Abstract
Patched 1 (Ptch1) has epithelial, stromal and systemic roles in murine mammary gland organogenesis, yet specific functions remain undefined. Cre-recombinase-mediated Ptch1 ablation in mammary epithelium increased proliferation and branching, but did not phenocopy transgenic expression of activated smoothened (SmoM2). The epithelium showed no evidence of canonical hedgehog signaling, and hyperproliferation was not blocked by smoothened (SMO) inhibition, suggesting a non-canonical function of PTCH1. Consistent with this possibility, nuclear localization of cyclin B1 was increased. In non-epithelial cells, heterozygous Fsp-Cre-mediated Ptch1 ablation increased proliferation and branching, with dysplastic terminal end buds (TEB) and ducts. By contrast, homozygous Ptch1 ablation decreased proliferation and branching, producing stunted ducts filled with luminal cells showing altered ovarian hormone receptor expression. Whole-gland transplantation into wild-type hosts or estrogen/progesterone treatment rescued outgrowth and hormone receptor expression, but not the histological changes. Bone marrow transplantation failed to rescue outgrowth. Ducts of Fsp-Cre;Ptch1fl/fl mice were similar to Fsp-Cre;SmoM2 ducts, but Fsp-Cre;SmoM2 outgrowths were not stunted, suggesting that the histology might be mediated by Smo in the local stroma, with systemic Ptch1 required for ductal outgrowth and proper hormone receptor expression in the mammary epithelium.
KEY WORDS: Epithelial-stromal interactions, Patched-1, Smoothened, Hedgehog signaling
Summary: Systemic and tissue-specific depletion of patched 1 in epithelial and stromal compartments of the mammary gland defines functions in ductal patterning, proliferation and gene expression.
INTRODUCTION
Organogenesis is the developmental process by which organs are constructed from undifferentiated germ layers. This process requires coordinated interactions between cells and tissues, and, for endocrine-targeted organs, cellular responses to extrinsic hormonal signals. These developmental processes are studied extensively, as they are often perturbed in cancer and other diseases.
The hedgehog signaling network regulates cellular and tissue interactions that are essential for metazoan organogenesis (Briscoe and Thérond, 2013; Johnson et al., 2011; Robbins et al., 2012). In ‘canonical’ mammalian hedgehog signaling, patched 1 (PTCH1) and patched 2 (PTCH2) inhibit downstream signaling by smoothened (SMO), an effector protein, in the absence of ligands. When SMO is inhibited, GLI3, and to a lesser extent GLI2, transcription factors are proteolytically cleaved into transcriptional repressors. With hedgehog ligand [sonic (SHH), indian (IHH) or desert (DHH) hedgehog] binding to PTCH1 and/or PTCH2 (PTCH1/2) on a responding cell, PTCH1/2-mediated inhibition of SMO is released, and GLI transcription factors (GLI1, GLI2 and GLI3) remain full-length transcriptional activators. GLI-mediated transcription regulates proliferation, survival, cell fate and autoregulatory feedback.
Some hedgehog network members function ‘non-canonically’, independent of the signaling cascade described above. For example, PTCH1 can sequester hedgehog ligand to restrict the range of signaling, sequester cyclin B1 in the cytoplasm to inhibit cell cycle progression, or induce caspase 9- or caspase 3-mediated apoptosis in the absence of hedgehog ligands (Barnes et al., 2001; Chen and Struhl, 1996; Mille et al., 2009). In mammary epithelial cells, SHH-stimulated PTCH1 promotes ERK1 and ERK2 phosphorylation independently of SMO (Chang et al., 2010). In the mouse mammary epithelium, constitutively activated Smo (SmoM2) acts as a G-protein-coupled receptor (GPCR) via Gαi2 to induce proliferation independently of GLI activity, as hyperproliferation was not blocked by pharmacological inhibition of GLI1 or GLI2 (Villanueva et al., 2015), consistent with observations by Riobo et al. (Riobo et al., 2006). TGFβ induces Gli2 to regulate osteolysis independently of Smo (Johnson et al., 2011), whereas K-Ras inhibits GLI2 function and GLI3 processing in the context of Smo activation (Lauth et al., 2010). A long non-coding RNA induced by the Twist transcription factor upregulates Gli1 and Gas1 (canonical hedgehog target genes) in vitro (Zhou et al., 2015). These non-canonical functions necessitate the evaluation of multiple network genes to fully understand hedgehog network function in a given organ.
The murine mammary gland is an excellent model for organogenesis (Daniel and Smith, 1999). In this system, organogenesis is initiated in the embryo, yielding a rudimentary ductal tree at birth, which remains relatively growth quiescent until puberty begins at 3-4 weeks of age. With puberty, systemic hormones (e.g. estrogen, progesterone and other hormones) drive ductal outgrowth via terminal end buds (TEBs). TEBs are transient structures that migrate and proliferate to produce a branched ductal tree that fills the mammary fat pad by 8-10 weeks of age. With conception, pregnancy hormones induce alveolar development to prepare for lactation. After lactation, the gland involutes and remodels to resemble the adult virgin (Hennighausen and Robinson, 2005; Macias and Hinck, 2012).
Previously, analysis of mammary glands from mice heterozygous for a germline knockout allele (Ptch1Δ/+), or homozygous for a hypomorphic Ptch1 allele (Ptch1mes), demonstrated distinct functions for Ptch1 in the mammary epithelium, local stroma and systemically (mammary gland extrinsic) during postnatal virgin development (Lewis et al., 1999; Moraes et al., 2009). Neither the specific functions of Ptch1, nor the association of these phenotypes with canonical hedgehog signaling was investigated. Here, we employ tissue compartment-specific ablation of Ptch1, transplantation and tissue-specific expression of an activated Smo allele, to specify epithelial, stromal and systemic Ptch1 functions in virgin mammary gland development.
RESULTS
Ptch1 inhibits proliferation and branching of mammary epithelium
To determine the null phenotype of Ptch1 in mammary epithelium, mTmG-tagged primary mammary epithelial cells homo- or heterozygous for a Ptch1 conditional ablation allele (Ptch1fl) were treated with Adenovirus-Cre (Ad-Cre) and transplanted into the mammary fat pads of SCID/bg recipients (wild-type for Ptch1). Ad-Cre-treated, Ptch1+/+, mTmG+ primary cells were transplanted to contralateral fat pads. This approach increased recombination compared with MMTV-Cre (Wagner et al., 2001).
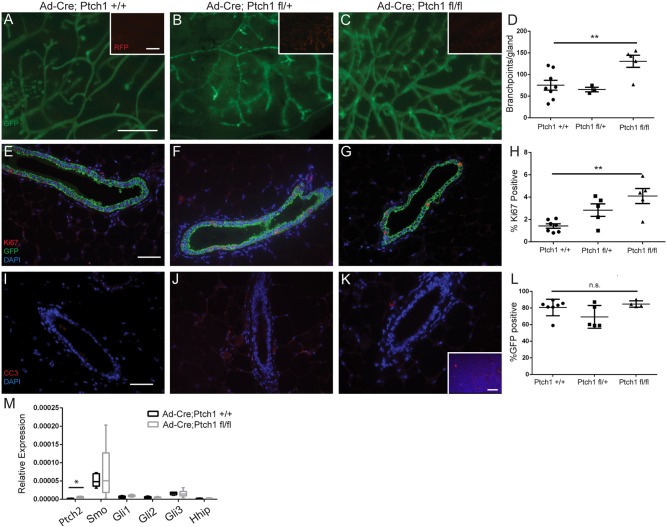
Eight weeks post-transplantation, we observed that whereas Ptch1+/+ glands had 75±11 branch points (Fig. 1A) and Ptch1fl/+ glands had a comparable 65±5 branch points (Fig. 1B), Ptch1fl/fl glands showed an increase in branch points with 131±14 (Fig. 1C) (P<0.011, paired t-test; quantification Fig. 1D). Increased branching was present with increased proliferation by Ki67 expression. Eight weeks post-transplantation, 1.4±0.2% of Ptch1+/+ cells were Ki67 positive (Fig. 1E). Ptch1fl/+ ducts were 2.8±0.6% Ki67 positive (Fig. 1F) (n.s., paired t-test), whereas proliferation in Ptch1fl/fl cells increased significantly to 4.1±0.7% Ki67 positive (Fig. 1G) (P<0.01, paired t-test; quantification, Fig. 1H). Apoptosis was comparable between Ptch1+/+ (Fig. 1I), Ptch1fl/+ (Fig. 1J) or Ptch1fl/fl ducts (Fig. 1K) using cleaved caspase 3 (CC3) staining, whereas CC3-positive cells were observed in positive control lymph nodes (Fig. 1K, inset).
Fig. 1.
