Abstract
Retinal degenerative diseases are the leading causes of blindness worldwide. Replacing lost retinal cells via stem cell-based therapies is an exciting, rapidly advancing area of translational research that has already entered the clinic. Here, we review the status of these clinical efforts for several significant retinal diseases, describe the challenges involved and discuss how basic developmental studies have contributed to and are needed to advance clinical goals.
KEY WORDS: Retinal development, Stem cell, Regenerative medicine, Degenerative retinal diseases, Macular degeneration, Retina pigment epithelial cells
Summary: This Review article summarizes the current state-of-the-art retinal stem cell therapies, and highlights the essential role of developmental biology in moving this field forward.
Introduction
The retina consists of the multi-layered neural retina (NR) and the monolayer of retinal pigment epithelium (RPE). As with other parts of the central nervous system, the adult mammalian retina has limited regenerative capacity, and thus NR or RPE cell death can lead to irreversible vision loss. Retinal degenerative diseases, such as glaucoma, retinitis pigmentosa (RP) and age-related macular degeneration (AMD), are characterized by the early loss of specific cell types: retinal ganglion cells (RGCs), photoreceptors or RPE cells, respectively (Davis et al., 2016; De Jong, 2006; Ferrari et al., 2011). Eventually, this leads to a common pathophysiology: the dysfunction of light-sensing photoreceptors, which results in untreatable blindness (Berson, 1993; De Jong, 2006; Quigley, 1993). Available therapies for some of these diseases can slow disease progression or relieve symptoms, but currently there are no effective treatments to restore vision. The rapidly increasing incidence of untreatable blindness worldwide due to age-associated degenerative disease and the burden of inherited retinal disease underscore the need for novel treatments (Quigley and Broman, 2006; Wong et al., 2014). Replacing dysfunctional retinal cells using stem cell-based therapies has the potential to alleviate symptoms or possibly cure these diseases, and represents a transformation in our approach to treating vision loss.
A key challenge for retinal cell therapy is to obtain the desired cell types robustly, efficiently and in large numbers. Stem cells, particularly human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs), are a valuable cell source for deriving retinal cell products. Typically, hPSCs must pass through a sequence of cell stages representing their in vivo development in order to differentiate appropriately into specialized retinal cells. This involves the formation of the anterior neural plate (ANP), then the eye field, then the optic vesicle, followed by the specification of naive NR versus RPE, before finally producing the various retinal cell types. Here, knowledge gained from basic developmental studies using model organisms has been of paramount importance for developing these differentiation protocols, and it is now possible to produce functional retinal cell types efficiently from hPSCs. Despite this progress, however, the production of human retinal cells at the quality and quantity required for clinical use remains challenging. A better understanding of the underlying mechanisms that control retinal development is fundamental for improvements to these protocols, and thus for the delivery of stem cell-based therapies for retinal disease.
In this Review, we summarize the current understanding of retinal development, with a particular emphasis on the key events that drive the specification of the RPE and the NR, the latter of which originates from retinal progenitor cells (RPCs). We then discuss how this knowledge has been applied to generate human retinal cells – RPE cells, photoreceptors and RGCs – in vitro from hPSCs. Some of these cells have already entered clinical trials for various retinal diseases, whereas others are still in the preclinical phase. We discuss existing treatments for retinal diseases such as AMD, RP and glaucoma, and consider how hPSC-derived retinal cells may represent a more attractive therapeutic alternative. Finally, we summarize some of the challenges facing stem cell therapies for retinal disease, for example maturation and integration of the hPSC-derived cells, as well as the possible immunogenicity of transplanted cells. Even in light of these and other challenges, it is clear that stem cell therapies hold tremendous promise for the treatment of some retinal diseases. With continuously refined protocols for differentiation and the possibility of genetic engineering, we expect this field will continue to move forward at an impressive rate.
Retinal development
Retinal development has been studied for many years using many different model organisms. The summary we present here is a general overview formed from studies in vertebrates, and focuses on those events that are key to the development and specification of the cell types most affected in human retinal disease. For a more detailed description of retinal development, we refer the reader to two review articles (Centanin and Wittbrodt, 2014; Heavner and Pevny, 2012).
Formation of the optic cup
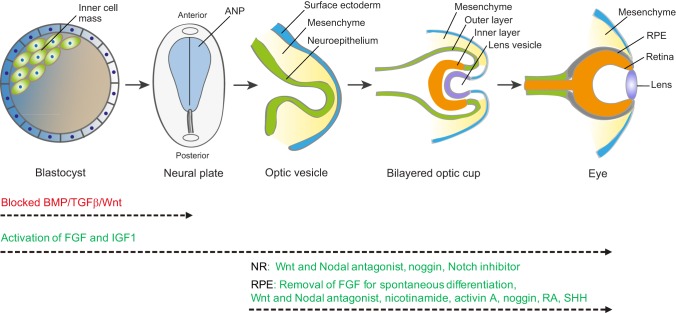
Gastrulation and neurulation result in the initial formation of the nervous system, in the form of the neural plate, and the specification of the eye field located within the ANP (Fig. 1) (Li et al., 1997). The eye field initially forms as a single domain in the early ANP but is subsequently split into two lateral eye primordia under the influence of the prechordal mesoderm. The two eye primordia then undergo extensive reorganization and evagination, resulting in the optic vesicles. Subsequent optic cup formation is the result of consecutive and reciprocal inductive interactions between the neuroepithelium of the ventral forebrain, surface ectoderm, and extraocular mesenchyme, which is both neural crest and mesoderm derived (Adler and Canto-Soler, 2007; Fuhrmann, 2010). As the evaginating optic vesicle makes contact with the mesenchyme and the ectoderm, it divides into a distal domain and the more proximal/ventral domains (Heavner and Pevny, 2012) (Fig. 1). The distal domain and its overlaying surface ectoderm become thickened and invaginated, forming the inner layer of the optic cup and the lens vesicle, respectively. Inductive signals including fibroblast growth factors (FGFs) and bone morphogenetic proteins (BMPs) from the overlaying lens placode drive the inner layer of the optic cup towards becoming NR (Kuribayashi et al., 2014; Pandit et al., 2015; Pittack et al., 1997; Zhao et al., 2001). The proximal domain of the optic vesicle becomes the outer layer of the optic cup and develops into the RPE layer under the influence of the extraocular mesenchyme and the nearby overlying surface ectoderm (Fuhrmann et al., 2000; Muller et al., 2007). Thus, a bilayered optic cup is formed. The most proximal/ventral domain of the optic vesicle narrows into the optic stalk, the cavity filling with RGC axons to create the optic nerve at later stages of retinal development (Fuhrmann, 2010; Heavner and Pevny, 2012; Molotkov et al., 2006) (Fig. 1).
Fig. 1.
Schematic of the key stages of retina development. Beginning with the blastocyst, which contains the pluripotent inner cell mass, gastrulation and neurulation lead to formation of the neural plate. The early eye field is located in the anterior neural plate (ANP) and develops into the optic vesicles. Blocking the activity of BMP, TGFβ and Wnt (red) promotes ANP development. Invagination of the optic vesicle leads to formation of the bilayered optic cup. The inner layer of the optic cup develops into the neural retina (NR) and the outer layer develops into the retinal pigment epithelium (RPE). Activation of FGF and IGF1 pathways (green) facilitates not only development of the ANP but also subsequent optic vesicle/cup formation and retina development. Wnt, FGF, BMP, Notch, SHH, RA and activin A signaling pathways (green) are involved in the specification of the RPE and NR. These factors have been used to promote NR and RPE production from stem cells in vitro, but the specific combinations and concentrations of each factor and the schedule of addition remain to be optimized for each lineage. It is also possible that additional factors not yet identified, and potentially specific to human retinal development, will aid neural retinal and RPE differentiation.
Specification of the RPE
At the optic vesicle stage, both distal and proximal domains are bipotential and capable of giving rise to either NR or RPE (Reh and Pittack, 1995). The basic helix-loop-helix (bHLH) transcription factor Mitf and homeobox protein Otx2 are the key regulators of RPE differentiation, and they work synergistically to control the RPE gene network and facilitate RPE differentiation (Martinez-Morales et al., 2003). Mitf is initially expressed throughout the optic vesicle, but becomes restricted to the proximal domain, the presumptive RPE, as the optic vesicle develops into optic cup (Nguyen and Arnheiter, 2000; Nguyen et al., 1997). The expression of Otx2 has been shown to be diffuse in early optic vesicles and later becomes confined to the region where RPE arises, after the formation of optic cup (Bovolenta et al., 1997). Morphogens, including Wnts and members of the TGFβ superfamily, also play important roles in RPE differentiation (Muller et al., 2007; Westenskow et al., 2009). As the proximal domain of the optic vesicle acquires an RPE identity, its distal domain develops into the NR under the influence of the overlying surface ectoderm. Both FGF2 and BMP4 signaling play key roles in activating Chx10 (Vsx2 – Mouse Genome Informatics), and this in turn antagonizes Mitf expression, which is essential for NR identity in the initially bipotential distal domain of the optic vesicle (Horsford et al., 2005; Huang et al., 2015; Nguyen and Arnheiter, 2000; Rowan et al., 2004).
Specification of the NR
The naive NR is a thin neuroepithelial layer composed of RPCs that can rise to various retinal neurons and Müller glial cells (Wetts and Fraser, 1988). Retinogenesis is a highly dynamic and precisely controlled process involving FGF2 (Hicks and Courtois, 1992), insulin-like growth factor (IGF) (Meyer-Franke et al., 1995), BMP (Lan et al., 2009), Nodal (Sakuma et al., 2002), Wnt (Ouchi et al., 2005) and Notch (Louvi and Artavanis-Tsakonas, 2006) signaling pathways, which transforms the naive NR into the mature retina with its characteristic laminar cytoarchitecture. There are three major layers in the mature retina: the outer nuclear layer (ONL), consisting of primary sensory neurons including rods and cones; the inner nuclear layer (INL), consisting of interneurons including horizontal, bipolar and amacrine cells, as well as the cell bodies of Müller glia; and the ganglion cell layer (GCL) consisting of long projection RGCs (Heavner and Pevny, 2012). The retina processes visual signals originating in photoreceptors and sends output to the brain via the RGC axons that run in the optic nerve to the optic chiasm where they partially decussate, parsing to run in the contra- and ipsilateral optic tracts. The bilateral optic tracts project posteriorly around the midbrain and form synapses at the lateral geniculate nucleus (LGN). LGN axons then travel through the deep brain as optic radiations and reach the primary visual cortex (Erskine and Herrera, 2007, 2014).
Most, if not all the RPCs in the naive neural retina, express all the eye field transcriptional factors, including ET (Tbx3), Rx (Rax), Pax6, Six3, Six6 (also known as Optx2), Tll (Nr2e1) and Lhx2 along with Chx10 and Sox2 (Heavner and Pevny, 2012 and references therein). Different expression profiles of additional transcription factors contribute to cell heterogeneity and generate bias in progenitor competency. For example, Otx2+ RPCs and Cdh6+ RPCs exist in the developing retina, and each subset exhibits bias towards generating a unique combination of retinal neurons (Cepko, 2014). Adding to this heterogeneity, RPCs display intrinsic changes in their competency to produce different retinal cell types as retinogenesis progresses (Cepko, 2014). This complex process yields numerous types of retinal progeny. In addition to the major cell classes, accumulating evidence suggests that there are multiple types of horizontal, bipolar, amacrine and RGCs based on cell morphology, transmitter expression profile and synaptic connectivity, indicating that we still have much to learn about the differentiation of retinal lineages (Masland, 2012). New techniques, such as single-cell transcriptomics, are advancing our understanding of RPC and retinal cell heterogeneity, with 39 distinct populations already identified in the mouse (Macosko et al., 2015; Young, 1985) and a greater diversity anticipated in humans.
