CASE SUMMARY
Hispanic G2P2002 female (i.e., gravida 2 para 2; two pregnancies with two living children) died at age 28 years, 32 days postoperative after cesarean delivery
Normal BMI: 23.9 kg/m2
Found unresponsive and in hypertensive crisis
Clinical history included gestational diabetes mellitus, preeclampsia with severe features, acute renal failure, chronic hypertension, and anemia
Admission glucose 259 mg/dL, pH 7.22, HCO3− 18.4 mEq/L, C-peptide 10.54 ng/mL, HbA1c not determined
Negative for pulmonary embolus, sepsis, and hemorrhagic stroke
Head computed tomography revealed anoxic encephalopathy with multiple infarcts in the dorsal midbrain and thalamus
Type 1 diabetes–associated autoantibodies: positive for glutamic acid decarboxylase antibodies (GADA; 1,068 units/mL with assay cutoff 20) and islet cell antibodies (ICA; 2,560 JDFU with assay cutoff 10) and negative for other autoantibodies (against insulin [IAA], insulinoma-associated protein 2 [IA-2A], and islet-specific zinc transporter 8 [ZnT8A])
HLA intermediate risk for type 1 diabetes: A*03:01, 30:01; DRB1*07:01, 11:02; DQA1*02:01, 05:01; DQB1*02:02, 03:19
Cause of death: anoxic encephalopathy of unknown etiology
CASE NARRATIVE
The patient (nPOD 6310) was a 28-year-old female, 32 days postoperative from a cesarean delivery. Her past medical history was significant for chronic hypertension, severe preeclampsia, acute renal failure, and gestational diabetes mellitus (GDM). She was brought to the emergency department after collapsing, with total downtime of 20–30 min. On arrival, she was admitted to the intensive care unit and found to be in hypertensive crisis. Her hospital course was complicated by renal failure requiring four rounds of hemodialysis, hypertension requiring multiple agents for control, and a degree of hyperglycemia requiring insulin. Computed tomography of the head demonstrated anoxic encephalopathy with multiple infarcts within the brain. The patient was declared brain-dead and, with authorization obtained from next of kin, became an organ donor. The pancreas, along with blood, was recovered for research through the Network for Pancreatic Organ Donors with Diabetes (nPOD) (1).
Diabetes is the most common medical complication of pregnancy, with potential for both maternal and fetal morbidity and mortality. Approximately 6–7% of pregnancies are complicated by diabetes, and approximately 90% of these cases are accounted for by GDM (2). GDM is a condition of carbohydrate intolerance with onset or first recognition during pregnancy. GDM places a mother at risk for developing overt diabetes outside of pregnancy, with up to 50% of mothers developing type 2 diabetes within 20–28 years (2,3) compared with as low as 2% incidence following a normoglycemic pregnancy (4).
Although the relationship between GDM and type 2 diabetes is well established, the relationship of GDM with type 1 diabetes is less clear. Indeed, this latter risk seems especially great when GDM is associated with type 1 diabetes autoantibodies (5,6). Nilsson et al. (6) demonstrated that among 385 women with GDM, 24 (6%) had β-cell–specific autoantibodies characteristic of type 1 diabetes. Of the 12 women who later developed this disease, 100% were glutamic acid decarboxlyase antibody (GADA)-positive during their pregnancy, although single, double, or triple autoantibody status for progressors was not reported. For the donor with history of GDM reported here, GADA and islet cell antibody (ICA) titers were exceedingly high (1,068 units/mL with assay cutoff 20 and 2,560 JDFU with assay cutoff 10, respectively), while autoantibodies against insulin (IAA), insulinoma-associated protein 2 (IA-2A), and islet-specific zinc transporter 8 (ZnT8A) were all negative by radioimmunoassay. It cannot be excluded that ICA reactivity detected in this donor may be attributable to the high-titer GADA, which has been shown to be a major antigen of the ICA reaction (7). Because of the polyclonal nature and indirect immunofluorescence detection methods (8), the ICA test clearly differs from other autoantibody testing via ELISA or radioimmunoassay in terms of antigen/epitope specificity. At this time, nPOD does not consider ICA for standard testing of autoantibodies (9–11). Future efforts are needed to determine the additional autoantibodies and their target antigens that contribute to ICA for more precise reporting of autoantibody status in subjects with positivity for both GADA and ICA.