Loss of Ptch1 in mammary epithelium increases branching and proliferation in adult virgin glands. (A-C) Fluorescent whole-mount (A) Ad-Cre;Ptch1+/+, (B) Ad-Cre;Ptch1fl/+ and (C) Ad-Cre;Ptch1fl/fl outgrowths. GFP identifies Cre+ cells. The insets show tdTomato Red+ Cre− cells. (D) Quantification showing increased branching in Ptch1fl/fl epithelium. (E-G) GFP- and Ki67-stained (E) Ad-Cre;Ptch1+/+, (F) Ad-Cre;Ptch1fl/+ and (G) Ad-Cre;Ptch1fl/fl ducts. (H) Quantification showing increased proliferation in Ptch1fl/fl epithelium. (I-K) CC3-stained (I) Ad-Cre; Ptch1+/+, (J) Ad-Cre;Ptch1fl/+ and (K) Ad-Cre;Ptch1fl/fl ducts – all negative for CC3. The inset shows a CC3-stained lymph node used as a positive control. (L) Quantification showing similar percentage GFP positivity in outgrowths of different genotypes. (M) Relative expression of hedgehog target genes in Ad-Cre;Ptch1+/+ and Ptch1fl/fl tissues. Data displayed as 2−dCt with minimum and maximum values. Ptch2 expression is significantly higher in Ptch1fl/fl tissues (unpaired t-test). Graphs show data as mean±s.e.m. Paired t-tests were used to compare Ptch1fl/fl glands with contralateral Ptch1+/+ controls. *P<0.05 and **P<0.01. Scale bars: 1 mm in A-C; 50 µm in E-G,I-K.
Previously, the histological defects of Ptch1Δ/+ or Ptch1mes ducts (Lewis et al., 1999; Moraes et al., 2009) were resolved with epithelial fragment transplantation. Consistently, histology was normal in Ad-Cre;Ptch1fl/+ and Ad-Cre;Ptch1fl/fl outgrowths (Fig. 1F-G), showing definitively that histological defects were not due to epithelial Ptch1 loss.
To ensure that the phenotypes were not due to differences in Cre-dependent recombination, we determined that GFP-positive cells contributed similarly to ductal outgrowths by immunofluorescence. An average of 81±4% of Ptch1+/+, 69±6% of Ptch1fl/+ and 85±2% of Ptch1fl/fl mammary epithelial cells were GFP positive (no difference, paired t-test) (Fig. 1L).
To investigate whether Ptch1fl/fl outgrowths displayed activated canonical hedgehog signaling due to reduced Smo inhibition, Ptch1+/+ and Ptch1fl/fl epithelium was evaluated by qPCR for hedgehog network gene expression. Of the genes evaluated, only Ptch2 mRNA was slightly upregulated (Fig. 2F) (P<0.016), suggesting that canonical hedgehog signaling was not activated.
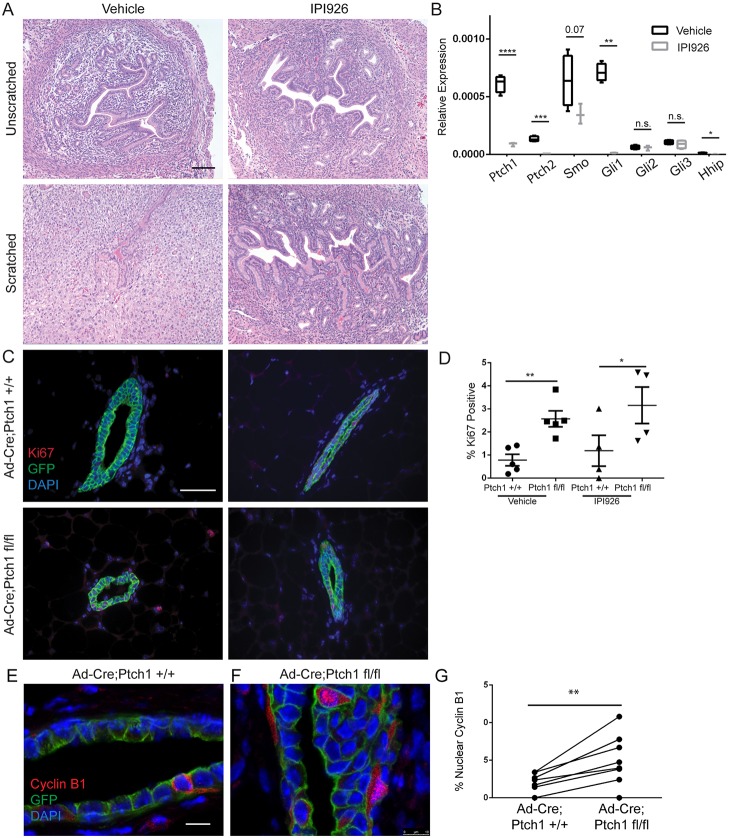
Fig. 2.
Hyperproliferation due to Ad-Cre-mediated Ptch1 loss is not due to SMO activation. (A) Hematoxylin and Eosin-stained vehicle-treated (left panels) and IPI926-treated (right panels) unscratched (upper panels) and scratched (lower panels) uterine tissue, showing decidualization in the vehicle-treated scratched uterus only. (B) qPCR of Ptch1+/+ and Ptch1fl/fl outgrowths showing no significant changes in hedgehog activation, aside from upregulation of Ptch2. Unpaired t-test are used for statistics. Data are represented as 2−dCt. (C) Ki67 and GFP co-stained vehicle-treated Ptch1+/+ (upper left), vehicle-treated Ptch1fl/fl (lower left), IPI926-treated Ptch1+/+ (upper right) and IPI926-treated Ptch1fl/fl ducts (lower right). (D) Quantification of the percentage of Ki67-positive cells by genotype, showing increased proliferation in vehicle and IPI926- treated mutant ducts relative to controls by paired t-test. (E,F) Confocal images of cyclin B1- stained Ptch1+/+ and Ptch1fl/fl ducts. (G) There is increased nuclear localization in Ptch1fl/fl ducts compared with controls (paired t-test). The scatterplot shows data as mean±s.e.m. Boxplots show data as mean±s.e.m., with minimum and maximum values. *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001. Scale bars: 50 µm in A,C; 10 µm in E,F.
Increased proliferation in Ad-Cre;Ptch1fl/fl ducts is not due to activated canonical hedgehog signaling
Gene expression analysis indicated that phenotypes from Ptch1 loss may not be due to increased SMO activity (Fig. 1M), consistent with unique mammary gland phenotypes elicited by epithelium-limited ablation of Ptch1 and activation of Smo (Visbal et al., 2011). To test whether hyperproliferation requires Smo activity, we evaluated hyperproliferation due to Ptch1 loss in the context of pharmacological inhibition of SMO.
To demonstrate SMO inhibitor (IPI926) efficacy, we tested whether IPI926 would blunt uterine scratch-induced decidualization, as canonical hedgehog signaling is required for decidualization (Matsumoto et al., 2002; Villanueva et al., 2015). The unscratched, vehicle- and IPI926-treated uteri displayed comparable histology (Fig. 2A). The scratched vehicle-treated tissue displayed histological changes consistent with decidualization (Fig. 2A) that were absent with IPI926 treatment (Fig. 2A). QPCR supported IPI926 efficacy: Ptch1, Ptch2, Gli1 and Hhip mRNA levels were significantly reduced in the IPI926-treated, scratched tissue relative to the vehicle-treated, scratched tissue (Fig. 2B).
Given the efficacy of IPI926 in vivo at the chosen dose, we treated mice bearing Ad-Cre;Ptch1+/+ and Ptch1fl/fl outgrowths with IPI926 3 days before harvest at 8 weeks. We assayed for Ki67 to determine whether hyperproliferation was blocked by SMO inhibition. Vehicle-treated Ptch1+/+ ducts were 0.8±0.25% Ki67 positive, and Ptch1fl/fl ducts increased to 2.6±0.35% Ki67 positive (Fig. 2C; P<0.0019, paired t-test). With IPI926 treatment, Ptch1+/+ ducts were 1.2±0.6% Ki67 positive, whereas IPI926-treated Ptch1fl/fl ducts retained increased proliferation with 3.2±0.71% Ki67-positive cells (Fig. 2C) (P<0.02, paired t-test). No significant differences were observed between vehicle and IPI926-treated Ptch1+/+, or vehicle and IPI926-treated Ptch1fl/fl outgrowths (quantification, Fig. 2D). As IPI926 did not block hyperproliferation, this phenotype is not likely due to SMO activation.
As the hyperproliferation with Ad-Cre-mediated Ptch1 loss was not blocked by SMO inhibition, we assayed whether the non-canonical function of PTCH1 in cytoplasmic retention of cyclin B1 could be involved (Barnes et al., 2001). Immunofluorescence showed that whereas Ptch1+/+ ducts displayed 0.17±0.05% cells with nuclear cyclin B1 (Fig. 2E), Ptch1fl/fl outgrowths showed a significant increase to 0.45±0.11% (Fig. 2F) (P<0.0085, paired t-test) (Fig. 2G).
Fsp-cre-mediated disruption of Ptch1 in non-epithelial cells alters mammary gland histology, proliferation and morphology
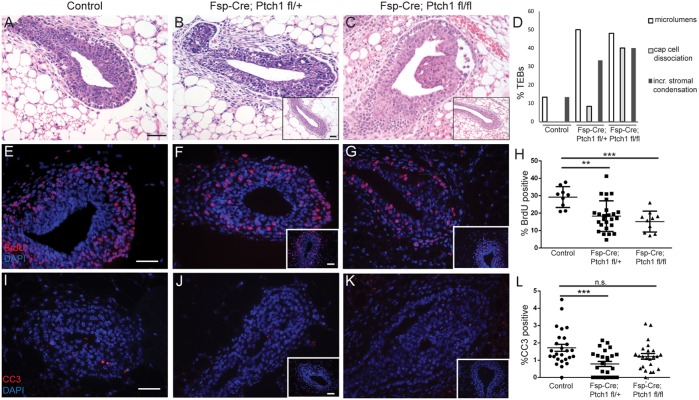
To investigate non-epithelial functions of Ptch1, we crossed mTmG- tagged, Ptch1fl/+ and Ptch1fl/fl with Fsp-Cre mice to ablate Ptch1 in fibroblasts and myeloid cells. At 6 weeks of age, control mice (Fsp-Cre;Ptch1+/+ and Ptch1fl/+ or Ptch1fl/fl mice lacking Fsp-Cre) displayed histologically normal TEBs (Fig. 3A), whereas many Fsp-Cre;Ptch1fl/+ TEBs had irregular shape, microlumens and an ill-defined cap cell layer (Fig. 3B). Histologically normal TEBs were also observed (Fig. 3B, inset). Additionally, Fsp-Cre;Ptch1fl/fl mice showed body cells detached from the cap cells (Fig. 3C), with few histologically normal TEBs (Fig. 3C, inset). Both mutants showed increased stromal condensation adjacent to TEBs relative to controls (Fig. 3B-D).