Generating human retinal cells in vitro
The substantial body of knowledge regarding retinal development in vivo, which has been acquired largely from animal models, can be used to guide efforts to produce human retinal cells from stem cell sources. Starting from hPSCs, the general approach is to apply specific molecules or growth factors at appropriate times to mimic the known in vivo retinogenesis signals. The first step is a period of induction towards the neural fate, and then further patterning and differentiation towards the desired retinal cell fate. The initial induction period often involves growing the PSCs in a 3D format as aggregations termed embryoid bodies or organoids, and, following this, their dissociation into a cell suspension that is then re-plated to create a cell monolayer, if this is desired. As described above, retinogenesis involves the FGF, IGF, BMP, Nodal, Wnt and Notch signaling pathways, and step-wise application of a combination of factors to modulate these pathways in vitro has successfully directed hPSCs towards a range of retinal fates.
Derivation of hPSC-RPE cells
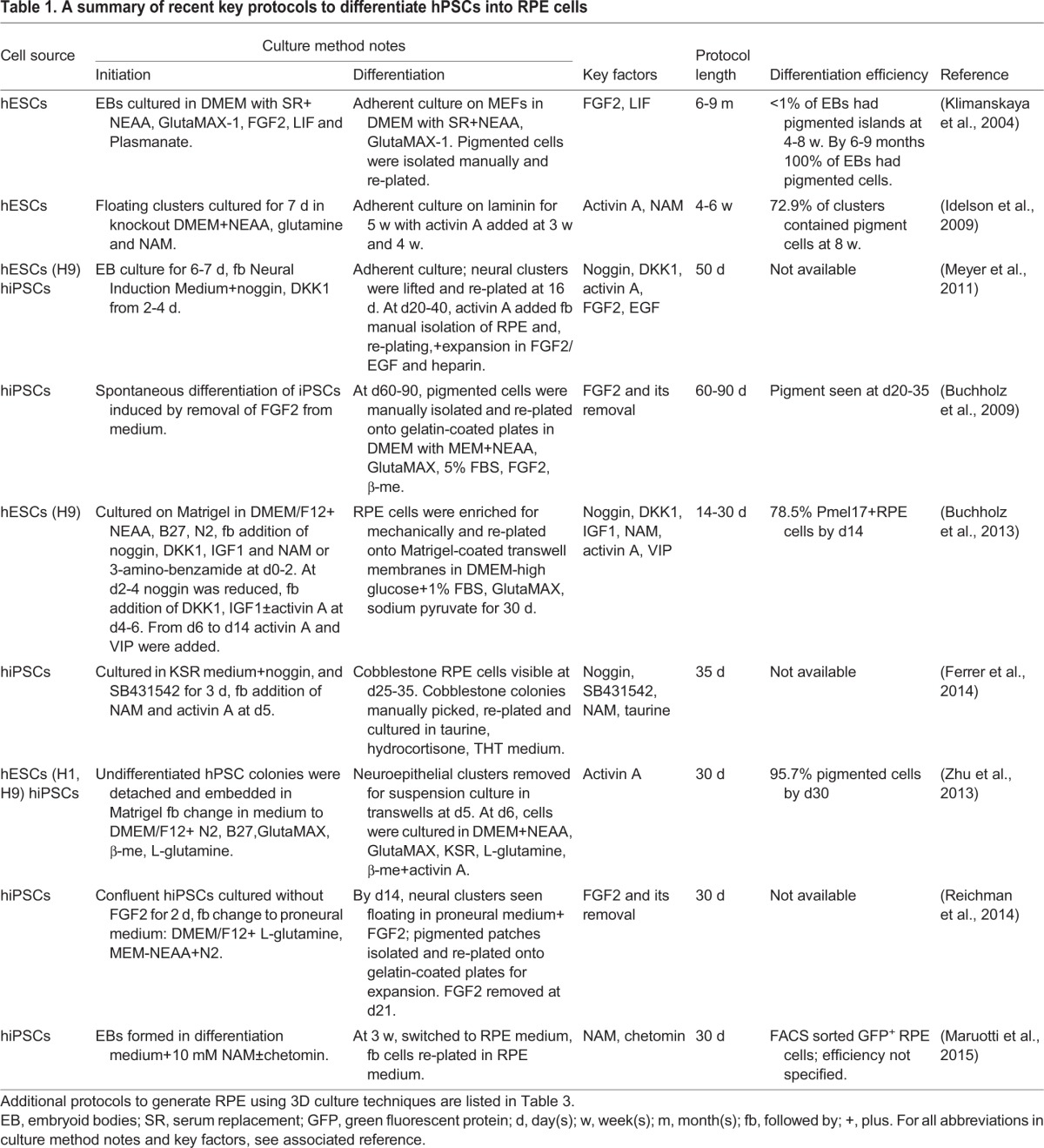
Numerous different protocols exist for the differentiation of hPSCs into RPE (Table 1). Remarkably, RPE cells can spontaneously differentiate from hESCs if FGF2 is removed from the culture medium (Klimanskaya et al., 2004), a finding later recapitulated using hiPSCs (Buchholz et al., 2009). This protocol can reliably derive RPE cells that grow on various culture substrates (Rowland et al., 2013) and in various culture media, but is inefficient (depending on the PSC line) and time-consuming (taking at least 2-3 months). To achieve higher efficiency, factors that regulate the formation of the RPE in vivo, such as Wnt and Nodal antagonists, have been applied at an appropriate time in the differentiation process (Osakada et al., 2008, 2009). Activin A is a TGFβ family member involved in the development of the RPE from the optic vesicle stage, and exposure to activin A for two weeks during the third or fourth week of differentiation dramatically increases the efficiency of RPE production from hESCs (Idelson et al., 2009). Zahabi et al. (2012) demonstrated that serial addition of noggin (a BMP4 antagonist), FGF2, retinoic acid (RA) and sonic hedgehog (SHH) to hiPSCs directed them to generate RPE cells at an efficiency of ∼60% after 2 months (Zahabi et al., 2012). Combined application of the retinal inducing factors IGF1, noggin, the Wnt inhibitor Dkk1 and FGF2, and the RPE inducers activin A and nicotinamide, was reported to facilitate rapid RPE generation, within 14 days after the onset of differentiation and with ∼80% efficiency (Buchholz et al., 2013). Small molecule approaches to stimulate hPSC-RPE generation have also been described (Maruotti et al., 2015). Despite these advances, even the most efficient protocols can result in residual iPSCs, but manual ‘picking’ under the microscope or enzyme-based selection of emerging RPE colonies followed by re-plating can lead to highly pure RPE cultures (Maruotti et al., 2013). Importantly, although hPSC-derived RPE cells produced by these different methods tend to appear morphologically similar and express appropriate markers, it is important to demonstrate that the cell products are properly differentiated as expected. Rigorous physiological testing, and comparison to native RPE cells as a ‘gold standard’ can help ensure that RPE products are functionally authentic (Miyagishima et al., 2016).
Table 1.
A summary of recent key protocols to differentiate hPSCs into RPE cells
Derivation of hPSC-photoreceptors
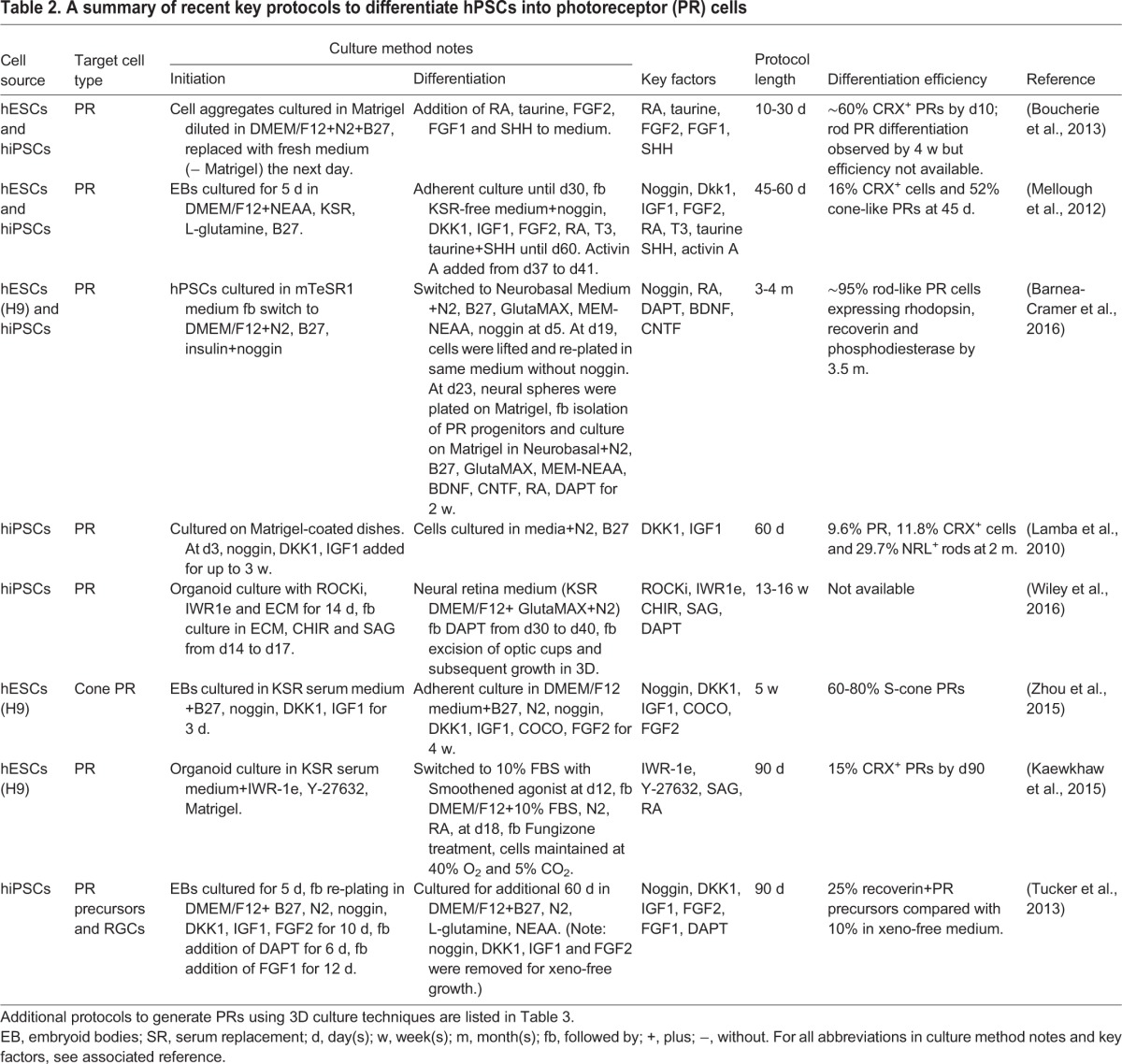
Derivation of photoreceptors in vitro from hPSCs has been challenging, especially to produce the mature outer segments – the delicate extension of photoreceptors that holds the light-sensitive pigment embedded in membrane stacks. Various different protocols exist, some of which have focused on generating RPCs that can differentiate more fully into photoreceptors in situ after transplantation (Table 2; Ikeda et al., 2005; Lamba et al., 2006). In vivo, both BMP and Wnt signaling must be inhibited to favor ANP development, whereas IGF1 is required to promote retinogenesis (Glinka et al., 1997; Mellough et al., 2012; Pera et al., 2001). In an attempt to recapitulate these signals, Lamba and colleagues used a combination of noggin (a BMP signaling inhibitor), Dkk1 (a Wnt signaling antagonist) and IGF1 (an inducer of the eye field) supplemented in the culture medium, which resulted in the first successful differentiation of hESCs into RPCs (Lamba et al., 2006). The resulting cells exhibited further differentiation into photoreceptors when co-cultured with mouse retinal tissues (Lamba et al., 2006). In a later study, a more homogeneous population of hiPSC-derived photoreceptors was obtained by fluorescence-activated cell sorting (FACS) for cells expressing GFP under the control of the human interphotoreceptor retinoid-binding protein (IRBP; also known as retinol binding protein 3) promoter, which is specific to photoreceptors (Lamba et al., 2010). Although this approach is suitable for obtaining more a homogeneous population of cells for the purpose of research, it is unlikely to be a strategy appropriate for clinical use due to the use of lentiviral vectors and proteins of non-human origin, which present a safety concern and could interfere with normal cell function. hESC-RPCs have also been successfully produced by inhibition of Wnt and Nodal signaling, and further differentiation into photoreceptors was achieved with supplementation of RA and taurine (Osakada et al., 2008). Additional refinement of the process by combining previously published methods into a three-step protocol has been reported to markedly increase the efficiency of photoreceptor generation (Mellough et al., 2012).
