Abstract
Replication-independent histone variants can replace the canonical replication-dependent histones. Vertebrates have multiple H2A variant histones, including H2AZ and H2AX that are present in most eukaryotes. H2AZ regulates transcriptional activation as well as the maintenance of gene silencing, while H2AX is important in DNA damage repair. The fruit fly Drosophila melanogaster has only one histone H2A variant (H2AV), which is a chimera of H2AZ and H2AX. In this study we found that lack of H2AV led to the formation of black melanotic masses in Drosophila third instar larvae. The formation of these masses was found in conjunction with a loss of the majority of the primary lymph gland lobes. Interestingly, the cells of the posterior signaling center were preserved in these mutants. Reduction of H2AV levels by RNAi knockdown caused a milder phenotype that preserved the lymph gland structure but that included precocious differentiation of the prohemocytes located within the medullary zone and the secondary lobes of the lymph gland. Mutant rescue experiments suggest that the H2AZ-like rather than the H2AX-like function of H2AV is primarily required for normal hematopoiesis.
KEY WORDS: Drosophila, H2AV, Hematopoiesis, Variant histone
Summary: A combination of homozygous mutant and RNAi analyses reveal that the variant histone H2AV is required for the normal differentiation of blood cells in Drosophila larval lymph glands.
INTRODUCTION
Within the nucleus the compaction of chromatin is important in not only protecting DNA from damage but also in controlling access of the transcriptional machinery to the genome (Lorch and Kornberg, 2015). Chromatin compaction is carried out with the aid of highly conserved histone proteins, which are assembled into the nucleosome structure. Each nucleosome is composed of 146 bp of DNA wrapped around an octamer of histones. This octamer includes two molecules each of histones H2A, H2B, H3 and H4 (Luger et al., 1997). In order for gene expression to occur, the compaction of chromatin must be relieved, allowing transcriptional machinery access to the DNA. One method whereby this is thought to be regulated is with the replacement of the replication-dependent canonical histones with replication-independent histone variants that can alter nucleosome structure and function (Luger et al., 1997).
Two variants of H2A that have been highly conserved in eukaryotes are H2AZ and H2AX (Malik and Henikoff, 2003). H2AZ can be found at gene regulatory regions and has been implicated in both the transcriptional activation of genes and the maintenance of gene silencing (Billon and Cote, 2013). By contrast, H2AX is important in the DNA damage repair response (Scully and Xie, 2013). In particular, phosphorylation of the C-terminal tail of H2AX is crucial for mediating the assembly of the repair machinery in response to double-strand breaks in chromosomal DNA. Unlike many other eukaryotes, the model organism Drosophila melanogaster has only one H2A variant, H2AV, which is a structural and functional chimera of H2AZ and H2AX (Baldi and Becker, 2013).
Previous studies of ATP-dependent chromatin-remodeling enzymes have shown a role for these complexes in Drosophila hematopoiesis (Badenhorst, 2014). NURF (nucleosome remodeling factor), a chromatin-remodeling complex that catalyzes nucleosome sliding, was found to play a role in hematopoiesis. Mutants of NURF display hemocyte-containing black melanotic masses, as well as increases in hemocyte number (Badenhorst, 2014; Kwon et al., 2008). Domino (Dom), a catalytic subunit of the Drosophila TIP60 complex (dTIP60), has also been implicated in the regulation of hematopoiesis. dTIP60 facilitates the acetylation and exchange of the phosphorylated H2A variant (H2AVph) with non-phosphorylated H2AV (Kusch et al., 2004). dom loss-of-function mutants in Drosophila larvae form black melanotic masses that are composed of necrotic prohemocytes (Braun et al., 1997). These results suggested a role for Dom in prohemocyte survival. This function appears to have been conserved in mice, where a Dom homolog was shown to be important both in primitive hematopoiesis in the embryo and in the maintenance of hematopoietic progenitor cells in the adult bone marrow (Fujii et al., 2010; Ueda et al., 2007).
Given the role that the dTIP60 complex plays in both H2AV exchange and hematopoiesis, we thought it would be informative to study a potential role for H2AV itself in hematopoiesis. Drosophila has three types of hemocytes that are most similar to members of the myeloid lineage of blood cells found in vertebrates (Evans et al., 2003). The most prevalent Drosophila blood cells are the plasmatocytes, which make up 90-95% of the mature hemocytes and are phagocytic in function. The second most prevalent blood cells are the crystal cells, which comprise ∼5% of the mature hemocytes and contain prominent cytoplasmic granules. Crystal cells aid in melanization, which is important for wound healing and the innate immune response. Finally, the rarest of blood cells are the lamellocytes, which are seldom seen under normal conditions. If an immune challenge is detected, the differentiation of these cells can be upregulated drastically. The role of the lamellocytes is to encapsulate and neutralize any objects too large to be phagocytized by plasmatocytes (Evans et al., 2003).
