Abstract
The relationships between diabetes and pancreatic ductal adenocarcinoma (PDAC) are complex. Longstanding type 2 diabetes (T2DM) is a risk factor for pancreatic cancer, but increasing epidemiological data point to PDAC as also a cause of diabetes due to unknown mechanisms. New-onset diabetes is of particular interest to the oncology community as the differentiation of new-onset diabetes caused by PDAC as distinct from T2DM may allow for earlier diagnosis of PDAC. To address these relationships and raise awareness of the relationships between PDAC and diabetes, a symposium entitled Diabetes, Pancreatogenic Diabetes, and Pancreatic Cancer was held at the American Diabetes Association’s 76th Scientific Sessions in June 2016. This article summarizes the data presented at that symposium, describing the current understanding of the interrelationships between diabetes, diabetes management, and pancreatic cancer, and identifies areas where additional research is needed.
Introduction
The most common and most lethal form of pancreatic cancer, referred to as pancreatic ductal adenocarcinoma (PDAC), has an extraordinary association with diabetes. In a recent series of 100 patients diagnosed with cancers of the lung, breast, colon, prostate, or pancreas, 68% of patients with PDAC had concurrent diabetes, whereas the prevalence of diabetes ranged from 15 to 21% in the other age-matched cancer cohorts and 24% in 100 age-matched control subjects (1). The increased incidence of pancreatic cancer in populations with diabetes has been observed repeatedly in epidemiological studies, with a relative risk or hazard ratio that ranges from 1.5 to 2.0 (2). Importantly, among patients with PDAC who also have diabetes, the diagnosis of diabetes occurred less than 24 months prior to the diagnosis of PDAC in 74–88% of patients (3). These facts illustrate that diabetes and PDAC demonstrate “dual causality,” in that both long-standing type 2 diabetes (T2DM) is a risk factor for the development of PDAC and, conversely, PDAC is a presumed cause of diabetes in a large number of cases. The mechanisms of these causal relationships are unclear, as are the diagnostic criteria for differentiating T2DM from diabetes that occurs as an early consequence of PDAC. These are important considerations clinically given that PDAC has an overall 5-year survival rate of 7–8% (4) and that it is projected to become the second leading cause of cancer-related deaths by 2020 (5). To explore these issues, a symposium was held at the 76th Scientific Sessions of the American Diabetes Association in 2016. The following represents a summary of the symposium presentations, with the purpose of providing more insight into these important relationships.
Diabetes as a Risk Factor for PDAC
The Centers for Disease Control and Prevention estimates that approximately 29 million people in the U.S. had T2DM in 2014, and about 8 million of these individuals have not yet been diagnosed. Moreover, approximately 86 million adults in the U.S. are believed to have prediabetes, defined by a fasting plasma glucose level of 100–125 mg/dL, a 2-h plasma glucose level of 140–199 mg/dL, or a glycohemoglobin (HbA1c) level of 5.7–6.4% (6). These statistics put into perspective the difficulties inherent in using glucose intolerance and diabetes as biomarkers for diagnosing PDAC-associated diabetes. The global spread of this huge health care burden further underscores the need to better understand the pathophysiology of T2DM and to distinguish it from pancreatogenic or type 3c diabetes (T3cDM), which has been classified as diabetes secondary to pancreatic exocrine disease.
Long-standing T2DM is a risk factor for PDAC (7). In addition, T2DM is often associated with obesity, and obesity per se also increases the risk for developing PDAC (8). T2DM is associated with resistance to the action of insulin to suppress hepatic glucose release. Concomitantly, there is failure to enhance peripheral (predominantly skeletal muscle) glucose utilization, with initially increased insulin levels, as the β-cell attempts to overcome the insulin resistance (9). With time, there is progressive β-cell failure leading to T2DM including aberrant activation of the unfolded protein response pathway, which may induce both apoptosis and senescence leading to a decreased β-cell mass (10). Nevertheless, both obesity and T2DM are likely to lead to long time periods in which such individuals have high intrapancreatic insulin levels due to an ongoing impetus by the β-cell to overcome the insulin-resistant state and maintain glucose homeostasis.