Fig. 3.
Pubertal animals (6 weeks) with Fsp-Cre-mediated loss of Ptch1 display dysmorphic hyperproliferative TEBs. (A-C) Hematoxylin and Eosin-stained (A) control, (B) Fsp-Cre;Ptch1fl/+ and (C) Fsp-Cre;Ptch1fl/fl TEBs. (D) Percentage perturbed TEBs by genotype. (E-G) BrdU-stained (E) control, (F) Fsp-Cre;Ptch1fl/+ and (G) Fsp-Cre;Ptch1fl/fl TEBs. (H) Quantification of BrdU in TEBs showing decreased proliferation in both mutants. One data point represents one TEB. (I-K) CC3-stained (I) control, (J) Fsp-Cre;Ptch1fl/+ and (K) Fsp-Cre;Ptch1fl/fl TEBs. (L) Quantification of CC3 by genotype. Only Fsp-Cre;Ptch1fl/+ has reduced apoptosis. Graphs show data as mean±s.e.m. **P<0.01 and ***P<0.001 by ANOVA/Tukey's test. n.s., not significant. Scale bars: 50 µm. Insets show histologically normal TEBs.
To test whether the dysmorphic TEBs had altered proliferation, we assayed BrdU labeling. Control TEBs were 29±2% BrdU positive (Fig. 3E), Fsp-Cre;Ptch1fl/+ TEBs were 18±2% positive (Fig. 3F) and Fsp-Cre;Ptch1fl/fl were 15±2% positive (Fig. 3G). Both mutants had less BrdU labeling than controls (controls versus Fsp-Cre;Ptch1fl/+ P<0.01, controls versus Fsp-Cre;Ptch1fl/fl P<0.001; ANOVA/Tukey's test, Fig. 3H).
With respect to apoptosis, control TEBs were 1.9±0.3% CC3 positive (Fig. 3I), whereas Fsp-Cre;Ptch1fl/+ mice had reduced (0.79±0.14%) CC3 positivity (Fig. 3J) (P<0.001, ANOVA/ Tukey's test). Fsp-Cre;Ptch1fl/fl TEBs had comparable apoptosis rates of 1.5±0.4% (Fig. 3K,L).
In 8-week-old mice, control glands displayed normal branching (66±8 per 2× field) (Fig. 4A). Despite reduced TEB proliferation, Fsp-Cre;Ptch1fl/+ glands were hyperbranched (129±11) (Fig. 4B). By contrast, Fsp-Cre;Ptch1fl/fl mice had reduced branching (18±4) (Fig. 4C). (Fig. 4A-C show part of the fat pad; quantification in Fig. S1). Control and Fsp-Cre;Ptch1fl/+ fat pads were 95±3% and 95±4% filled with epithelium, respectively, whereas Fsp-Cre;Ptch1fl/fl ducts were dramatically stunted with 39±5% fat pad filled (P<0.0001, ANOVA/Tukey's test) (Fig. 4D). Time points after 8 weeks were not evaluated owing to skin phenotypes and low mutant survival.
Fig. 4.
Eight-week-old control, Fsp-Cre;Ptch1fl/+ and Fsp-Cre;Ptch1fl/fl animals display altered branching, histology and epithelial proliferation. (A-C) Whole-mount (A) control, (B) Fsp-Cre;Ptch1fl/+ and (C) Fsp-Cre;Ptch1fl/fl glands showing branching. Heterozygotes are hyperbranched, whereas homozygotes display reduced branching. (D) Quantification of fat pad filling, showing that Fsp-Cre;Ptch1fl/fl outgrowths are severely stunted. (E-G) Hematoxylin and Eosin-stained (E) control, (F) Fsp-Cre;Ptch1fl/+ and (G) Fsp-Cre;Ptch1fl/fl ducts showing frequent partial filling in heterozygotes, and complete filling in homozygotes. (H) Quantification of ductal filling frequency by genotype. (I-K) BrdU-stained (I) control, (J) Fsp-Cre;Ptch1fl/+ and (K) Fsp-Cre;Ptch1fl/fl ducts. (L) Quantification of BrdU showing hyperproliferation in heterozygotes and hypoproliferation in homozygotes. (M-O) ERα-stained (M) control, (N) Fsp-Cre;Ptch1fl/+ and (O) Fsp-Cre;Ptch1fl/fl ducts showing upregulated ERα expression in Fsp-Cre;Ptch1fl/fl ducts. (P) Quantification of ERα in the mammary epithelium. (Q-S) PR-stained (Q) control (R) Fsp-Cre;Ptch1fl/+ and (S) Fsp-Cre;Ptch1fl/fl ducts showing ablation of PR in Fsp-Cre;Ptch1fl/fl ducts. (T) Quantification of PR in the mammary epithelium. Graphs show data as mean±s.e.m. Insets display histologically normal ducts. *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001 by ANOVA/Tukey's test. Scale bars: 1 mm in A-C; 50 µm in E-G,I-K,M-O,Q-S.
Control ducts had normal histology at 8 weeks (Fig. 4E). However, Fsp-Cre;Ptch1fl/+ ducts displayed microlumens and partially filled ducts (Fig. 4F). Histologically normal ducts were also observed (Fig. 4F, inset). Fsp-Cre;Ptch1fl/fl ducts were more frequently filled (Fig. 4G), with less frequent normal histology (Fig. 4G, inset). There was increased ductal filling in Fsp-Cre;Ptch1fl/+ (P<0.01) or Fsp-Cre;Ptch1fl/fl ducts (P<0.001, ANOVA/ Tukey's test) (Fig. 4H).
Ductal filling was confirmed by confocal microscopy of control (Fig. S2A), Fsp-Cre;Ptch1fl/+ (Fig. S2B) and Fsp-Cre;Ptch1fl/fl glands (Fig. S2C) (Movies 1 and 2 for control and Fsp-Cre;Ptch1fl/fl ducts). To determine which cell type filled the ducts, we performed immunostaining for cytokeratin 8 (K8) (luminal cells) and cytokeratin 5 (K5) (basal cells). Control ducts had K8+ cells surrounded by K5+ cells as expected (Fig. S2D). Fsp-Cre;Ptch1fl/+ (Fig. S2E) and Fsp-Cre;Ptch1fl/fl ducts (Fig. S2F) displayed K8+ cells filling ducts. Insets show histologically normal ducts. ZO-1 (zona occludens 1) expression, which stains tight junctions and apical surfaces of luminal cells, was also assayed by immunofluorescence. Although ZO-1 stained the control duct lumens as expected (Fig. S2G), stained Fsp-Cre;Ptch1fl/+ ducts confirmed the presence of microlumens (Fig. S2H), whereas Fsp-Cre;Ptch1fl/fl ducts displayed abnormal concentric patterning (Fig. S2I).
We observed a significant reduction in mammary gland mass at 8 weeks of age in homozygous mutants (0.04±0.01 g) versus controls (0.16±0.02 g) or heterozygotes (0.13±0.03 g) (Fig. S3A) (P<0.01, ANOVA/Tukey's test). Mammary glands of homozygous mutants were also smaller than controls when normalized to body weight (Fig. S3B) (P<0.05). Fsp-Cre;Ptch1fl/fl body weights (14±0.5 g) were also decreased versus controls (23±1.2 g) and heterozygotes (22±0.7 g) (P<0.0001, Fig. S3C). Heterozygotes displayed no significant changes.
With respect to proliferation at 8 weeks, control ducts were 2.0±0.8% BrdU positive (Fig. 4I), whereas Fsp-Cre;Ptch1fl/+ ducts were hyperproliferative (5.4±1.2%) (P<0.0334, ANOVA/Tukey's test) (Fig. 4J). By contrast, ducts of Fsp-Cre;Ptch1fl/fl mice showed virtually no proliferation (0.04±0.03%) (Fig. 4K) (P<0.0001, ANOVA/Tukey's test; quantification Fig. 4L). Thus, Fsp-Cre;Ptch1fl/+ mammary ducts had increased proliferation and branching, whereas the stunted ducts of Fsp-Cre;Ptch1fl/fl animals lacked proliferation.
Given that Fsp-Cre induces recombination in mammary gland extrinsic cells, and that the stunted duct and hypoproliferation phenotypes observed in Fsp-Cre;Ptch1fl/fl mice were similar to the stunted hypoproliferative ducts of estrogen receptor α (ERα) knockout mice, and reduced side branching and proliferation similar to the progesterone receptor (PR) knockout mice, we hypothesized that hormone signaling in Fsp-Cre;Ptch1fl/fl mice was disrupted (Bocchinfuso and Korach, 1997; Lydon et al., 1995).