The larval hematopoietic organ, the lymph gland, is a bilaterally symmetric structure that flanks either side of the dorsal vessel or heart tube (Fig. 1). Multiple lobes are located on either side of the heart and are termed primary, secondary and tertiary lobes. The primary lobes contain not only undifferentiated prohemocytes, but also differentiated hemocytes. The secondary and tertiary lobes commonly contain undifferentiated prohemocytes during larval stages. The primary lobes of the lymph gland, which are located at the anteriormost region of the hematopoietic organ, can each be subdivided into three distinct zones. The differentiated hemocytes are located in the outermost region called the cortical zone. The undifferentiated prohemocytes are located in the central region called the medullary zone. Finally, there is a small subset of cells located in the posterior medial portion of each primary lobe called the posterior signaling center (PSC) (Jung et al., 2005). It is the role of the PSC to send signals to the other cells of the lymph gland, thereby orchestrating their differentiation. The PSC sends out multiple signals that not only keep the prohemocytes of the medullary zone in an undifferentiated state, but that also lead to the differentiation of hemocytes within the cortical zone (Krzemien et al., 2007; Mandal et al., 2007). We now show that the absence of H2AV causes a variety of hematopoietic abnormalities in Drosophila larvae, including the formation of melanotic masses.
Fig. 1.
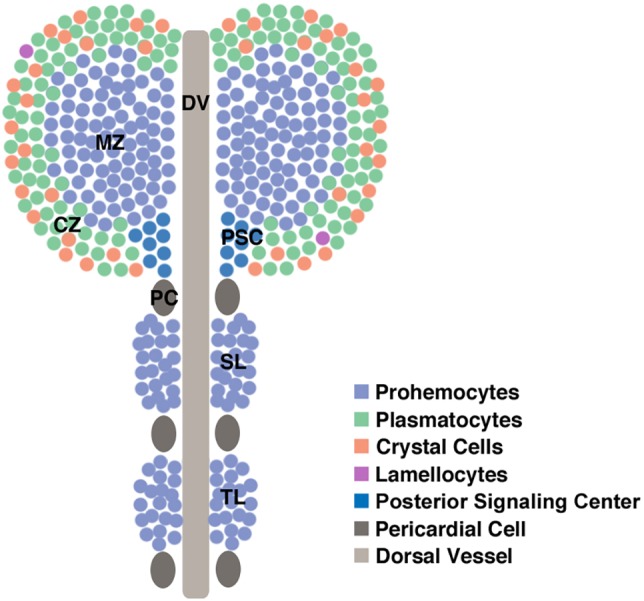
Architecture of a Drosophila third instar larval lymph gland. Differentiated plasmatocytes and crystal cells are shown in the cortical zone. Owing to their rarity, lamellocytes have not been depicted. CZ, cortical zone; DV, dorsal vessel; MZ, medullary zone; PC, pericardial cell; PSC, posterior signaling center; SL, secondary lobe; TL, tertiary lobe.
RESULTS
Loss of H2AV causes melanotic masses in Drosophila larvae
Unlike most other eukaryotes studied thus far, D. melanogaster has only one histone H2A variant, H2AV. This protein is thought to be a functional chimera of the H2AX and H2AZ proteins present in most other eukaryotes, including humans (Baldi and Becker, 2013). Comparison of the Drosophila H2AV protein and the two most common H2A variants in humans reveals that H2AV is much more closely related to human H2AZ than to human H2AX (Fig. 2A,B). However, the C-terminal tail of Drosophila H2AV does contain an H2AX-like SQXY peptide that can be phosphorylated in response to DNA damage (Madigan et al., 2002). The rest of the human H2AX protein is far more closely related to the canonical H2A proteins of human and Drosophila, than to human H2AZ and Drosophila H2AV. These observations are consistent with a model in which the H2AZ gene family arose via duplication of, and divergence from, an ancestral H2A-like gene early during eukaryotic evolution (Malik and Henikoff, 2003). On the other hand, genes encoding H2AX appear to have arisen multiple times during eukaryotic evolution. This most frequently occurred by a more recent duplication of an H2A-encoding gene followed by the addition of sequences encoding an SQXY-containing C-terminal tail to one duplicated copy. In D. melanogaster, sequences encoding an SQXY-containing tail appear instead to have been added to an unduplicated H2AZ-encoding gene, thereby giving rise to the gene encoding H2AV.
Fig. 2.
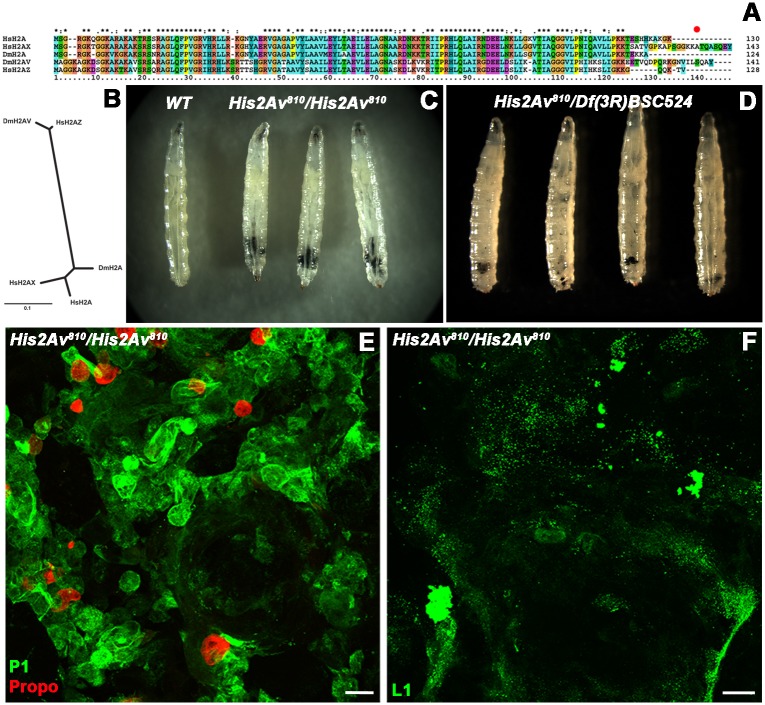
Lack of H2AV causes melanotic masses in larvae. (A) Sequence alignment of H. sapiens H2A, H2AX and H2AZ and D. melanogaster H2A and H2AV proteins. The red dot indicates the site of serine phosphorylation in H2AV. (B) Phylogenetic tree showing relatedness of H. sapiens H2A, H2AX and H2AZ and D. melanogaster H2A and H2AV proteins. (C) Comparison of wild-type (WT) and homozygous His2Av810 mutant third instar larvae. Black melanotic masses can be seen in the homozygous His2Av810 mutant larvae. (D) Larvae hemizygous for the His2Av810 mutation over the chromosomal deletion Df(3R)BSC524 also show black melanotic masses. (E) Dissected black melanotic masses from homozygous His2Av810 mutant larvae immunostained for plasmatocytes (P1, green) and crystal cells (Propo, red). (F) Dissected black melanotic masses from homozygous His2Av810 mutant larvae immunostained for lamellocytes (L1, green). Scale bars: 23 µm in E; 32 µm in F.