Table 2.
A summary of recent key protocols to differentiate hPSCs into photoreceptor (PR) cells
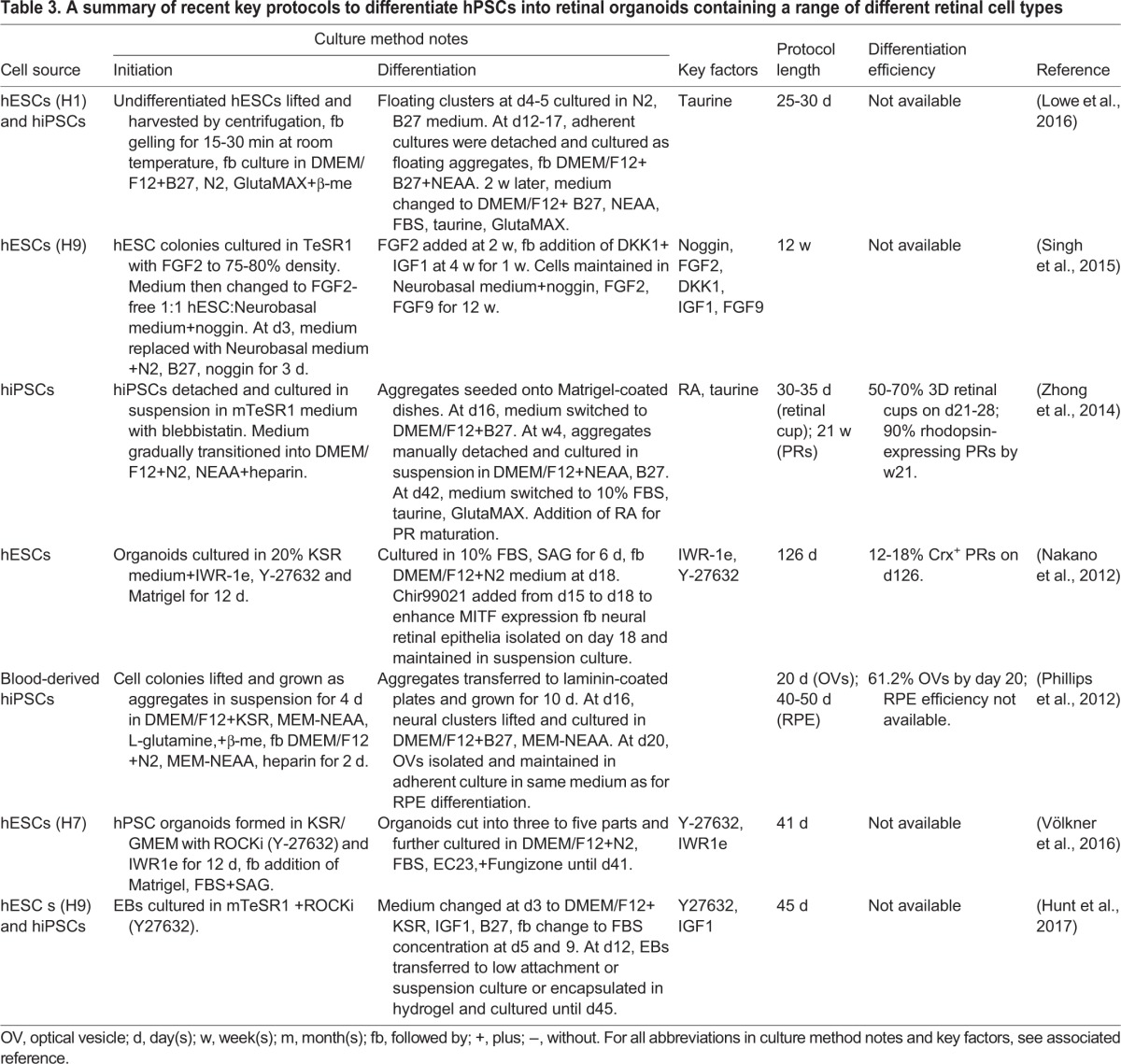
Given the difficulty of differentiating mature photoreceptors from hPSCs in 2D cultures, approaches that use 3D retinal organoid cultures might be met with more success (see Table 3), owing to the more complex environment that resembles the in vivo retinal anlage. In an early study using mouse ESCs, Hirano and colleagues demonstrated evidence for the formation of 3D ocular structures (Hirano et al., 2003). Subsequently, Yoshiki Sasai's group made considerable progress in growing self-organized 3D optic cups from hESCs, and showed the formation of photoreceptors with reasonable inner segments and connecting cilia (Nakano et al., 2012). In their study, a serum-free and growth-factor-reduced medium was used to create embryoid body-like aggregates, and treatment with the Notch inhibitor DAPT over days 29-43 dramatically accelerated photoreceptor differentiation, which was 40-78% on day 43 but only 12-18% on day 126 in the absence of DAPT (Nakano et al., 2012). Zhong et al. (2014) reported successful induction of fully laminated 3D retinal tissue from hiPSCs, including photoreceptors with outer segment discs that showed some response to light (Zhong et al., 2014). In this protocol, RA was added to the culture medium over various time periods to stimulate photoreceptor production. Addition of RA over weeks 10-14 induced ∼33% photoreceptors by week 17, increasing to 90% by week 21 (Zhong et al., 2014). The differentiated photoreceptors were shown by electron microscopy to have formed outer segments and they were able to respond to light by week 25-27, as assessed by voltage-clamp recordings (Zhong et al., 2014). The progress in 3D retinal organoid culture and the success of photoreceptor differentiation with advanced functional maturation make it possible to study human photoreceptors, and explore the application of this lineage in cell transplantation therapeutic applications.
Table 3.
A summary of recent key protocols to differentiate hPSCs into retinal organoids containing a range of different retinal cell types
Derivation of hPSC-RGCs
As with RPE and photoreceptor differentiation, the addition of small molecules and growth factors to the hPSCs grown in culture can promote RGC development (see Table 4). In the early steps of RGC specification, RPC generation is promoted by noggin, DKK1 and IGF1 (Lamba et al., 2006; Tucker et al., 2013), then RGC differentiation ensues. Riazifar et al. reported functional RGC differentiation from both hESCs and hiPSCs with an efficiency of 30% using a Notch inhibitor (Riazifar et al., 2014). Later, a self-induction protocol of RGCs from hiPSCs modified from a 3D retinal generation protocol was reported (Tanaka et al., 2015). In this protocol, 3D optic vesicle-like structures were first generated, and these were then attached in adherent 2D cultures, enabling the RGCs to grow long axons by day 50. RA was added 3 days before attachment to promote RGC axon growth (Tanaka et al., 2015). Successful differentiation of RGCs from a hESC cell line was obtained by removing the factors that favor photoreceptor induction and instead adding forskolin, an adenylate cyclase activator known to increase RGC neurite outgrowth, probably via inhibition of the SHH signaling pathway, which itself is an inhibitor of RGC differentiation (Sluch et al., 2015). This protocol employed a clustered regularly interspaced short palindromic repeats (CRISPR)-engineered reporter that allowed subsequent purification of differentiated RGCs by FACS. More recently, RGC production from hPSCs was accomplished using a stepwise differentiation protocol starting with embryoid bodies (EBs) that were grown in neural induction medium, followed by retinal neurosphere isolation and subsequent RGC differentiation (Ohlemacher et al., 2016). Of the resulting cells, 36.1% expressed the RGC-associated transcription factor BRN3 (POU4F1) within the first 40 days of differentiation (Ohlemacher et al., 2016).
Table 4.
A summary of recent key protocols to differentiate hPSCs into RGCs

In summary, successful differentiation of hPSCs into retinal cells is possible by supplementation of a combination of growth factors or small molecules that mimic the signals that occur during in vivo retinogenesis, as well as the use of either 2D or 3D culture approaches, depending on the specific cell type to be produced. Still, issues remain to be resolved, including the ability to enrich for specific retinal subtypes, given that methods currently produce a mix of different cell types. Although FACS enrichment has achieved ∼80-90% purity in the research setting, introduction of markers or antibody surface labeling might not be desirable for clinical use. The risk of tumor formation caused by contamination of undifferentiated stem and prolific progenitor cells is still a potential problem in cell transplantation application, and further emphasizes the need to optimize protocols. Methods to accelerate the process and produce enriched cell populations with high purity by direct reprograming are also being pursued. In a recent study, fibroblast-to-RPE conversion was achieved by the overexpression of key transcription factors PAX6, LHX2, OTX2, SOX9, MITF, SIX3, ZNF92, GLIS3 and FOXD1 (D'Alessio et al., 2015). Direct reprograming is an exciting advance; however, the onus is then on ensuring that the cell product generated in this manner is authentic and stable. Regardless of the method, once optimized protocols to obtain clinical grade cells are established, the manufacturing process can move towards good manufacturing practice (GMP) compliance. Cells can then be delivered from the GMP manufacturing site to the clinical location for transplantation. However, in order to start clinical trials, it is necessary to obtain animal study data supporting the efficacy and safety of the cell product (see Box 1).
Box 1. The research pipeline for stem cell therapy.
