β-galactosidase (β-gal) antibody (1:200) (Medical and Biological Laboratories, Nagoya, Japan); chicken anti–β-gal antibody (1:200) (abcam); and chicken anti–green fluorescent protein (GFP) antibody (1:500) (abcam). Primary antibodies were detected with donkey-raised secondary antibody–conjugated fluorescein at a 1:500 dilution. Fluorescent images were captured using an Olympus FV1000-D confocal microscope. The images shown are representative of our analysis of at least three independently derived mice unless otherwise specified.
The total number of insulin-, glucagon-, PP-, and β-gal–positive cells in five sections per pancreas from at least three mice per genotype were manually counted in the Ngn3-Cre (Figs. 1D and 2B) and Gcg-Cre (Fig. 3D) lines. Approximately 500 cells were counted in the 6-week-old pancreas and 200 cells in the P0.5 pancreas. The data are presented as the ratio of each hormone-positive cell or hormone-positive–to–β-gal–positive cell for each genotype.
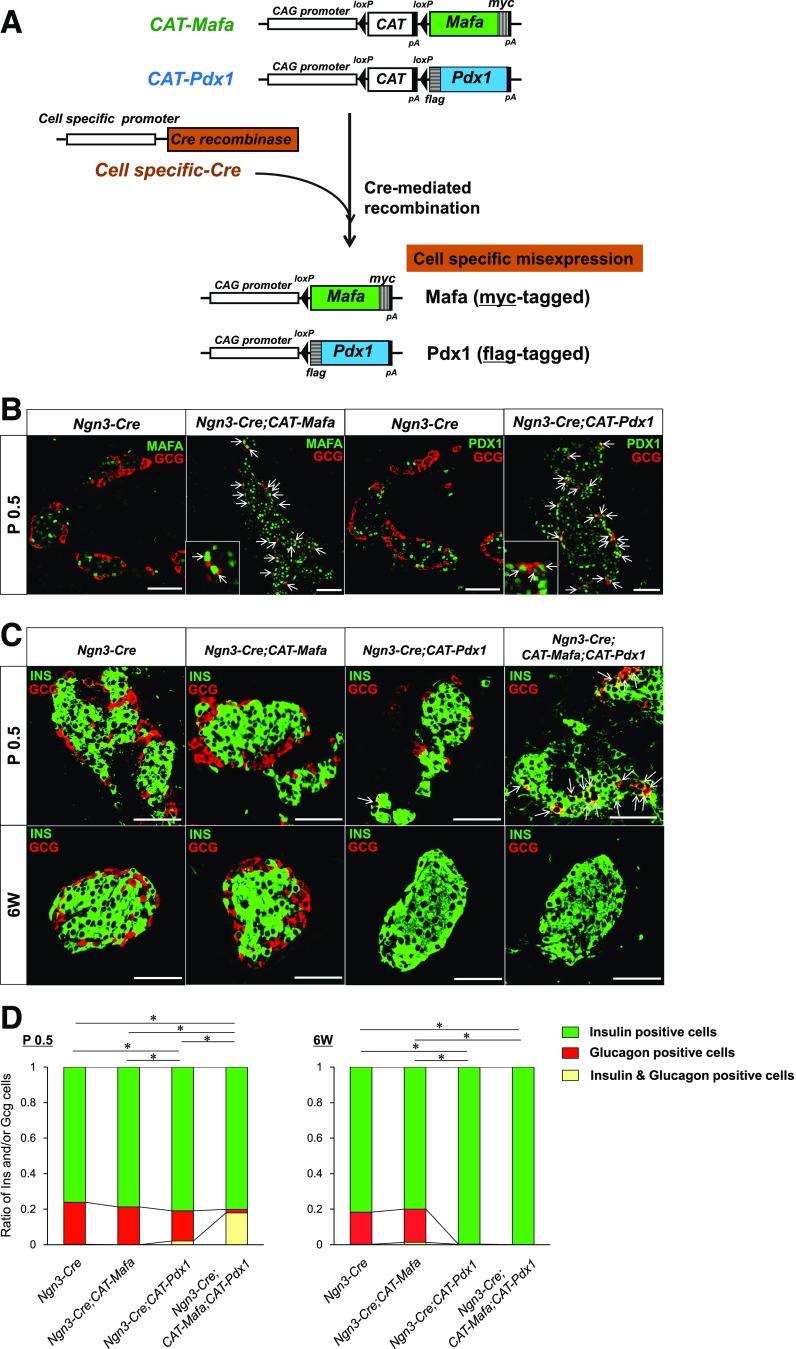
Figure 1.