In order to study the role of the His2Av gene that encodes H2AV in D. melanogaster, we examined His2Av810/His2Av810 homozygous mutants and observed a previously unreported formation of black melanotic masses during larval stages (Fig. 2C). His2Av810 is a null allele that contains a 311 bp deletion that removes the region surrounding and including the second exon of the gene (van Daal and Elgin, 1992). His2Av810/His2Av810 mutants arrest in development during late third instar or prepupal stages (Kotova et al., 2011). In order to test whether the formation of the melanotic masses was due to the loss of H2AV, rather than some other unknown mutation in the genetic background of the homozygous animals, we crossed His2Av810/+ heterozygotes with flies heterozygous for Df(3R)BSC524, a large deletion mutant with molecularly defined endpoints in the same region of the genome (Cook et al., 2012). This chromosomal deficiency (Df) lacks 312,855 contiguous base pairs on the right arm (R) of chromosome 3, including the entire His2Av gene. In heterozygous His2Av810/Df(3R)BSC524 larvae we observed the formation of similar melanotic masses, implying that the absence of H2AV is indeed the cause of this phenotype (Fig. 2D).
To further test whether the absence of H2AV alone is responsible for the observed melanotic masses, we introduced a transgene expressing an H2AV-RFP fusion protein under the control of the His2Av promoter into His2Av810/His2Av810 animals. No melanotic masses were observed (data not shown). These results again imply that the absence of H2AV is indeed the cause of this phenotype.
The formation of melanotic masses in Drosophila has been commonly linked to the immune response (Minakhina and Steward, 2006; Rodriguez et al., 1996). Generally, hemocytes undergo melanization as an immune response to a present threat. However, in certain mutants, this response occurs without the presence of any discernable immune challenge (Harrison et al., 1995; Lemaitre et al., 1995; Minakhina and Steward, 2006; Zettervall et al., 2004). In order to determine whether the melanotic masses we observed included hemocytes and, if so, which types of hemocytes, we immunostained the melanotic masses using antibodies against three different hemocyte cell types (Evans et al., 2014). A Nimrod (P1) antibody was used to detect plasmatocytes, a Prophenyloxidase (Propo) antibody to detect crystal cells, and an Atilla (L1) antibody to detect lamellocytes. We identified plasmatocytes and crystal cells within the melanotic masses (Fig. 2E), but did not find any lamellocytes (Fig. 2F). These results imply that the melanotic tumors observed in the absence of H2AV are composed of hemocytes.
Loss of H2AV results in premature dispersal of the prohemocytes and hemocytes found within the larval lymph gland
In order to understand whether the absence of H2AV was having an effect on the cells of the lymph gland and whether the lymph gland was a possible source of the hemocytes that resulted in the formation of the melanotic masses, we first looked for the expression of H2AV in wild-type lymph gland lobes. We wished to ascertain whether H2AV would be found at varying expression levels within specific zones and cell types of the lymph gland. However, immunostaining revealed ubiquitous and uniform expression of H2AV in all of the different zones within the primary lymph gland lobes (Fig. 3A). We also looked at H2AV phosphorylation in the lymph gland in order to discern whether significant levels of DNA damage were present. We saw only small numbers of cells within the lymph gland that were positive for H2AVph under normal conditions (Fig. 3B,B′). In order to ascertain whether the cells of the lymph gland might be contributing to the hemocytes found within the melanotic masses that formed in the absence of H2AV, we looked at the primary lymph gland lobes of His2Av810/His2Av810 and His2Av810/+ third instar larvae.
Fig. 3.