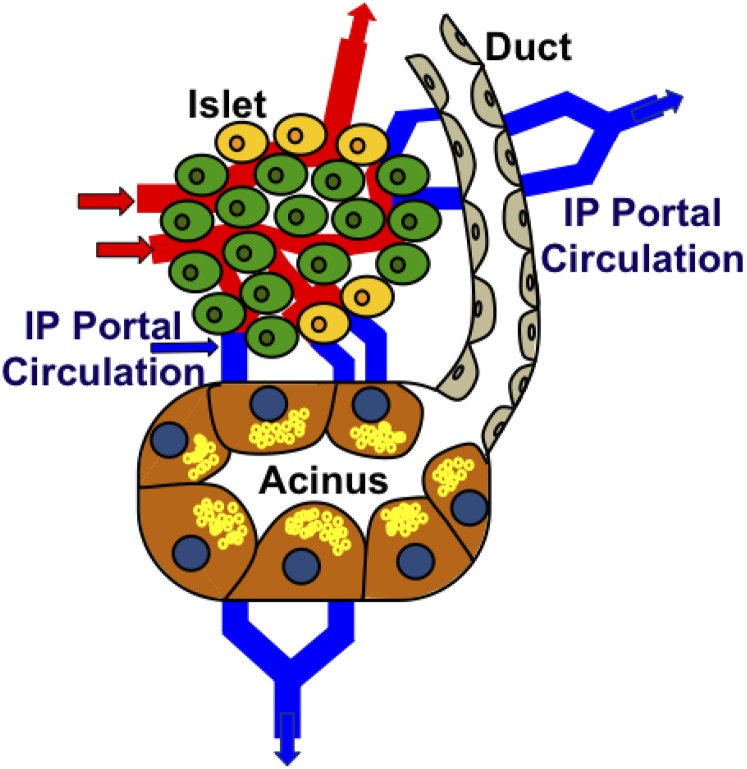
Insulin released by the β-cells is discharged into an intrapancreatic portal circulation that provides blood to acinar and ductal cells adjacent to the islets. Both cell types can be adjacent to the islets and may be supplied by blood from the intrapancreatic portal circulation (Fig. 1). This proximity enables high levels of islet hormones to directly reach groups of acinar and ductal cells, exerting what has been termed proxicrine effects on insulin receptors that are present on acinar cells and on IGF-I receptors in any transformed cells that may arise in this region, thereby promoting their survival and proliferation. Thus, hyperinsulinemia, particularly intrapancreatic, as a result of obesity and insulin resistance in prediabetes or early T2DM may plausibly contribute to the observed increased risk in PDAC.
Figure 1.
Schematic representation of the islet–acinar–ductal axis. An endocrine islet consisting mostly of insulin-producing β-cells (green) but also other endocrine cell types such as glucagon (yellow) is shown receiving an arterial blood supply (red). Some of this arterial blood drains into an intrapancreatic (IP) portal circulation that bathes adjacent acinar and ductal cells. The insulin-rich blood supply exerts trophic effects on these cells that are most evident in the acinar cells making up one such acinus. The acinar cells are large and have many enzyme-rich zymogen granules (shown as small yellow circles within each acinar cell).
Additionally, poor glycemic control is associated with increased levels of advanced glycation end products (AGE) that activate a receptor for AGE termed RAGE (11). This receptor belongs to the immunoglobulin super family and binds several ligands in addition to AGE, including certain inflammatory cytokines and members of the S100 family of proteins that have been implicated in inflammation and cancer, including PDAC (11). Moreover, RAGE activation contributes to the development of obesity and its associated proinflammatory profile (12). Conceivably, therefore, excessive RAGE activation may also contribute to the higher incidence of PDAC in T2DM, as well as to the increased incidence of colorectal cancer, breast cancer, and other cancers that may occur with T2DM and obesity.
Genomic Associations of Diabetes, Chronic Pancreatitis, and Pancreatic Cancer
Although there is strong clinical and epidemiological evidence that links the risk of PDAC to long-standing T2DM or to chronic pancreatitis (CP) (the most frequent cause of T3cDM), the genetic basis of susceptibility among these three diseases varies widely, with little overlap. Indeed, genetic heterogeneity of all three is the rule. Through extensive studies of case series and families and using a variety of study designs, catalogs of genes have been identified for each condition. Interestingly, what is known about the mechanism by which these genes influence susceptibility is uneven because of the different methods used to identify the genes.
All three diseases share the following characteristics: 1) all have subsets of patients who report family history or familial clustering, which are indicators of shared genetic and/or environmental etiologies, 2) variation in age at diagnosis has been linked to familial risk in some patients, and 3) Mendelian segregation analyses provide a formal demonstration that in some families there is evidence for a hereditary component (typically due to a major gene). In addition, there are epidemiological risk factors (e.g., obesity [diabetes], alcohol intake [CP], and smoking [PDAC]) that may interact with genetic factors to enhance risk.
Numerous approaches have been used to discover susceptibility genes, from family-based studies to case-control and cohort studies that use a small list of candidate genes; large, agnostic genome-wide association studies (GWAS) that search for associated single nucleotide polymorphisms (SNPs) that are typically noncoding; or next-generation sequencing. The needed analytic and bioinformatics methods have kept pace and have provided the statistical tools for interrogating the resulting data. The genetic basis of T2DM is best characterized as polygenic, with over 50 genes implicated (13). No single major gene explains the genetic risk of T2DM except in very limited subsets (14). In contrast, there are well-documented reports of CP and PDAC kindred that can be delineated as due to mutations in major genes, while overall GWAS studies have identified many low-penetrance common SNPs that are associated with increased risk.
Among the three conditions, T2DM has been the most intensively studied, and considerably more resources have been invested in uncovering the genetics of diabetes, consistent with the high prevalence and economic burden of this disease. However, genetic discoveries have not led to ready clinical application. It is clear from dozens of studies that diabetes is a multifactorial/polygenic disease and is genetically heterogeneous. Variants of over 50 genes have been found to confer genetic risk, each with a modest effect (e.g., PPARG and KDNJ11, identified through the candidate gene approach; and TCF7L2, WFS1, HDF1B, FTO, CDKN2A, and SLC20A8, among others, identified through association and GWAS approaches). Researchers have developed new strategies in the search for diabetes predisposition genes by further characterizing the genetic basis of diabetes through subclinical or related phenotypes (15). The outcome of research to date is that susceptibility to diabetes fits a polygenic risk model, with each genetic variant having a small effect. Interestingly, despite these efforts, the genetic variants do not significantly improve risk assessment over common risk factors such as age, sex, family history, BMI, and clinical measures (16).