Mafa and Pdx1 enhance production of insulin-positive cells from glucagon-positive cells when coexpressed in pancreatic endocrine precursor cells. A: Schematic representation of Mafamyc, Pdx1flag, and Cre-mediated recombination. Exogenous myc-tagged Mafa or flag-tagged Pdx1 is expressed via the excision of the stuffer chloramphenicol acetyltransferase (CAT) cassette upon production in islet cell precursors of Ngn3-Cre or of Gcg-Cre in islet α-cells. B: MafA and Pdx1 antibodies were used to show ectopically expressed Mafamyc and Pdx1flag in essentially all Ngn3-Cre;CAT-Mafa (left) or Ngn3-Cre;CAT-Pdx1 (right) islet glucagon-positive cells at P0.5. The arrows illustrate Mafa or Pdx1 expressing glucagon-positive cells. C: Insulin-positive and glucagon-positive cells in representative islets of the Ngn3-Cre, Ngn3-Cre;CAT-Mafa, Ngn3-Cre;CAT-Pdx1, and Ngn3-Cre;CAT-Mafa;CAT-Pdx1 pancreas at P0.5 or 6 weeks. The arrows depict insulin and glucagon–copositive cells, which are only detected in the Ngn3-Cre;CAT-Mafa;CAT-Pdx1 pancreas at P0.5. D: Quantitative analysis of the islet insulin-, glucagon-, or cohormone-positive production at P0.5 and 6-week-old cells in the Ngn3-Cre, Ngn3-Cre;CAT-Mafa, Ngn3-Cre;CAT-Pdx1, and Ngn3-Cre;CAT-Mafa;CAT-Pdx1 pancreas. The ratio of the insulin-, glucagon-, or double-positive cells is presented relative to the total hormone-positive cell number. The ratio of the double hormone–positive cells to glucagon-positive cells was compared at P0.5 and of glucagon-positive cells to insulin-positive cells at 6 weeks. n = 3–6. 6W, six weeks; CAG, chicken β-actin promoter and cytomegalovirus enhancer; INS, insulin. *P < 0.001. Scale bars: 50 μm.
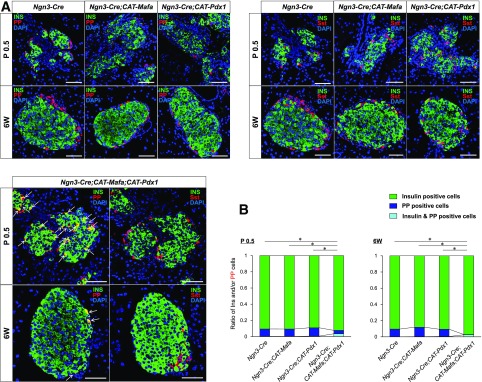
Figure 2.
Insulin-positive cells are produced from PP cells upon coexpression of Mafa and Pdx1 in pancreatic endocrine precursor cells. A: Immunohistochemical analysis of pancreata from Ngn3-Cre, Ngn3-Cre;CAT-Mafa, Ngn3-Cre;CAT-Pdx1, and Ngn3-Cre;CAT-Mafa;CAT-Pdx1 mice at P0.5 and 6 weeks for insulin, PP, and somatostatin (Sst). Nuclei were stained with DAPI. There were PP cells coexpressing PP and insulin at P0.5 and 6 weeks. Arrows depict insulin and PP–copositive cells. Scale bars: 50 μm. B: The ratio of insulin and somatostatin–positive cells to somatostatin-positive cells was compared at P0.5; the somatostatin-positive cell–to–insulin-positive cell ratio was compared at 6 weeks. n = 3–6. 6W, 6 weeks; INS, insulin. *P < 0.001.