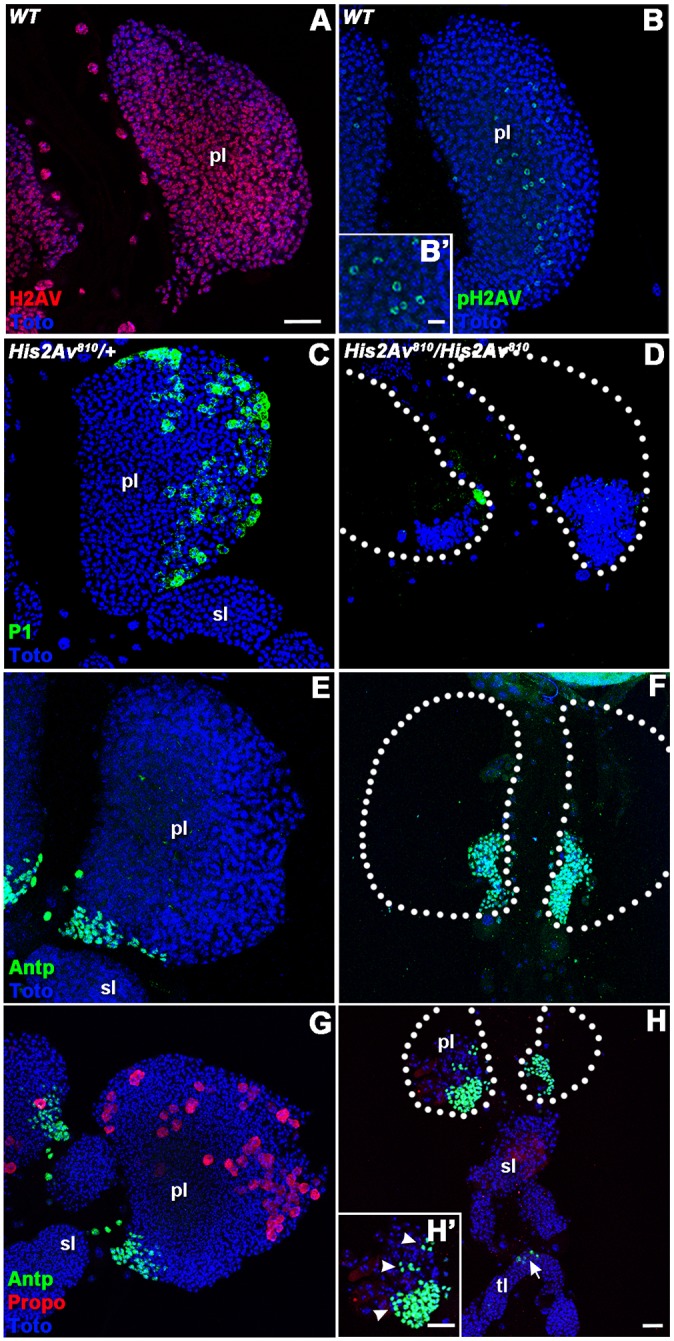
H2AV is required for normal larval hematopoiesis. (A,B) WT larval lymph gland immunostained for H2AV (red) and phospho-H2AV (pH2AV, green). Inset (B′) shows a magnified area of pH2AV staining in a central region of a primary lobe. (C-H) His2Av810/+ (C,E,G) and His2Av810/His2Av810 (D,F,H) larval lymph gland lobes immunostained for plasmatocytes (P1, green; C,D), the PSC (Antp, green; E-H′) and crystal cells (Propo, red; G-H′). Nuclei were labeled with a DNA dye (Toto, blue; A-H). Prospective ‘missing’ areas of His2Av810/His2Av810 primary lymph gland lobes are outlined (white dotted lines in D,F,H). Inset (H′) is a magnified area of the primary lymph gland lobe shown in H. Discontinuous areas of the PSC are marked with arrowheads (H′). Note that H is shown at lower magnification than other panels in order to display the secondary and tertiary lobes, which normally contain no Antp-positive cells. Arrow in H shows aberrant Antp expression in the tertiary lobe. pl, primary lobe; sl, secondary lobe; tl, tertiary lobe. Scale bars: 20 µm, except 10 µm in B′.
We found that the heterozygous mutants had larval lymph glands very similar to those of wild-type (WT) animals. Further investigation using antibodies against specific zones and cell types within the lymph glands of heterozygotes showed a WT pattern of expression of these markers (Fig. 3C,E,G). P1 was used to label plasmatocytes within the cortical zone (Fig. 3C), while Antennapedia (Antp) and Propo were used to label the PSC and the crystal cells, respectively (Fig. 3E,G).
In contrast to the WT-like phenotype seen in heterozygous larvae, examination of the His2Av810/His2Av810 larvae revealed a structural loss of most of the primary lymph gland lobes (Fig. 3D,F,H). A majority of the differentiated plasmatocytes and crystal cells had dispersed from the primary lobes in homozygous mutant larvae (Fig. 3D,H), along with a majority of the undifferentiated prohemocytes normally found within the medullary zone of the primary lobes (Fig. 3D,F,H). Although some crystal cell staining could still be seen within the primary and secondary lobes of the lymph gland (Fig. 3H), the staining for Propo appeared hazy, suggesting that the crystal cells might have burst and released the Propo protein into the surrounding area.
Interestingly, the one portion of the lymph gland that appeared to remain intact in His2Av810/His2Av810 larvae was the Antp-positive PSC (Fig. 3F,H). Some of the homozygous mutant larvae did contain ectopic clusters of Antp-positive cells distinct from the PSC within the primary lobes (Fig. 3H′), as well as the abnormal presence of Antp-positive cells within the secondary or tertiary lobes of the lymph gland (Fig. 3H). Normally, Antp is restricted to a discrete region within the primary lobes of the lymph gland. Although the area occupied by the PSC appeared to be expanded within some of the homozygous mutant larvae relative to heterozygotes, the number of Antp-positive cells was similar in the primary lobes of the lymph glands in both genotypes (Fig. S1). Rather, the proportion of the primary lobe occupied by Antp-positive PSC cells increased dramatically from a mean of 4% in heterozygotes to 79% in homozygotes (Fig. S1).