At 8 weeks of age, ER and PR expression was perturbed in Fsp-Cre;Ptch1fl/fl mice. Although controls had 36±6% ERα-positive cells (Fig. 4M) and heterozygotes had comparable levels (38±6%) (Fig. 4N), ERα expression in Fsp-Cre;Ptch1fl/fl ducts increased to 62±2% (Fig. 4O) (P<0.05, ANOVA/Tukey's test; quantification, Fig. 4P). Control ducts were 18±3% PR positive (Fig. 4Q) and heterozygotes were comparable (27±4%) (Fig. 4R). However, PR expression was abolished in homozygotes (0.9±0.3%) (Fig. 4S) (P<0.01, ANOVA/Tukey's test; quantified in Fig. 4T).
Whole-gland transplantation rescues ductal growth and ER/PR expression, but not histological defects of Fsp-Cre;Ptch1fl/fl mice
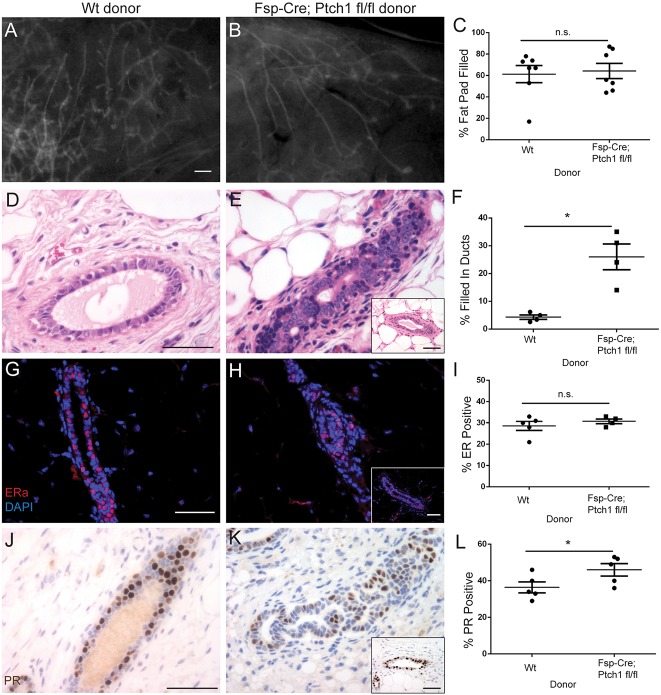
To determine whether phenotypes caused by Fsp-Cre-mediated disruption of Ptch1 were due to Ptch1 functions in mammary gland extrinsic cells, Cre- control and Fsp-Cre;Ptch1fl/fl donor glands were transplanted contralaterally into pre-pubertal recipient SCID/bg animals that were wild type for Ptch1. Eight weeks post-transplantation, the stunted duct phenotype was rescued, with similar fat pad filling between Cre- (61±11%) (Fig. 5A) and Fsp-Cre;Ptch1fl/fl donor glands (64±7%) (Fig. 5B) (quantification, Fig. 5C; P<0.8285, paired t-test). In contrast to 8-week-old homozygous mutants from genetic crosses (Fig. 4C), TEBs were observed in transplanted Fsp-Cre;Ptch1fl/fl glands (Fig. 5B). Although Cre- donor ducts displayed normal histology (Fig. 5D), Fsp-Cre;Ptch1fl/fl glands were frequently filled-in (Fig. 5E), with some histologically normal ducts (Fig. 5E inset). Cre- donor ducts were 4±0.8% filled, whereas ducts of Fsp-Cre;Ptch1fl/fl donors were 26±5% filled (P<0.0252, paired t-test) (quantification, Fig. 5F). Thus, the filled-in duct phenotype is due to loss of Ptch1 in the local mammary stroma, whereas the stunted duct growth was due to Ptch1 disruption in mammary gland extrinsic Fsp-positive cells.
Fig. 5.
Whole-gland transplantation rescues stunted ducts and ER/PR levels, but not histology, of Fsp-Cre;Ptch1fl/fl animals. Genotype indicates donor glands transplanted to SCID/bg recipients that are wild type for Ptch1. (A,B) Fluorescent whole-mount (A) control and (B) Fsp-Cre;Ptch1fl/fl donor glands, 8 weeks post-transplantation. (C) Quantification of fat pad filling, indicating no difference between groups. (D,E) Hematoxylin and Eosin-stained (D) control and (E) Fsp-Cre;Ptch1fl/fl donor ducts. (F) Quantification of ductal filling, showing increased ductal filling in mutant donors. (G,H) ERα-stained (G) control and (H) Fsp-Cre;Ptch1fl/fl donor ducts. (I) ERα quantification showing similar expression between groups. (J,K) PR-stained (G) control and (H) Fsp-Cre;Ptch1fl/fl donor ducts. (L) PR quantification showing a small increase in Fsp-Cre;Ptch1fl/fl donor ducts. Graphs show data as mean±s.e.m. Insets display histologically normal ducts. *P<0.05 by paired t-test. Insets display histologically normal ducts (E,H,K). n.s., not significant. Scale bars: 0.5 mm in A,B; 50 µm in D,E,G,H,J,K.
In whole-gland transplants, ERα positivity was comparable between Cre- (29±2%) (Fig. 5G) and Fsp-Cre;Ptch1fl/fl donor ducts by immunostaining (32±1%) (Fig. 5H) (n.s., paired t-test; quantification, Fig. 5I). Similarly, ducts of Cre- donors were 36±3% PR positive (Fig. 5J), whereas Fsp-Cre;Ptch1fl/fl donors were 46±3% (Fig. 5K) (quantification, Fig. 5L). This modest increase was significant (P<0.023, paired t-test). The normalization of ER and PR levels by whole-gland transplantation demonstrates that mammary gland extrinsic Ptch1 regulates ductal outgrowth and the characteristic ER/PR patterning of the mammary epithelium.
Stunted ducts, but not histological defects, of Fsp-Cre;Ptch1fl/fl mutants are rescued by E+P treatment
As whole-gland transplantation showed that ‘systemic’ Ptch1 regulates mammary ductal elongation (Fig. 5) and Fsp-Cre;Ptch1fl/fl mutants had altered ER/PR patterning (Fig. 4), we tested whether estrogen and progesterone (E+P) treatment would rescue the stunted ducts. Relative to the control vehicle-treated glands, the E+P-treated control glands had increased tertiary branching (Fig. 6A), as expected. Here, Fsp-Cre;Ptch1+/+ and Ptch1fl/+ or Ptch1fl/fl mice lacking Fsp-Cre were used as controls. Although the vehicle-treated control fat pads were 86±4% filled, vehicle-treated mutants displayed reduced fat pad filling (57±4%) and side branching, as previously described (Fig. 6A) (P<0.01, ANOVA/Tukey's versus control). E+P-treated control fat pads were 90±3% filled and E+P-treated Fsp-Cre;Ptch1fl/fl fat pads were 85±6% filled, consistent with rescue of the stunted ducts (quantification, Fig. 6B; P<0.01, ANOVA/Tukey's test versus vehicle-treated mutants; no difference, E+P-treated control versus E+P-treated mutants). E+P-treated Fsp-Cre;Ptch1fl/fl outgrowths still displayed reduced branching compared with the E+P-treated controls (Fig. 6A). Thus, Ptch1 may regulate estrogen and/or progesterone production to drive pubertal ductal elongation.
Fig. 6.
Ptch1 may regulate estrogen/progesterone production, but not myeloid cell function, to promote mammary ductal elongation. (A) Whole-mount vehicle- or E+P-treated control or Fsp-Cre;Ptch1fl/fl glands. E+P increases branching (compare top and bottom panels). (B) Quantification showing E+P-mediated rescue of stunted ducts of Fsp-Cre;Ptch1fl/fl mutants (‘Mutant’). (C) BrdU labeling quantification in vehicle- or E+P-treated control and Fsp-Cre;Ptch1fl/fl ducts. E+P induced proliferation, albeit attenuated, in Fsp-Cre;Ptch1fl/fl mutants. (D) Whole-mount glands of control to control (upper left), control to Fsp-Cre;Ptch1fl/fl (upper right) or Fsp-Cre;Ptch1fl/fl to control (lower left) bone marrow-transplanted animals. Inset: inguinal mammary lymph node of Cre- recipient showing colonization by Cre+, mTmG+ cells. Lower right: quantification showing that donor bone marrow does not change mammary ductal outgrowth. Graphs show data as mean±s.e.m. Scale bars: 0.5 mm in A,D; 50 µm in D, inset. **P<0.01, ****P<0.0001 by ANOVA/Tukey's test.
We also evaluated proliferation in response to E+P. While vehicle-treated, control glands displayed 1.3±0.3% BrdU positivity, vehicle-treated Fsp-Cre;Ptch1fl/fl mutants displayed reduced positivity (0±0%) as previously (Fig. 6C). E+P-treated, control ducts were 14.5±1.8% BrdU positive (P<0.0001, ANOVA/Tukey's test versus vehicle-treated controls), whereas E+P-treated Fsp-Cre;Ptch1fl/fl ducts were 2.5±0.5% BrdU positive (Fig. 6C) (significantly reduced versus E+P-treated controls, P<0.0001 by ANOVA). Thus, although E+P induced proliferation and branching, the response in Fsp-Cre;Ptch1fl/fl mutants was attenuated compared with controls. The attenuated proliferation and tertiary branching displayed by the Fsp-Cre;Ptch1fl/fl mutants in response to E+P suggests that alveologenesis would be perturbed in these animals. Consistent with E+P rescue of ductal outgrowth suggesting functional defects in the ovary, Fsp-Cre;Ptch1fl/fl animals displayed a disrupted estrous cycle (Fig. S4A-C) and dramatically reduced fertility over 6.5 weeks (Fig. S4D).