Family-based strategies and knowledge of the pathophysiology of CP facilitated early success in elucidating its genetic heterogeneity and established PRSS1, SPINK1, CTRC, CFTR, and CASR as susceptibility loci for CP (17). Much of the variability in susceptibility to recurrent acute and chronic pancreatitis is related to genetic differences between patients. A large two-stage GWAS analysis by Whitcomb et al. (18) identified and replicated PRSS1-PRSS2 and X-linked CLDN2 as susceptibility loci, with the latter gene’s variants potentially interacting with alcohol consumption. Alcohol was long thought to be the primary causative agent, but genetic contributions have been of interest since the discovery that rare PRSS1, CFTR, and SPINK1 variants were associated with pancreatitis risk. Thus, a single factor rarely causes pancreatitis, and the majority of patients with recurrent acute and chronic pancreatitis have multiple variants in a gene, or epistatic interactions between multiple genes, coupled with environmental stressors.
The study of genetic predisposition to PDAC has been particularly challenging because of the logistics of recruiting and collecting biospecimens for analysis from patients with poor survival. Like diabetes and CP, PDAC is genetically heterogeneous. Susceptibility genes that have been identified range from rare mutations in genes associated with cancer syndromes to common SNPs. Designs that include family-based and case-control studies have uncovered mutations in known syndrome-related genes, such as BRCA1, BRCA2, CDKN2A, and CFTR. Next-generation sequencing has identified additional mutations such as PALB2 (19) and ATM (20).
More recently, patients with apparent sporadic PDAC have been reported to carry germline mutations in major genes as well (21); this is likely to change our current paradigm for risk assessment. GWAS studies of large numbers of sporadic cases of pancreatic cancer and control subjects have implicated SNPs in regions that harbor ABO, TERT, and CLPTM1L, among dozens of other genes. However, risk modeling that utilizes GWAS SNPs has not shown that the genetic information improves prediction in the general population (22).
The Role of Obesity- and Pancreatitis-Associated Inflammation in the Development of PDAC
There is strong evidence that obesity is associated with an increased risk of cancer, including pancreatic cancer (23). In fact, the anticipated increase in pancreatic cancer incidence and deaths may be at least partially attributed to the obesity endemic. There are many possible mechanisms by which obesity leads to (pancreatic) cancer, including insulin resistance with resulting hyperinsulinemia and inflammation (24). Nonsteroidal anti-inflammatory drugs can attenuate pancreatic cancer development in a genetically engineered mouse model, indicating an important role of tissue inflammation in this disease (25). Obesity-associated tissue inflammation is thought to create a fertile microenvironment conducive to tumor initiation and/or promotion. Recent evidence indicates that in addition to measures of general obesity, e.g., BMI, visceral adiposity carries a strong association to metabolic diseases and gastrointestinal cancers, including pancreatic cancer (26).
The mesenteric adipose tissue adjacent to the pancreas (peri-pancreatic depot) showed a substantially enhanced proinflammatory response to a high-fat, high-calorie diet compared with the peri-gonadal depot (27). A precise knowledge of adipose tissue depot-specific responses to diet-induced obesity and their distinct effects on cancer development are critically important to explain the association of certain body compositions to cancer risk and to understand possible sex differences. Mechanistically, a high-fat, high-calorie diet accelerates the progression of pancreatic intraepithelial neoplasia, a known precursor of pancreatic cancer, and increases the incidence of invasive and metastatic pancreatic cancer in the conditional KrasG12D mouse model (28,29).
Antidiabetes Medications and the Risk of PDAC
Because various antidiabetes medications can directly affect the key factors mediating the association between T2DM and PDAC, some of these medications may have an impact on PDAC development, progression, and outcome.
High levels of insulin activate the IGF receptor, thereby acquiring the potential to exert mitogenic and tumor-promoting effects. Epidemiological studies have found that new use of insulin was associated with an increased risk of PDAC (30). Sulfonylureas (insulin secretagogues) stimulate endogenous release of insulin by inhibiting the K-ATP channel of the pancreatic β-cells. Use of sulfonylureas has been associated with an increased risk of pancreatic cancer but to a less extent than that for use of insulin. Thiazolidinediones reduce insulin resistance by activating peroxisome proliferator–activated receptor γ, a nuclear receptor regulating glucose and lipid metabolism. Use of thiazolidinediones was not associated with risk of PDAC according to a recent review and meta-analysis (31).
Incretin-based therapies include glucagon-like peptide 1 (GLP-1) receptor agonists and dipeptidyl peptidase 4 (DPP-4) inhibitors. GLP-1 stimulates insulin secretion by the β-cell through activation of the GLP-1 receptor resulting in increased production of cAMP. It also increases insulin mRNA stability via upregulation of the transcription factor PDX-1 and, in rodents, promotes β-cell growth and survival. DPP-4 inhibitors increase the endogenous levels of GLP-1 and the second principal incretin hormone glucose-dependent insulinotropic polypeptide (GIP). Therefore, GLP-1 mimetics and DPP-4 inhibitors may have an indirect trophic effect on pancreatic ductal cells. The association of incretin-based therapy and acute pancreatitis has been raised in clinical studies, and as a result, the U.S. Food and Drug Administration has issued a warning regarding this relationship. Considering the long latency nature of human malignancies, further studies are required to clarify the association of incretin use and PDAC.