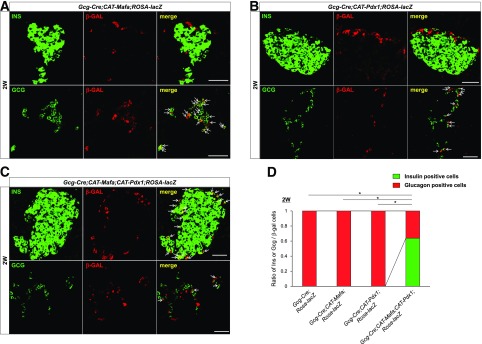
Figure 3.
Mafa + Pdx1 produces insulin-positive cells from α-cells. Insulin, glucagon, and β-gal staining was performed in the Gcg-Cre;CAT-Mafa;ROSA-LacZ (A), Gcg-Cre;CAT-Pdx1;ROSA-LacZ (B), and Gcg-Cre;CAT-Mafa;CAT-Pdx1;ROSA-LacZ (C) pancreas. The arrows in A and B illustrate that Gcg-Cre activates ROSA-LacZ reporter expression in only α-cells. Because Gcg-Cre was expressed in only ∼35% of α-cells, a nonrepresentative islet image is shown to more clearly illustrate the extent of glucagon-positive–to–insulin-positive cell conversion in Gcg-Cre;CAT-Mafa;CAT-Pdx1;ROSA-LacZ islets. The arrows show that a large fraction of β-gal–positive cells express insulin by 2 weeks in the Gcg-Cre;CAT-Mafa;CAT-Pdx1;ROSA-LacZ pancreas (C). D: The ratio of insulin-positive, glucagon-positive, and insulin + glucagon–positive cells within β-gal–positive cells to total β-gal–positive cells at 2 weeks in Gcg-Cre;ROSA-LacZ, Gcg-Cre;CAT-Mafa;ROSA-LacZ, Gcg-Cre;CAT-Pdx1;ROSA-LacZ, and Gcg-Cre;CAT-Mafa;CAT-Pdx1;ROSA-LacZ is displayed, and the ratio of insulin-positive cells is compared. n = 4. 2W, 2 weeks; INS, insulin. *P < 0.001. Scale bars: 50 μm.
Statistical Analysis
Statistical analysis was performed using one-way ANOVA followed by the Fisher test. A value of P < 0.05 was considered to be statistically significant.
Results
Mafa + Pdx1 More Efficiently Programs Endocrine Ngn3-Positive Precursors to β-Cells Than Pdx1 Alone
To explore how Mafa influences the ability of Pdx1 to convert α-cells into β-cells, we developed mouse lines that allow for conditional and heritably overproduction of either Mafa, Pdx1, or Mafa + Pdx1 upon expression of Cre recombinase (Fig. 1A). Antibodies to Mafa and Pdx1 and their incorporated antigenic tags were used to immunohistochemically detect the levels of the transgenic proteins, termed Mafamyc and Pdx1flag. Both of these islet β-cell–enriched proteins (27–29) were now found throughout the islet cell population upon activation in embryonic endocrine precursors with Ngn3-driven Cre, which were termed the Ngn3-Cre;CAT-Mafa and Ngn3-Cre;CAT-Pdx1 lines. For example, flag-tagged Pdx1 was produced in essentially all islet cells in P0.5 and 6-week-old Ngn3-Cre;CAT-Pdx1 mice; the penetrance rate was ∼95% in the insulin-positive and glucagon-positive cell populations (Supplementary Fig. 1). Notably, neither Mafamyc nor Pdx1flag was expressed in the surrounding acinar or ductal cells of the pancreas (Fig. 1B). While there was no overt effect on α-cell fate in Ngn3-Cre;CAT-Mafa islets (Fig. 1C and D), insulin was expressed in ∼10% of Ngn3-Cre;CAT-Pdx1 glucagon-positive cells at P0.5. These double hormone–positive cells resolved to become only insulin-positive by 6 weeks, with essentially no islet glucagon-positive cells remaining (Fig. 1C), as previously reported (19). Moreover, there was no evidence for apoptosis within Mafamyc– and Pdx1flag–positive islet glucagon-positive or insulin-positive cells, with the TUNEL-positive signal detected exclusively from red blood cells (Supplementary Fig. 2). These results suggested that resolution of the Mafamyc + Pdx1flag–positive cells to monohormonal insulin production reflects transdifferentiation of α- to β-like cells.