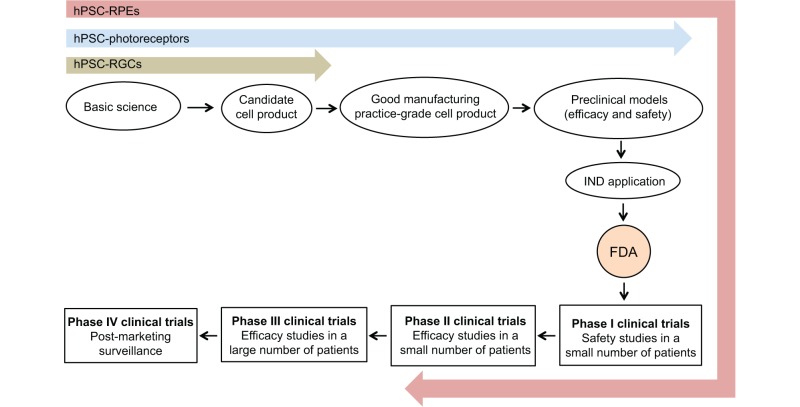
The research pipeline for stem cell therapy, using the USA system as an example, involves producing a clinical grade cell product, typically via good manufacturing practice (GMP) procedures, and evaluating the efficacy and safety of the cell product in animal models of the targeted disease. These preclinical data are included in an Investigational New Drug Application (IND) to the Food and Drug Administration (FDA) along with a clinical trial design. The FDA then has 30 days to issue a decision whether to allow the trial to proceed, or to put it on hold for further evaluation. Clinical trials proceed through four major stages: Phase I, safety in a small number of patients; Phase II, efficacy in a small number of patients; Phase III, efficacy in a larger patient population; and Phase IV, post-marketing surveillance. The current state of progress of stem cell-derived RGC (green), photoreceptor (blue) and RPE (pink) cell transplantation is indicated.
Candidate retinal degenerative diseases and stem cell therapies
Degenerative retinopathies that lead to permanent blindness include AMD, RP and glaucoma. The primary cell loss in each of these major diseases is RPE, photoreceptors and RGCs, respectively. By focusing on progress made towards the treatment of each disease, we will illustrate the status of the field and highlight the challenges that need to be addressed.
AMD and RPE transplantation
AMD is the major cause of irreversible blindness in the elderly in the developed world, with an incidence predicted to reach 200 million globally by 2020 (Ambati and Fowler, 2012a; Wong et al., 2014). It is a degenerative retinal disease that affects the central, macular region of the eye, the region responsible for high acuity color vision. AMD is classified into a dry or wet form based on lack or presence of choroidal neovascularization, respectively (De Jong, 2006). Dry AMD accounts for about 90% of the AMD cases in the USA and Europe and is characterized by the presence of lipid and protein aggregates termed ‘drusen’ that gradually accumulate between the RPE and its basal substrate, Bruch's membrane (Ambati and Fowler, 2012b; Johnson et al., 2001, 2005). Drusen deposits lead to a thickening of Bruch's membrane, which inhibits nutrient diffusion from plasma to the RPE and waste removal in the opposite direction, contributing to RPE degeneration (Abdelsalam et al., 1999). RPE dysfunction/death leads to insufficient phagocytosis of photoreceptor outer segments and subsequent photoreceptor death in the macula, resulting in central vision loss (Sparrow et al., 2010). As the disease progresses, in some patients (∼10% of total AMD cases), choroidal blood vessels invade the retina, leading to wet AMD and a rapid, devastating loss of central vision (De Jong, 2006). Currently, vitamin supplementation is recommended to slow dry AMD progression, but this has limited efficacy (Chew et al., 2013), and anti-vascular endothelial growth factor (VEGF) antibodies are injected intra-ocularly to manage choroidal neovascularization and hemorrhaging in wet AMD (Heier et al., 2012). There is no effective treatment to reverse dry AMD or to stop it from progressing to wet AMD. Replacement of RPE cells from stem cell sources has the potential to rescue RPE function as an AMD therapeutic. Surgical translocation of RPE from healthy areas into diseased areas has demonstrated positive benefit, but these are difficult surgeries to perform and complications can arise (Stanga et al., 2002). Nevertheless, these findings provide a proof of concept that transplantation of healthy RPE cells subretinally into diseased areas can be beneficial.
Preclinical studies of RPE cell transplantation into animal models started about 10 years ago, with the Royal College of Surgeons (RCS) rats as the most widely used model of RPE-based retinal degeneration (D'Cruz et al., 2000; Dowling and Sidman, 1962; Ramsden et al., 2013). RCS rats have a mutation in the receptor tyrosine kinase Mertk gene, which impairs outer segment phagocytosis by the RPE layer and leads to secondary photoreceptor death and vision deterioration. Hence, they are frequently used as a model of inherited retinal degeneration. Primate ESC-derived RPEs have been transplanted into the subretinal space of RCS rats and recovery of retinal function was observed (Haruta et al., 2004). Later, RPE cells were successfully generated from hESCs and subretinal transplantation of these cells into RCS rats resulted in cell survival, photoreceptor rescue and visual function improvement (Idelson et al., 2009; Lund et al., 2006; Vugler et al., 2008).
In 2011, Advanced Cell Technology, (later Ocata and now Astellas), in the USA launched the first Phase I/II human clinical trial using hESC-derived RPE, and the data indicated a good safety profile (Schwartz et al., 2012). The three-year follow-up study of three dosage cohorts (50,000 cells, 100,000 cells and 150,000 cells) corroborated good safety and indicated increased general and peripheral vision of the injected eye by visual field testing; visual acuity increased by 11-15 letters in AMD patients at 6 months and 12 months after transplantation, with the fellow uninjected eye showing no vision improvement at these two time points (Schwartz et al., 2015). A Phase II study with more patients to assess efficacy is expected to report results imminently.
Additional clinical trials with a similar design have been launched in different countries (Table 5). RPE cells injected in a cell suspension, as in the ongoing Asteallas trial, might be able to re-establish function, whereas other groups are using patches of previously polarized RPE cells (Falkner-Radler et al., 2011; Seiler and Aramant, 2012). Inserting a patch requires a larger cut in the retina, which can cause additional complications, and as it is a foreign agent, the substrate itself may cause complications. There could, however, be advantages to transplanting a pre-formed polarized monolayer, as the RPE cells are correctly orientated and have pre-formed tight junctions. A clinical trial (NCT01691261) investigating the use of a hESC-derived RPE monolayer immobilized on a polyester membrane for wet AMD patients has treated two patients, sponsored by The London Project in collaboration with Pfizer (Table 5). A similar approach is being used by Regenerative Patch Technologies using hESCs on a parylene membrane. In these studies, the supportive membrane is permanent, and if cells die then the membrane will form a barrier between the retina and endogenous RPE, which could cause retinal cell death. Other groups are using a biodegradable matrix so that once the cells are in position, the matrix will dissolve (Bharti, 2013). This might be the best option; however, it is not yet known how the products of matrix degradation will affect human retinal function, an important question that will be resolved by an anticipated clinical trial.
Table 5.
A summary of current clinical trials that involve the use of stem or progenitor cells for treating glaucoma, RP and AMD

iPSCs obtained from patients' somatic cells offer the potential for immune-compatibility. The first Phase I clinical trial of autologous iPSC-derived RPE sheets for wet AMD patients was launched by RIKEN (Rikagaku Kenkyusho Institute), Japan, in 2014. The investigators performed the surgery on the first patient in September 2014 and it was reported that the patient did not experience any serious side effects (Reardon and Cyranoski, 2014; Mandai et al., 2017). The study was put on hold because genetic mutations were found in cells from the second patient (https://www.newscientist.com/article/dn27986/). More recently, the approach has been modified towards using allogeneic iPSCs and the clinical trial has resumed.
In addition to these pluripotent sources, cells extracted from the adult human RPE layer can be activated in vitro into a stem cell state, termed RPE stem cells (RPESCs) (Salero et al., 2012). Importantly, the RPE cells derived from RPESCs in vitro are polarized, express RPE markers and have the key physiological properties expected of native RPE cells (Blenkinsop et al., 2015). As RPESCs can be obtained readily from eyes donated to eye banks, and are not pluripotent but restricted and poised to make RPE, they may have a safety margin over hPSC-derived products, making them a promising candidate for future cell transplantation therapy for RPE-based retinal diseases.
RP and photoreceptor transplantation
RP is a group of inherited retinal degenerative diseases that are associated with more than 40 genes and inherited in an autosomal dominant (AD), autosomal recessive (AR) or X-linked recessive (XR) pattern (Ferrari et al., 2011). Approximately 1 in 4000 individuals are affected with RP worldwide (National Eye Institute, USA) and it is the leading cause of inherited blindness (Boughman et al., 1980). The most common RP subtype is caused by mutations in the RHO gene, which encodes the critical phototransduction protein rhodopsin and accounts for ∼30-40% of AD cases (Ferrari et al., 2011). In this RP subtype, the primary pathological change affects the photoreceptors: the rods and cones. Initial degeneration of the rods is followed by cone degeneration and, later, the entire inner retina degenerates as the disease progresses, resulting in disruption of retinal structure (Fahim et al., 2000; Jones et al., 2003; Strettoi and Pignatelli, 2000; Strettoi et al., 2003). Because of the pathological changes, the clinical manifestation of this RP subtype is characterized by dynamic vision changes, typically with initial loss of night vision in the teenage years followed by a progressive decrease in the peripheral visual field with severe vision damage by 40-50 years of age, and eventually complete blindness (Hartong et al., 2006). There are no disease-altering treatments for RP, hence significant efforts are being made to replace lost photoreceptors with exogenous cells derived from stem cells.
Rods and cones are afferent sensory neurons with only one direction of synaptic connection with the next cell layer within the retina, so it might be easier to achieve incorporation into the native neuronal network than for cells that require substantial and long-distance connectivity. To replace dysfunctional or dead photoreceptors, various forms of transplant have been applied in animal models, including full-thickness retina, photoreceptor sheets (sliced by laser or vibratome), dissociated cells including photoreceptors or the RPCs capable of producing them, and hPSC-derived cells.
Subretinal transplantation of full-thickness retina or photoreceptor sheets is technically difficult. Cell integration and synaptic re-connection of full retina transplantation was found to be less effective compared with dissociated cell transplantation (Aramant and Seiler, 2004; Ghosh et al., 2004). In vitro expanded RPCs originally isolated from the NR of postnatal day 1 mouse were injected into the subretinal space of mature mice with a degenerating retina. These cells matured to express photoreceptor markers, were able to integrate into the host inner retina and rescue degenerative photoreceptors, and showed improved light-mediated behavior (Klassen et al., 2004). In another study, hESC-derived photoreceptor precursor cells were transplanted subretinally into the Crx−/− mouse model and an improved light response was observed with cell integration in the host retina (Lamba et al., 2009). Similarly, hiPSC-derived photoreceptors survive and integrate into the wild-type mouse retina (Lamba et al., 2010). Tucker et al. reported similar findings in a degenerative mouse model, where they showed that subretinal injection of mouse iPSC-derived photoreceptor precursors integrated into the retinal ONL and improved electroretinography responses (a measurement of light-mediated trans-retinal function across the whole retina) (Tucker et al., 2011). A recent mouse 3D retinal organoid study showed that transplanted organoid-derived photoreceptors can survive in the subretinal space and differentially integrate into the retina of mouse models with cone-rod degeneration (Santos-Ferreira et al., 2016). Interestingly, effective or poor integration was observed in mice with incomplete or complete photoreceptor loss, respectively, indicating that the stage of disease progression has an impact on cell integration. However, some studies have called into question whether transplanted photoreceptors actually integrate, and evidence suggests that they might instead fuse with existing photoreceptors (Singh et al., 2014). These data demonstrate the importance of understanding the specific cell integration process. Still, if fusion occurs to some extent, and enables exchange of cell components from healthy donor to diseased host cells and produces effective restoration of vision, then this could still be a viable therapeutic avenue. Overall, the progress in preclinical studies of photoreceptor transplantation in animal models indicates that cell replacement therapy for severe photoreceptor degeneration might be a possibility. More needs to be done to optimize the stage of cells being used for transplantation and to understand and optimize cell integration in order to improve outcomes.
