Abstract
Ten-week-old Zucker diabetic fatty (ZDF) rats at an early stage of diabetes embody metabolic characteristics of obese human patients with type 2 diabetes, such as severe insulin and glucose intolerance in muscle and the liver, excessive postprandial excursion of plasma glucose and insulin, and a loss of metabolic flexibility with decreased lipid oxidation. Metabolic flexibility and glucose flux were examined in ZDF rats during fasting and near-normal postprandial insulinemia and glycemia after correcting excessive postprandial hyperglycemia using treatment with a sodium–glucose cotransporter 2 inhibitor (SGLT2-I) for 7 days. Preprandial lipid oxidation was normalized, and with fasting, endogenous glucose production (EGP) increased by 30% and endogenous glucose disposal (E-Rd) decreased by 40%. During a postprandial hyperglycemic-hyperinsulinemic clamp after SGLT2-I treatment, E-Rd increased by normalizing glucose effectiveness to suppress EGP and stimulate hepatic glucose uptake; activation of glucokinase was restored and insulin action was improved, stimulating muscle glucose uptake in association with decreased intracellular triglyceride content. In conclusion, SGLT2-I treatment improves impaired glucose effectiveness in the liver and insulin sensitivity in muscle by eliminating glucotoxicity, which reinstates metabolic flexibility with restored preprandial lipid oxidation and postprandial glucose flux in ZDF rats.
Introduction
Type 2 diabetes (T2D) is a progressive disorder characterized by ongoing deterioration of glycemic control and gradual decline in insulin secretion in response to nutrient loads. As the syndrome progresses, most, if not all, patients with T2D initially develop excessive postprandial excursion of blood glucose levels while maintaining near-normal fasting glycemia (1). Postprandial hyperglycemic spikes have recently received much attention because they may be relevant to the pathophysiology of late diabetes complications (2,3). Therefore, reducing postprandial hyperglycemic excursions is one of the main goals in the management of patients with T2D. Sodium–glucose cotransporter 2 inhibitors (SGLT2-Is), a recently available class of antihyperglycemic agents, reduce hyperglycemia by shunting a substantial amount of glucose into urine (4); this action differs from that of former therapeutic strategies that stimulate endogenous glucose disposal (E-Rd) and/or suppress endogenous glucose production (EGP).
In normal subjects, an increase in plasma glucose and insulin acts to minimize postprandial hyperglycemia via suppression of net hepatic glucose production and stimulation of glucose uptake with glucose storage in the liver and peripheral tissues. While the abnormal excursion of postprandial hyperglycemia in T2D results from decreased glucose effectiveness and insulin action to stimulate glucose disposal and suppress EGP (5–8), glucose disposal and suppression of EGP during the postprandial state in T2D are near normal (9). In patients with T2D, suppression of EGP and glucose disposal by insulin and glucose show a rightward shift of the dose response curve (reduced insulin sensitivity) but normal maximal suppression (no maximal efficacy defect) (10–12). These results suggest that excessively elevated postprandial hyperglycemia and insulinemia compensate for blunted glucose effectiveness and insulin action to stimulate glucose disposal and suppress EGP in these patients. Such compensation may be attenuated by treatment with an SGLT2-I. Indeed, it has been reported that correcting hyperglycemia in patients with T2D using treatment with an SGLT2-I increased EGP and decreased E-Rd when in a preprandial state (13,14). Nevertheless, no reports of severe deficiency of glucose storage exist, and it is not well understood how postprandial glucose flux is altered in patients with T2D treated with SGLT2-Is. Interestingly, Hawkins et al. (15) reported that patients with T2D with good glycemic control exhibit better glucose effectiveness compared with patients with T2D with poor glycemic control, suggesting the possibility that postprandial glucose disposal can be enhanced by improving glucose effectiveness by correcting hyperglycemia using treatment with SGLT2-Is.
Zucker diabetic fatty (ZDF) rats (10 weeks old), a model of the early stage of T2D associated with obesity, are characterized by postprandial hyperglycemia and hyperinsulinemia that result from severe insulin resistance and glucose intolerance, despite near-normal fasting glycemia (16). Using this model, we report that correcting the excessive excursion of postprandial hyperglycemia using an SGLT2-I improves postprandial glucose disposal by restoring glucose effectiveness in the liver and improving insulin resistance in skeletal muscle by eliminating glucotoxicity.
Research Design and Methods
Animals and Surgical Procedures
Six-week-old male ZDF rats (ZDF-GmiCrl-fa/fa) and their lean male littermates (ZCL; ZDF/GmiCrl-+/fa) were purchased from Charles River Laboratory, Inc. (Wilmington, MA), fed the 5008 Formulab Diet (Purina Mills, St. Louis, MO), and given water ad libitum in an environmentally controlled room with a 12-h light/12-h dark cycle. Two weeks before each study (at 8 weeks of age), rats underwent surgery to place catheters in the ileal vein, left carotid artery, and right jugular vein, as previously described (17–19). All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health, and all protocols were approved by the Vanderbilt University Institutional Animal Care and Use Committee.
Treatment With the SGLT2-I
Animals were dosed by gavage with either vehicle (0.2% carboxymethyl cellulose containing 0.2% Tween 80) at 5 mL/kg or an SGLT2-I (canagliflozin; Mitsubishi Tanabe Pharma Corp., Saitama, Japan) (20) at 10 mg/kg in a 5 mL/kg volume. To assess the acute effect of the SGLT2-I, 10-week-old ZDF rats were fasted from 6:00 a.m. and dosed once at 8:00 a.m. with either the SGLT2-I (ZDF-CA1) or vehicle (ZDF-V1) (Supplementary Fig. 1). Likewise, age-matched ZCL rats were dosed once with vehicle (ZCL-V1). To examine the effect of more chronic treatment with the SGLT2-I, rats, from 9 weeks of age, were given the drug (ZDF-CA7) or vehicle (ZDF-V7 or ZCL-V7) at 4:00 p.m. daily for 7 days (Supplementary Fig. 1).
Measurement of Postprandial Metabolic Flux
A mixed-meal tolerance test (MTT) was performed beginning at noon (Supplementary Fig. 1). Each MTT consisted of a 30-min control/basal period (−30 to 0 min) and a 60-min test period (0 to 60 min). At 0 min, animals were given by gavage a liquid mixed meal (5 mL/kg) containing (per 5 mL) 1.55 g glucose (1.65 g of Polycose [Abbott Nutrition, Columbus, OH]), 0.75 g protein (0.87 g of Beneprotein [Nestlé Health Science, Florham Park, NJ]), and 0.21 g lipid (0.42 mL of Microlipid [Nestlé Health Science, Florham Park, NJ]).
Measurement of the Daily Rhythm of Energy Metabolism
Food intake, oxygen consumption, and carbon dioxide production were monitored using an Oxymax-CLAMS (Columbus Instruments, Columbus, OH) in ZCL-V7, ZDF-V7, and ZDF-CA7. Body composition was measured immediately after this using an EchoMRI 700 (Echo Medical System, Houston, TX) (Supplementary Fig. 2), as previously described (21).