Mafa expression in endocrine precursor cells augmented the actions of Pdx1 in the Ngn3-Cre;CAT-Mafa;CAT-Pdx1 line. Thus, there was a roughly ninefold increase in the number of insulin and glucagon–copositive cells in these islets at birth compared with Ngn3-Cre;CAT-Pdx1 islets (17.9 vs. 2.0%) (Fig. 1D). Insulin was also clearly detected in Ngn3-Cre;CAT-Mafa;CAT-Pdx1 PP cells at P0.5, while no induction was found in Ngn3-Cre;CAT-Mafa or Ngn3-Cre;CAT-Pdx1 islets (Fig. 2A and Supplementary Fig. 3A). Notably, insulin and PP–copositive cells were still detectible in 6-week-old Ngn3-Cre;CAT-Mafa;CAT-Pdx1 islets (Fig. 2A). In addition and as reported earlier, insulin was produced in very few somatostatin hormone–expressing Ngn3-Cre;CAT-Pdx1 islet δ-cells (19) or Ngn3-Cre;CAT-Mafa;CAT-Pdx1 islet δ-cells (Supplementary Fig. 3). These results are consistent with lineage-tracing experiments indicating that islet β- and PP cells are derived from a common PP-positive precursor (22) and illustrate the differential plasticity of islet lineages to Mafa and Pdx1 reprogramming.
Mafa + Pdx1 Can Effectively Convert α-Cells Into Islet β-Cells
Pdx1 alone is incapable of converting α-cells into islet β-cells in vivo (19). However, since cosynthesized Mafa + Pdx1 favored the production of β-cells at the expense of α-cells when coproduced in Ngn3-positive progenitors (Fig. 1D), we wondered whether these factors would have such facilitating properties if exclusively expressed in α-cells. Glucagon enhancer/promoter–driven Cre transgenic mice (30) (Gcg-Cre) were used to overexpress Mafa, Pdx1, and Mafa + Pdx1 in α-cells, with concomitant excision of the loxP site flanked LacZ or GFP expression cassettes in the ROSA26 locus used for cell lineage labeling. Cre-mediated recombination was observed in roughly 35% of islet glucagon-positive cells in Gcg-Cre;CAT-Mafa (Fig. 3A) and Gcg-Cre;CAT-Pdx1 (Fig. 3B) islets. Moreover, insulin, somatostatin-positive, and PP-positive cells were not β-gal positive (Supplementary Fig. 4) (data not shown).
The effect on islet insulin-positive cell formation of forced Mafa + Pdx1 expression in α-cells was compared among Gcg-Cre;CAT-Mafa;CAT-Pdx1, Gcg-Cre;CAT-Mafa, and Gcg-Cre;CAT-Pdx1 islets. Neither Mafa nor Pdx1 could independently produce insulin in α-cells (Fig. 3A and B), a result consistent with earlier data examining forced Pdx1 expression (19). In contrast, insulin staining was clearly detectable in Gcg-Cre;CAT-Mafa;CAT-Pdx1 α-cells marked by β-gal, myc tag, and flag tag by 2 weeks; the analysis was performed at this time point rather than P0.5 owing to the later developmental expression of Gcg-Cre relative to Ngn3-Cre. Notably, glucagon was no longer detectible in the 2-week-old lineage-labeled insulin-positive cell population (Fig. 3C and D and Supplementary Fig. 5A and B), and there was not any overt change in number of these cells in 6-week-old Gcg-Cre;CAT-Mafa;CAT-Pdx1 islets (Supplementary Fig. 6) (data not shown). As observed in the insulin-positive cell population generated by Mafamyc and Pdx1flag expression in Ngn3 islet progenitors, there was no evidence of cell death within Gcg-Cre;CAT-Mafa;CAT-Pdx1 islets (Supplementary Figs. 2 and 7). The remaining glucagon-positive cells in Gcg-Cre;CAT-Mafa;CAT-Pdx1 islets never expressed Mafamyc or Pdx1flag—a reflection of the poor penetrance of Gcg-Cre (Supplementary Fig. 8).