Together, these results suggest that H2AV is required within prohemocytes and its absence might alter the response of these cells to signals originating from the PSC. Consistent with this hypothesis, the Hedgehog (Hh) ligand that prevents differentiation of prohemocytes was detected by immunostaining in the PSC cells of both heterozygous and homozygous His2Av810 mutant lymph glands (Fig. S2A,B) (Mandal et al., 2007). Likewise, levels of Wingless (Wg) protein expression appeared similar in heterozygous and homozygous His2Av810 mutant lymph gland lobes (Fig. S2C,D). Past studies have shown that Wg plays a dual role within the lymph gland, maintaining undifferentiated prohemocytes and dictating PSC cell number (Sinenko et al., 2009).
An H2AV-RFP fusion protein is able to partially rescue the His2Av810 homozygous mutant lymph gland phenotype
In order to determine whether the His2Av810/His2Av810 lymph gland phenotype could be rescued, we introduced a transgene expressing an H2AV-RFP fusion protein into the homozygous mutant background. We were able to rescue most of the dispersal phenotype of the lymph glands of these larvae (Fig. S3A-F). However, we did observe an increase in plasmatocyte differentiation in both the primary and secondary lobes (Fig. S3C,D). Crystal cells, present in both primary and secondary lobes, appeared to have burst (Fig. S3E,F, arrowheads). It is possible that the incomplete nature of the rescue was due to the RFP domain that is fused to the H2AV protein. In this regard, others have previously reported that a similar fusion of GFP can disrupt some of the normal functions of H2AV in vivo (Kotova et al., 2011).
Reducing levels of H2AV in prohemocytes results in their premature differentiation within the medullary zone, but not in the early dispersal of hemocytes from the primary lymph gland lobes
In order to determine whether the premature differentiation of prohemocytes in the absence of H2AV was a cell-autonomous property, we drove expression of UAS-His2AvHMO5177 RNAi with a domeless-GAL4 driver. A UAS-GFP transgene acted as a reporter for the location of domeless-GAL4 and UAS-His2Av RNAi expression. Importantly, domeless-GAL4 is expressed in the prohemocytes but not in the PSC of the primary lobes of the lymph gland (Jung et al., 2005). domeless-GAL4 expression initially occurs in a majority of the prohemocytes of the primary lobe, but continued expression then becomes restricted to the undifferentiated prohemocytes within the medullary zone as the cells within the cortical zone start to differentiate. domeless is also expressed in the prohemocytes of the secondary lobes. In order to test whether we were achieving a significant reduction of H2AV protein levels, we carried out immunostaining with an anti-H2AV antibody (Fig. 4A-B′). We observed a dramatic reduction of H2AV protein levels in a majority of the prohemocytes found within the primary lobes of the lymph gland, whereas H2AV protein was still expressed in the cells of the PSC, as expected (Fig. 4A,A′).
Fig. 4.
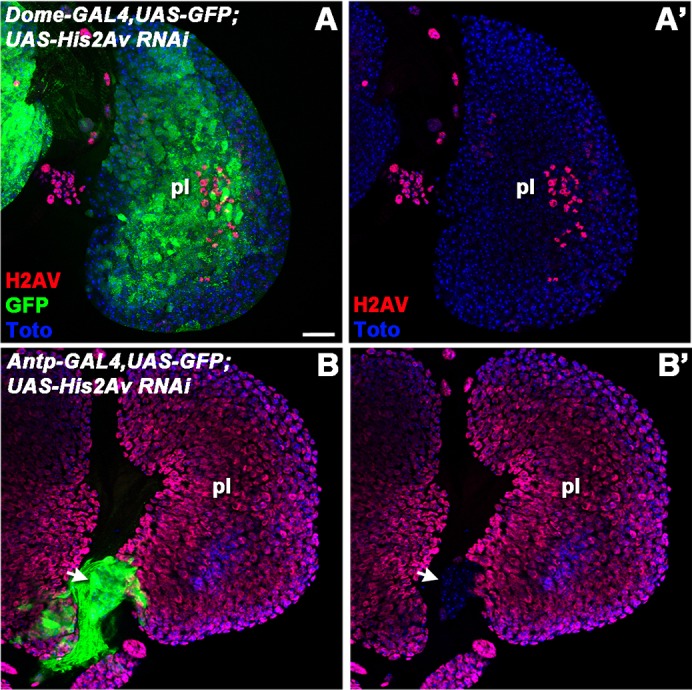
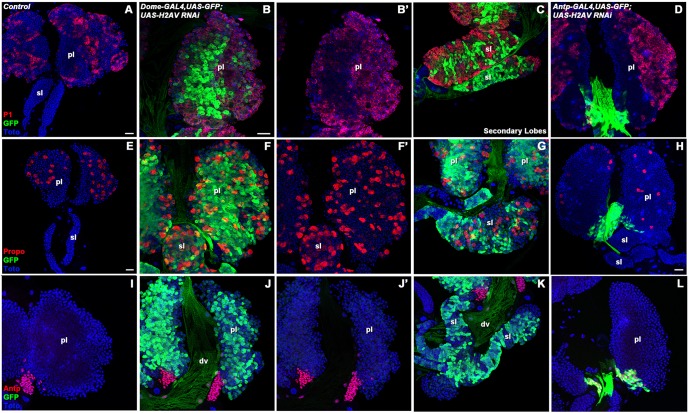
Cell type-specific knockdown of H2AV with GAL4-driven RNAi. domeless-GAL4 (A,A′) or Antp-GAL4 (B,B′) was used to drive UAS-GFP and UAS-His2Av RNAiHM05177 in lymph glands. GFP expression indicates areas in which the drivers are active (green; A,B). H2AV protein was detected by immunolabeling (red; A-B′). Nuclei were labeled with a DNA dye (Toto, blue; A-B′). Arrow indicates an area in which H2AV protein is absent and the Antp-GAL4 driver is active (B,B′). pl, primary lobe. Scale bar: 20 µm.