Measurement of Glucose Flux
ZCL-V7, ZDF-V7, and ZDF-CA7 were fasted from 6:00 a.m. and clamp studies were performed between 11:00 a.m. and 3:30 p.m. (Supplementary Fig. 3). Each study consisted of a 90-min tracer equilibrium period (−150 to −60 min), a 1-h basal period (−60 to 0 min), and a 2-h test period (0 to 120 min). At −150 min, both [2-3H]- and [3-3H]-glucose were administered at 60 µCi in a bolus, followed by continuous infusion of 0.6 µCi ⋅ min−1 into the systemic circulation through the jugular vein catheter. During the test period in the pancreatic and glycemic clamp studies, somatostatin was infused into the systemic circulation at 5 µg ⋅ kg−1 ⋅ min−1 to inhibit endogenous insulin and glucagon secretion. Recombinant glucagon (GlucaGen; Novo Nordisk A/S, Bagsvaerd, Denmark) was infused into the hepatic portal system through the ileal catheter at 1.8 ng ⋅ kg−1 ⋅ min−1 to maintain basal plasma glucagon levels. Human recombinant insulin (Novolin R; Novo Nordisk Inc., Plainsboro, NJ) was infused into the hepatic portal system through the ileal catheter at 4 and 16 mU ⋅ kg−1 ⋅ min−1 during the basal insulinemic and hyperinsulinemic clamps, respectively. Blood glucose levels were maintained at desired levels by infusing 50% dextrose solution (D50; Hospira, Inc., Lake Forest, IL) into the systemic circulation. Blood samples were taken from the arterial catheter.
Tissue Collection
At the conclusion of every energy measurement or clamp experiment, the animal was anesthetized with an intravenous infusion of pentobarbital sodium (50 mg ⋅ kg−1) and a laparotomy was performed immediately. Any urine accumulated in the bladder was collected. The median lobe of the liver was excised and dropped into ice-cold buffered 4% paraformaldehyde for immunohistochemical analysis. The left lobe of the liver and skeletal muscle (vastus lateralis, gastrocnemius-plantaris, and soleus) were frozen using Wollenberg tongs precooled in liquid nitrogen.
Measurement of Metabolites in Blood and Tissue
Plasma insulin, glucagon, triglycerides, and nonesterified free fatty acids (FFAs); blood lactate and alanine; urine glucose; and liver and skeletal muscle glycogen and triglycerides were determined as previously described (17,18,21). [2-3H]- and [3-3H]-glucose radioactivity in plasma glucose and in glycogen glucose were determined by selective enzymatic detritiation of [2-3H]-glucose (18,22).
Measurement of mRNA Levels and Protein and Enzyme Activity in the Liver
Western blotting was performed to quantify the levels of glucokinase (GK), GK regulatory protein (GKRP), phosphoenolpyruvate carboxykinase (PEPCK), glucose-6-phosphatase (G6Pase), AKT and its phosphorylated forms (p-AKTThr308 and p-AKTSer473), and β-actin in the liver, as reported previously (21). RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA) for the synthesis of cDNA (iScript; Bio-Rad Laboratories) and was assessed with quantitative real-time PCR using SYBER Green Supermix (Bio-Rad Laboratories) and primer pair probes specific to genes of interest (listed in Supplementary Table 1). Levels of specific mRNAs of interest were normalized to that of ribosomal protein L13a to control for nonspecific sample-to-sample variation. Glycogen synthase and phosphorylase a activities in the liver were measured using methods described previously (21). The immunoreactivities of both GK and GKRP within the nucleus and cytoplasm of each parenchymal cell were measured as described previously (17,18), with some modifications (Supplementary Data).
Calculations
Rates of glucose appearance (Ra), total glucose disappearance (Rd), E-Rd, glucose cycling (GC), EGP, the fractional detritiation of [2-3H]-glucose-6-phosphate (G6P), and glycogen synthesis via the direct pathway were quantified as described previously (22), with some modifications (Supplementary Data).
Statistical Analyses
The data collected are expressed as means ± SEMs. The significance of the differences in time-course data between the groups were analyzed using two methods: a two-way, repeated-measures ANOVA and a one-way ANOVA or Student t test. Differences were considered significant at P < 0.05.
Results
SGLT2-I Corrects Excessive Postprandial Hyperglycemia in ZDF Rats
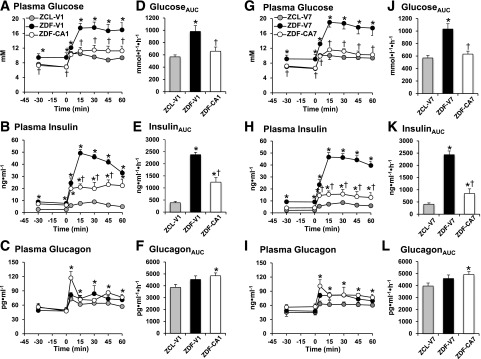
We first examined whether acute treatment with an SGLT2-I could correct excessive postprandial hyperglycemia in ZDF rats. Four hours after treatment with vehicle (ZCL-V1 and ZDF-V1 in Fig. 1A–F), ZDF-V1 exhibited higher levels of plasma glucose (9.4 ± 1.1 vs. 7.2 ± 0.3 mmol/L) and insulin (8.2 ± 0.8 vs. 2.5 ± 0.3 ng/mL) than ZCL-V1 during the basal period. After administration of the mixed meal, a markedly larger excursion of plasma glucose and insulin occurred in ZDF-V1 compared with ZCL-V1. No significant difference was found in plasma glucagon levels during the basal period or after the MTT between ZCL-V1 and ZDF-V1. These parameters were not altered by daily treatment with vehicle for 7 days (ZCL-V7 and ZDF-V7 in Fig. 1G–L). On the other hand, after 4 h of SGLT2-I treatment (ZDF-CA1; Fig. 1A–F), the basal level and the MTT-induced increment of plasma glucose in ZDF-V1 were nearly normalized. While basal hyperinsulinemia (6.7 ± 0.5 ng/mL) was not reduced, the excursion of plasma insulin on the MTT was reduced by 50% compared with ZDF-V1. After 7 days of daily treatment with the SGLT2-I (ZDF-CA7), basal and postprandial glycemia remained close to the levels seen in ZCL-V7. In addition, basal (4.3 ± 0.5 ng/mL) and MTT-induced levels of insulin were further decreased toward normal. Plasma glucagon levels during the MTT were increased by 20% with either 4 h or 7 days of treatment with the SGLT2-I compared with vehicle-treated ZCL. These results indicate that SGLT2-I treatment normalizes both basal and postprandial hyperglycemia, which was accompanied by partial reduction of hyperinsulinemia and elevation of plasma glucagon levels in ZDF rats.
Figure 1.
Plasma glucose, insulin, and glucagon levels before and after a gavage of a liquid mixed meal in ZCL and ZDF rats treated with an SGLT2-I for 4 h (A–F) or 7 days of daily dosing (G–L). ZDF rats were treated with vehicle or the SGLT2-I for 4 h (ZDF-V1 and ZDF-CA1) or for 7 days (ZDF-V7 and ZDF-CA7) before the MTT. ZCL rats were treated with vehicle for 4 h (ZCL-V1) or for 7 days (ZCL-V7) before the MTT. Animals were fasted for 6 h before the MTT. Areas under the curve (AUCs) of the plasma glucose (D and J), insulin (E and K), and glucagon (F and L) levels were calculated for a 60-min period after gavage administration of the mixed meal. Values are the mean ± SEM of data from six animals in each group. *Significant difference from the corresponding values of the ZCL-V1 group (P < 0.05); †significant difference from the corresponding values of the ZDF-V1 group (P < 0.05).