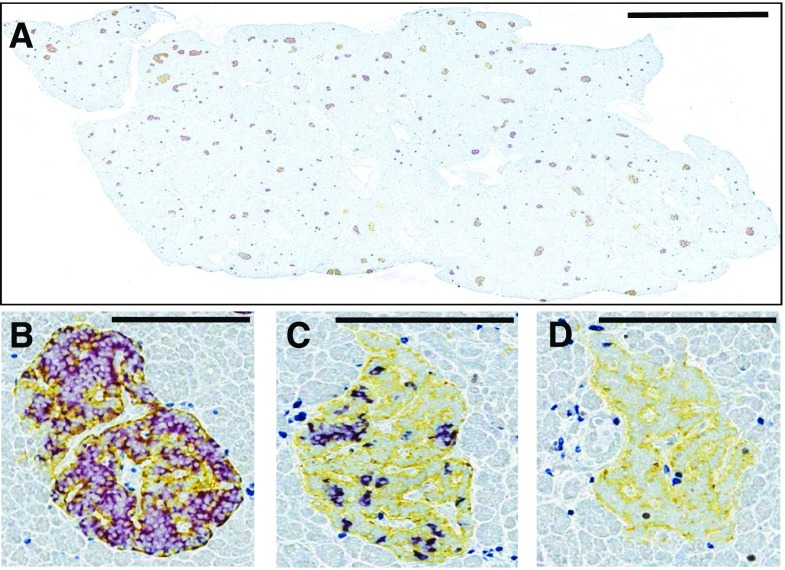
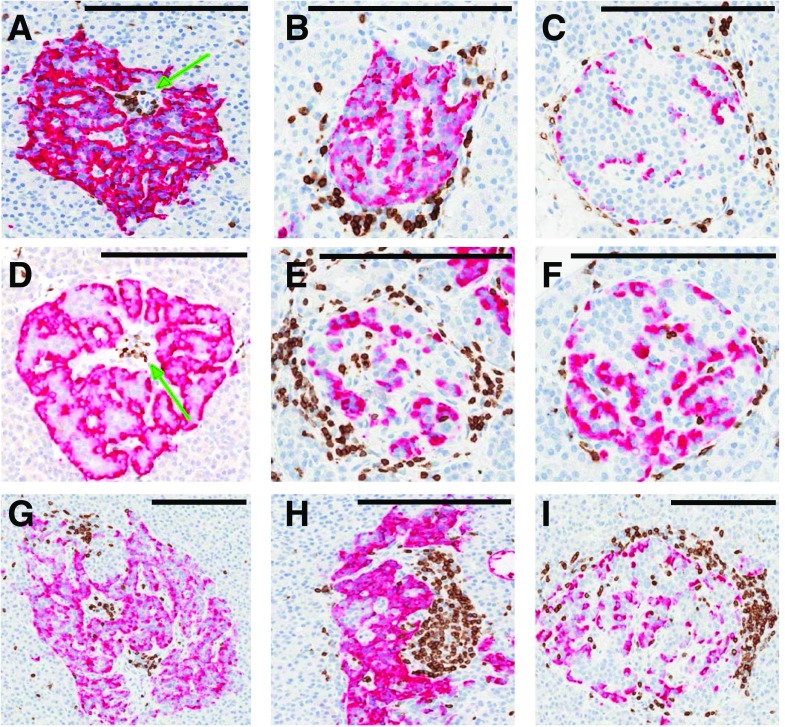
Previous studies, albeit limited and largely focused on the natural history of type 1 diabetes, have demonstrated immunohistological changes in pancreatic islets, including insulitis, to be rare among autoantibody-positive adults (10–12). Thus, it was of great interest to evaluate whether this patient’s GADA positivity, in the presence of GDM, associated with unique immunohistological features of type 1 diabetes in the pancreatic islets and to compare the findings observed in the pancreata of GADA-positive persons without diabetes or individuals with recent-onset type 1 diabetes. For this purpose, the pancreas was evaluated for islet expression of insulin and islets with ≥6 CD3+ T cells. The pancreas of this patient showed numerous islets with insulin (Fig. 1A and B), an observation consistent with the noted serum C-peptide level of 10.54 ng/mL, indicating the potential for active insulin production. However, islets with low β-cell to α-cell ratios (Fig. 1C) and “pseudoatrophic” islets (i.e., insulin-negative) (Fig. 1D) were also seen. This latter finding was most intriguing because pseudoatrophic islets are considered a pathognomonic feature of the type 1 diabetes pancreas, reflective of β-cell destruction (11). Moreover, 44 of 2,026 (2.2%) pancreatic islets examined (methods as previously described by Campbell-Thompson et al. [11]) from nine blocks encompassing the pancreas head, body, and tail regions demonstrated CD3+ infiltration (insulitis), another classic feature of type 1 diabetes (13). The observed insulitis was mild. The average number of CD3+ cells per infiltrated islet in the donor 6310 pancreas was 9.2 ± 0.9 cells (mean ± SEM; range 6–21) inside (intrainsulitis) (Fig. 2A) or in the islet periphery (peri-insulitis) (Fig. 2B and C) compared with 0.1 ± 0.05 CD3+ cells per islet in control pancreata from three Hispanic female organ donors aged 21, 24, and 26 years. Other noteworthy findings included multifocal, mild chronic interstitial fibrosis, which has also been reported in GADA+ single-autoantibody pancreas donors (12). The residual β-cell mass was found to be 763 mg (methods as previously described by Campbell-Thompson et al. [11]), with a pancreas weight of 82.83 g and a relative pancreas weight of 1.24 g/kg (calculated as previously described by Campbell-Thompson et al. [14]). Approximately 5–10% of GDM cases may be associated with autoantibody positivity (5), and it is reasonable to suspect that the presence of autoantibodies reflects autoimmune destruction of pancreatic β-cells (2). However, direct histological evidence within the literature is lacking. The T-cell infiltration observed in this patient clearly resembles the insulitis found in individuals without diabetes positive for multiple type 1 diabetes–associated autoantibodies (e.g., GADA+IA-2A+ nPOD 6267 [Fig. 2D–F]) and as represented by a patient with recent-onset type 1 diabetes (e.g., GADA+ nPOD 6362 [Fig. 2G–I]).
Figure 1.