Lymph glands in which levels of H2AV protein were greatly reduced in the prohemocytes, via domeless-GAL4-driven RNAi, displayed a marked increase in the differentiation of plasmatocytes and crystal cells in comparison to controls (Fig. 5A-C,E-G). This increased differentiation of plasmatocytes and crystal cells was seen not only in the medullary zone of the primary lobes, but was also present extensively within the secondary lobes of the lymph gland (Fig. 5C,G). By contrast, control lymph glands did not display differentiation within the secondary lobes at similar stages (Fig. 5A,E). We also looked for differentiation of lamellocytes, but did not see any substantial increase in the number of differentiating lamellocytes in comparison to controls (data not shown). Since differentiation can be regulated by the presence of the PSC, we also looked at the expression of Antp within these lymph glands using an Antp-specific antibody (Fig. 5I-K). However, we did not see any discernable differences in the number or location of Antp-positive cells in the PSC of domeless-GAL4-driven UAS-His2Av RNAi lymph glands. These results are consistent with a cell-autonomous function of H2AV within the prohemocytes of the lymph gland.
Fig. 5.
RNAi knockdown of H2AV in the lymph gland causes abnormal hemocyte differentiation. domeless (dome)-GAL4-driven UAS-GFP (and, by inference, UAS-His2Av RNAi) expression is shown in green in the primary (B,F,J) and secondary (C,G,K) lobes of the lymph gland. Expanded differentiation of plasmatocytes (P1, red) can be seen via immunostaining in the primary (B,B′) and secondary (C) lobes in comparison to the control (A). Crystal cell differentiation, as shown via immunostaining (Propo, red), is also expanded in the primary (F,F′) and secondary (G) lobes in comparison to the control (E). The PSC, as visualized by immunostaining (Antp, red), appeared comparable in control lymph glands (I) and those with dome-GAL4-driven UAS-His2Av RNAi (J-K). Antp-GAL4 was used to drive UAS-His2Av RNAi as well as a UAS-GFP reporter (green) in the PSC (D,H,L). Plasmatocyte differentiation in an Antp-GAL4-driven knockdown (D) was comparable to controls (A). However, crystal cell numbers (H) appeared reduced in these knockdown lymph glands in comparison to the control (E). PSC size itself (L) was comparable to the control (I). dv, dorsal vessel; pl, primary lobe; sl, secondary lobe. Scale bars: 20 µm (in A for A; in E for E; in B for B-D,F-G,I-L; in H for H).
In order to test whether H2AV was also required within cells of the PSC for normal prohemocyte differentiation, we drove UAS-His2Av RNAi and UAS-GFP expression with an Antp-GAL4 driver that is expressed in the PSC cells but not in the other cells of the lymph gland (Mandal et al., 2007). Expression of the Antp-Gal4 driver matched Antp protein expression as could be seen by immunostaining (Fig. 5L). We used anti-H2AV immunostaining to verify that the expected reduction in H2AV protein levels occurred only within the cells of the PSC and not in other cells within the lymph gland (Fig. 4B,B′). In lymph glands with greatly reduced levels of H2AV in the PSC, plasmatocyte differentiation appeared normal and the medullary zone was maintained (Fig. 5D). However, a decrease in crystal cells was observed in comparison to controls (Fig. 5H). Given the possible bursting of the crystal cells observed in the His2Av810/His2Av810 mutants and the reduction in crystal cell number when an Antp-GAL4 driver was used to greatly reduce levels of H2AV in the PSC, it is possible that proper H2AV expression within the PSC is required for crystal cell differentiation and maintenance.