Glaucoma and RGC transplantation
Glaucoma is a leading cause of blindness worldwide (Quigley and Broman, 2006). It is a chronic and multifactorial retinopathy characterized by progressive RGC loss and optic nerve damage. The most common type is primary open-angle glaucoma (POAG), in which increased intraocular pressure (IOP) causes progressive damage of RGCs and degeneration of the optic nerves (Nickells et al., 2012). Current treatments for POAG are mainly focused on lowering IOP. However, this strategy cannot always prevent disease progression and some glaucoma patients may not have increased IOP. To improve management of glaucoma, stop the irreversible disease progression and restore vision in these patients, researchers have begun to consider using stem cell-derived RGCs to rebuild the visual perception pathway and restore sight (Sluch and Zack, 2014; Sun et al., 2015).
Unlike unidirectional photoreceptors, RGCs are projection neurons that need to integrate into a complex synaptic network, extend long processes down the optic nerve and form appropriate connections to achieve functionality. Because of these challenges, RGC transplantation is still at an early stage of preclinical study compared with RPE or photoreceptor transplantation. Mouse ESCs and iPSCs have been successfully differentiated towards the RGC fate in vitro (Chen et al., 2010; Jagatha et al., 2009), but after intravitreal transplantation, iPSC-derived RGCs do not integrate into the 5-week-old RGC-injured mouse retina, although the cells survived (Chen et al., 2010). In contrast, mouse ESC-derived RGC-like cells were able to integrate into the retina of postnatal day 7 rats and differentiate into cells with RGC characteristics in situ (Jagatha et al., 2009). These studies suggest that the developmental stage of the host is an important factor when considering cell replacement strategies, but why this difference occurs is not yet clear. It could be that the early postnatal niche contains specific factors that are permissive to RGC integration, which are otherwise absent in the adult. Understanding the molecular basis might help to recreate the optimal environment for RGC transplantation in the adult, which is an important hurdle to overcome for effective treatment of glaucoma patients. In contrast to these stem cell-derived RGC transplantation studies, Venugopalan and colleagues have demonstrated that primary RGCs obtained from early postnatal mice that were injected intravitreally were able to integrate into the retina of adult rats (1-3 months), with >60% extending axon-like processes towards the host optic nerve head and forming morphological synapses 1-4 weeks after transplantation (Venugopalan et al., 2016). This study strengthens the possibility of using stem cell-derived RGCs to replace those lost in the adult retina, and indicates that a comparison of stem cell-derived RGCs and primary RGCs at the early postnatal stage would be valuable to help identify cell stage-specific parameters that promote integration into the mature retina. Despite successful engraftment in some cases, studies have yet to show any obvious functional improvement after RGC transplantation, perhaps because of lack of full cell integration and axon growth. If these obstacles can be overcome, stem cell-derived RGCs or RGC precursors could someday become a viable approach for treating patients with glaucoma.
Immunogenicity challenges in retinal stem cell therapy
Cell survival and transplantation success are determined not only by cell migration and integration, but also by the extent of immune rejection. Although the subretinal space and the intravitreal cavity are relatively immune privileged sites, damage to the blood–retina barrier, leaky blood vessels and activated microglia cells that are present in diseased or injured eyes or induced by the surgery itself can cause immune rejection and inflammatory responses (Enzmann et al., 1998; Langmann, 2007). Indeed, immune rejection and inflammatory reactions have been observed after cell transplantation (Boyd et al., 2005). Earlier studies of retinal transplantation showed that most NR or RPE grafts were eventually subject to chronic immune rejection, even though they were initially accepted (Jiang et al., 1995; Zhang and Bok, 1998). ESCs express no major histocompatibility complex (MHC) II and only a low level of MHC I, but MHC I was found to be upregulated after transplantation and cell maturation in vivo (Drukker et al., 2002). Even autologous iPSCs, which should be less immunogenic – and indeed previous studies showed no immune response after transplantation of iPSC-derived cells (Araki et al., 2013; Guha et al., 2013) – have been reported to produce an immune response when retroviruses were used for reprogramming (Okita et al., 2011; Zhao et al., 2011). Transplantation of iPSC-derived RPE cells from MHC homozygous donors into MHC-matched histocompatible recipients, in contrast, elicited no signs of immune rejection, indicating that MHC matching is beneficial for a successful allogeneic stem cell transplantation (Sugita et al., 2016). A thorough understanding of the immunogenicity of stem cells and the optimal immunosuppression regime is essential for future clinical applications.
Conclusion
Much progress has been made towards translating stem cell technology into therapies for retinal disease. Attempts to differentiate retinal cells from hPSCs have been largely successful, guided substantially by knowledge of in vivo retinal development gained from animal models. However, more defined differentiation protocols are required to improve efficiency and to obtain high-quality enriched retinal cells, and cells of the desired stage, given that PSC-derived products tend to reflect a fetal rather than adult stage. Further knowledge, specifically of human retinal development, might help to identify additional key factors that are important for the specification of various human retinal lineages. Applying this knowledge would in turn add to the efficiency and precision of the in vitro cell differentiation process. Such insight into human retinal development has been aided tremendously by the advent of 3D cell culture techniques, guided most prominently by the work of Yoshiki Sasai and colleagues (Nakano et al., 2012). This work has spurred progress in complex retinal tissue development and has provided the opportunity to study more fully developed retinal cells. In addition to carefully controlling culture conditions with more sophisticated, sequential treatments and 3D cultures that better mimic in vivo development, genetic modification of hPSCs or other somatic cells may prove to be a viable approach to generate specific populations of retinal cells.
With protocols already well developed to manufacture highly enriched populations of human RPE cells, the transplantation of stem cell-derived RPE cells has already entered early stage clinical trials, and is demonstrating safety and indications of efficacy. The impressive progress in photoreceptor production and vision restoration after transplantation into animals indicates that photoreceptor transplantation is likely to be the next candidate retinal cell entering the clinic. RGC production and transplantation still pose significant challenges, and solving these will hopefully pave the way to the introduction of other retinal cell types and more complex 3D retinal tissues. Undeniably, further studies are required to understand how retinal neurons can effectively integrate and achieve functional maturation, especially in a degenerating retinal environment in which the disease process perturbs the cell environment and causes synaptic rearrangements and alterations in retinal circuitry that could be difficult to reverse. Lastly, the challenge of immune rejection of transplants needs to be addressed, and here the possibility of using autologous iPSC products is particularly promising, if we can address the current high cost of producing personalized cells. The road to the clinic is undeniably long, but the exciting progress made in these pioneering studies gives us hope that stem cell-based therapies might someday be part of the clinical arsenal to combat blinding disorders.
Acknowledgements
We are grateful to Dr Heinrich Medicus for encouragement and support.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
The authors were supported by the New York State Empire Stem Cell Fund (NYSTEM C028504), the National Eye Institute (R01EY022079), the Macula Vision Research Foundation, and the Neural Stem Cell Institute Regenerative Research Foundation. The opinions expressed are solely those of the authors and not necessarily those of the Empire State Stem Cell Board, the New York State Department of Health, the State of New York, or any other funding agency. Deposited in PMC for release after 12 months.
References
- Abdelsalam A., Del Priore L. and Zarbin M. A. (1999). Drusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation-induced regression. Surv. Ophthalmol. 44, 1-29. 10.1016/S0039-6257(99)00072-7 [DOI] [PubMed] [Google Scholar]
- Adler R. and Canto-Soler M. V. (2007). Molecular mechanisms of optic vesicle development: complexities, ambiguities and controversies. Dev. Biol. 305, 1-13. 10.1016/j.ydbio.2007.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambati J. and Fowler B. J. (2012a). Mechanisms of age-related macular degeneration. Neuron 75, 26-39. 10.1016/j.neuron.2012.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambati J. and Fowler B. J. (2012b). Mechanisms of agerelated macular degeneration. Neuron 75, 26-39. 10.1016/j.neuron.2012.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki R., Uda M., Hoki Y., Sunayama M., Nakamura M., Ando S., Sugiura M., Ideno H., Shimada A., Nifuji A. et al. (2013). Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature 494, 100-104. 10.1038/nature11807 [DOI] [PubMed] [Google Scholar]
- Aramant R. B. and Seiler M. J. (2004). Retinal transplantation – advantages of intact fetal sheets. Eye Res. 23, 475-494. [DOI] [PubMed] [Google Scholar]
- Barnea-Cramer A., Wang W., Lu S.-J., Singh M., Luo C., Huo H., McClements M., Barnard A. R., MacLaren R. E. and Lanza R. (2016). Function of human pluripotent stem cell-derived photoreceptor progenitors in blind mice. Sci. Rep. 6, 29784 10.1038/srep29784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson E. (1993). Retinitis pigmentosa: the Friedenwald lecture. Invest. Ophthalmol. Vis. Sci. 34, 1659-1676. [PubMed] [Google Scholar]
- Bharti K. (2013). Developing cell-based therapy for macular degeneration using iPS cell derived RPE tissue on biodegradable scaffolds. Cytotherapy 15, S19 10.1016/j.jcyt.2013.01.068 [DOI] [Google Scholar]