SGLT2-I Restores Metabolic Flexibility
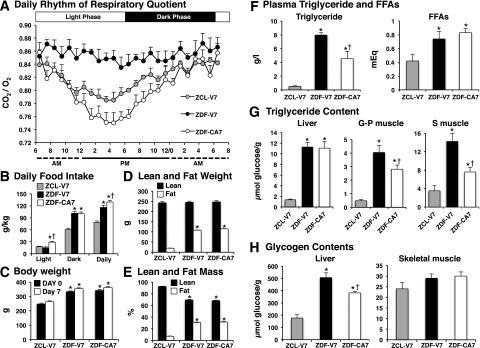
The effects of SGLT2-I treatment on metabolic flexibility, the capacity of the body to switch between lipid oxidation during fasting and carbohydrate oxidation after feeding and vice versa (23), were examined in ZDF rats (Fig. 2). The daily rhythms associated with a 12-h light/12-h dark cycle were seen in the respiratory exchange ratio (RER) and food intake in ZCL-V7. The RER was highest at the end of the dark phase, then progressively declined during the light phase (Fig. 2A). Of total daily food intake, 75% was consumed during the dark phase (Fig. 2B). The daily rhythm of RER was markedly blunted in ZDF-V7 compared with ZCL-V7, indicating low lipid oxidation. Further, food intake during the light phase was similar in ZCL-V7 and ZDF-V7, but in the dark phase it was 60% greater in ZDF-V7. The daily rhythm of RER was restored in ZDF-CA7 compared with ZDF-V7; the profile was similar to that of ZCL-V7, peaking near the end of the dark phase then gradually declining during the light phase, indicating restored lipid oxidation during the light phase. Food intake during the light phase was doubled, and total food intake was 10% higher in ZDF-CA7 than in ZDF-V7. Body weight (Fig. 2C), fat weight (Fig. 2D), and percentage fat mass (Fig. 2E); plasma triglyceride and FFA levels (Fig. 2F); triglyceride content in skeletal muscle and the liver (Fig. 2G); and glycogen content in the liver (Fig. 2H) were markedly higher in ZDF-V7 compared with ZCL-V7. While SGLT2-I treatment (ZDF-CA7) did not affect body weight or lean and fat mass, the treatment reduced the triglyceride content of plasma and skeletal muscle, but not content in the liver, and reduced glycogen content in the liver, but not in skeletal muscle, in ZDF rats.
Figure 2.
Food intake, body weight, body composition, daily rhythm of respiratory quotient, plasma triglycerides and FFAs, and glycogen content in liver and skeletal muscle after 7 days of daily treatment with an SGLT2-I. ZDF and ZCL rats were treated from 9 weeks of age with vehicle (ZDF-V7 and ZCL-V7, respectively) or an SGLT2-I (ZDF-CA7) once daily for 7 days. A: Oxygen consumption and CO2 production were monitored at 1-min intervals every 15 min for 24 h (from 6:00 a.m. on day 7 to 8:00 a.m. on day 8). B: Food intake during the light phase (6:00 a.m. to 6:00 p.m.) and the dark phase (6:00 p.m. to 6:00 a.m.) on day 7 of the treatments. C: Body weight before initiation of the treatment (at 9:00 a.m. on day 1) and after 7 days of the treatment (at 9:00 a.m. on day 7). D and E: Body composition measured between 9:00 and 11:00 a.m. on day 7 of treatment. F: Plasma triglycerides and FFAs. G: Triglyceride content in the liver and skeletal muscle. G-P, gastrocnemius-plantaris; S, soleus. H: Glycogen content in the liver and skeletal muscle (vastus lateralis). Blood and these tissues were collected between 12:00 and 1:00 p.m. on day 8. Values are the mean ± SEM of data from eight animals in each group. *Significant difference from the corresponding values of the ZCL-V7 group (P < 0.05); †significant difference from the corresponding values of the ZDF-V7 group (P < 0.05).
Effect of the SGLT2-I Treatment on Glucose Flux in a Fasting Condition
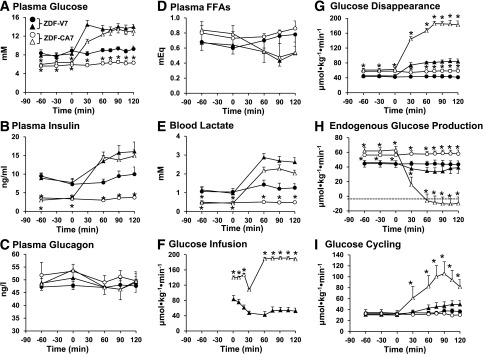
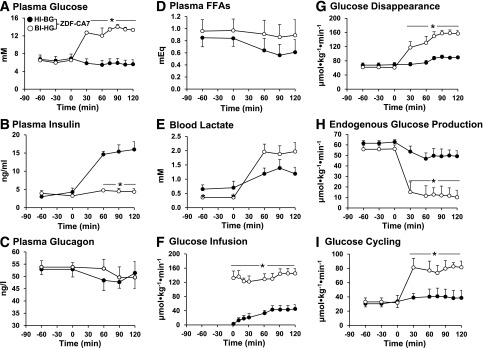
We measured glucose flux during a portion of the light phase, between 12:00 and 3:00 p.m. Under basal (no clamp) conditions (Fig. 3 and Table 1), plasma glucose and insulin levels were markedly lower and plasma glucagon levels tended to be higher (P < 0.10) in ZDF-CA7 compared with ZDF-V7. Blood levels of lactate and alanine were lower by 60% and 25%, respectively, whereas plasma FFAs were somewhat higher. E-Rd was approximately half (Table 1). The metabolic changes caused by the SGLT2-I resemble those caused by fasting. Interestingly, EGP was increased by 30% (Table 1). These metabolic changes associated with SGLT2-I treatment of ZDF rats are quite similar to those reported to occur in patients with T2D who received an SGLT2-I (4,13,14).
Figure 3.
Plasma levels of glucose (A), insulin (B), glucagon (C), and FFAs (D); blood lactate (E); and rates of glucose infusion (F), glucose disappearance (G), EGP (H), and GC (I) under basal conditions and during a hyperinsulinemic-hyperglycemic clamp within the early stage of diabetes in ZDF rats. ZDF rats were given vehicle (ZDF-V7) or an SGLT2-I (ZDF-CA7) once every day for 7 days before each study. Animals were fasted for 6 h before each study. •, ZDF-V7 in the basal (no clamp) condition; ○, ZDF-CA7 in the basal (no clamp) condition; ▲, ZDF-V7 with a hyperinsulinemic-hyperglycemic clamp from 0 to 120 min; △, ZDF-CA7 with a hyperinsulinemic-hyperglycemic clamp from 0 to 120 min. Values are the mean ± SEM of data from six animals in each group. *Significant difference from the corresponding values of the ZDF-V7 group (P < 0.05).
Table 1.