A mutant H2AV protein lacking the C-terminal H2AX-like SQAY motif can partially rescue larval hematopoiesis
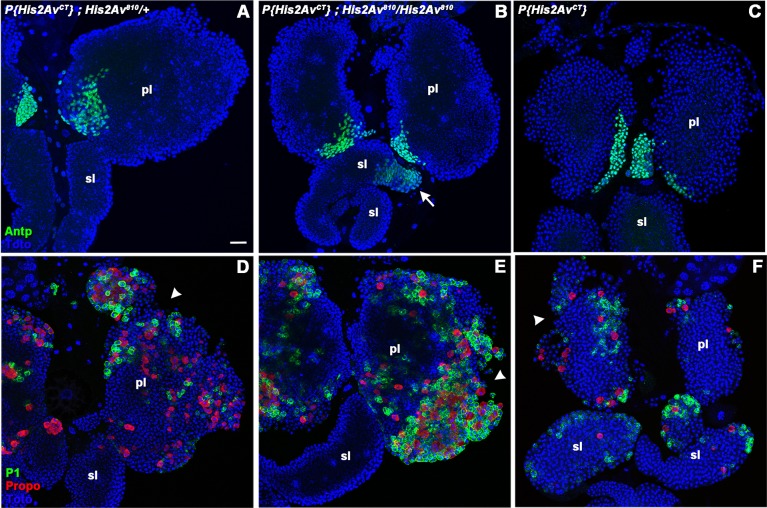
In Drosophila, the H2AX-like function of H2AV requires a C-terminal SQAY motif that is phosphorylated on the serine when double-strand breaks in DNA are present nearby (Madigan et al., 2002). In order to determine whether loss of the H2AX-like function of H2AV led to the hematopoietic phenotypes seen in His2Av810/His2Av810 larvae, we tested for rescue with a previously described H2AV mutant protein that lacks the C-terminal 14 amino acids including the SQAY motif (H2AVCT) (Clarkson et al., 1999). This mutant protein was also able to rescue most of the lymph gland dispersal phenotype (Fig. 6B,E). The number of Antp-positive PSC cells in the H2AVCT-rescued His2Av810/His2Av810 mutant larvae primary lobes was similar to that in heterozygous larvae with or without the H2AVCT-encoding transgene (Fig. S1). However, some abnormalities were present in the H2AVCT-rescued homozygous mutant larvae, including the presence of Antp-positive cells in the secondary lobes (Fig. 6B). Crystal cell and plasmatocyte differentiation appeared to be increased, and some shedding of hemocytes was still observed (Fig. 6E), although not to the extent seen in unrescued His2Av810/His2Av810 mutants.
Fig. 6.
A mutant H2AV protein lacking the C-terminal H2AX-like phosphorylation site partially rescues larval lymph gland structure and function in a His2Av null mutant. A transgene that expresses an H2AV protein lacking 14 C-terminal amino acids including the SQAY motif, P{His2AvCT}, was tested in an otherwise wild-type His2Av background (C,F), in a His2Av810/His2Av810 background (B,E) and in a His2Av810/+ background (A,D). The PSC was labeled by immunostaining (Antp, green; A-C). Arrow (B) marks area of Antp expansion into the secondary lobe. Plasmatocytes (P1, green) and crystal cells (Propo, red) were also labeled by immunostaining (D-F). Arrowheads mark areas of hemocyte dispersal from the primary lymph gland lobes. Nuclei were labeled with a DNA dye (Toto, blue; A-F). pl, primary lobe; sl, secondary lobe. Scale bar: 20 µm.
Because the rescue of normal larval hematopoiesis by H2AVCT was incomplete, we wondered whether this mutant protein might itself have a dominant phenotype. Expression of H2AVCT protein altered the normally WT lymph gland phenotype of His2Av810/+larvae (Fig. 6A,D). H2AVCT caused crystal cell and plasmatocyte differentiation to no longer be restricted to the cortical zone, and some early shedding of differentiated hemocytes was seen (Fig. 6D).
The phenotype seen when H2AVCT is expressed in a H2AV810/+ background suggested a possible dominant effect. To further test this hypothesis, we examined larvae containing the H2AVCT protein in a His2Av wild-type background (Fig. 6C,F). We found that H2AVCT again caused a phenotype that included differentiation of crystal cells and plasmatocytes within the secondary lobes (Fig. 6F), and shedding of hemocytes from the primary lobes.
We wished to distinguish among different hypotheses that might explain the dominant lymph gland phenotypes caused by the presence of the mutant H2AVCT protein described above. The observed dominant H2AVCT phenotype could be due to the absence of a short peptide upstream of the SQAY motif. Alternately, the phenotype might be caused by the dilution of wild-type H2AV proteins capable of responding to DNA damage by mutant H2AVCT proteins, thereby causing increased levels of cell death within the organism. This in turn might result in increased mobilization of hemocytes from the lymph gland in response to cell damage and/or cell death. Finally, it is possible that an increased dosage of mutant or wild-type H2AV might cause a dominant phenotype in larval hematopoiesis.
Overexpression of wild-type H2AV protein in the lymph gland via either a domeless-GAL4 or an Antp-GAL4 driver resulted in less cohesion of the Antp-positive cells of the PSC (Fig. S4C-E). Overexpression of wild-type H2AV in the PSC via Antp-GAL4 also resulted in lower numbers of differentiated plasmatocytes and crystal cells (Fig. S4D,E). However, a similar alteration in differentiated hemocyte numbers was not observed when the overexpression of wild-type H2AV was driven via a domeless-GAL4 driver (Fig. S4A,B). Similar alterations in hemocyte differentiation or PSC cell cohesion were also observed when we drove expression of either a mutant H2AV protein that mimicked constitutive phosphorylation of the serine 137 residue within the SQAY motif (H2AVSE; Fig. S4F-J) or a mutant H2AV protein that was non-phosphorylatable due to a serine-to-alanine substitution (H2AVSA; Fig. S4K-O) with domeless-GAL4 or Antp-GAL4 (Kotova et al., 2011). Taken together, these results show that increasing the dosage of H2AV proteins, whether wild type or mutant, can also cause alterations in larval hematopoiesis.