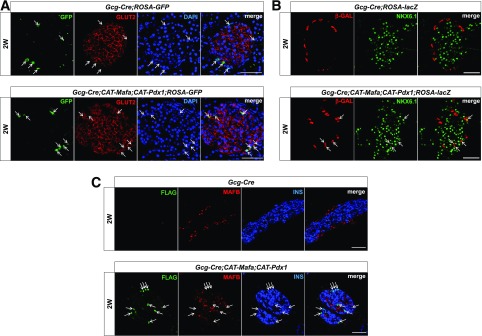
For further assessment of the change to β-cell identity of the α-cells made to coexpress Mafa and Pdx1, immunostaining was performed with various islet α-cell– (i.e., Mafb and Arx) and β-cell– (i.e., Glut2, Nkx6.1, and Urocortin3 [Ucn3] [31]) enriched protein antibodies on this new insulin-positive cell population (Fig. 4 and Supplementary Fig. 9). Significantly, many of the β-cell–enriched products were made in these cells, including the principal β-cell glucose transporter, Glut2 (i.e., 80% of GFP-traced recombinated cells), the Nkx6.1 transcription factor (36% of β-gal–traced recombinated cells), and Ucn3 (46% of Flag-traced recombinated cells). In contrast, Mafb (27), a transcription factor normally produced in immature islet β-cells (15), was not present in Mafamyc + Pdx1flag–produced insulin-positive cells (Fig. 4C). Similarly, Arx (32), a transcription factor essential for α-cell formation (32), was expressed poorly in α-cell–derived insulin-positive cells (i.e., ∼21%) (Supplementary Fig. 9B). Taken together, these findings demonstrate that Pdx1 + Mafa can transdifferentiate α-cells into β-like cells by inducing key features of the β-cell and suppressing those of the α-cell.
Figure 4.
Islet β-cell identity marker expression is gained at the expense of α in Gcg-Cre;CAT-Mafa;CAT-Pdx1 islets. Islet β-cell–enriched Glut2 (A) and Nkx6.1 (B) production is specifically induced in Gcg-Cre;CAT-Mafa;CAT-Pdx1;ROSA α-cell lineage–labeled GFP (A) and β-gal (B) cells at the rate of 80% and 36% among labeled cells, respectively. In contrast, the production of α-cell identity (C) Mafb is lost in this α-lineage–marked cell population. The arrows illustrate Glut2-positive, Nkx6.1-positive, or Mafb-negative cells labeled with the lineage marker and presumably expressing Mafamyc and/or Pdx1flag. 2W, 2 weeks; INS, insulin. Scale bars: 50 μm.
Discussion
Several distinct islet-enriched transcription factors are capable of reprogramming other pancreatic cell types into β-like cells, including Pdx1, Ngn3, and Mafa in exocrine acinar cells (3); Pdx1 (19) as well as Nkx6.1 in Ngn3-positive precursors (33); and Pax4 in ductal cells (34). Such observations illustrate the tremendous cellular plasticity within the pancreas that could be exploited therapeutically to sustain islet β-cell activity in patients with T1D. Interestingly, islet α-cells, the principal remaining pancreatic islet cell population in these individuals, convert to β-cells upon ablation of this insulin-positive cell population in adult mice. However, little is known about the factors that initiate this cell fate change. Here we show that islet β-cell–enriched Mafa not only potentiates the ability of Pdx1 to convert Ngn3-positive cells to insulin-positive cells but also permits Pdx1 to reprogram α-cells into β-cells. These findings illustrate the mutually supportive abilities of these transcription factors to promote the β-cell fate choice.