Plasma glucose, insulin, glucagon, and FFA concentrations; blood lactate and alanine concentrations; glucose infusion rate; and Rd, EGP, GC, and glucose excretion into urine rates during the test periods over 7 days in the ZDF-V7 and ZDF-CA7 groups
| Parameters | ZDF-V7 |
ZDF-CA7 |
||||
|---|---|---|---|---|---|---|
| Basal (no clamp) (n = 6) | HI-HG (n = 6) | Basal (no clamp) (n = 6) | HI-HG (n = 6) | HI-BG (n = 6) | BI-HG (n = 6) | |
| Plasma glucose (mmol/L) | 8.9 ± 0.5 | 13.8 ± 0.8† | 6.2 ± 0.4* | 12.6 ± 0.8† | 5.7 ± 0.3‡ | 13.2 ± 0.7†# |
| Plasma insulin (ng · mL−1) | 9.1 ± 1.4 | 15.1 ± 2.3† | 3.4 ± 0.6* | 14.4 ± 1.2† | 15.3 ± 1.6† | 4.5 ± 0.7‡# |
| Plasma glucagon (pg · mL−1) | 47.5 ± 1.5 | 47.5 ± 3.0 | 49.9 ± 2.2 | 47.6 ± 4.1 | 49.2 ± 4.3 | 50.8 ± 4.3 |
| Blood lactate (mmol/L) | 1.3 ± 0.3 | 2.73 ± 0.24† | 0.49 ± 0.04* | 2.16 ± 0.19*† | 1.26 ± 0.24†‡ | 1.94 ± 0.29†# |
| Blood alanine (mmol/L) | 0.69 ± 0.08 | 0.96 ± 0.10 | 0.51 ± 0.09 | 0.62 ± 0.04* | 0.46 ± 0.06‡ | 0.78 ± 0.13‡ |
| Plasma FFAs (mEq) | 0.65 ± 0.14 | 0.53 ± 0.10 | 0.96 ± 0.15 | 0.53 ± 0.21 | 0.60 ± 0.20 | 0.89 ± 0.23 |
| GIR (µmol · kg−1 · min−1) | 0 | 27 ± 4 | 0 | 235 ± 6* | 71 ± 12† | 180 ± 16†‡ |
| Total Rd (µmol · kg−1 · min−1) | 43 ± 4 | 62 ± 5† | 58 ± 4* | 226 ± 10†* | 120 ± 7†‡ | 192 ± 13†‡ |
| Cumulated UG (mmol · kg−1 · 2 h−1) | 0 | 0.05 ± 0.02 | 3.66 ± 0.31* | 11.60 ± 1.18*† | 4.86 ± 0.48† | 11.95 ± 1.17†‡ |
| Total Rd – AUC (mmol · kg−1 · 2 h−1) | 5.17 ± 0.49 | 6.99 ± 0.58† | 6.86 ± 0.50* | 23.11 ± 1.05*† | 12.89 ± 0.79†‡ | 19.54 ± 1.88†# |
| E-Rd – AUC (mmol · kg−1 · 2 h−1) | 5.17 ± 0.49 | 6.93 ± 0.58† | 3.20 ± 0.48* | 11.50 ± 0.45*† | 8.03 ± 0.59†‡ | 7.59 ± 0.88†‡ |
| EGP (µmol · kg−1 · min−1) | 44 ± 5 | 35 ± 8 | 58 ± 4* | −9 ± 11*† | 49 ± 6‡ | 12 ± 8†‡# |
| Detritiation efficiency (%) | 74 ± 5 | 66 ± 8 | 80 ± 4 | 56 ± 11† | 73 ± 6‡ | 52 ± 6†# |
| GC (µmol · kg−1 · min−1) | 42 ± 5 | 51 ± 11† | 36 ± 6 | 166 ± 23*† | 54 ± 17†‡ | 152 ± 24†# |
| G6Pase (µmol · kg−1 · min−1) | 85 ± 10 | 87 ± 19 | 94 ± 10 | 157 ± 34† | 103 ± 23 | 164 ± 30† |
Values are means ± SEMs of an average of six studies for each group. The reported test period values are the averages of measurements taken at 60, 90, and 120 min for plasma insulin, glucagon, and FFAs and for blood lactate and alanine and at 60, 75, 90, 105, and 120 min for glucose infusion rate (GIR), Rd, EGP, and GC. AUC, area under the curve; BI-HG, basal insulinemic–hyperglycemic clamp; HI-BG, hyperinsulinemic–basal glycemic clamp; HI-HG, hyperinsulinemic-hyperglycemic clamp; UG, glucose excretion into urine. *Significantly different from the corresponding values in the ZDF-V7 group (P < 0.05). †Significantly different from the corresponding values under basal conditions (no clamp) for the identical group (P < 0.05). ‡Significantly different from the corresponding values under HI-HG clamp conditions for the identical group (P < 0.05). #Significantly different from the corresponding values under HI-BG clamp conditions for the identical group (P < 0.05).
SGLT2-Is Restore Glucose Flux in Response to a Normal Postprandial Condition
In response to the clamp that created near-normal postprandial hyperinsulinemic-hyperglycemia, EGP, G6Pase flux, GC, and GC fraction in G6Pase flux were not altered significantly from that of the basal (no clamp) condition in ZDF-V7 (Fig. 3 and Table 1), although the incorporation of plasma glucose into hepatic glycogen via the direct pathway increased approximately threefold (Table 2). In ZDF-CA7, on the other hand, EGP was completely suppressed during the clamp. In addition, GC and G6Pase flux increased 4.5- and 2-fold, respectively, and G6Pase flux was equivalent to GC. The incorporation of plasma glucose into glycogen in the liver increased 11-fold with the activation of glycogen synthase and was 3.5 times larger than in ZDF-V7 (Table 2). These results indicate that treatment with an SGLT2-I restores the response to normal postprandial conditions in ZDF rats, including the suppression of EGP and increased glucose phosphorylation, with incorporation of G6P derived from plasma glucose into glycogen. In addition, E-Rd and the incorporation of plasma glucose into skeletal muscle glycogen during the hyperinsulinemic-hyperglycemia clamp was two times greater in ZDF-CA7 compared with ZDF-V7. These results suggest that treatment with an SGLT2-I improves the blunted response of EGP and glucose storage to a normal postprandial condition.
Table 2.
Glycogen synthase and phosphorylase activities in the liver, glycogen in the liver and skeletal muscle, and incorporation of plasma glucose into glycogen via the direct pathway in liver and skeletal muscle at the end of the test periods over 7 days in the ZDF-V7 and ZDF-CA7 groups
| Parameters | Tissue | ZDF-V7 |
ZDF-CA7 |
||||
|---|---|---|---|---|---|---|---|
| Basal (no clamp) (n = 6) | HI-HG (n = 6) | Basal (no clamp) (n = 6) | HI-HG (n = 6) | HI-BG (n = 6) | BI-HG (n = 6) | ||
| Glycogen synthase (mU/g) | |||||||
| Active form | Liver | 4.1 ± 0.9 | 7.0 ± 1.5† | 3.5 ± 0.6 | 18.4 ± 2.8*† | 6.4 ± 0.6†‡ | 15.3 ± 2.5†# |
| Total | Liver | 56 ± 7 | 67 ± 6 | 54 ± 4 | 64 ± 5 | 64 ± 3 | 59 ± 4 |
| Glycogen phosphorylase a (units/g) | Liver | 1.67 ± 0.22 | 1.40 ± 0.22 | 1.39 ± 0.36 | 1.41 ± 0.11 | 1.58 ± 0.29 | 1.28 ± 0.32 |
| Glycogen content (µmol glucose/g) | Liver | 293 ± 43 | 325 ± 36 | 193 ± 36* | 300 ± 33† | 217 ± 31‡ | 299 ± 27†# |
| Muscle | 23.1 ± 1.4 | 26.3 ± 1.9 | 21.4 ± 0.3 | 27.8 ± 3.8† | 29.1 ± 2.6† | 23.9 ± 1.8‡# | |
| Glucose incorporation into glycogen (µmol glucose/g/2 h) | Liver | 3.2 ± 0.6 | 13.3 ± 5.8† | 0.4 ± 0.1* | 46.1 ± 8.1*† | 7.7 ± 2.6†‡ | 39.9 ± 6.2†# |
| Muscle | 0.40 ± 0.05 | 1.30 ± 0.21† | 0.13 ± 0.03* | 2.83 ± 0.45*† | 2.52 ± 0.38† | 0.82 ± 0.21†‡# | |
Values are means ± SEMs of six studies for each group. Liver and muscle were collected at the end of the test period. BI-HG, basal insulinemic–hyperglycemic clamp. *Significantly different from the corresponding values of the ZDF-V7 group (P < 0.05). †Significantly different from the corresponding values under basal conditions for the identical group. ‡Significantly different from the corresponding values under the hyperinsulinemic-hyperglycemic clamp (HI-HG) for the identical group. #Significantly different from the corresponding values under the hyperinsulinemic–basal glycemic clamp (HI-BG) for the identical group.