- Blenkinsop T. A., Saini J. S., Maminishkis A., Bharti K., Wan Q., Banzon T., Lotfi M., Davis J., Singh D., Rizzolo L. et al. (2015). Human adult retinal pigment epithelial stem cell–derived RPE monolayers exhibit key physiological characteristics of native tissue. Invest. Ophthalmol. Vis. Sci. 56, 7085-7099. 10.1167/iovs.14-16246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucherie C., Mukherjee S., Henckaerts E., Thrasher A. J., Sowden J. C. and Ali R. R. (2013). Brief report: self-organizing neuroepithelium from human pluripotent stem cells facilitates derivation of photoreceptors. Stem Cells 31, 408-414. 10.1002/stem.1268 [DOI] [PubMed] [Google Scholar]
- Boughman J. A., Conneally P. M. and Nance W. E. (1980). Population genetic studies of retinitis pigmentosa. Am. J. Hum. Genet. 32, 223-235. [PMC free article] [PubMed] [Google Scholar]
- Bovolenta P., Mallamaci A., Briata P., Corte G. and Boncinelli E. (1997). Implication of OTX2 in pigment epithelium determination and neural retina differentiation. J. Neurosci. 17, 4243-4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A., Higashi Y. and Wood K. (2005). Transplanting stem cells: potential targets for immune attack. Modulating the immune response against embryonic stem cell transplantation. Adv. Drug. Deliver. Rev. 57, 1944-1969. 10.1016/j.addr.2005.08.004 [DOI] [PubMed] [Google Scholar]
- Buchholz D. E., Hikita S. T., Rowland T. J., Friedrich A. M., Hinman C. R., Johnson L. V. and Clegg D. O. (2009). Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells 27, 2427-2434. 10.1002/stem.189 [DOI] [PubMed] [Google Scholar]
- Buchholz D. E., Pennington B. O., Croze R. H., Hinman C. R., Coffey P. J. and Clegg D. O. (2013). Rapid and efficient directed differentiation of human pluripotent stem cells into retinal pigmented epithelium. Stem Cells Transl. Med. 2, 384-393. 10.5966/sctm.2012-0163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanin L. and Wittbrodt J. (2014). Retinal neurogenesis. Development 141, 241-244. 10.1242/dev.083642 [DOI] [PubMed] [Google Scholar]
- Cepko C. (2014). Intrinsically different retinal progenitor cells produce specific types of progeny. Nat. Rev. Neurosci. 15, 615-627. 10.1038/nrn3767 [DOI] [PubMed] [Google Scholar]
- Chen M., Chen Q., Sun X., Shen W., Liu B., Zhong X., Leng Y., Li C., Zhang W., Chai F. et al. (2010). Generation of retinal ganglion-like cells from reprogrammed mouse fibroblasts. Invest. Ophthalmol. Vis. Sci. 51, 5970-5978. 10.1167/iovs.09-4504 [DOI] [PubMed] [Google Scholar]
- Chew E., Clemons T., Agrón E., Sperduto R., SanGiovanni J., Kurinij N. and Davis M. (2013). Long-term effects of vitamins C, E, beta-carotene and zinc on age-related macular degeneration. AREDS report No. 35. Ophthalmology 120, 1604-1611. 10.1016/j.ophtha.2013.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessio A. C., Fan Z. P., Wert K. J., Baranov P., Cohen M. A., Saini J. S., Cohick E., Charniga C., Dadon D., Hannett N. M. et al. (2015). A systematic approach to identify candidate transcription factors that control cell identity. Stem Cell Rep 5, 763-775. 10.1016/j.stemcr.2015.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. M., Crawley L., Pahlitzsch M., Javaid F. and Cordeiro M. F. (2016). Glaucoma: the retina and beyond. Acta Neuropathol. 132, 807-826. 10.1007/s00401-016-1609-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Cruz P. M., Yasumura D., Weir J., Matthes M. T., Abderrahim H., LaVail M. M. and Vollrath D. (2000). Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet. 9, 645-651. 10.1093/hmg/9.4.645 [DOI] [PubMed] [Google Scholar]
- de Jong P. T. V. M. (2006). Age-related macular degeneration. N. Engl. J. Med. 355, 1474-1485. 10.1056/NEJMra062326 [DOI] [PubMed] [Google Scholar]
- Dowling J. E. and Sidman R. (1962). Inherited retinal dystrophy in the rat. J. Cell Biol. 14, 73-109. 10.1083/jcb.14.1.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drukker M., Katz G., Urbach A., Schuldiner M., Markel G., Itskovitz-Eldor J., Reubinoff B., Mandelboim O. and Benvenisty N. (2002). Characterization of the expression of MHC proteins in human embryonic stem cells. Proc. Natl. Acad. Sci. 99, 9864-9869. 10.1073/pnas.142298299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzmann V., Faude F., Wiedemann P. and Kohen L. (1998). Immunological problems of transplantation into the subretinal space. Acta Anat (Basel). 162, 178-183. 10.1159/000046484 [DOI] [PubMed] [Google Scholar]
- Erskine L. and Herrera E. (2007). The retinal ganglion cell axon's journey: insights into molecular mechanisms of axon guidance. Dev. Biol. 308, 1-14. 10.1016/j.ydbio.2007.05.013 [DOI] [PubMed] [Google Scholar]
- Erskine L. and Herrera E. (2014). Connecting the retina to the brain. ASN Neuro. 6 10.1177/1759091414562107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim A. T., Daiger S. P. and Weleber R. G. (2000). Nonsyndromic retinitis pigmentosa overview. In: GeneReviews [Internet] (ed. Pagon R. A., Adam M. P., Ardinger H. H., et al. ). Seattle (WA): University of Washington, Seattle. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1417/. [Google Scholar]
- Falkner-Radler C. I., Krebs I., Glittenberg C., Povazay B., Drexler W., Graf A. and Binder S. (2011). Human retinal pigment epithelium (RPE) transplantation: outcome after autologous RPE-choroid sheet and RPE cell-suspension in a randomised clinical study. Br. J. Ophthalmol. 95, 370-375. 10.1136/bjo.2009.176305 [DOI] [PubMed] [Google Scholar]
- Ferrari S., Iorio E., Barbaro V., Ponzin D., Sorrentino F. S. and Parmeggiani F. (2011). Retinitis pigmentosa: genes and disease mechanisms. Curr. Genomics 12, 238-249. 10.2174/138920211795860107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer M., Corneo B., Davis J., Wan Q., Miyagishima K. J., King R., Maminishkis A., Marugan J., Sharma R., Shure M. et al. (2014). A multiplex high-throughput gene expression assay to simultaneously detect disease and functional markers in induced pluripotent stem cell-derived retinal pigment epithelium. Stem Cells Transl. Med. 3, 911-922. 10.5966/sctm.2013-0192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann S. (2010). Eye morphogenesis and patterning of the optic vesicle. Curr. Top. Dev. Biol. 93, 61-84. 10.1016/B978-0-12-385044-7.00003-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann S., Levine E. M. and Reh T. A. (2000). Extraocular mesenchyme patterns the optic vesicle during early eye development in the embryonic chick. Development 127, 4599-4609. [DOI] [PubMed] [Google Scholar]
- Ghosh F., Wong F., Johansson K., Bruun A. and Petters R. M. (2004). Transplantation of full-thickness retina in the rhodopsin transgenic pig. Retina 24, 98-109. 10.1097/00006982-200402000-00014 [DOI] [PubMed] [Google Scholar]
- Gill K. P., Hung S. S. C., Sharov A., Lo C. Y., Needham K., Lidgerwood G. E., Jackson S., Crombie D. E., Nayagam B. A., Cook A. L. et al. (2016). Enriched retinal ganglion cells derived from human embryonic stem cells. Sci. Rep. 6, 30552 10.1038/srep30552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka A., Wu W., Onichtchouk D., Blumenstock C. and Niehrs C. (1997). Head induction by simultaneous repression of Bmp and Wnt signalling in Xenopus. Nature 389, 517-519. 10.1038/39092 [DOI] [PubMed] [Google Scholar]
- Guha P., Morgan J. W., Mostoslavsky G., Rodrigues N. P. and Boyd A. S. (2013). Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell. 12, 407-412. 10.1016/j.stem.2013.01.006 [DOI] [PubMed] [Google Scholar]
- Hartong D. T., Berson E. L. and Dryja T. P. (2006). Retinitis pigmentosa. Lancet 368, 1795-1809. 10.1016/S0140-6736(06)69740-7 [DOI] [PubMed] [Google Scholar]
- Haruta M., Sasai Y., Kawasaki H., Amemiya K., Ooto S., Kitada M., Suemori H., Nakatsuji N., Ide C., Honda Y. et al. (2004). In vitro and in vivo characterization of pigment epithelial cells differentiated from primate embryonic stem cells. Invest Ophthalmol. Vis. Sci. 45, 1020-1025. 10.1167/iovs.03-1034 [DOI] [PubMed] [Google Scholar]
- Heavner W. and Pevny L. (2012). Eye development and retinogenesis. Cold Spring Harb. Perspect. Biol. 4 10.1101/cshperspect.a008391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heier J. S., Brown D. M., Chong V., Korobelnik J.-F., Kaiser P. K., Nguyen Q. D., Kirchhof B., Ho A., Ogura Y., Yancopoulos G. D. et al. (2012). Intravitreal aflibercept (VEGF trap-eye) in wet agerelated macular degeneration. Ophthalmology 119, 2537-2548. 10.1016/j.ophtha.2012.09.006 [DOI] [PubMed] [Google Scholar]
- Hicks D. D. and Courtois Y. Y. (1992). Fibroblast growth factor stimulates photoreceptor differentiation in vitro. J. Neurosci. 12, 2022-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M., Yamamoto A., Yoshimura N., Tokunaga T., Motohashi T., Ishizaki K., Yoshida H., Okazaki K., Yamazaki H., Hayashi S.-I. et al. (2003). Generation of structures formed by lens and retinal cells differentiating from embryonic stem cells. Dev. Dyn. 228, 664-671. 10.1002/dvdy.10425 [DOI] [PubMed] [Google Scholar]
- Horsford D. J., Nguyen M. T., Sellar G. C., Kothary R., Arnheiter H. and McInnes R. R. (2005). Chx10 repression of Mitf is required for the maintenance of mammalian neuroretinal identity. Development 132, 177-187. 10.1242/dev.01571 [DOI] [PubMed] [Google Scholar]
- Huang J., Liu Y., Oltean A. and Beebe D. C. (2015). Bmp4 from the optic vesicle specifies murine retina formation. Dev. Biol. 402, 119-126. 10.1016/j.ydbio.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt N. C., Hallam D., Karimi A., Mellough C. B., Chen J., Steel D. H. W. and Lako M. (2017). 3D culture of human pluripotent stem cells in RGD-alginate hydrogel improves retinal tissue development. Acta Biomater. 49, 329-343. 10.1016/j.actbio.2016.11.016 [DOI] [PubMed] [Google Scholar]
- Idelson M., Alper R., Obolensky A., Ben-Shushan E., Hemo I., Yachimovich-Cohen N., Khaner H., Smith Y., Wiser O., Gropp M. et al. (2009). Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell 5, 396-408. 10.1016/j.stem.2009.07.002 [DOI] [PubMed] [Google Scholar]
- Ikeda H., Osakada F., Watanabe K., Mizuseki K., Haraguchi T., Miyoshi H., Kamiya D., Honda Y., Sasai N., Yoshimura N. et al. (2005). Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proc. Natl. Acad. Sci. USA 102, 11331-11336. 10.1073/pnas.0500010102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagatha B., Divya M. S., Sanalkumar R., Indulekha C. L., Vidyanand S., Divya T. S., Das A. V. and James J. (2009). In vitro differentiation of retinal ganglion-like cells from embryonic stem cell delivery neural progenitors. Biochem. Biophys. Res. Commun. 380, 230-235. 10.1016/j.bbrc.2009.01.038 [DOI] [PubMed] [Google Scholar]
- Jiang L. Q., Jorquera M., Streilein J. W. and Ishioka M. (1995). Unconventional rejection of neural retinal allografts implanted into the immunologically privileged site of the eye. Transplantation 59, 1201-1207. 10.1097/00007890-199504270-00021 [DOI] [PubMed] [Google Scholar]