Heritably expressing Mafa and Pdx1 into Ngn3-positive cells profoundly impacted the fraction of α-cells converted to insulin and glucagon–coproducing cells at birth, with essentially all α-cells undergoing a cell fate switch in Ngn3-Cre;CAT-Mafa;CAT-Pdx1 islets (Fig. 1D). This population appears to be completely reprogrammed to β-cells by 6 weeks in Ngn3-Cre;CAT-Pdx1 (19) and Ngn3-Cre;CAT-Mafa;CAT-Pdx1 islets (Fig. 1D), as illustrated by the gain in β-cell identity marker expression and loss of α (Fig. 4 and Supplementary Fig. 9). The significant enhancement by Mafa of Pdx1-mediated transdifferentiation at postnatal 0.5 day (P0.5) implies that both factors act simultaneously to change the chromatin architecture of α-cell progenitors to a β-like fate. In addition, Mafa + Pdx1 induced insulin production in islet PP cells and silenced PP hormone expression (Fig. 2A). Unfortunately, the lack of distinctive maturation markers for PP cell markers precludes us from determining the extent of reprogramming.
Notably, while Nkx6.1 also effectively converts Ngn3-positive progenitors to insulin-positive cells, there are clear difference in how this transcription factor acts in relation to Mafa + Pdx1. For example, Nkx6.1 reprogrammed all neonatal islet cell types to express the insulin hormone (i.e., α, δ, ε, and PP) and not simply the α- and PP cells (33). However, developmental Nkx6.1 expression is also unable to effectively convert α-cell progenitors to insulin producing, and it would be of interest to determine whether Mafa has the same enabling capabilities. Even more profound mechanistic differences were observed upon expression of Pax4 in embryonic Ngn3-positive cells or α-cells (34), which produced β-like cells from pancreatic ductal cells that resulted in oversized islets, β-cell dysfunction, and diabetes. Each of these reprogramming factors presumably initiates the β-cell–fate switch by binding to target sequences in silent chromatin (35). The differences in their potential presumably reflect their ability to embed in nucleosomal DNA and the functional properties of recruited coregulators, as illustrated for the Oct4, Sox2, Klf4, and c-Myc transcription in generating pluripotent stem cells (36).
Gene induction involves the recruitment of transcriptional coregulators by enhancer-bound transcription factors like Mafa and Pdx1. These protein-protein interactions ultimately lead to assembly at the promoter of the RNA polymerase II transcriptional machinery. Coregulators can have positive (coactivator) and/or negative (corepressor) actions on target gene transcription, thus conferring a second level of specificity to the transcriptional response. For example, the islet-cell–enriched Nkx2.2 transcription recruits the DNMT3a, Grg3, and HDAC1 corepressors to silence transcription of the Arx gene in developing β-cells (37). It will be interesting to determine how various recently described coactivators of Pdx1 and Mafa impact their reprogramming abilities, as one would predict that the chromatin-modifying activities of Swi/Snf4 (i.e., Pdx1 [38]) and MLL4 (i.e., Mafa [39]) to be of importance.
Collectively, these results strongly suggest that Pdx1 and Mafa are principal mediators of β-cell production from non–β-cells, including in the context of developing α-cells as well as adult acinar (3), stomach (5), and intestinal (4) cells. In consideration of these observations, we predict that these transcription factors also play a principal role in the induction of the newly formed β-cells produced from α- (16) and δ- (17) cells after almost complete ablation of the islet β-cell population by diphtheria toxin expression. Importantly, islet α-, PP, and δ-cells appear to be preserved in the context of the depleted β-cell mass in patients with T1D (40–42) and T2D (43,44) and, consequently, are potentially reprogrammable by transcription factor expression (e.g., Mafa + Pdx1, Pax4 [34]) or drug treatment (18). Collectively, these findings further illuminate the significance of understanding the mechanisms involved in regulating islet cell identity and function in diabetes treatment.
Supplementary Material
Article Information
Acknowledgments. The authors thank Yuko Sasaki for excellent technical assistance and Chikayo Yokogawa for excellent secretarial assistance, both from the Osaka University Graduate School of Medicine.