Bone marrow transplantation does not rescue outgrowth of Fsp-Cre;Ptch1fl/fl mutants
Myeloid cells regulate pubertal ductal outgrowth of the mammary epithelium (Gouon-Evans et al., 2000), a subset of which are Fsp-Cre positive (Bhowmick et al., 2004). We therefore tested whether bone marrow transplantation could rescue ductal elongation in the Fsp-Cre;Ptch1fl/fl mutants. Six weeks after transplantation, control recipients of control bone marrow displayed 89±1.5% fat pad filled, and a normal ductal structure (Fig. 6D). Here, controls consisted of Fsp-Cre;Ptch1+/+ and Ptch1fl/+ or Ptch1fl/fl mice lacking Fsp-Cre. We observed engraftment of the transplanted cells, as GFP+ cells were present with transplantation of Fsp-Cre;Ptch1+/+ cells to a Cre- recipient (Fig. 6D). Control bone marrow transplanted to Fsp-Cre;Ptch1fl/fl recipients filled only 61±2% of the fat pad (Fig. 6D). Glands from control recipients of Fsp-Cre;Ptch1fl/fl bone marrow displayed 89±1.2% of the fat pad filled (Fig. 6D). Control bone marrow transplanted to Fsp-Cre;Ptch1fl/fl mutants displayed reduced fat pad filling relative to the other groups (Fig. 6D; P<0.0001, ANOVA/Tukey's test). The inability of control bone marrow to rescue the mutant phenotype or of mutant bone marrow to induce stunted ducts in controls indicates that Ptch1 does not regulate ductal elongation in myeloid cells.
Fsp-Cre-mediated expression of activated SMO phenocopies histological defects in Fsp-Cre;Ptch1fl/fl mice
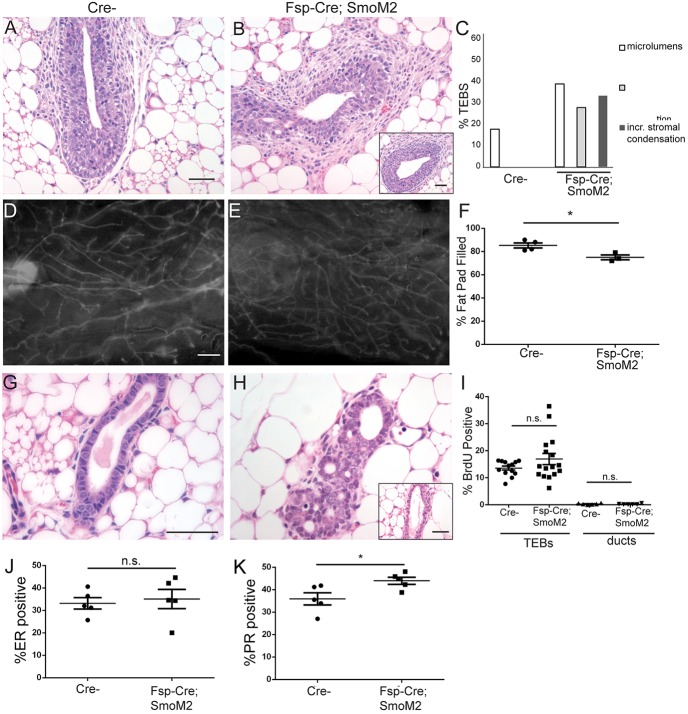
To evaluate whether the non-epithelial effects of Ptch1 loss were possibly mediated by Smo, we assessed whether Fsp-Cre-mediated expression of activated Smo could recapitulate phenotypes in Fsp-Cre;Ptch1fl/fl mice. At 6 weeks of age, Cre- control TEBs had normal histology (Fig. 7A), whereas Fsp-Cre;SmoM2 TEBs displayed dysmorphia (Fig. 7B). Dysmorphic TEBs had irregular shape, microlumens and increased periductal stromal condensations (Fig. 7C). These TEBs were similar to Fsp-Cre;Ptch1fl/+ (Fig. 3B), Fsp-Cre;Ptch1fl/fl (Fig. 3C) and Ptch1Δ/+ TEBs (Lewis et al., 1999).
Fig. 7.
Aberrant Fsp-Cre;Ptch1fl/fl histology may be due to activated canonical hedgehog signaling. (A,B) Hematoxylin and Eosin-stained (A) Cre- and (B) Fsp-Cre; SmoM2 TEBs from 6-week-old mice, showing perturbed histology and increased stromal condensation. (C) Quantification of perturbed TEBs. (D,E) Fluorescent mount of (D) Cre- and (E) Fsp-Cre; SmoM2 glands at 8 weeks. (F) Quantification showing a slight reduction of fat pad filling in mutants. (G,H) Hematoxylin and Eosin-stained (G) Cre- and (H) filled-in Fsp-Cre; SmoM2 ducts. (I) BrdU quantification showing no difference in TEBs at 6 weeks and ducts at 8 weeks. (J) ERα quantification showing no difference at 8 weeks. (K) PR quantification showing a small increase in mutants at 8 weeks. Data are displayed as mean±s.e.m. Unpaired t-test was used for analysis. *P<0.05. n.s., not significant. Insets (B,H) show histologically normal structures. Scale bars: 50 µm in A,B,G,H; 0.5 mm in D,E.
At 8 weeks of age, whole mounts of Cre- (Fig. 7D) and Fsp-Cre;SmoM2+ glands (Fig. 7E) were comparable. Cre- fat pads were 85±2% filled and Fsp-Cre;SmoM2+ fat pads were slightly less filled(74±2%) (Fig. 7F) (P<0.0225, t-test). This reduction was less than in Fsp-Cre;Ptch1fl/fl mutants, which displayed ∼40% filled fat pads at 8 weeks (Fig. 4D). While Cre- ducts at 8 weeks displayed normal histology (Fig. 7G), Fsp-Cre;SmoM2+ ducts often contained extra cells and microlumens (Fig. 7H), with some ducts appearing normal (Fig. 7H). Neither mutant TEBs nor mature ducts displayed altered proliferation relative to Cre- ducts (Fig. 7I).
We tested whether the ERα and PR expression phenotypes of the Fsp-Cre;Ptch1fl/fl mutants are phenocopied by the Fsp-Cre;SmoM2 mutants. At 8 weeks of age, Cre- ducts were 33.2±2.5% ER positive and Fsp-Cre;SmoM2 ducts were 35.1±2.3% positive (Fig. 7J) (not different by t-test). PR positivity was 35.9±2.7% in Cre- ducts, whereas Fsp-Cre;SmoM2 ducts displayed slightly higher PR positivity (44.0±1.6%) (Fig. 7K, P<0.033, unpaired t-test). Thus, Fsp-Cre;SmoM2 mutants do not display the increased ER or reduced PR expression present in the Fsp-Cre;Ptch1fl/fl mutants.
DISCUSSION
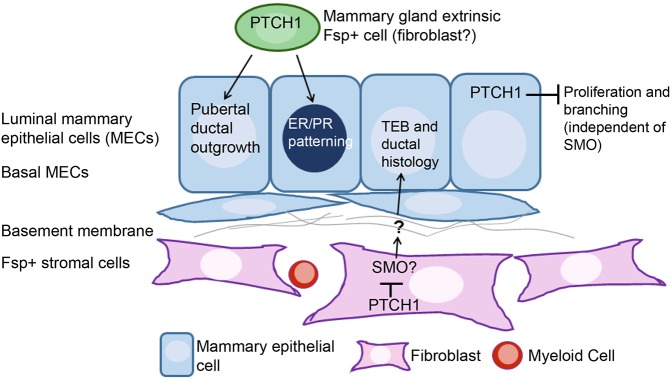
Here, we elucidate tissue compartment-specific roles of Ptch1 in virgin mammary gland development using improved mouse models, and offer insight into signaling downstream of Ptch1. Ptch1 loss in the mammary epithelium elicits hyperproliferation and hyperbranching, likely independent of Smo. Data from Fsp-Cre;Ptch1fl/fl mutants indicate Ptch1 in Fsp+ fibroblasts regulates ductal histology, perhaps via Smo. We also show the crucial systemic roles of Ptch1 in ductal elongation and ER/PR expression in the mammary epithelium (see Fig. 8, model).
Fig. 8.
Ptch1 functions in mammary gland morphogenesis and histogenesis. PTCH1 in the mammary epithelium inhibits proliferation and branching, independently of SMO. PTCH1 is essential in a mammary gland extrinsic Fsp-positive cell (fibroblast) for mammary ductal ER/PR patterning and for pubertal outgrowth. Ptch1 acts locally in an Fsp-positive stromal cell (likely a fibroblast) to inhibit SMO and elicit normal TEB and ductal histology.