Treatment With an SGLT2-I Restores Glucose Effectiveness in the Liver and Improves Insulin Sensitivity in Muscle
We investigated whether the improved hepatic glucose flux and muscle glucose disposal in response to a postprandial condition with SGLT2-I treatment of ZDF rats results from improvement of insulin sensitivity and/or glucose effectiveness.
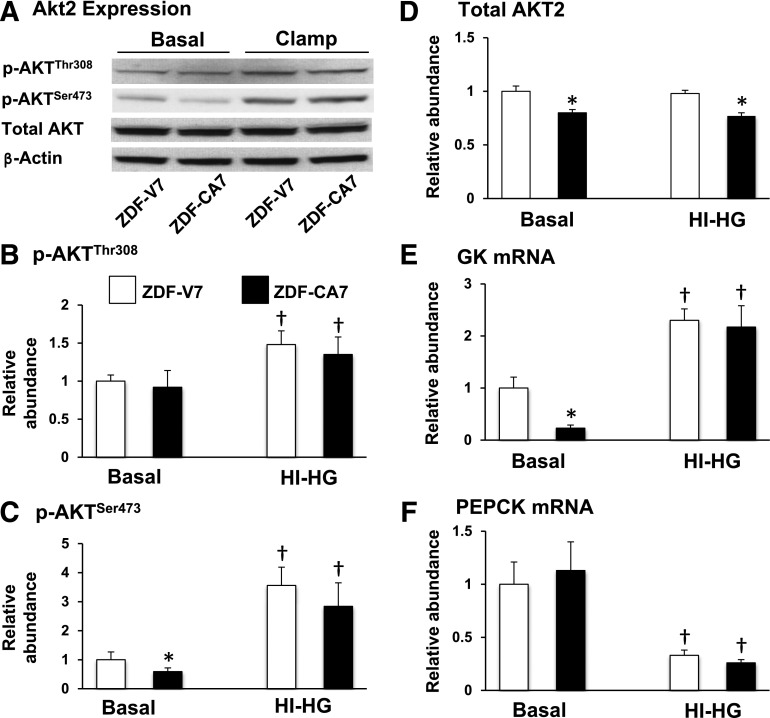
Insulin regulates hepatic glucose metabolism and some gene expression processes via the activation of Akt2/PKBβ by two upstream kinases, PDK1 and mammalian target of rapamycin complex 2, via phosphorylation of the T-loop of the catalytic domain (Thr308) and the carboxyl terminal hydrophobic domain (Ser473), respectively (24). The downstream targets of AKT activation in the liver include the stimulation of GK and the inhibition of PEPCK gene expression (25,26). As shown in Fig. 4, p-AKTSer473 (panels A–C), total AKT (panels A and D), and GK mRNA (panel E) levels were lower in ZDF-CA7 compared with ZDF-V7, whereas PEPCK mRNA levels (panel F) were similar under the basal (no clamp) condition. In response to the hyperinsulinemic-hyperglycemic clamp, on the other hand, p-AKTThr308, p-AKTSer473 (panels B and C), and GK mRNA (panel E) levels increased and PEPCK mRNA (panel F) levels decreased to similar levels in both groups. In addition, in ZDF-CA7, the alterations of EGP, GC, and G6Pase flux (Fig. 3 and Table 1), and the incorporation of plasma glucose into glycogen in the liver (Table 2), as seen during a hyperinsulinemic-hyperglycemic clamp, were reproduced during a basal insulinemic-hyperglycemic clamp but not during a hyperinsulinemic–basal glycemic clamp (Fig. 5 and Tables 1 and 2). These results suggest that improved hepatic glucose flux in response to a postprandial condition by SGLT2-I treatment was associated with restored glucose effectiveness, not with alteration of insulin resistance in the liver. On the other hand, improved incorporation of glucose into glycogen in skeletal muscle that occurred during a hyperinsulinemic-hyperglycemic clamp was reproduced during a hyperinsulinemic–basal glycemic clamp, but not during a basal insulinemic–hyperglycemic clamp (Table 2), indicating improved insulin sensitivity in skeletal muscle after the SGLT2-I treatment.
Figure 4.
Effect of 7 days of SGLT2-I treatment on hepatic insulin sensitivity. ZDF rats were given (from 9 weeks of age) vehicle (ZDF-V7) or an SGLT2-I (ZDF-CA7) daily for 7 days before a clamp study. Animals were fasted for 6 h before each clamp study, and livers were collected at the end of the test period (a basal [no clamp] period or a hyperinsulinemic-hyperglycemic [HI-HG] clamp) (Fig. 2). A: Representative images from Western blotting of protein extracts prepared from livers that were probed with antibodies for the detection of total Akt, p-AktThr308, p-AktSer473, and β-actin and measurement of the relative abundance of p-AktThr308 (B), p-AktSer473 (C), Akt (D), and GK (E) and PEPCK (F) mRNA. Quantitative results were normalized to the average basal value of ZDF-V7 for relative abundance. Values are the mean ± SEM of data from six animals in each group. *Significant difference from the corresponding values of the ZDF-V7 group within each treatment (P < 0.05); †significant difference from the basal values of the identical group (P < 0.05).
Figure 5.
Plasma levels of glucose (A), insulin (B), glucagon (C), and FFAs (D); blood lactate (E); and rates of glucose infusion (F), glucose disappearance (G), EGP (H), and GC (I) before and during a hyperinsulinemic–basal glycemic clamp (HI-BG, •) and basal insulinemic-hyperglycemic clamp (BI-HG, ○) within the early stage of diabetes in ZDF rats. ZDF rats were given an SGLT2-I (ZDF-CA7) daily for 7 days before each study. Animals were fasted for 6 h before each study. Values are the mean ± SEM of data from six animals in each group. *Significant difference from the corresponding values under the hyperinsulinemic–basal glycemic clamp (P < 0.05).