- Johnson L. V., Leitner W. P., Staples M. K. and Anderson D. H. (2001). Complement activation and inflammatory processes in drusen formation and age related macular degeneration. Exp. Eye Res. 73, 887-896. 10.1006/exer.2001.1094 [DOI] [PubMed] [Google Scholar]
- Johnson P. T., Brown M. N., Pulliam B. C., Anderson D. H. and Johnson L. V. (2005). Synaptic pathology, altered gene expression, and degeneration in photoreceptors impacted by drusen. Invest. Ophthalmol. Vis. Sci. 46, 4788-4795. 10.1167/iovs.05-0767 [DOI] [PubMed] [Google Scholar]
- Jones B. W., Watt C. B., Frederick J. M., Baehr W., Chen C.-K., Levine E. M., Milam A. H., Lavail M. M. and Marc R. E. (2003). Retinal remodeling triggered by photoreceptor degenerations. J. Comp. Neurol. 464, 1-16. 10.1002/cne.10703 [DOI] [PubMed] [Google Scholar]
- Kaewkhaw R., Kaya K. D., Brooks M., Homma K., Zou J., Chaitankar V., Rao M. and Swaroop A. (2015). Transcriptome dynamics of developing photoreceptors in three-dimensional retina cultures recapitulates temporal sequence of human cone and rod differentiation revealing cell surface markers and gene networks. Stem Cells 33, 3504-3518. 10.1002/stem.2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen H. J., Ng T. F., Kurimoto Y., Kirov I., Shatos M., Coffey P. and Young M. J. (2004). Multipotent retinal progenitors express developmental markers, differentiate into retinal neurons, and preserve light-mediated behavior. Invest. Ophthalmol. Vis. Sci. 45, 4167-4173. 10.1167/iovs.04-0511 [DOI] [PubMed] [Google Scholar]
- Klimanskaya I., Hipp J., Rezai K. A., West M., Atala A. and Lanza R. (2004). Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells 6, 217-245. 10.1089/clo.2004.6.217 [DOI] [PubMed] [Google Scholar]
- Kuribayashi H., Baba Y. and Watanabe S. (2014). BMP signaling participates in late phase differentiation of the retina, partly via upregulation of Hey2. Dev. Neurobiol. 74, 1172-1183. 10.1002/dneu.22196 [DOI] [PubMed] [Google Scholar]
- Lamba D. A., Karl M. O., Ware C. B. and Reh T. A. (2006). Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 103, 12769-12774. 10.1073/pnas.0601990103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba D. A., Gust J. and Reh T. A. (2009). Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell 4, 73-79. 10.1016/j.stem.2008.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba D. A., McUsic A., Hirata R. K., Wang P.-R., Russell D. and Reh T. A. (2010). Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS ONE 5, e8763 10.1371/journal.pone.0008763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan L., Vitobello A., Bertacchi M., Cremisi F., Vignali R., Andreazzoli M., Demontis G. C., Barsacchi G. and Casarosa S. (2009). Noggin elicits retinal fate in Xenopus animal cap embryonic stem cells. Stem Cells. 27, 2146-2152. 10.1002/stem.167 [DOI] [PubMed] [Google Scholar]
- Langmann T. (2007). Microglia activation in retinal degeneration. J. Leukoc. Biol. 81, 1345-1351. 10.1189/jlb.0207114 [DOI] [PubMed] [Google Scholar]
- Li H., Tierney C., Wen L., Wu J. Y. and Rao Y. (1997). A single morphogenetic field gives rise to two retina primordia under the influence of the prechordal plate. Development 124, 603-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvi A. and Artavanis-Tsakonas S. (2006). Notch signalling in vertebrate neural development. Nat. Rev. Neurosci. 7, 93-102. 10.1038/nrn1847 [DOI] [PubMed] [Google Scholar]
- Lowe A., Harris R., Bhansali P., Cvekl A. and Liu W. (2016). Intercellular adhesion-dependent cell survival and ROCK-regulated actomyosin-driven forces mediate self-formation of a retinal organoid. Stem Cell Rep. 6, 743-756. 10.1016/j.stemcr.2016.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund R., Wang S., Klimanskaya I., Holmes T., Ramos-Kelsey R., Lu B., Girman S., Bischoff N., Sauvé Y. and Lanza R. (2006). Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells 8, 189-199. 10.1089/clo.2006.8.189 [DOI] [PubMed] [Google Scholar]
- Macosko E. Z., Basu A., Satija R., Nemesh J., Shekhar K., Goldman M., Tirosh I., Bialas A. R., Kamitaki N., Martersteck E. M. et al. (2015). Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202-1214. 10.1016/j.cell.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai M., Watanabe A., Kurimoto Y., Hirami Y., Morinaga C., Daimon T., Fujihara M., Akimaru H., Sakai N., Shibata Y. et al. (2017). Autologous induced stem-cell–derived retinal cells for macular degeneration. New Engl. J. Med. 376, 1038-1046. 10.1056/NEJMoa1608368 [DOI] [PubMed] [Google Scholar]
- Martinez-Morales J. R., Dolez V., Rodrigo I., Zaccarini R., Leconte L., Bovolenta P. and Saule S. (2003). OTX2 activates the molecular network underlying retina pigment epithelium differentiation. J. Biol. Chem. 278, 21721-21731. 10.1074/jbc.M301708200 [DOI] [PubMed] [Google Scholar]
- Maruotti J., Wahlin K., Gorrell D., Bhutto I., Lutty G. and Zack D. (2013). A simple and scalable process for the differentiation of retinal pigment epithelium from human pluripotent stem cells. Stem Cells Transl. Med. 2, 341-354. 10.5966/sctm.2012-0106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruotti J., Sripathi S., Bharti K., Fuller J., Wahlin K., Ranganathan V., Sluch V., Berlinicke C., Davis J., Kim C. et al. (2015). Small-molecule-directed, efficient generation of retinal pigment epithelium from human pluripotent stem cells. Proc. Natl. Acad. Sci. USA 112, 10950-10955. 10.1073/pnas.1422818112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland R. H. (2012). The neuronal organization of the retina. Neuron 76, 266-280. 10.1016/j.neuron.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellough C. B., Sernagor E., Moreno-Gimeno I., Steel D. H. W. and Lako M. (2012). Efficient stage-specific differentiation of human pluripotent stem cells toward retinal photoreceptor cells. Stem Cells 30, 673-686. 10.1002/stem.1037 [DOI] [PubMed] [Google Scholar]
- Meyer-Franke A., Kaplan M. R., Pfieger F. W. and Barres B. A. (1995). Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron 15, 805-819. 10.1016/0896-6273(95)90172-8 [DOI] [PubMed] [Google Scholar]
- Meyer J. S., Howden S. E., Wallace K. A., Verhoeven A. D., Wright L. S., Capowski E. E., Pinilla I., Martin J. M., Tian S., Stewart R. et al. (2011). Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells 29, 1206-1218. 10.1002/stem.674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagishima K., Wan Q., Corneo B., Sharma R., Lotfi M., Boles N., Hua F., Maminishkis A., Zhang C., Blenkinsop T. et al. (2016). In pursuit of authenticity: induced pluripotent stem cell-derived retinal pigment epithelium for clinical applications. Stem Cells Trans. Med. 5, 1562-1574. 10.5966/sctm.2016-0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molotkov A., Molotkova N. and Duester G. (2006). Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development 133, 1901-1910. 10.1242/dev.02328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller F., Rohrer H. and Vogel-Hopker A. (2007). Bone morphogenetic proteins specify the retinal pigment epithelium in the chick embryo. Development 134, 3483-3493. 10.1242/dev.02884 [DOI] [PubMed] [Google Scholar]
- Nakano T., Ando S., Takata N., Kawada M., Muguruma K., Sekiguchi K., Saito K., Yonemura S., Eiraku M. and Sasai Y. (2012). Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 10, 771-785. 10.1016/j.stem.2012.05.009 [DOI] [PubMed] [Google Scholar]
- Nguyen M. and Arnheiter H. (2000). Signaling and transcriptional regulation in early mammalian eye development: a link between FGF and MITF. Development 127, 3581-3591. [DOI] [PubMed] [Google Scholar]
- Nguyen M. T. T., Nakayama A., Chen C., Opdecamp K. and Arnheiter H. (1997). Mitf, a basic helix-loop-helix-zipper transcription factor regulating development of the retinal pigment epithelium (RPE). Dev. Biol. 186, B25. [DOI] [PubMed] [Google Scholar]
- Nickells R., Howell G., Soto I. and John S. (2012). Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu. Rev. Neurosci. 35, 153-179. 10.1146/annurev.neuro.051508.135728 [DOI] [PubMed] [Google Scholar]
- Ohlemacher S., Sridhar A., Xiao Y., Hochstetler A., Sarfarazi M., Cummins T. and Meyer J. (2016). Stepwise differentiation of retinal ganglion cells from human pluripotent stem cells enables analysis of glaucomatous neurodegeneration. Stem Cells 34, 1553-1562. 10.1002/stem.2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K., Nagata N. and Yamanaka S. (2011). Immunogenicity of induced pluripotent stem cells. Circ. Res. 109, 720-721. 10.1161/RES.0b013e318232e187 [DOI] [PubMed] [Google Scholar]
- Osakada F., Ikeda H., Mandai M., Wataya T., Watanabe K., Yoshimura N., Akaike A., Sasai Y. and Takahashi M. (2008). Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat. Biotechnol. 26, 215-224. 10.1038/nbt1384 [DOI] [PubMed] [Google Scholar]
- Osakada F., Jin Z.-B., Hirami Y., Ikeda H., Danjyo T., Watanabe K., Sasai Y. and Takahashi M. (2009). In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J. Cell Sci. 122, 3169-3179. 10.1242/jcs.050393 [DOI] [PubMed] [Google Scholar]
- Ouchi Y., Tabata Y., Arai K.-I. and Watanabe S. (2005). Negative regulation of retinal-neurite extension by beta-catenin signaling pathway. J. Cell Sci. 118, 4473-4483. 10.1242/jcs.02575 [DOI] [PubMed] [Google Scholar]
- Pandit T., Jidigam V. K., Patthey C. and Gunhaga L. (2015). Neural retina identity is specified by lens-derived BMP signals. Development 142, 1850-1859. 10.1242/dev.123653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera E. M., Wessely O., Li S.-Y. and De Robertis E. M. (2001). Neural and head induction by insulin-like growth factor signals. Dev. Cell 1, 655-665. 10.1016/S1534-5807(01)00069-7 [DOI] [PubMed] [Google Scholar]
- Phillips M., Wallace K., Dickerson S., Miller M., Verhoeven A., Martin J., Wright L., Shen W., Capowski E., Percin E. et al. (2012). Blood-derived human iPS cells generate optic vesicle–like structures with the capacity to form retinal laminae and develop synapses. Invest. Ophthalmol. Vis. Sci. 53, 2007-2019. 10.1167/iovs.11-9313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittack C., Grunwald G. and Reh T. (1997). Fibroblast growth factors are necessary for neural retina but not pigmented epithelium differentiation in chick embryos. Development 124, 805-816. [DOI] [PubMed] [Google Scholar]
- Quigley H. A. (1993). Open-angle glaucoma. N. Engl. J. Med. 328, 1097-1106. 10.1056/NEJM199304153281507 [DOI] [PubMed] [Google Scholar]
- Quigley H. A. and Broman A. T. (2006). The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 90, 262-267. 10.1136/bjo.2005.081224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden C. M., Powner M. B., Carr A.-J. F., Smart M. J. K., da Cruz L. and Coffey P. J. (2013). Stem cells in retinal regeneration: past, present and future. Development 140, 2576-2585. 10.1242/dev.092270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon S. and Cyranoski D. (2014). Japan stem-cell trial stirs envy: researchers elsewhere can't wait to test iPS cells in humans. Nature 513, 278-288. 10.1038/513287a [DOI] [PubMed] [Google Scholar]