The Ad-Cre;Ptch1fl/fl model displayed hyperbranching and hyperproliferation in adult virgins. Whereas mammary glands expressing SmoM2 also displayed hyperproliferation and hyperbranching (Moraes et al., 2009; Visbal et al., 2011), the Ptch1 loss and SmoM2 phenotypes diverge. SmoM2 expression yielded hyperproliferation and hyperbranching via a mixture of SmoM2+ and SmoM2– cells (Visbal et al., 2011), and elicited precocious alveolar budding – which are not the case with Ptch1 loss. Recently, we found that SmoM2-dependent hyperproliferation in the mammary gland requires Gαi2-dependent signaling (Villanueva et al., 2015). Hyperproliferation was blocked by inhibiting some Gαi subunits, but not by inhibiting GLI1 and GLI2 (Villanueva et al., 2015). The differences between these models suggests that Ptch1 loss increases proliferation independently of Smo. However, we cannot exclude the possibility that divergent phenotypes could be due to different functions of SmoM2 [an allele identified in human basal cell carcinoma (Xie et al., 1998)] versus endogenous Smo. The phenotypic differences between SmoM2 conditional expression and Ptch1 loss in the mammary epithelium agree with the lack of canonical hedgehog target gene upregulation in Ad-Cre;Ptch1fl/fl ducts, and the inability of IPI926 to block hyperproliferation (Figs 1, 2), suggesting that hyperproliferation is SMO independent. These data fit with reports that SMO (Moraes et al., 2009) and activated hedgehog signaling are absent from the normal mammary epithelium (Chang et al., 2010; Hatsell and Cowin, 2006). From our data, it is possible that Ptch1 loss-induced hyperproliferation is due to reduced sequestration of cyclin B1 outside the nucleus.
Data here confirm that non-epithelial Ptch1 regulates ductal histology. Analysis of Ptch1Δ/+ (Lewis et al., 1999) and Ptch1mes/mes animals (Moraes et al., 2009) indicated that Ptch1 mediates ductal development; virgin Ptch1Δ/+ mice had dysmorphic TEBs and filled-in ducts (Lewis et al., 1999). Whole Ptch1Δ/+ glands transplanted to a wild-type host displayed filled-in ducts, whereas transplanted epithelial fragments did not, indicating that local stromal Ptch1 controls histology. From the Fsp-Cre model and transplantation experiments, we conclude that Ptch1 in the mammary fat pad fibroblasts – not myeloid cells – regulates histology. Based on the similar histology of Fsp-Cre;Ptch1fl/fl and Fsp-Cre;SmoM2 ducts, it seems that Ptch1 may regulate histology via Smo. Taken together, the Fsp-Cre and Ad-Cre studies indicate that most phenotypes of the Ptch1mes/mes mice, including altered TEB and ductal histology, and defective ductal elongation, were due to non-epithelial functions of Ptch1.
Aside from defining local stromal Ptch1 function, we have uncovered a role for mammary extrinsic, non-epithelial Ptch1 in pubertal mammary ductal outgrowth and ER/PR patterning in the mammary epithelium. The Fsp-Cre;Ptch1fl/fl mutant diverges from the Ptch1mes/mes mutant (Moraes et al., 2009), which displayed reduced ER and PR expression in stunted ducts. The differences between the Ptch1mes/mes and Fsp-Cre;Ptch1fl/fl models could be due to conditional ablation versus a hypomorphic allele, and/or global genetic manipulation versus loss of Ptch1 in Fsp-positive cells. Altered ER/PR patterning may be due to abrogated hormone production by the ovary or pituitary, which may have been differentially affected in these models.
We have also further defined the ‘systemic’ function of Ptch1. As E+P rescued the stunted ducts, Ptch1 may regulate E+P production and ovarian function to regulate pubertal outgrowth and proliferation. Indeed, the Fsp-Cre;Ptch1fl/fl mutants displayed functional defects, including abrogated cycling and fertility. As the stunted duct phenotype was not rescued by bone marrow transplantation, Ptch1 does not function in myeloid cells to control ductal elongation.
As Fsp-Cre-mediated Ptch1 loss reduced mammary gland mass, and the mammary fat pad consists primarily of adipocytes, it could be hypothesized that off-target Cre activity in adipocytes contributed to stunted ductal outgrowth. Mice with loss of adipocytes displayed stunted ducts (Landskroner-Eiger et al., 2010). Although we cannot exclude the possibility that changes in the mutant adipocytes contributed to the stunted ducts, we did not observe Cre-dependent GFP expression in adipocytes, consistent with previous reports (Cheng et al., 2005); thus, such effects would likely be due to paracrine signaling.
Data here show stroma-to-epithelium and epithelium intrinsic Ptch1 functions in mammary gland development. It would be pertinent to determine whether bi-directional hedgehog-mediated tissue interactions exist in other organs where only unidirectional signaling is reported, e.g. prostate and pancreas (Hebrok et al., 2000; Wang et al., 2003). Dissecting these tissue-tissue interactions is crucial, as these developmental programs are inappropriately re-activated in cancer, and correlate with poor prognosis, e.g. in prostate and pancreatic cancer (Bailey et al., 2009; Fan et al., 2004).
Implications for Ptch1 and Smo in breast cancer
The hedgehog network is misregulated in many cancers, including breast (Moraes et al., 2007; Rubin and de Sauvage, 2006). Although hedgehog network activation induces basal cell carcinoma and medulloblastoma, data connecting hedgehog signaling and breast tumorigenesis are largely correlative, although Gli1 overexpression in mice induces tumorigenesis (Fiaschi et al., 2009).
PTCH1 protein levels are reduced in 50% of DCIS and invasive breast cancer (IBC), whereas 70% of DCIS and 30% of IBC display aberrant SMO, suggesting that hedgehog activation occurs frequently and early in human breast cancer (Moraes et al., 2007). Furthermore, PTCH1 underexpression correlated with Ptch1 promoter methylation (Wolf et al., 2007). However, neither Ptch1Δ/+ nor MMTV-SmoM2 mice show mammary tumors (Moraes et al., 2007, 2009). Our data suggest that perhaps, in the case of Ptch1Δ/+, the opposing functions of epithelial and systemic Ptch1 offset one another. These observations may explain why breast cancer incidence in individuals with Gorlin syndrome (Gorlin, 1987), who are heterozygous for germline Ptch1 loss-of-function and display higher risk for other cancers, is not higher than in the general population. Our Ad-Cre;Ptch1fl/+ data suggest that Ptch1 heterozygosity would not alter mammary epithelial histology or proliferation.
Previous data suggest that high hedgehog ligand expression in tumor epithelium induces GLI1 (which is indicative of activated hedgehog signaling) in the adjacent stroma, which correlates with invasiveness and poor patient prognosis (O'Toole et al., 2011). As local stromal loss of Ptch1 and non-epithelial activation of Smo promote a DCIS-like phenotype in mammary epithelium, perhaps stromal Ptch1 loss promotes cancer-associated phenotypes. The data presented here suggest that loss of Ptch1 in fibroblasts may increase survival, reduce non-apoptotic cell death or alter lumen formation. It would be interesting to determine whether Ptch1 heterozygosity correlates with DCIS in patients.
MATERIALS AND METHODS
Animal models
Mice carrying Ptch1c, here termed Ptch1fl, Cre-dependent conditional ablation allele were a gift from Dr Brandon Wainwright (University of Queensland, Australia) (Ellis et al., 2003). Mice expressing Cre-recombinase under the Fsp1 (S100A4) promoter were a gift from Dr Eric Neilson (Vanderbilt University, Nashville, TN, USA). These mice express Cre in fibroblasts and myeloid-derived leukocytes (Bhowmick et al., 2004). Mice carrying the Gt(ROSA)26Sortm1(Smo/YFP)Amc/J SmoM2 conditional activation allele were obtained from Jackson Labs (#005130) (Jeong et al., 2004). All animals were genetically tagged with the mTmG Cre-dependent reporter at the Rosa26 locus, Gt(ROSA)26Sortm4(ACTB-tdTomato,−EGFP)Luo/J. Cells lacking Cre-recombinase express tdTomato Red, whereas cells expressing Cre-recombinase display membrane-bound eGFP (Jackson Labs, #007576) (Muzumdar et al., 2007).
For studies of Ptch1fl, Fsp-Cre;Ptch1fl/+ males were crossed to Ptch1fl/+ or Ptch1fl/fl females. Fsp-Cre;SmoM2 mice were obtained by crossing Fsp-Cre, mTmG-positive males to SmoM2+/− females (Xie et al., 1998). Genotyping for Ptch1fl, SmoM2 and Fsp-Cre was performed as previously described (Bhowmick et al., 2004; Ellis et al., 2003; Jeong et al., 2004). CB.17/IcrHsd-Prkdc-scid-Lyst-bg (SCID/beige) mice (Harlan Laboratories) used for transplantation were from a breeding colony at Baylor College of Medicine. Animals were maintained according to the NIH Guide for the Care and Use of Experimental Animals with approval from Baylor College of Medicine Institutional Animal Care and Use Committee. For some analyses, 5-Bromo-2′-deoxyuridine (BrdU) (Sigma, B5002) in PBS was administered intraperitoneally 2 h prior to harvest at 250 mg/kg.