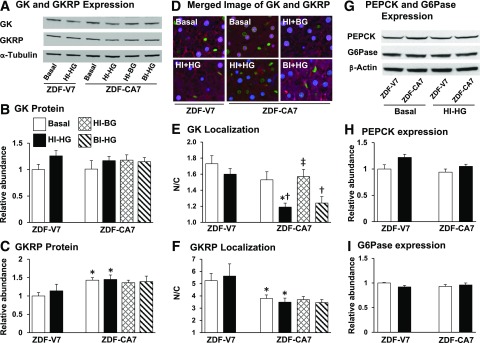
Restored Glucose Effectiveness in Intracellular GK Translocation
We reported previously that a blunted increase in glucose phosphorylation and glycogen synthesis in response to the postprandial condition seen at an early stage of diabetes in ZDF rats was associated with impaired dissociation of GK from its regulatory protein in the nucleus and a lack of subsequent translocation of GK from the nucleus to the cytoplasm (18,27). As shown in Fig. 6, GK protein levels were similar (panels A and B), whereas GKRP protein levels were higher (panels A and C), in ZDF-CA7 compared with ZDF-V7. While GK and GKRP immunoreactivities under a basal condition were predominantly localized in the nucleus in both ZDF-V7 and ZDF-CA7 (panels D–F), the nuclear-to-cytoplasmic ratio of the immunoreactivity (N/C) of GK tended to be lower and the N/C of GKRP was significantly lower in ZDF-CA7 compared with ZDF-V7. In response to a hyperinsulinemic-hyperglycemic clamp, the N/C of GK did not change in ZDF-V7 but decreased markedly in ZDF-CA7, indicating that the translocation of GK was restored by SGLT2-I treatment. This reduction of the N/C was also observed in response to a basal insulinemic–hyperglycemic clamp, but not a hyperinsulinemic–basal glycemic clamp, indicating that the GK translocates in response to hyperglycemia, not hyperinsulinemia, which was restored by SGLT2-I treatment. On the other hand, the expression of gluconeogenic enzymes, PEPCK (Fig. 6G and H) and G6Pase (Fig. 6G and I), was not altered by treatment with an SGLT2-I.
Figure 6.
Hepatic intracellular localization of GK and GKRP and expression of GK, GKRP, PEPCK, and G6Pase at the end of the test period (basal [no clamp condition, hyperinsulinemic-hyperglycemic [HI-HG], hyperinsulinemic–basal glycemic [HI-BG], and basal insulinemic–hyperglycemic clamp [BI- HG]) in ZDF rats treated with either vehicle (ZDF-V7) or an SGLT2-I (ZDF-CA7) for 7 days before each study. A: Representative Western blot images of GK, GKRP, and α-tubulin. B and C: Quantitative analysis of Western blot images of GK (B) and GKRP (C). D: Merged images of the immunoreactivity associated with GK and GKRP. E and F: Nuclear-to-cytosolic ratio of immunoreactivity of GK (E) and GKRP (F). G: Western blot images of PEPCK, G6Pase, and β-actin. H and I: Quantitative analysis of Western blot images of PEPCK (H) and G6Pase (I). GK, GKRP, PEPCK, and G6Pase levels are normalized to the average basal value of ZDF-V7. Values are the mean ± SEM of data from six animals in each group. *Significant difference from the corresponding values of the ZDF-V7 group within each treatment (P < 0.05); †significant difference from the basal values of the identical group (P < 0.05); ‡significant difference from the corresponding values under the HI-HG clamp of the identical group (P < 0.05).
Discussion
This study focused on the early stage of diabetes in ZDF rats and demonstrates that the correction of a markedly abnormal excursion in postprandial hyperglycemia by treatment with an SGLT2-I was associated with increased lipid oxidation and EGP in the preprandial state. SGLT2-I treatment also improved E-Rd in the postprandial state by normalizing the attenuated effectiveness of glucose to suppress hepatic glucose production and stimulate hepatic glycogen synthesis (glucose uptake), and by ameliorating insulin action to stimulate glucose utilization in skeletal muscle. The normalization of glucose effectiveness in liver was associated with restoration of its effect to accelerate glucose phosphorylation by activating GK allosterically. The improvement of insulin resistance in skeletal muscle was accompanied by decreased intracellular lipid content.
Metabolic Alterations Caused by SGLT2-I Treatment in ZDF Rats Resemble Those Reported in Patients With T2D
In patients with T2D, the reduction of hyperglycemia by treatment with an SGLT2-I was accompanied by a reduction in the ratio of plasma insulin to plasma glucagon (13,14), increased lipid oxidation (4), higher levels of plasma FFAs and lower levels of plasma triglycerides (4), and decreased E-Rd (13,14). These metabolic changes resemble those of long-term fasting and, interestingly, EGP increases (4,13,14). Elevated EGP was considered to be a consequence of lower plasma glucose and insulin levels in the presence of elevated glucagon levels (13,14). It has been reported that weight loss in patients with T2D who are treated with an SGLT2-I is much less than that predicted from the observed calorie loss via glucosuria (28). This maintenance of body weight may be the result of increased calorie intake (28). In this study, the metabolic alterations by SGLT2-I treatment reported for patients with T2D were reproduced in 10-week-old ZDF rats, a model of an early stage of obesity-related T2D.
Correction of Excessive Hyperglycemia Improves Insulin Sensitivity in Skeletal Muscle but Not in Liver
Merovic et al. (13) and Ferrannini et al. (14) reported that SGLT2-I treatment improves whole-body insulin resistance in patients with T2D. In the current study, we confirm the improvement of E-Rd in response to insulin after SGLT2-I treatment at an early stage of diabetes in ZDF rats, although there is potential risk for an error in measurement of E-Rd resulting from imprecise estimates of glucose loss in the urine. Further, we demonstrate that the improvement in insulin resistance caused by SGLT2-I treatment takes place in skeletal muscle by reducing intracellular lipid content and increasing lipid oxidation. The combination of increased intracellular lipids and a low oxidative capacity are key features in the development of muscular insulin resistance in obese patients with T2D (4,29,30). Chronic exposure of rats (31) and human myotubes (32) to a high concentration of glucose has been reported to cause insulin resistance in skeletal muscle by increasing intracellular amounts of triglycerides and diacylglycerol. Increased diacylglycerol increases membrane protein kinase C isozyme protein or kinase activity, and impairs insulin signaling (33). While the contribution of enhanced acyl-CoA:1,2-diacylglycerol acyltransferase 1 activity (32) and decreased diacylglycerol kinase activity (34) have been proposed for glucose-induced lipid accumulation and insulin resistance in skeletal muscle, the mechanism by which increased extracellular glucose concentration alters the activities of these enzymes remains unknown. Interestingly, the correction of diabetic hyperglycemia by SGLT2-I treatment did not lower liver intracellular lipid content or improve hepatic insulin resistance. Considine et al. (35) reported that normalization of circulating glucose using phlorizin in 10- to 12-week-old ZDF rats did not result in an improvement of insulin receptor phosphorylation nor a reduction in increased membrane protein kinase C isozyme protein or kinase activity. The mechanism behind the difference in the effect of correcting hyperglycemia on insulin resistance and lipid metabolism between skeletal muscle and liver remains for a future study.
Correction of Excessive Hyperglycemia Restores Glucose Effectiveness to Regulate GK Activity in the Liver
It has been reported that glucose-induced suppression of net hepatic glucose production was associated with increased glucose phosphorylation (36,37) but was not primarily a result of reduced carbon flow through G6Pase (36,38) or the gluconeogenic pathway (38). In this study, the restoration of normal glucose-induced suppression of EGP by correcting postprandial hyperglycemia in ZDF rats was accompanied by a simultaneous increase in G6Pase flux, GC, GC fraction in G6Pase flux, and incorporation of plasma glucose into glycogen via the direct pathway, indicating an increased flux of plasma glucose toward the G6P pool and a consequent increase in the occupancy of G6P derived from plasma glucose in the G6P pool. These results suggest that restoration of glucose effectiveness on hepatic glucose flux in ZDF rats treated with an SGLT2-I is associated with its effect to stimulate glucose phosphorylation.