There is strong experimental evidence to support an antitumor activity of metformin. The fundamental mechanism of metformin action involves two possible avenues: decreased circulating insulin and IGF levels and inhibition of mitochondrial oxidative phosphorylation, which consequently leads to energy stress, AMPK activation, and inhibition of mTOR signaling. A study in 2014 identified mitochondrial glycerophosphate dehydrogenase as the major molecular target of metformin (32). Inhibition of this enzyme resulted in an altered hepatocellular redox state, reduced conversion of lactate and glycerol to glucose, and decreased hepatic gluconeogenesis.
To date, at least 12 observational studies have investigated the association of metformin use and risk of PDAC with inconsistent data. A meta-analysis of these studies showed a summary risk ratio of 0.73 (95% CI 0.56–0.96, P = 0.023) among metformin users (33). Notably, two randomized clinical trials comparing metformin with active glucose-lowering therapy or placebo/usual care in T2DM patients did not find any significant impact of metformin use on cancer events. These trials were not designed with cancer as an end point, and the follow-up times were somewhat short. Even if the protective effect of metformin is confirmed in future epidemiological studies, more work needs to be done to identify eligible subpopulations that would benefit from metformin for PDAC prevention.
Overall, the causal relationship between antidiabetes therapies and the risk of PDAC cannot be established by the current observational studies. Many of the reported observational studies suffered from limitations of insufficient power and inadequate outcome validation, incomplete covariate ascertainment, and inadequate confounding control. Better designed epidemiological investigations and systematic capture of data on pancreatitis and PDAC from cardiovascular outcome trials and other ongoing clinical trials should facilitate meta-analyses and accumulation of further knowledge.
T3cDM: Prevalence, Differentiation From T2DM, and Importance of Recognition
The most common form of T3cDM is diabetes associated with recurrent acute or chronic pancreatitis, although the best recognized is cystic fibrosis–related diabetes. Diabetes is also associated with pancreatic cancer, and the most obvious form of T3cDM is that following partial or complete surgical resection of the pancreas. In recurrent acute or chronic pancreatitis and in cystic fibrosis, the development of exocrine insufficiency typically predates the development of the endocrine insufficiency and diabetes (34). Any episode of acute pancreatitis can cause a transient diabetic state and increases the risk of the subsequent development of diabetes (35).
The pathophysiology of T3cDM most commonly involves pancreatic glandular inflammation and subsequent irreversible fibrotic damage leading to islet cell loss. Unlike other types of diabetes, the islet loss involves not only the β-cells but also the pancreatic polypeptide (PP) cells early in the disease course and the α-cells late in the disease course (Table 1). Because of concomitant pancreatic exocrine insufficiency, there is also maldigestion of nutrients with consequent impairment in incretin secretion, and pancreatic enzyme replacement can improve incretin and insulin secretion as well as glucose tolerance. Typically, islet β-cell secretory capacity is preserved until the majority of pancreatic exocrine function is lost (36); however, given how common overweight and obesity are now in the population, traditional T2DM risk factors and insulin resistance may accelerate the presentation of diabetes in the context of pancreatic disease.
Table 1.
Clinical and laboratory findings in types of diabetes
| Parameter | T1DM | T2DM | T3cDM |
|---|---|---|---|
| Ketoacidosis | Common | Rare | Rare |
| Hypoglycemia | Common | Rare | Common |
| Peripheral insulin sensitivity | Normal or decreased | Decreased | Normal or increased |
| Hepatic insulin sensitivity | Normal or decreased | Decreased | Normal or decreased |
| Insulin levels | Low or absent | High or “normal” | “Normal” or low |
| Glucagon levels | Normal or high | Normal or high | “Normal” or low |
| PP levels | Normal or low (late) | Normal or high | Low or absent |
| GIP levels | Normal or low | Variable | Low |
| GLP-1 levels | Normal | Variable | Variable |
| Typical age of onset | Childhood or adolescence | Adulthood | Any |
| Typical etiology | Autoimmune | Obesity, age | CP, cystic fibrosis, postoperative |
“Normal,” inappropriate in the context of elevated glucose. Adapted from Cui and Andersen (47).
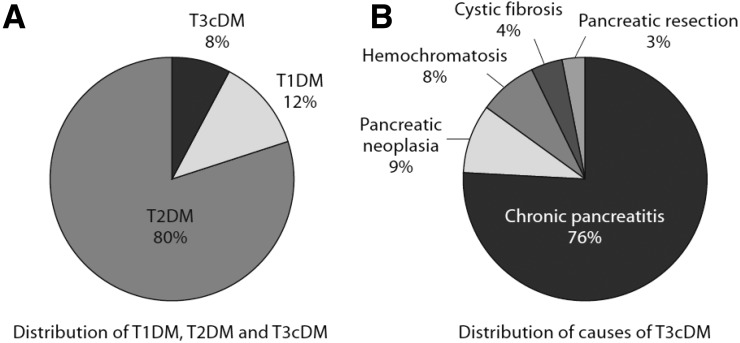
Importantly, pancreatic disease remains underrecognized as an underlying etiology of diabetes. At an academic referral center in Germany, almost 10% of all patients with diabetes could be classified as T3cDM, with CP being the most common etiology affecting 80% of cases (37) (Fig. 2). In most of these cases, T3cDM was initially misclassified as T2DM, leading the authors to propose diagnostic criteria for T3cDM (38). As outlined in Table 2, the diagnosis of T3cDM requires 1) the presence of pancreatic exocrine insufficiency, 2) evidence of pathological pancreatic imaging, and 3) the absence of type 1 diabetes (T1DM)-associated autoimmune markers; the diagnosis may be further supported by evidence of PP, incretin, or insulin secretory defects in the absence of clinical or biochemical evidence of overt insulin resistance. Even with this guidance, discriminating T3cDM secondary to pancreatitis from T2DM can still be challenging. When ambiguity remains, confirmation of T3cDM can be made by documentation of an absent PP response to mixed-nutrient ingestion, which best discriminates the pathological islet response from that of T2DM (39).