Funding. This work was supported by grants from JDRF (Career Development Awards 2-2005-946 to T.Ma. and 46-2010-755, 26-2007-928, and 17-2012-389 to P.L.H.); KAKENHI (10379258 to T.Ma.); the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (DK-089566 and DK-089572 to P.L.H. and DK-50203 and DK-090570 to R.S.); and the Swiss National Science Foundation (to P.L.H.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. T.Ma. designed experiments. T.Ma., T.Mi., and P.L.H. generated the mice. T.Ma. and S.K. performed immunohistochemistry. T.Ma., S.K., S.S., N.S., and S.T. performed mouse experiments. T.Ma., T.Mi., and R.S. wrote the manuscript. N.K., D.K., H.K., R.S., and I.S. supervised the project. T.Ma. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-0887/-/DC1.
References
- 1.Rezania A, Bruin JE, Arora P, et al. . Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 2014;32:1121–1133 [DOI] [PubMed] [Google Scholar]
- 2.Pagliuca FW, Millman JR, Gürtler M, et al. . Generation of functional human pancreatic β cells in vitro. Cell 2014;159:428–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 2008;455:627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen YJ, Finkbeiner SR, Weinblatt D, et al. . De novo formation of insulin-producing “neo-β cell islets” from intestinal crypts. Cell Reports 2014;6:1046–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ariyachet C, Tovaglieri A, Xiang G, et al. . Reprogrammed stomach tissue as a renewable source of functional β cells for blood glucose regulation. Cell Stem Cell 2016;18:410–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao T, McKenna B, Li C, et al. . Pdx1 maintains β cell identity and function by repressing an α cell program. Cell Metab 2014;19:259–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahlgren U, Jonsson J, Edlund H. The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1-deficient mice. Development 1996;122:1409–1416 [DOI] [PubMed] [Google Scholar]
- 8.Offield MF, Jetton TL, Labosky PA, et al. . PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development 1996;122:983–995 [DOI] [PubMed] [Google Scholar]
- 9.Grapin-Botton A, Majithia AR, Melton DA. Key events of pancreas formation are triggered in gut endoderm by ectopic expression of pancreatic regulatory genes. Genes Dev 2001;15:444–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 1994;371:606–609 [DOI] [PubMed] [Google Scholar]
- 11.Gradwohl G, Dierich A, LeMeur M, Guillemot F. Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A 2000;97:1607–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 2002;129:2447–2457 [DOI] [PubMed] [Google Scholar]
- 13.Johansson KA, Dursun U, Jordan N, et al. . Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev Cell 2007;12:457–465 [DOI] [PubMed] [Google Scholar]
- 14.Zhang C, Moriguchi T, Kajihara M, et al. . MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol 2005;25:4969–4976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Artner I, Hang Y, Mazur M, et al. . MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes 2010;59:2530–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorel F, Népote V, Avril I, et al. . Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 2010;464:1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chera S, Baronnier D, Ghila L, et al. . Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers. Nature 2014;514:503–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bramswig NC, Everett LJ, Schug J, et al. . Epigenomic plasticity enables human pancreatic α to β cell reprogramming. J Clin Invest 2013;123:1275–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang YP, Thorel F, Boyer DF, Herrera PL, Wright CV. Context-specific α- to-β-cell reprogramming by forced Pdx1 expression. Genes Dev 2011;25:1680–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuoka TA, Kaneto H, Kawashima S, et al. . Preserving Mafa expression in diabetic islet β-cells improves glycemic control in vivo. J Biol Chem 2015;290:7647–7657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyatsuka T, Kaneto H, Kajimoto Y, et al. . Ectopically expressed PDX-1 in liver initiates endocrine and exocrine pancreas differentiation but causes dysmorphogenesis. Biochem Biophys Res Commun 2003;310:1017–1025 [DOI] [PubMed] [Google Scholar]
- 22.Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development 2000;127:2317–2322 [DOI] [PubMed] [Google Scholar]