Adenoviral transduction and transplantation
For epithelial ablation of Ptch1, mammary epithelial cells were harvested from glands 1, 3, 4 and 5 of 8-week-old Ptch1+/+ and Ptch1fl/fl females with the lymph nodes removed. Glands were minced, digested with collagenase A (Roche Applied Science) and 0.05% trypsin-EDTA, and strained into single cells (Visbal et al., 2011). Cells were infected at MOI 50 with Adenovirus-Cre (Ad-Cre) from the Vector Development Laboratory Core Facility at Baylor College of Medicine. Cells were recounted, resuspended in 50% PBS/50% Matrigel (BD Biosciences) and 100,000 Ptch1+/+ and Ptch1fl/+ or Ptch1fl/fl cells were injected contralaterally into epithelium-free ‘cleared’ inguinal fat pads of 3-week-old SCID/beige recipient mice (Deome et al., 1959) using a Hamilton syringe. Outgrowths were harvested 8 weeks later.
Whole-mount analysis
For fluorescent whole-mount analysis, glands were agitated in 1 ml of 50% PBS/50% glycerol solution at 4°C overnight as described previously (Landua et al., 2009), and imaged using a Leica MZFL16 fluorescence stereomicroscope with a DFC300 FX camera. Branch points were counted manually using Metamorph software. Confocal microscopy was performed with a Leica TCS SP5 microscope. Non-fluorescent whole mounts were analyzed using Neutral Red (Sigma) staining and imaged with a Leica MZ12.5 stereomicroscope with a Lumenera Infinity 1 camera, as described previously (Landua et al., 2009).
Immunofluorescence
Tissues were fixed in 4% paraformaldehyde in PBS for 3 h at 4°C, embedded in paraffin wax and sectioned at 3 μm. Slides were rehydrated using decreasing concentrations of ethanol. Immunostaining was carried out using antigen retrieval in 0.1 M sodium citrate buffer (pH 6.0) and heating to 120°C in a decloaker (Biocare Medical). Primary antibodies were incubated overnight at 4°C with 8% MOM protein reagent (Vector Labs, BMK2202) and 1.5% goat serum. See Table S1 for antibody information. Micrographs were taken with a Zeiss Leica Axioskop 2 Plus with an AxioCam MRm FX camera. Cells from ten 40× fields, or ∼1000 mammary epithelial cells were quantified per animal using Metamorph software. Each TEB was a data point, with ∼300 cells/TEB.
Whole-gland transplantation
Control (Ptch1fl/fl only or Fsp-Cre only) and Fsp-Cre;Ptch1fl/fl donor glands at 3 weeks of age were transplanted contralaterally into 3-week-old SCID/bg recipient mice as described previously (Lewis et al., 2001; Moraes et al., 2009). Glands were analyzed 8 weeks after transplantation.
Estrogen and progesterone treatment
Daily subcutaneous treatments of 1 µg β-estradiol (Sigma) and 1 mg (Sigma) progesterone in sesame oil, or sesame oil only, were administered for 14 days prior to animal harvest.
IPI926 treatment (inhibition of SMO)
Either IPI926 (Infinity) dissolved in 13% ethanol in Tween-20 (Sigma) or vehicle alone were administered by oral gavage. IPI926 doses were 40 mg/kg. For the mammary gland experiment, three daily treatments of vehicle or IPI926 were given prior to harvest.
Uterine scratch
After ovarectomy post-weaning and a 1-week rest, a prescribed course of estrogen (0.1 µg in 100 µl sesame oil for 3 days), 2 days rest, then estrogen+progesterone (1 mg progesterone+6.7 ng estrogen daily until harvest) was administered prior to scratch of one uterine horn by blunted needle as described previously (Finn and Martin, 1972). Vehicle or IPI926 was administered for 7 days prior to, and the day of harvest 9 days after the first estrogen treatment. Hormone and IPI926 doses were timed as described previously (Villanueva et al., 2015).
QPCR
Tissues were collected into RNA Later (Qiagen) and frozen at −80°C. RNA was extracted with the Qiagen RNeasy Kit, and cDNA was synthesized with the Superscript III kit (Thermo Fisher) using random hexamers. The cDNA was analyzed using an Applied Biosystems 7500-Fast thermocycler for TaqMan quantitative PCR under standard conditions. Product accumulation was represented as 2−ΔCt, with ANOVA of ΔCt values used for statistical comparison. 18S rRNA was used for normalization. See Table S2 for primers.
Bone marrow transplantation
Recipient animals 4-5 weeks of age received Baytril water 24 h prior to irradiation and up to 6 days post-irradiation. Recipients received a dose of 400 centigray, and 24 h later, bone marrow cells were harvested and isolated from 4-5-week-old donor mice. Irradiated recipients received 2 million donor cells injected retro-orbitally. Recipients were harvested 6 weeks post-transplantation.
Acknowledgements
We thank Andrew Ta, Rupali Sood and Sydnee Spruiell for technical assistance; Dr. Brandon Wainwright (University of Queensland) and Dr Eric Neilson (Vanderbilt University) for mouse lines; and Drs Tao Wang and Susan Hilsenbeck for advice regarding statistical analyses. We acknowledge Dr. Chad Shaw, Lukas Simon and David Henke for bioinformatics assistance. We thank Yi Athena Ren and JoAnne Richards for critical advice, and Dr Hugo Villanueva for experimental assistance.
Footnotes
Competing interests
M.T.L. is a founder of, and Limited Partner in, StemMed Ltd, and is a Manager of StemMed Holdings LLC, its General Partner.
Author contributions
J.D.L. participated in imaging, animal work and bone marrow transplantation. A.P.V. assisted with experimental design, animal acquisition and breeding. T.M. performed all other experiments, statistics, interpretation and manuscript preparation. M.T.L. participated in project conception, experimental design, data interpretation and manuscript preparation, and was principle investigator.
Funding
This work was supported by the National Institutes of Health and National Cancer Institute (RO1 CA127857 to M.T.L. and P30 CA125123 to the Dan L. Duncan Cancer Center to C. Kent Osborne). This project was supported by the Cytometry and Cell Sorting Core at Baylor College of Medicine with funding from the National Institutes of Health/National Institutes of Allergy and Infectious Disease, National Cancer Institute and National Center for Research Resources (P30AI036211, P30CA125123 and S10RR024574 to Joel M. Sederstrom, Director). Deposited in PMC for immediate release.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.140434.supplemental
References
- Bailey J. M., Mohr A. M. and Hollingsworth M. A. (2009). Sonic hedgehog paracrine signaling regulates metastasis and lymphangiogenesis in pancreatic cancer. Oncogene 28, 3513-3525. 10.1038/onc.2009.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes E. A., Kong M., Ollendorff V. and Donoghue D. J. (2001). Patched1 interacts with cyclin B1 to regulate cell cycle progression. EMBO J. 20, 2214-2223. 10.1093/emboj/20.9.2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick N. A., Chytil A., Plieth D., Gorska A. E., Dumont N., Shappell S., Washington M. K., Neilson E. G. and Moses H. L. (2004). TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303, 848-851. 10.1126/science.1090922 [DOI] [PubMed] [Google Scholar]
- Bocchinfuso W. P. and Korach K. S. (1997). Mammary gland development and tumorigenesis in estrogen receptor knockout mice. J. Mammary Gland Biol. Neoplasia. 2, 323-334. 10.1023/A:1026339111278 [DOI] [PubMed] [Google Scholar]
- Briscoe J. and Thérond P. P. (2013). The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 14, 416-429. 10.1038/nrm3598 [DOI] [PubMed] [Google Scholar]
- Chang H., Li Q., Moraes R. C., Lewis M. T. and Hamel P. A. (2010). Activation of Erk by sonic hedgehog independent of canonical hedgehog signalling. Int. J. Biochem. Cell Biol. 42, 1462-1471. 10.1016/j.biocel.2010.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. and Struhl G. (1996). Dual roles for patched in sequestering and transducing Hedgehog. Cell 87, 553-563. 10.1016/S0092-8674(00)81374-4 [DOI] [PubMed] [Google Scholar]
- Cheng N., Bhowmick N. A., Chytil A., Gorksa A. E., Brown K. A., Muraoka R., Arteaga C. L., Neilson E. G., Hayward S. W. and Moses H. L. (2005). Loss of TGF-beta type II receptor in fibroblasts promotes mammary carcinoma growth and invasion through upregulation of TGF-alpha-, MSP- and HGF-mediated signaling networks. Oncogene 24, 5053-5068. 10.1038/sj.onc.1208685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel C. W. and Smith G. H. (1999). The mammary gland: a model for development. J. Mammary Gland Biol. Neoplasia. 4, 3-8. 10.1023/A:1018796301609 [DOI] [PubMed] [Google Scholar]
- Deome K. B., Faulkin L. J. Jr, Bern H. A. and Blair P. B. (1959). Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res 19, 515-520. [PubMed] [Google Scholar]