Figure 2.
Prevalence of types of diabetes. Distribution of types of diabetes (A) and causes of T3cDM (pancreatogenic) diabetes (B) based on studies of 1,922 patients with diabetes reported by Hardt et al. (49). Reproduced from Cui and Andersen (47).
Table 2.
Diagnostic criteria for T3cDM
| Major criteria (all must be fulfilled) |
| Presence of exocrine pancreatic insufficiency (according to monoclonal fecal elastase 1 or direct function tests). |
| Pathological pancreatic imaging (by endoscopic ultrasound, MRI, or computed tomography). |
| Absence of T1DM-associated autoimmune markers. |
| Minor criteria |
| Impaired β-cell function (e.g., as measured by HOMA-B, C-peptide/glucose ratio). |
| No excessive insulin resistance (e.g., as measured by HOMA of insulin resistance). |
| Impaired incretin (e.g., GIP) or PP secretion. |
| Low serum levels of lipid soluble vitamins (A, D, E, or K). |
Adapted from Ewald and Bretzel (38). HOMA-B, HOMA of β-cell function.
Recognition of T3cDM is important for identification of underlying pancreatic disease that may require specific intervention. Oral pancreatic enzyme replacement to correct maldigestion, particularly of fat, is necessary to optimize incretin secretion and enable the absorption of vitamin D and other fat-soluble vitamins. Insulin therapy is ultimately needed for most patients, and metformin should be considered when concomitant insulin resistance is present. Since metformin may reduce the risk of pancreatic cancer in patients with T2DM, its use is strongly encouraged when evidence of insulin resistance is present (34).
Inaccurate classification of diabetes in clinical practice has limited our understanding of the prevalence of T3cDM, and even our current diagnostic criteria still require prospective validation before we can appreciate their limitations as well as the health consequences of misdiagnosis. Most importantly, randomized clinical trial evidence is needed for specified interventions to prevent diabetes from developing in patients with pancreatic disease and how to best treat T3cDM in those affected.
Mechanisms of Diabetes in Pancreatic Cancer
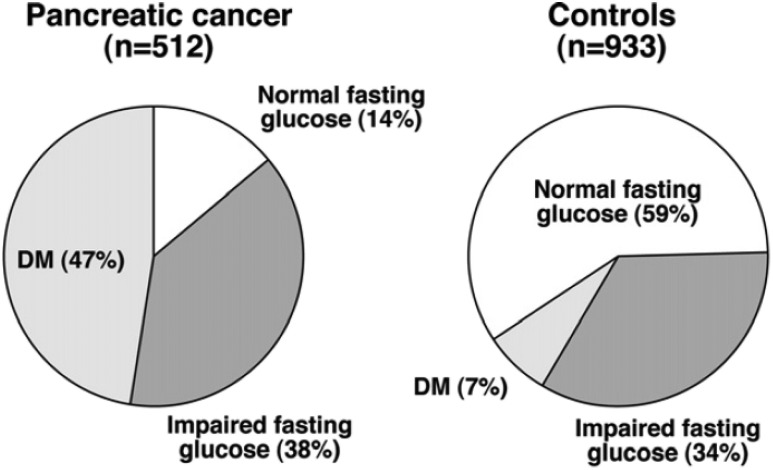
The prevalence of diabetes among patients with PDAC is extraordinarily high. In a study of 512 PDAC patients and 933 control subjects, diabetes was found in 47% of PDAC patients compared with only 7% of control subjects, and although a normal fasting glucose was present in 59% of control subjects, it was found in only 14% of PDAC patients (3) (Fig. 3). In 74% of the PDAC patients with diabetes, the diagnosis of diabetes was made within 24 months before the diagnosis of PDAC, frequently at a time when the tumor was radiographically occult (40). This suggests that in many patients, new-onset diabetes is caused by the tumor and may be a useful “biomarker” for the diagnosis of PDAC. The following observations support this concept.
Figure 3.
Prevalence of diabetes (DM) in PDAC. The prevalence of diabetes and impaired fasting glucose in 512 pancreatic cancer patients and 933 control subjects. Reproduced from Pannala et al. (3).