Representative images from nPOD donor 6310. Formalin-fixed, paraffin-embedded tissue sections (4 μm) from the pancreas head, body, and tail regions were stained by four-color immunohistochemistry for insulin (purple), glucagon (yellow), CD3 (blue), and Ki67 (black). Numerous insulin+glucagon+ islets, some large, were present as seen in a representative whole-tissue cross-section (A). Normal islets (B), islets with low β-cell to α-cell ratio (C), and pseudoatrophic (insulin-negative) islets (D) were observed. Scale bars: 4 mm (A), 200 μm (B–D).
Figure 2.
Comparison of insulitic islets from nPOD donor 6310 with multiple (GADA+IA-2A+) autoantibody–positive nPOD donor 6267 and GADA+ nPOD donor 6362 with new-onset type 1 diabetes. Representative images from donors 6310 (A–C), 6267 (D–F), and 6362 (G–I) are shown. Formalin-fixed, paraffin-embedded tissue sections (4 μm) from the pancreas head, body, and tail regions were stained by double immunohistochemistry for CD3 (brown) and glucagon (red). The insulitis found in donor 6310 was mild. The majority of infiltrating cells were found to be inside the donor 6310 islets (intrainsulitis, green arrow) (A). Lymphocytic infiltration was also observed in the donor 6310 islet periphery (peri-insulitis), showing focal aggregation (B) and direct contact with the peripheral islet cells (C). Intrainsulitis (D) was seen in the donor 6267 pancreas (green arrow), but the insulitic lesions with peri-insulitis (E and F) were more abundant. The insulitis seen in the pancreas of donor 6362 with recent-onset type 1 diabetes was more robust, with higher numbers of lymphocytes observed inside (G) and on the periphery (H and I) of many islets. Scale bars: 200 μm (all panels).
It is well known that at the onset of type 1 diabetes, a majority of patients have circulating autoantibodies against single or multiple islet cell antigens and demonstrate insulitis in the pancreas. The autoantibodies can be detected in the circulation many years before disease onset. However, their role in initiating and perpetuating the autoimmune process in pancreatic islets is not completely established (13). Here, we present what we believe is an extremely rare, histology-based report of a patient positive for GADA and ICA who, in the presence of GDM, developed insulitis, as well as pseudoatrophic islets reflective of autoimmune type 1 diabetes–like loss of β-cells. Without prior medical history and longitudinal autoantibody measurements, it is not possible to ascertain from this single case a role for GDM in contributing to anti–β-cell autoimmunity, but taken together, it seems plausible to question whether the major physiological changes related to pregnancy and/or GDM may have been a potential factor that enhanced diabetes progression to the degree that eventually, had she not died of complications related to pregnancy, she would have subsequently developed overt type 1 diabetes or latent autoimmune diabetes in adults (LADA) (15). Future clinical and histopathological research of autoantibody-positive GDM is needed to improve our understanding of autoimmune diabetes during and following pregnancy.