Glucose phosphorylation is mediated by GK in the liver. When the glucose concentration is low, GKRP binds GK, inhibits its activity by decreasing its affinity for glucose (39), and sequesters GK in the nuclear compartment (40,41). Increased glucose concentrations increase the binding of glucose per se to the catalytic site in GK and induce global conformational changes of GK (42) that dissociate GK from GKRP, followed by GK transport to the cytoplasm (17,18,43,44). We previously reported that a blunted increase in glucose phosphorylation in response to normal postprandial conditions at an early stage of diabetes in ZDF rats was associated with defective dissociation of the GK-GKRP complex in the nucleus (18,27). The restoration of glucose effectiveness to stimulate glucose phosphorylation in ZDF rats treated with an SGLT2-I was accompanied by restored GK translocation from the nucleus to the cytoplasm. It is therefore likely that glucotoxicity impairs the action of glucose to dissociate the GK-GKRP complex.
Effect of Correcting Hyperglycemia Is Not Secondary to Reduced Plasma Insulin Levels
The correction of postprandial hyperglycemia in ZDF rats was accompanied by a marked reduction in postprandial hyperinsulinemia. Persistent hyperinsulinemia causes insulin resistance and impaired glucose effectiveness (45–48) via desensitization of insulin signaling per se (47). We previously reported that in the late phase of diabetes in ZDF rats, the reduction of fasting and postprandial hyperglycemia by SGLT2-I treatment improved skeletal muscle glucose uptake and hepatic glucose flux in response to normal postprandial conditions, without altering plasma insulin levels (21). Hepatic insulin sensitivity was not improved by SGLT2-I treatment. Therefore, it is unlikely that correcting hyperglycemia using SGLT-I treatment restores glucose effectiveness in the liver or improves insulin sensitivity in skeletal muscle via reduced hyperinsulinemia.
SGLT2-I Treatment Recovers Metabolic Flexibility by Eliminating Glucotoxicity in T2D
Insulin resistance and T2D are accompanied by a blunting of metabolic flexibility, the capacity of the body to switch between lipid oxidation in the fasting state to carbohydrate oxidation in the fed state (a glucose-/insulin-stimulated condition) and vice versa (23). It has been reported that impaired metabolic switching is an intrinsic property of skeletal muscle, with lowered fasting lipid oxidation (49,50) and decreased glucose disposal rate related to defective glucose transport (51). This study demonstrates that the correction of diabetic hyperglycemia by SGLT2-I treatment restores normal metabolic flexibility by increasing lipid oxidation in skeletal muscle, improving insulin resistance in skeletal muscle, and restoring normal glucose effectiveness in the liver so it stores glucose as glycogen. The restored metabolic flexibility may save carbohydrate utilization in the preprandial state and accelerate carbohydrate storage as glycogen in the postprandial state. Consequently, subjects with T2D treated with an SGLT2-I do not develop a deficient level of carbohydrate storage, despite correcting hyperglycemia by shunting glucose into urine.
Supplementary Material
Article Information
Acknowledgments. The authors acknowledge Dr. Alan D. Cherrington, Vanderbilt University School of Medicine, for his careful reading of the manuscript.
Funding. This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grant DK-60667) and in part through the use of the Cell Imaging Shared Resource (supported by National Institutes of Health grants CA68485, DK020593, DK58404, DK59637, and EY08126) and the Diabetes Research and Training Center Metabolic Physiology Shared Resource (supported by NIDDK grant DK020593). Mitsubishi Tanabe Pharma Corporation provided canagliflozin for use in this project.
Duality of Interest. K.U. is an employee of Mitsubishi Tanabe Pharma Corporation. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. T.P.O., E.C.J., S.K.E., A.V.C., K.U., T.D.F., A.E.P., L.L.S., and R.L.P. performed experiments and acquired data. L.L.S. and R.L.P. edited the manuscript. M.S. designed the study, performed experiments, analyzed and interpreted data, and wrote the manuscript. All authors approved the final version of the manuscript. M.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-1410/-/DC1.
T.P.O. and E.C.J. are co–first authors.
K.U. is currently affiliated with Sales & Marketing Division, Mitsubishi Tanabe Pharma Corporation, Osaka, Japan.
References
- 1.Ohlson LO, Larsson B, Björntorp P, et al. . Risk factors for type 2 (non-insulin-dependent) diabetes mellitus. Thirteen and one-half years of follow-up of the participants in a study of Swedish men born in 1913. Diabetologia 1988;31:798–805 [DOI] [PubMed] [Google Scholar]
- 2.Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes 2005;54:1–7 [DOI] [PubMed] [Google Scholar]
- 3.Madsbad S. Impact of postprandial glucose control on diabetes-related complications: how is the evidence evolving? J Diabetes Complications 2016;30:374–385 [DOI] [PubMed] [Google Scholar]
- 4.Monica Reddy RP, Inzucchi SE. SGLT2 inhibitors in the management of type 2 diabetes. Endocrine 2016;53:364–372 [DOI] [PubMed] [Google Scholar]
- 5.Mitrakou A, Kelley D, Mokan M, et al. . Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N Engl J Med 1992;326:22–29 [DOI] [PubMed] [Google Scholar]
- 6.Ludvik B, Nolan JJ, Roberts A, et al. . Evidence for decreased splanchnic glucose uptake after oral glucose administration in non-insulin-dependent diabetes mellitus. J Clin Invest 1997;100:2354–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mevorach M, Giacca A, Aharon Y, Hawkins M, Shamoon H, Rossetti L. Regulation of endogenous glucose production by glucose per se is impaired in type 2 diabetes mellitus. J Clin Invest 1998;102:744–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basu A, Basu R, Shah P, et al. . Effects of type 2 diabetes on the ability of insulin and glucose to regulate splanchnic and muscle glucose metabolism: evidence for a defect in hepatic glucokinase activity. Diabetes 2000;49:272–283 [DOI] [PubMed] [Google Scholar]
- 9.Basu A, Dalla Man C, Basu R, Toffolo G, Cobelli C, Rizza RA. Effects of type 2 diabetes on insulin secretion, insulin action, glucose effectiveness, and postprandial glucose metabolism. Diabetes Care 2009;32:866–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck-Nielsen H, Hother-Nielsen O, Staehr P. Is hepatic glucose production increased in type 2 diabetes mellitus? Curr Diab Rep 2002;2:231–236 [DOI] [PubMed] [Google Scholar]
- 11.Groop LC, Bonadonna RC, DelPrato S, et al. . Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest 1989;84:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Prato S, Matsuda M, Simonson DC, et al. . Studies on the mass action effect of glucose in NIDDM and IDDM: evidence for glucose resistance. Diabetologia 1997;40:687–697 [DOI] [PubMed] [Google Scholar]
- 13.Merovci A, Solis-Herrera C, Daniele G, et al. . Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014;124:509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrannini E, Muscelli E, Frascerra S, et al. . Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014;124:499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawkins M, Gabriely I, Wozniak R, Reddy K, Rossetti L, Shamoon H. Glycemic control determines hepatic and peripheral glucose effectiveness in type 2 diabetic subjects. Diabetes 2002;51:2179–2189 [DOI] [PubMed] [Google Scholar]