Despite the fact that canonical risk factors for diabetes such as age, obesity, and a family history of diabetes are also risk factors for PDAC, the incidence of diabetes in PDAC is much higher than the incidence of diabetes among other common malignancies. In a study of the prevalence of diabetes among patients with the most common solid tumors, diabetes was found in 68% of patients with PDAC compared with 14.8–23.5% of patients with breast, colon, lung, and prostate carcinoma and in age-matched control subjects (1). Further, although insulin resistance is a common finding in patients with both PDAC and diabetes, most patients with PDAC report weight loss rather than weight gain. The clinical features of deteriorating glycemic control in conjunction with weight loss that accompanies PDAC prior to its diagnosis are atypical for T2DM; this combination of features should alert the clinician to the possibility of PDAC-associated diabetes.
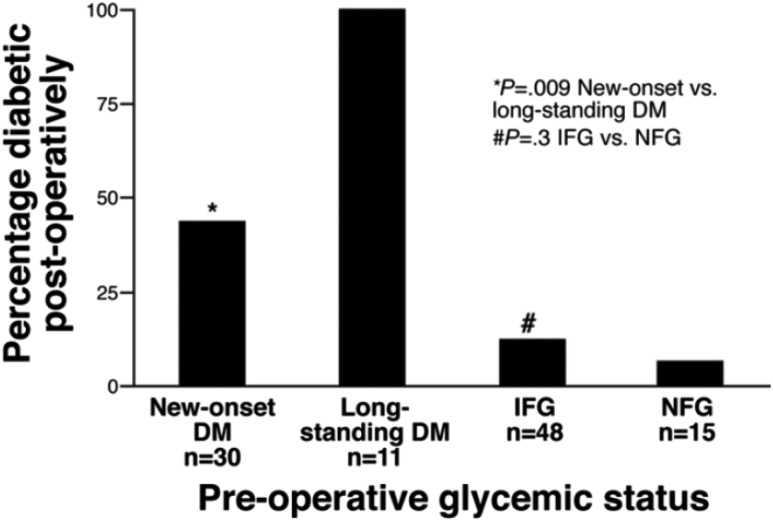
Perhaps most compelling are the data showing that new-onset diabetes associated with PDAC may resolve following tumor resection, as long as there are sufficient islets left in the residual pancreatic tissue. Pancreatic resection is a recognized cause of T3cDM, due to loss of islet mass, and is seen in about half of all subjects after proximal pancreatectomy. Earlier reports had observed an actual improvement in diabetes after resection of some pancreatic cancers (41), but it was unclear whether this was a consequence of nutritional or other factors. In a study of 104 patients who underwent PDAC resection, of whom 41 had diabetes at the time of surgery, it was found that 57% of the patients with new-onset diabetes had resolution of their diabetes postoperatively, whereas all of the patients with long-standing diabetes had a persistence of diabetes after pancreatic resection (3) (Fig. 4). These observations strongly suggest that new-onset diabetes associated with PDAC may be a paraneoplastic phenomenon, where one or more factors induced by the malignancy interfere with insulin secretion or insulin action, leading to diabetes.
Figure 4.
Prevalence of diabetes (DM) after pancreaticoduodenectomy for PDAC. The prevalence of diabetes after resection of PDAC in new-onset diabetes (<2 years duration), long-standing diabetes (>2 years duration), impaired fasting glucose (IFG) (100–125 mg/dL) preoperatively, and normal fasting glucose (NFG) (≤99 mg/dL) preoperatively. Reproduced from Pannala et al. (3).
Numerous studies have attempted to identify the mechanism(s) or the genomic and/or protein markers of the diabetes caused by PDAC. Connexin 26, a gap junction protein, has been described as being highly overexpressed in islets of PDAC patients with diabetes (42), and a PDAC-derived S-100A8 N-terminal peptide has been described as a diabetogenic agent by Basso et al. (43). Huang et al. (44) described two upregulated genes in 27 patients with PDAC associated with new-onset diabetes, vanin-1 and matrix metalloproteinase 9, and a variety of microRNA fragments have been suggested as possibly having predictive usefulness (45). Chari and colleagues (46) at the Mayo Clinic have suggested that adrenomedullin, carried within PDAC-derived exosomes, acts as a peptide mediator of the impaired insulin sensitivity and secretion seen in PDAC-associated diabetes. Adrenomedullin is a multifunctional vasoactive peptide that has been implicated in inflammation and sepsis and is highly overexpressed in PDAC (46). Together with vanin-1, which is also expressed in response to inflammation, these findings suggest that mediators of inflammation may play a role in altered islet function and insulin action in PDAC.
A deficiency of PP release has been documented in T3cDM associated with pancreatic resection, CP, and cystic fibrosis (47), and a recent pilot study suggested that impaired PP release may be a marker of PDAC-associated diabetes (48). PP release is increased in T2DM, and a deficient PP response to nutrient ingestion may differentiate new-onset diabetes that is caused by pancreatic exocrine disease from T2DM. In the pilot study by Hart et al. (48), basal and meal-stimulated PP release was significantly decreased in patients with diabetes associated with PDAC localized to the head of the pancreas compared with patients with T2DM. Further studies sponsored by the National Institutes of Health–sponsored Chronic Pancreatitis, Diabetes, Pancreatic Cancer consortium are currently under way to determine whether a deficient PP response to nutrients can identify a high-risk group with new-onset diabetes that includes patients with early-stage PDAC.