Article Information
Acknowledgments. The authors acknowledge the nPOD staff members and organ procurement organizations that partner with nPOD to recover organ donors. Additional donor details can be obtained through the JDRF nPOD website (www.jdrfnpod.org). Donor data sets are available through nPOD DataShare, an online database for collaborative communication organized around the nPOD specimen repository. Images in this manuscript were obtained from the nPOD online pathology site. The authors would also like to thank William Patrick Duff (Division of Maternal & Fetal Medicine, University of Florida) for his review of the clinical data and opinion on case presentation, Janelle Noble (Children’s Hospital Oakland Research Institute) for review of the high-resolution HLA genotype, and Mark A. Atkinson (Departments of Pathology and Pediatrics, University of Florida) for manuscript review and thoughtful discussions.
Funding. This work was supported by JDRF (25-2013-268, 17-2012-3, and 25-2012-516 to M.C.-T. and I.K.), the National Institutes of Health (PO1-AI-42288), and the National Institute of Diabetes and Digestive and Kidney Diseases (1DP3-DK-101120-01 and 1UC4-DK-104155-01 to M.C.-T.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.J. researched the data and wrote the manuscript. A.P. contributed to discussion and reviewed and edited the manuscript. M.C.-T. conceived of the study and reviewed and edited the manuscript. I.K. researched the data and wrote the manuscript. I.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Pugliese A, Yang M, Kusmarteva I, et al. The Juvenile Diabetes Research Foundation Network for Pancreatic Organ Donors with Diabetes (nPOD) Program: goals, operational model and emerging findings. Pediatr Diabetes 2014;15:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest 2005;115:485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitzmiller JL, Dang-Kilduff L, Taslimi MM. Gestational diabetes after delivery. Short-term management and long-term risks. Diabetes Care 2007;30(Suppl. 2):S225–S235 [DOI] [PubMed] [Google Scholar]
- 4.Feig DS, Zinman B, Wang X, Hux MJ. Risk of development of diabetes mellitus after diagnosis of gestational diabetes. CMAJ; 2008;179:229–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haller-Kikkatalo K, Uibo R. Clinical recommendations for the use of islet cell autoantibodies to distinguish autoimmune and non-autoimmune gestational diabetes. Clin Rev Allergy Immunol 2016;50:23–33 [DOI] [PubMed] [Google Scholar]
- 6.Nilsson C, Ursing D, Törn C, Aberg A, Landin-Olsson M. Presence of GAD antibodies during gestational diabetes mellitus predicts type 1 diabetes. Diabetes Care 2007;30:1968–1971 [DOI] [PubMed] [Google Scholar]
- 7.Atkinson MA, Kaufman DL, Newman D, Tobin AJ, Maclaren NK. Islet cell cytoplasmic autoantibody reactivity to glutamate decarboxylase in insulin-dependent diabetes. J Clin Invest 1993;91:350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bingley PJ, Bonifacio E, Mueller PW. Diabetes Antibody Standardization Program: first assay proficiency evaluation. Diabetes 2003;52:1128–1136 [DOI] [PubMed] [Google Scholar]
- 9.Wasserfall C, Montgomery E, Yu L, et al. Validation of a rapid type 1 diabetes autoantibody screening assay for community-based screening of organ donors to identify subjects at increased risk for the disease. Clin Exp Immunol 2016;185:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell-Thompson M. Organ donor specimens: what can they tell us about type 1 diabetes? Pediatr Diabetes 2015;16:320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell-Thompson M, Fu A, Kaddis JS, et al. Insulitis and β-cell mass in the natural history of type 1 diabetes. Diabetes 2016;65:719–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiberg A, Granstam A, Ingvast S, et al. Characterization of human organ donors testing positive for type 1 diabetes-associated autoantibodies. Clin Exp Immunol 2015;185:278–288 [DOI] [PMC free article] [PubMed]
- 13.Campbell-Thompson ML, Atkinson MA, Butler AE, et al. The diagnosis of insulitis in human type 1 diabetes. Diabetologia 2013;56:2541–2543 [DOI] [PubMed] [Google Scholar]
- 14.Campbell-Thompson ML, Kaddis JS, Wasserfall C, et al. The influence of type 1 diabetes on pancreatic weight. Diabetologia 2016;59:217–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laugesen E, Østergaard JA, Leslie RD; Danish Diabetes Academy Workshop and Workshop Speakers . Latent autoimmune diabetes of the adult: current knowledge and uncertainty. Diabet Med 2015;32:843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]