- Ellis T., Smyth I., Riley E., Graham S., Elliot K., Narang M., Kay G. F., Wicking C. and Wainwright B. (2003). Patched 1 conditional null allele in mice. Genesis 36, 158-161. 10.1002/gene.10208 [DOI] [PubMed] [Google Scholar]
- Fan L., Pepicelli C. V., Dibble C. C., Catbagan W., Zarycki J. L., Laciak R., Gipp J., Shaw A., Lamm M. L. G., Munoz A. et al. (2004). Hedgehog signaling promotes prostate xenograft tumor growth. Endocrinology 145, 3961-3970. 10.1210/en.2004-0079 [DOI] [PubMed] [Google Scholar]
- Fiaschi M., Rozell B., Bergström A. and Toftgård R. (2009). Development of mammary tumors by conditional expression of GLI1. Cancer Res. 69, 4810-4817. 10.1158/0008-5472.CAN-08-3938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn C. A. and Martin L. (1972). Endocrine control of the to timing of endometrial stimulus sensitivity a decidual to implantation importance hormones later. Biol. Reprod. 7, 82-86. 10.1093/biolreprod/7.1.82 [DOI] [PubMed] [Google Scholar]
- Gorlin R. J. (1987). Nevoid basal cell carcinoma syndrome. Medicine 66, 98-113. 10.1097/00005792-198703000-00002 [DOI] [PubMed] [Google Scholar]
- Gouon-Evans V., Rothenberg M. E. and Pollard J. W. (2000). Postnatal mammary gland development requires macrophages and eosinophils. Development 127, 2269-2282. [DOI] [PubMed] [Google Scholar]
- Hatsell S. J. and Cowin P. (2006). Gli3-mediated repression of Hedgehog targets is required for normal mammary development. Development 133, 3661-3670. 10.1242/dev.02542 [DOI] [PubMed] [Google Scholar]
- Hebrok M., Kim S. K., St Jacques B., McMahon A. P. and Melton D. A. (2000). Regulation of pancreas development by hedgehog signaling. Development 127, 4905-4913. [DOI] [PubMed] [Google Scholar]
- Hennighausen L. and Robinson G. W. (2005). Information networks in the mammary gland. Nat. Rev. Mol. Cell Biol. 6, 715-725. 10.1038/nrm1714 [DOI] [PubMed] [Google Scholar]
- Jeong J., Mao J., Tenzen T., Kottmann A. H. and McMahon A. P. (2004). Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 18, 937-951. 10.1101/gad.1190304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. W., Nguyen M. P., Padalecki S. S., Grubbs B. G., Merkel A. R., Oyajobi B. O., Matrisian L. M., Mundy G. R. and Sterling J. A. (2011). TGF-beta promotion of Gli2-induced expression of parathyroid hormone-related protein, an important osteolytic factor in bone metastasis, is independent of canonical Hedgehog signaling. Cancer Res. 71, 822-831. 10.1158/0008-5472.CAN-10-2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landskroner-Eiger S., Park J., Israel D., Pollard J. W. and Scherer P. E. (2010). Morphogenesis of the developing mammary gland: stage-dependent impact of adipocytes. Dev. Biol. 344, 968-978. 10.1016/j.ydbio.2010.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landua J. D., Visbal A. P. and Lewis M. T. (2009). Methods for preparing fluorescent and neutral red-stained whole mounts of mouse mammary glands. J. Mammary Gland Biol. Neoplasia. 14, 411-415. 10.1007/s10911-009-9155-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauth M., Bergström Å., Shimokawa T., Tostar U., Jin Q., Fendrich V., Guerra C., Barbacid M. and Toftgård R. (2010). DYRK1B-dependent autocrine-to-paracrine shift of Hedgehog signaling by mutant RAS. Nat. Struct. Mol. Biol. 17, 718-725. 10.1038/nsmb.1833 [DOI] [PubMed] [Google Scholar]
- Lewis M. T., Ross S., Strickland P. A., Sugnet C. W., Jimenez E., Scott M. P. and Daniel C. W. (1999). Defects in mouse mammary gland development caused by conditional haploinsufficiency of Patched-1. Development 126, 5181-5193. [DOI] [PubMed] [Google Scholar]
- Lewis M. T., Ross S., Strickland P. A., Sugnet C. W., Jimenez E., Hui C.-c. and Daniel C. W. (2001). The Gli2 transcription factor is required for normal mouse mammary gland development. Dev. Biol. 238, 133-144. 10.1006/dbio.2001.0410 [DOI] [PubMed] [Google Scholar]
- Lydon J. P., DeMayo F. J., Funk C. R., Mani S. K., Hughes A. R., Montgomery C. A., Shyamala G., Conneely O. M. and O'Malley B. W. (1995). Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 9, 2266-2278. 10.1101/gad.9.18.2266 [DOI] [PubMed] [Google Scholar]
- Macias H. and Hinck L. (2012). Mammary gland development. Wiley Interdiscip. Rev. Dev. Biol. 1, 533-557. 10.1002/wdev.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H., Zhao X., Das S. K., Hogan B. L. M. and Dey S. K. (2002). Indian hedgehog as a progesterone-responsive factor mediating epithelial-mesenchymal interactions in the mouse uterus. Dev. Biol. 245, 280-290. 10.1006/dbio.2002.0645 [DOI] [PubMed] [Google Scholar]
- Mille F., Thibert C., Fombonne J., Rama N., Guix C., Hayashi H., Corset V., Reed J. C. and Mehlen P. (2009). The Patched dependence receptor triggers apoptosis through a DRAL-caspase-9 complex. Nat. Cell Biol. 11, 739-746. 10.1038/ncb1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes R. C., Zhang X., Harrington N., Fung J. Y., Wu M.-F., Hilsenbeck S. G., Allred D. C. and Lewis M. T. (2007). Constitutive activation of smoothened (SMO) in mammary glands of transgenic mice leads to increased proliferation, altered differentiation and ductal dysplasia. Development 134, 1231-1242. 10.1242/dev.02797 [DOI] [PubMed] [Google Scholar]
- Moraes R. C., Chang H., Harrington N., Landua J. D., Prigge J. T., Lane T. F., Wainwright B. J., Hamel P. A. and Lewis M. T. (2009). Ptch1 is required locally for mammary gland morphogenesis and systemically for ductal elongation. Development 136, 1423-1432. 10.1242/dev.023994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar M. D., Tasic B., Miyamichi K., Li L. and Luo L. (2007). A global double-fluorescent Cre reporter mouse. Genesis 605, 593-605. 10.1002/dvg.20335 [DOI] [PubMed] [Google Scholar]
- O'Toole S. A., Machalek D. A., Shearer R. F., Millar E. K. A., Nair R., Schofield P., McLeod D., Cooper C. L., McNeil C. M., McFarland A. et al. (2011). Hedgehog overexpression is associated with stromal interactions and predicts for poor outcome in breast cancer. Cancer Res. 71, 4002-4014. 10.1158/0008-5472.CAN-10-3738 [DOI] [PubMed] [Google Scholar]
- Riobo N. A., Saucy B., Dilizio C. and Manning D. R. (2006). Activation of heterotrimeric G proteins by Smoothened. Proc. Natl. Acad. Sci. USA 103, 12607-12612. 10.1073/pnas.0600880103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins D. J., Fei D. L. and Riobo N. A. (2012). The Hedgehog signal transduction network. Sci. Signal. 5, re6 10.1126/scisignal.2002906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin L. L. and de Sauvage F. J. (2006). Targeting the Hedgehog pathway in cancer. Nat. Rev. Drug Discov. 5, 1026-1033. 10.1038/nrd2086 [DOI] [PubMed] [Google Scholar]
- Villanueva H., Visbal A. P., Obeid N. F., Ta A. Q., Faruki A. A., Wu M.-F., Hilsenbeck S. G., Shaw C. A., Yu P., Plummer N. W. et al. (2015). An essential role for Gα(i2) in Smoothened-stimulated epithelial cell proliferation in the mammary gland. Sci. Signal. 8, ra92 10.1126/scisignal.aaa7355 [DOI] [PubMed] [Google Scholar]
- Visbal A. P., LaMarca H. L., Villanueva H., Toneff M. J., Li Y., Rosen J. M. and Lewis M. T. (2011). Altered differentiation and paracrine stimulation of mammary epithelial cell proliferation by conditionally activated Smoothened. Dev. Biol. 352, 116-127. 10.1016/j.ydbio.2011.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K.-U., Ward T., Davis B., Wiseman R. and Hennighausen L. (2001). Spatial and temporal expression of the Cre gene under the control of the MMTV-LTR in different lines of transgenic mice. Transgenic Res. 10, 545-553. 10.1023/A:1013063514007 [DOI] [PubMed] [Google Scholar]
- Wang B. E., Shou J., Ross S., Koeppen H., De Sauvage F. J. and Gao W.-Q. (2003). Inhibition of epithelial ductal branching in the prostate by sonic hedgehog is indirectly mediated by stromal cells. J. Biol. Chem. 278, 18506-18513. 10.1074/jbc.M300968200 [DOI] [PubMed] [Google Scholar]
- Wolf I., Bose S., Desmond J. C., Lin B. T., Williamson E. A., Karlan B. Y. and Koeffler H. P. (2007). Unmasking of epigenetically silenced genes reveals DNA promoter methylation and reduced expression of PTCH in breast cancer. Breast Cancer Res. 105, 139-155. 10.1007/s10549-006-9440-4 [DOI] [PubMed] [Google Scholar]
- Xie J., Murone M., Luoh S.-M., Ryan A., Gu Q., Zhang C., Bonifas J. M., Lam C.-W., Hynes M., Goddard A. et al. (1998). Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature 391, 90-92. 10.1038/34201 [DOI] [PubMed] [Google Scholar]
- Zhou M., Hou Y., Yang G., Zhang H., Tu G., Du Y., Wen S., Xu L., Tang X., Tang S. et al. (2015). LncRNA-Hh strengthen cancer stem cells generation in twist-positive breast cancer via activation of hedgehog signaling pathway. Stem Cells 34, 55-66. 10.1002/stem.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]