Conclusions
Long-standing T2DM and CP (that may be associated with T3cDM) are risk factors for the development of PDAC. Obesity accompanying T2DM may also increase the risk of PDAC due to hyperinsulinemia, the actions of adipokines, or other factors. PDAC is also a cause of diabetes due to as yet unknown mechanism(s), and the differentiation of new-onset diabetes caused by PDAC, which is distinct from long-standing T2DM or T3cDM associated with CP, may allow for the diagnosis and intervention in early-stage disease at a point at which curative therapy is possible. Much information needs to be acquired, however, to understand the mechanisms of both the contributions of long-standing T2DM and obesity on the development of PDAC and the cause of PDAC-associated diabetes. The ability to distinguish PDAC-associated diabetes from T2DM is critical for recognition that new-onset diabetes in patients aged 50 years or older may be a harbinger of PDAC leading to earlier PDAC detection and improved therapeutic outcome in this deadly malignancy.
Article Information
Funding. This work was supported in part by National Institutes of Health grants RO1-CA-075059 (to M.K.), P01-CA-163200 (to G.E.), R01-DK-97830 (to M.R.R.), U01-DK-108288 (to S.T.C.), and P50-CA-014236 (to J.L.A.). The authors acknowledge travel support from the Pancreatic Cancer Action Network.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
References
- 1.Aggarwal G, Kamada P, Chari ST. Prevalence of diabetes mellitus in pancreatic cancer compared to common cancers. Pancreas 2013;42:198–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA 1995;273:1605–1609 [PubMed] [Google Scholar]
- 3.Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology 2008;134:981–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30 [DOI] [PubMed] [Google Scholar]
- 5.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–2921 [DOI] [PubMed] [Google Scholar]
- 6.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 2015;314:1021–1029 [DOI] [PubMed] [Google Scholar]
- 7.Wolpin BM, Bao Y, Qian ZR, et al. . Hyperglycemia, insulin resistance, impaired pancreatic β-cell function, and risk of pancreatic cancer. J Natl Cancer Inst 2013;105:1027–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang H, Dong X, Hassan M, Abbruzzese JL, Li D. Body mass index and obesity- and diabetes-associated genotypes and risk for pancreatic cancer. Cancer Epidemiol Biomarkers Prev 2011;20:779–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003;46:3–19 [DOI] [PubMed] [Google Scholar]
- 10.Dooley J, Tian L, Schonefeldt S, et al. . Genetic predisposition for beta cell fragility underlies type 1 and type 2 diabetes. Nat Genet 2016;48:519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leclerc E, Vetter SW. The role of S100 proteins and their receptor RAGE in pancreatic cancer. Biochim Biophys Acta 2015;1852:2706–2711 [DOI] [PMC free article] [PubMed]
- 12.Song F, Hurtado del Pozo C, Rosario R, et al. . RAGE regulates the metabolic and inflammatory response to high-fat feeding in mice. Diabetes 2014;63:1948–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herder C, Roden M. Genetics of type 2 diabetes: pathophysiologic and clinical relevance. Eur J Clin Invest 2011;41:679–692 [DOI] [PubMed] [Google Scholar]
- 14.Fuchsberger C, Flannick J, Teslovich TM, et al. . The genetic architecture of type 2 diabetes. Nature 2016;536:41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeggini E, Scott LJ, Saxena R, et al.; Wellcome Trust Case Control Consortium . Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet 2008;40:638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meigs JB, Shrader P, Sullivan LM, et al. . Genotype score in addition to common risk factors for prediction of type 2 diabetes. N Engl J Med 2008;359:2208–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitcomb DC. Genetic aspects of pancreatitis. Annu Rev Med 2010;61:413–424 [DOI] [PubMed] [Google Scholar]
- 18.Whitcomb DC, LaRusch J, Krasinskas AM, et al.; Alzheimer’s Disease Genetics Consortium . Common genetic variants in the CLDN2 and PRSS1-PRSS2 loci alter risk for alcohol-related and sporadic pancreatitis. Nat Genet 2012;44:1349–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones S, Hruban RH, Kamiyama M, et al. . Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science 2009;324:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts NJ, Jiao Y, Yu J, et al. . ATM mutations in patients with hereditary pancreatic cancer. Cancer Discov 2012;2:41–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu C, Hart SN, Bamlet WR, et al. . Prevalence of pathogenic mutations in cancer predisposition genes among pancreatic cancer patients. Cancer Epidemiol Biomarkers Prev 2016;25:207–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein AP, Lindström S, Mendelsohn JB, et al. . An absolute risk model to identify individuals at elevated risk for pancreatic cancer in the general population. PLoS One 2013;8:e72311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer 2015;15:484–498 [DOI] [PubMed] [Google Scholar]
- 24.Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol 2014;10:455–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Funahashi H, Satake M, Dawson D, et al. . Delayed progression of pancreatic intraepithelial neoplasia in a conditional Kras(G12D) mouse model by a selective cyclooxygenase-2 inhibitor. Cancer Res 2007;67:7068–7071 [DOI] [PubMed] [Google Scholar]