- Reh T. A. and Pittack C. (1995). Transdifferentiation and retinal regeneration. Semin. Cell Biol. 6, 137-142. 10.1006/scel.1995.0019 [DOI] [PubMed] [Google Scholar]
- Reichman S., Terray A., Slembrouck A., Nanteau C., Orieux G., Habelerd W., Nandrot E., Sahel J.-A., Monville C. and Goureaua O. (2014). From confluent human iPS cells to self-forming neural retina and retinal pigmented epithelium. Proc. Natl. Acad. Sci. USA. 111, 8518-8523. 10.1073/pnas.1324212111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazifar H., Jia Y., Chen J., Lynch G. and Huang T. (2014). Chemically induced specification of retinal ganglion cells from human embryonic and induced pluripotent stem cells. Stem Cells Transl. Med. 3, 424-432. 10.5966/sctm.2013-0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan S., Chen C. M.-A., Young T. L., Fisher D. E. and Cepko C. L. (2004). Transdifferentiation of the retina into pigmented cells in ocular retardation mice defines a new function of the homeodomain gene Chx10. Development 131, 5139-5152. 10.1242/dev.01300 [DOI] [PubMed] [Google Scholar]
- Rowland T. J., Blaschke A. J., Buchholz D. E., Hikita S. T., Johnson L. V. and Clegg D. O. (2013). Differentiation of human pluripotent stem cells to retinal pigmented epithelium in defined conditions using purified extracellular matrix proteins. J. Tissue Eng. Regen. Med 7, 642-653. 10.1002/term.1458 [DOI] [PubMed] [Google Scholar]
- Sakuma R., Ohnishi Y.-Y., Meno C., Fujii H., Juan H., Takeuchi J., Ogura T., Li E., Miyazono K. and Hamada H. (2002). Inhibition of Nodal signalling by Lefty mediated through interaction with common receptors and efficient diffusion. Genes Cells. 7, 401-412. 10.1046/j.1365-2443.2002.00528.x [DOI] [PubMed] [Google Scholar]
- Salero E., Blenkinsop T. A., Corneo B., Harris A., Rabin D., Stern J. H. and Temple S. (2012). Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives. Cell Stem Cell 10, 88-95. 10.1016/j.stem.2011.11.018 [DOI] [PubMed] [Google Scholar]
- Santos-Ferreira T., Volkner M., Borsch O., Haas J., Cimalla P., Vasudevan P., Carmeliet P., Corbeil D., Michalakis S., Koch E. et al. (2016). Stem cell-derived photoreceptor transplants differentially integrate into mouse models of cone-rod dystrophy. Invest. Ophthalmol. Vis. Sci. 57, 3509-3520. 10.1167/iovs.16-19087 [DOI] [PubMed] [Google Scholar]
- Schwartz S. D., Hubschman J.-P., Heilwell G., Franco-Cardenas V., Pan C. K., Ostrick R. M., Mickunas E., Gay R., Klimanskaya I. and Lanza R. (2012). Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 379, 713-720. 10.1016/S0140-6736(12)60028-2 [DOI] [PubMed] [Google Scholar]
- Schwartz S., Regillo C., Lam B., Eliott D., Rosenfeld P., Gregori N., Hubschman J.-P., Davis J., Heilwell G. and Spirn M. (2015). Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 385, 509-516. 10.1016/S0140-6736(14)61376-3 [DOI] [PubMed] [Google Scholar]
- Seiler M. J. and Aramant R. B. (2012). Cell replacement and visual restoration by retinal sheet transplants. Prog. Retin. Eye Res. 31, 661-687. 10.1016/j.preteyeres.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M., Aslam S., Duncan I., Cramer A., Barnard A. and MacLaren R. (2014). Cell fusion following photoreceptor transplantation into the non-degenerate retina. Invest. Ophthalmol. Vis. Sci. 55, 3989. [Google Scholar]
- Singh R. K., Mallela R. K., Cornuet P. K., Reifler A. N., Chervenak A. P., West M. D., Wong K. Y. and Nasonkin I. O. (2015). Characterization of three-dimensional retinal tissue derived from human embryonic stem cells in adherent monolayer cultures. Stem Cells Dev. 24, 2778-2795. 10.1089/scd.2015.0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluch V. and Zack D. (2014). Stem cells, retinal ganglion cells and glaucoma. Dev. Opthalmol. 8, 111-121. 10.1159/000358409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluch V., Davis C., Ranganathan V., Kerr J., Krick K., Martin R., Berlinicke C., Marsh-Armstrong N., Diamond J., Mao H.-Q. et al. (2015). Differentiation of human ESCs to retinal ganglion cells using a CRISPR engineered reporter cell line. Sci. Rep. 5, 16595 10.1038/srep16595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow J., Hicks D. and Hamel C. (2010). The retinal pigment epithelium in health and disease. Curr. Mol. Med. 10, 802-823. 10.2174/156652410793937813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanga P. E., Kychenthal A., Fitzke F. W., Halfyard A. S., Chan R., Bird A. C. and Aylward G. W. (2002). Retinal pigment epithelium translocation after choroidal neovascular membrane removal in age-related macular degeneration. Opthalmology 109, 1492-1498. 10.1016/S0161-6420(02)01099-0 [DOI] [PubMed] [Google Scholar]
- Strettoi E. and Pignatelli V. (2000). Modifications of retinal neurons in a mouse model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 97, 11020-11025. 10.1073/pnas.190291097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E., Pignatelli V., Rossi C., Porciatti V. and Falsini B. (2003). Remodeling of second-order neurons in the retina of rd/rd mutant mice. Vision Res. 43, 867-877. 10.1016/S0042-6989(02)00594-1 [DOI] [PubMed] [Google Scholar]
- Sugita S., Iwasaki Y., Makabe K., Kamao H., Mandai M., Shiina T., Ogasawara K., Hirami Y., Kurimoto Y. and Takahashi M. (2016). Successful transplantation of retinal pigment epithelial cells from MHC homozygote iPSCs in MHC-matched models. Stem Cell Rep. 7, 635-648. 10.1016/j.stemcr.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Williams A., Waisbourd M. and Lacovitti L. and Katz L. J. (2015). Stem cell therapy for glaucoma: science or snake oil? Surv. Ophthalmol. 60, 93-105. 10.1016/j.survophthal.2014.07.001 [DOI] [PubMed] [Google Scholar]
- Tanaka T., Yokoi T., Tamalu F., Watanabe S.-I., Nishina S. and Azuma N. (2015). Generation of retinal ganglion cells with functional axons from human induced pluripotent stem cells. Sci. Rep. 5, 8344 10.1038/srep08344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teotia P., Chopra D. A., Dravid S. M., Van Hook M. J., Qiu F., Morrison J., Rizzino A. and Ahmad I. (2016). Generation of functional human retinal ganglioncells with target specificity from pluripotent stemcells by chemically defined recapitulation of developmental mechanism. Stem Cells 35, 572-585. 10.1002/stem.2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker B. A., Park I.-H., Qi S. D., Klassen H. J., Jiang C., Yao J., Redenti S., Daley G. Q. and Young M. J. (2011). Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS ONE 6, e18992 10.1371/journal.pone.0018992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker B. A., Anfinson K. R., Mullins R. F., Stone E. M. and Young M. J. (2013). Use of a synthetic xeno-free culture substrate for induced pluripotent stem cell induction and retinal differentiation. Stem Cells Transl. Med. 2, 16-24. 10.5966/sctm.2012-0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopalan P., Wang Y., Nguyen T., Huang A., Muller K. J. and Goldberg J. L. (2016). Transplanted neurons integrate into adult retinas and respond to light. Nat. Commun. 7, 10472 10.1038/ncomms10472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völkner M., Zschätzsch M., Rostovskaya M., Overall R., Busskamp V., Anastassiadis K. and Karl M. (2016). Retinal organoids from pluripotent stem cells efficiently recapitulate retinogenesis. Stem Cell Rep. 6, 525-538. 10.1016/j.stemcr.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vugler A., Carr A.-J., Lawrence J. Chen L. L., Burrell K., Wright A., Lundh P., Semo M., Ahmado A., Gias C. et al. (2008). Elucidating the phenomenon of HESC-derived RPE: anatomy of cell genesis, expansion and retinal transplantation. Exp. Neurol. 214, 347-361. 10.1016/j.expneurol.2008.09.007 [DOI] [PubMed] [Google Scholar]
- Westenskow P., Piccolo S. and Fuhrmann S. (2009). Beta-catenin controls differentiation of the retinal pigment epithelium in the mouse optic cup by regulating Mitf and Otx2 expression. Development 136, 2505-2510. 10.1242/dev.032136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetts R. and Fraser S. (1988). Multipotent precursors can give rise to all major cell types of the frog retina. Science 239, 1142-1145. 10.1126/science.2449732 [DOI] [PubMed] [Google Scholar]
- Wiley L., Burnight E., DeLuca A., Anfinson K., Cranston C., Kaalberg E., Penticoff J., Affatigato L., Mullins R., Stone E. et al. (2016). cGMP production of patient-specific iPSCs and photoreceptor precursor cells to treat retinal degenerative blindness. Sci. Rep. 6, 30742 10.1038/srep30742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W. L., Su X., Li X., Cheung C. M. G., Klein R., Cheng C.-Y. and Wong T. Y. (2014). Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Global Health 2, 106-116. 10.1016/S2214-109X(13)70145-1 [DOI] [PubMed] [Google Scholar]
- Young R. W. (1985). Cell differentiation in the retina of the mouse. Anat. Rec. 212, 199-205. 10.1002/ar.1092120215 [DOI] [PubMed] [Google Scholar]
- Zahabi A., Shahbazi E., Ahmadieh H., Hassani S. N., Totonchi M., Taei A., Masoudi N., Ebrahimi M., Aghdami N., Seifinejad A. et al. (2012). A new efficient protocol for directed differentiation of retinal pigmented epithelial cells from normal and retinal disease induced pluripotent stem cells 12, 2262-2272. 10.1089/scd.2011.0599 [DOI] [PubMed] [Google Scholar]
- Zhang X. and Bok D. (1998). Transplantation of retinal pigment epithelial cells and immune response in the subretinal space. Invest. Ophthalmol. Vis. Sci. 39, 1021-1027. [PubMed] [Google Scholar]
- Zhao S., Hung F.-C., Colvin J. S., White A., Dai W., Lovicu F. J., Ornitz D. M. and Overbeek P. A. (2001). Patterning the optic neuroepithelium by FGF signaling and Ras activation. Development 128, 5051-5060. [DOI] [PubMed] [Google Scholar]
- Zhao T., Zhang Z.-N., Rong Z. and Xu Y. (2011). Immunogenicity of induced pluripotent stem cells. Nature 474, 212-215. 10.1038/nature10135 [DOI] [PubMed] [Google Scholar]
- Zhong X., Gutierrez C., Xue T., Hampton C., Vergara N., Cao L.-H., Peters A., Park T., Zambidis E., Meyer J. et al. (2014). Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat. Commun. 5, 4047 10.1038/ncomms5047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Flamier A., Abdouh M., Tétreault N., Barabino A., Wadhwa S. and Bernier G. (2015). Differentiation of human embryonic stem cells into cone photoreceptors through simultaneous inhibition of BMP, TGFβ and Wnt signaling. Development 142, 3294-3306. 10.1242/dev.125385 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Carido M., Meinhardt A., Kurth T., Karl M., Ader M. and Tanaka E. (2013). Three-dimensional neuroepithelial culture from human embryonic stem cells and its use for quantitative conversion to retinal pigment epithelium. PLoS ONE 8, e54552 10.1371/journal.pone.0054552 [DOI] [PMC free article] [PubMed] [Google Scholar]