- 23.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 1999;21:70–71 [DOI] [PubMed] [Google Scholar]
- 24.Mao X, Fujiwara Y, Chapdelaine A, Yang H, Orkin SH. Activation of EGFP expression by Cre-mediated excision in a new ROSA26 reporter mouse strain. Blood 2001;97:324–326 [DOI] [PubMed] [Google Scholar]
- 25.Matsuoka TA, Kaneto H, Miyatsuka T, et al. . Regulation of MafA expression in pancreatic beta-cells in db/db mice with diabetes. Diabetes 2010;59:1709–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuoka T, Kajimoto Y, Watada H, et al. . Glycation-dependent, reactive oxygen species-mediated suppression of the insulin gene promoter activity in HIT cells. J Clin Invest 1997;99:144–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuoka TA, Zhao L, Artner I, et al. . Members of the large Maf transcription family regulate insulin gene transcription in islet beta cells. Mol Cell Biol 2003;23:6049–6062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuoka TA, Artner I, Henderson E, Means A, Sander M, Stein R. The MafA transcription factor appears to be responsible for tissue-specific expression of insulin. Proc Natl Acad Sci U S A 2004;101:2930–2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J 1993;12:4251–4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herrera PL, Orci L, Vassalli JD. Two transgenic approaches to define the cell lineages in endocrine pancreas development. Mol Cell Endocrinol 1998;140:45–50 [DOI] [PubMed] [Google Scholar]
- 31.Li C, Chen P, Vaughan J, Lee KF, Vale W. Urocortin 3 regulates glucose-stimulated insulin secretion and energy homeostasis. Proc Natl Acad Sci U S A 2007;104:4206–4211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collombat P, Mansouri A, Hecksher-Sorensen J, et al. . Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev 2003;17:2591–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaffer AE, Taylor BL, Benthuysen JR, et al. . Nkx6.1 controls a gene regulatory network required for establishing and maintaining pancreatic Beta cell identity. PLoS Genet 2013;9:e1003274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collombat P, Xu X, Ravassard P, et al. . The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell 2009;138:449–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwafuchi-Doi M, Zaret KS. Pioneer transcription factors in cell reprogramming. Genes Dev 2014;28:2679–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soufi A, Garcia MF, Jaroszewicz A, Osman N, Pellegrini M, Zaret KS. Pioneer transcription factors target partial DNA motifs on nucleosomes to initiate reprogramming. Cell 2015;161:555–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papizan JB, Singer RA, Tschen SI, et al. . Nkx2.2 repressor complex regulates islet β-cell specification and prevents β-to-α-cell reprogramming. Genes Dev 2011;25:2291–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKenna B, Guo M, Reynolds A, Hara M, Stein R. Dynamic recruitment of functionally distinct Swi/Snf chromatin remodeling complexes modulates Pdx1 activity in islet β cells. Cell Reports 2015;10:2032–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scoville DW, Cyphert HA, Liao L, et al. . MLL3 and MLL4 methyltransferases bind to the MAFA and MAFB transcription factors to regulate islet β-cell function. Diabetes 2015;64:3772–3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gepts W, De Mey J. Islet cell survival determined by morphology. An immunocytochemical study of the islets of Langerhans in juvenile diabetes mellitus. Diabetes 1978;27(Suppl. 1):251–261 [DOI] [PubMed]
- 41.Somoza N, Vargas F, Roura-Mir C, et al. . Pancreas in recent onset insulin-dependent diabetes mellitus. Changes in HLA, adhesion molecules and autoantigens, restricted T cell receptor V beta usage, and cytokine profile. J Immunol 1994;153:1360–1377 [PubMed] [Google Scholar]
- 42.Gómez Dumm CL, Cónsole GM, Luna GC, Dardenne M, Goya RG. Quantitative immunohistochemical changes in the endocrine pancreas of nonobese diabetic (NOD) mice. Pancreas 1995;11:396–401 [DOI] [PubMed] [Google Scholar]
- 43.Yoon KH, Ko SH, Cho JH, et al. . Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab 2003;88:2300–2308 [DOI] [PubMed] [Google Scholar]
- 44.Henquin JC, Rahier J. Pancreatic alpha cell mass in European subjects with type 2 diabetes. Diabetologia 2011;54:1720–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.