- 16.Shiota M, Printz RL. Diabetes in Zucker diabetic fatty rat. Methods Mol Biol 2012;933:103–123 [DOI] [PubMed] [Google Scholar]
- 17.Chu CA, Fujimoto Y, Igawa K, et al. . Rapid translocation of hepatic glucokinase in response to intraduodenal glucose infusion and changes in plasma glucose and insulin in conscious rats. Am J Physiol Gastrointest Liver Physiol 2004;286:G627–G634 [DOI] [PubMed] [Google Scholar]
- 18.Fujimoto Y, Donahue EP, Shiota M. Defect in glucokinase translocation in Zucker diabetic fatty rats. Am J Physiol Endocrinol Metab 2004;287:E414–E423 [DOI] [PubMed] [Google Scholar]
- 19.Shiota M. Measurement of glucose homeostasis in vivo: combination of tracers and clamp techniques. Methods Mol Biol 2012;933:229–253 [DOI] [PubMed] [Google Scholar]
- 20.Nomura S, Sakamaki S, Hongu M, et al. . Discovery of canagliflozin, a novel C-glucoside with thiophene ring, as sodium-dependent glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes mellitus. J Med Chem 2010;53:6355–6360 [DOI] [PubMed] [Google Scholar]
- 21.Ueta K, O’Brien TP, McCoy GA, et al. . Glucotoxicity targets hepatic glucokinase in Zucker diabetic fatty rats, a model of type 2 diabetes associated with obesity. Am J Physiol Endocrinol Metab 2014;306:E1225–E1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres TP, Fujimoto Y, Donahue EP, et al. . Defective glycogenesis contributes toward the inability to suppress hepatic glucose production in response to hyperglycemia and hyperinsulinemia in Zucker diabetic fatty rats. Diabetes 2011;60:2225–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 2000;49:677–683 [DOI] [PubMed] [Google Scholar]
- 24.Lu M, Wan M, Leavens KF, et al. . Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med 2012;18:388–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iynedjian PB. Molecular physiology of mammalian glucokinase. Cell Mol Life Sci 2009;66:27–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gabbay RA, Sutherland C, Gnudi L, et al. . Insulin regulation of phosphoenolpyruvate carboxykinase gene expression does not require activation of the Ras/mitogen-activated protein kinase signaling pathway. J Biol Chem 1996;271:1890–1897 [DOI] [PubMed] [Google Scholar]
- 27.Shin JS, Torres TP, Catlin RL, Donahue EP, Shiota M. A defect in glucose-induced dissociation of glucokinase from the regulatory protein in Zucker diabetic fatty rats in the early stage of diabetes. Am J Physiol Regul Integr Comp Physiol 2007;292:R1381–R1390 [DOI] [PubMed] [Google Scholar]
- 28.Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care 2015;38:1730–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelley DE, Goodpaster BH, Storlien L. Muscle triglyceride and insulin resistance. Annu Rev Nutr 2002;22:325–346 [DOI] [PubMed] [Google Scholar]
- 30.Befroy DE, Petersen KF, Dufour S, et al. . Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes 2007;56:1376–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laybutt DR, Schmitz-Peiffer C, Saha AK, Ruderman NB, Biden TJ, Kraegen EW. Muscle lipid accumulation and protein kinase C activation in the insulin-resistant chronically glucose-infused rat. Am J Physiol 1999;277:E1070–E1076 [DOI] [PubMed] [Google Scholar]
- 32.Aas V, Kase ET, Solberg R, Jensen J, Rustan AC. Chronic hyperglycaemia promotes lipogenesis and triacylglycerol accumulation in human skeletal muscle cells. Diabetologia 2004;47:1452–1461 [DOI] [PubMed] [Google Scholar]
- 33.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med 2014;371:2237–2238 [DOI] [PubMed] [Google Scholar]
- 34.Chibalin AV, Leng Y, Vieira E, et al. . Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell 2008;132:375–386 [DOI] [PubMed] [Google Scholar]
- 35.Considine RV, Nyce MR, Allen LE, et al. . Protein kinase C is increased in the liver of humans and rats with non-insulin-dependent diabetes mellitus: an alteration not due to hyperglycemia. J Clin Invest 1995;95:2938–2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossetti L, Giaccari A, Barzilai N, Howard K, Sebel G, Hu M. Mechanism by which hyperglycemia inhibits hepatic glucose production in conscious rats. Implications for the pathophysiology of fasting hyperglycemia in diabetes. J Clin Invest 1993;92:1126–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barzilai N, Hawkins M, Angelov I, Hu M, Rossetti L. Glucosamine-induced inhibition of liver glucokinase impairs the ability of hyperglycemia to suppress endogenous glucose production. Diabetes 1996;45:1329–1335 [DOI] [PubMed] [Google Scholar]
- 38.Hellerstein MK, Neese RA, Schwarz JM, Turner S, Faix D, Wu K. Altered fluxes responsible for reduced hepatic glucose production and gluconeogenesis by exogenous glucose in rats. Am J Physiol 1997;272:E163–E172 [DOI] [PubMed] [Google Scholar]
- 39.van Schaftingen E, Vandercammen A, Detheux M, Davies DR. The regulatory protein of liver glucokinase. Adv Enzyme Regul 1992;32:133–148 [DOI] [PubMed] [Google Scholar]
- 40.de la Iglesia N, Veiga-da-Cunha M, Van Schaftingen E, Guinovart JJ, Ferrer JC. Glucokinase regulatory protein is essential for the proper subcellular localisation of liver glucokinase. FEBS Lett 1999;456:332–338 [DOI] [PubMed] [Google Scholar]
- 41.Shiota C, Coffey J, Grimsby J, Grippo JF, Magnuson MA. Nuclear import of hepatic glucokinase depends upon glucokinase regulatory protein, whereas export is due to a nuclear export signal sequence in glucokinase. J Biol Chem 1999;274:37125–37130 [DOI] [PubMed] [Google Scholar]
- 42.Kamata K, Mitsuya M, Nishimura T, Eiki J, Nagata Y. Structural basis for allosteric regulation of the monomeric allosteric enzyme human glucokinase. Structure 2004;12:429–438 [DOI] [PubMed] [Google Scholar]
- 43.Brown KS, Kalinowski SS, Megill JR, Durham SK, Mookhtiar KA. Glucokinase regulatory protein may interact with glucokinase in the hepatocyte nucleus. Diabetes 1997;46:179–186 [DOI] [PubMed] [Google Scholar]
- 44.Fernández-Novell JM, Castel S, Bellido D, Ferrer JC, Vilaró S, Guinovart JJ. Intracellular distribution of hepatic glucokinase and glucokinase regulatory protein during the fasted to refed transition in rats. FEBS Lett 1999;459:211–214 [DOI] [PubMed] [Google Scholar]
- 45.Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care 1990;13:610–630 [DOI] [PubMed] [Google Scholar]
- 46.Unger RH, Grundy S. Hyperglycaemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: implications for the management of diabetes. Diabetologia 1985;28:119–121 [DOI] [PubMed] [Google Scholar]
- 47.Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care 2008;31(Suppl. 2):S262–S268 [DOI] [PubMed] [Google Scholar]
- 48.Rizza RA, Mandarino LJ, Genest J, Baker BA, Gerich JE. Production of insulin resistance by hyperinsulinaemia in man. Diabetologia 1985;28:70–75 [DOI] [PubMed] [Google Scholar]
- 49.Corpeleijn E, Mensink M, Kooi ME, Roekaerts PM, Saris WH, Blaak EE. Impaired skeletal muscle substrate oxidation in glucose-intolerant men improves after weight loss. Obesity (Silver Spring) 2008;16:1025–1032 [DOI] [PubMed] [Google Scholar]
- 50.Ferrannini E, Baldi S, Frascerra S, et al. . Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes 2016;65:1190–1195 [DOI] [PubMed] [Google Scholar]
- 51.Galgani JE, Heilbronn LK, Azuma K, et al.; Look AHEAD Adipose Research Group . Metabolic flexibility in response to glucose is not impaired in people with type 2 diabetes after controlling for glucose disposal rate. Diabetes 2008;57:841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.