- 26.Vongsuvanh R, George J, Qiao L, van der Poorten D. Visceral adiposity in gastrointestinal and hepatic carcinogenesis. Cancer Lett 2013;330:1–10 [DOI] [PubMed] [Google Scholar]
- 27.Hertzer KM, Xu M, Moro A, et al. . Robust early inflammation of the peripancreatic visceral adipose tissue during diet-induced obesity in the KrasG12D model of pancreatic cancer. Pancreas 2016;45:458–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dawson DW, Hertzer K, Moro A, et al. . High-fat, high-calorie diet promotes early pancreatic neoplasia in the conditional KrasG12D mouse model. Cancer Prev Res (Phila) 2013;6:1064–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philip B, Roland CL, Daniluk J, et al. . A high-fat diet activates oncogenic Kras and COX2 to induce development of pancreatic ductal adenocarcinoma in mice. Gastroenterology 2013;145:1449–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colmers IN, Bowker SL, Tjosvold LA, Johnson JA. Insulin use and cancer risk in patients with type 2 diabetes: a systematic review and meta-analysis of observational studies. Diabetes Metab 2012;38:485–506 [DOI] [PubMed] [Google Scholar]
- 31.Bosetti C, Rosato V, Buniato D, Zambon A, La Vecchia C, Corrao G. Cancer risk for patients using thiazolidinediones for type 2 diabetes: a meta-analysis. Oncologist 2013;18:148–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madiraju AK, Erion DM, Rahimi Y, et al. . Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 2014;510:542–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z, Lai ST, Xie L, et al. . Metformin is associated with reduced risk of pancreatic cancer in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract 2014;106:19–26 [DOI] [PubMed] [Google Scholar]
- 34.Rickels MR, Bellin M, Toledo FGS, et al.; PancreasFest Recommendation Conference Participants . Detection, evaluation and treatment of diabetes mellitus in chronic pancreatitis: recommendations from PancreasFest 2012. Pancreatology 2013;13:336–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das SL, Kennedy JI, Murphy R, Phillips AR, Windsor JA, Petrov MS. Relationship between the exocrine and endocrine pancreas after acute pancreatitis. World J Gastroenterol 2014;20:17196–17205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Domschke S, Stock KP, Pichl J, Schneider MU, Domschke W. Beta-cell reserve capacity in chronic pancreatitis. Hepatogastroenterology 1985;32:27–30 [PubMed] [Google Scholar]
- 37.Ewald N, Kaufmann C, Raspe A, Kloer HU, Bretzel RG, Hardt PD. Prevalence of diabetes mellitus secondary to pancreatic diseases (type 3c). Diabetes Metab Res Rev 2012;28:338–342 [DOI] [PubMed] [Google Scholar]
- 38.Ewald N, Bretzel RG. Diabetes mellitus secondary to pancreatic diseases (type 3c)--are we neglecting an important disease? Eur J Intern Med 2013;24:203–206 [DOI] [PubMed] [Google Scholar]
- 39.Andersen DK. The practical importance of recognizing pancreatogenic or type 3c diabetes. Diabetes Metab Res Rev 2012;28:326–328 [DOI] [PubMed] [Google Scholar]
- 40.Pelaez-Luna M, Takahashi N, Fletcher JG, Chari ST. Resectability of presymptomatic pancreatic cancer and its relationship to onset of diabetes: a retrospective review of CT scans and fasting glucose values prior to diagnosis. Am J Gastroenterol 2007;102:2157–2163 [DOI] [PubMed] [Google Scholar]
- 41.Permert J, Ihse I, Jorfeldt L, von Schenck H, Arnquist HJ, Larsson J. Improved glucose metabolism after subtotal pancreatectomy for pancreatic cancer. Br J Surg 1993;80:1047–1050 [DOI] [PubMed] [Google Scholar]
- 42.Pfeffer F, Koczan D, Adam U, et al. . Expression of connexin26 in islets of Langerhans is associated with impaired glucose tolerance in patients with pancreatic adenocarcinoma. Pancreas 2004;29:284–290 [DOI] [PubMed] [Google Scholar]
- 43.Basso D, Greco E, Fogar P, et al. . Pancreatic cancer-derived S-100A8 N-terminal peptide: a diabetes cause? Clin Chim Acta 2006;372:120–128 [DOI] [PubMed] [Google Scholar]
- 44.Huang H, Dong X, Kang MX, et al. . Novel blood biomarkers of pancreatic cancer-associated diabetes mellitus identified by peripheral blood-based gene expression profiles. Am J Gastroenterol 2010;105:1661–1669 [DOI] [PubMed] [Google Scholar]
- 45.He XY, Yuan YZ. Advances in pancreatic cancer research: moving towards early detection. World J Gastroenterol 2014;20:11241–11248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sah RP, Nagpal SJS, Mukhopadhyay D, Chari ST. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol 2013;10:423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cui Y, Andersen DK. Pancreatogenic diabetes: special considerations for management. Pancreatology 2011;11:279–294 [DOI] [PubMed] [Google Scholar]
- 48.Hart PA, Baichoo E, Bi Y, Hinton A, Kudva YC, Chari ST. Pancreatic polypeptide response to a mixed meal is blunted in pancreatic head cancer associated with diabetes mellitus. Pancreatology 2015;15:162–166 [DOI] [PubMed] [Google Scholar]
- 49.Hardt PD, Brendel MD, Kloer HU, Bretzel RG. Is pancreatic diabetes (type 3c diabetes) underdiagnosed and misdiagnosed? Diabetes Care 2008;31(Suppl. 2):S165–S169 [DOI] [PubMed] [Google Scholar]