DISCUSSION
We discovered that absence of the variant histone protein H2AV results in the formation of larval melanotic masses containing plasmatocytes and crystal cells. Previous studies have proposed that the formation of melanotic masses can be due either to the response of a normal immune system to abnormal tissue formed during development, or to a developmental defect in the hemocytes of the lymph gland (Rizki and Rizki, 1980; Rodriguez et al., 1996; Watson et al., 1991). Our data showing the loss of a majority of the primary lymph gland lobes in the His2Av810 null mutant, as well as the early differentiation of the medullary zone and secondary lobe prohemocytes when H2AV levels were reduced via RNAi, are consistent with the latter model. Our results demonstrate an important role for H2AV during normal hemocyte differentiation and dispersal. Interestingly, studies using a human histiocytic lymphoma cell line or normal macrophages differentiated with macrophage colony stimulating factor (M-CSF; CSF1) have shown an upregulation of the His2Av-related human H2A.Z (H2AFZ) gene during macrophage differentiation (Baek et al., 2009). These results imply an evolutionarily conserved role for the closely related H2AV and H2AZ histone variants in blood cell differentiation.
The presence of black melanotic masses in Drosophila larvae is not restricted to His2Av mutants. This phenotype has previously been observed in mutants of two different ATP-dependent chromatin-remodeling complexes. Dom, which is a catalytic subunit of the dTip60 complex, plays a role in H2A variant exchange in nucleosomes, as well as in DNA damage repair (Kusch et al., 2004). dom loss-of-function mutants display black melanotic masses that are composed of melanized lymph glands (Braun et al., 1997). Mutants have shown that the vertebrate homolog of dom is required for both embryonic and adult hematopoiesis in the laboratory mouse (Fujii et al., 2010; Ueda et al., 2007). Loss of a subunit of another ATP-dependent chromatin-remodeling complex, NURF, also causes melanotic masses (Badenhorst, 2014). In addition, melanotic masses have been observed in mutations that affect various signaling pathways. For example, constitutive activation of the JAK-STAT pathway via the dominant gain-of-function HopTUM mutation results in the formation of melanotic masses (Hanratty and Dearolf, 1993). Constitutive activation of the Toll pathway via the dominant gain-of-function Tl10b mutation also causes melanotic masses (Lemaitre et al., 1995). These observations raise the question of whether the closely related variant histones H2AV and H2AZ might be required to repress these evolutionarily conserved signaling pathways in hematopoietic cells.
Although the majority of the cells in the primary lymph gland lobes in His2Av mutants are lost, the Antp-positive cells comprising the PSC are spared and can be seen adjacent to the cardioblasts of the dorsal vessel. In addition, these cells express the Hh ligand that normally prevents premature differentiation of hemocyte precursors. The presence of Antp-positive cells can also be observed in posterior lymph gland lobes, where Antp is not normally expressed. In this regard, previous studies have shown that His2Av can function as a Polycomb Group (PcG) gene, and PcG proteins are known to be important for repressing the transcription of homeotic genes such as Antp (Swaminathan et al., 2005). In particular, it has been reported that Antp expression is expanded in the central nervous system of larvae that are mutant for His2Av. Reduction of H2AV levels via RNAi in the prohemocytes of the primary lobes, as well as in the secondary lobes, led to increased differentiation of plasmatocytes and crystal cells. This suggests that H2AV also acts downstream of the signals that originate from the PSC and that maintain the prohemocytes of the medullary zone in an undifferentiated state.
Reduction of H2AV levels via RNAi causes a less severe phenotype than that of His2Av810 null mutants, in that the primary lobes of the lymph gland are preserved. However, there is a loss of the undifferentiated prohemocytes found within the medullary zone, as these cells differentiate into mature hemocytes. Previous studies in the testis of Drosophila have shown an important role for H2AV in the maintenance of both the germline and cyst stem cells (Morillo Prado et al., 2013). Together, these results suggest a possible role for H2AV in the transcriptional control of genes important for stem cell maintenance in general. In this regard, the closely related H2AZ protein of mammals has been reported to be important for the differentiation of embryonic stem cells in culture (Creyghton et al., 2008).
H2AV might be exerting its effects on the lymph gland through various signaling pathways that have been shown to orchestrate prohemocyte differentiation. Two pathways that might be affected are the Hh and Wg signaling pathways. Hh has been implicated in maintaining prohemocytes in an undifferentiated state (Mandal et al., 2007). However, we observed Hh expression in the PSC of both heterozygous and homozygous mutant His2Av810 larval lymph glands. Wg has been reported to not only maintain the prohemocyte population in an undifferentiated state, but also to dictate PSC cell number (Sinenko et al., 2009). We did not detect any significant differences in the staining of prohemocytes and PSC cells with anti-Wg antibodies in homozygous versus heterozygous mutant His2Av810 larval lymph glands. These results suggest that loss of H2AV might alter the intracellular responses to these ligands rather than their expression.
Drosophila H2AV is a chimeric protein that plays the roles of two widely conserved variant histones, H2AX and H2AZ (Baldi and Becker, 2013). H2AX is important for the DNA damage repair response (Scully and Xie, 2013), while H2AZ is important for both transcriptional activation and gene silencing (Billon and Cote, 2013). Previous studies have shown that H2AVCT, which lacks H2AX function, is able to rescue the lethal phenotype seen in His2Av810 null mutants, allowing the organisms to progress to pupation and adulthood (Clarkson et al., 1999). We found that H2AVCT was able to partially rescue the His2Av810 null larval hematopoietic phenotype, arguing that an H2AZ-like function rather than an H2AX-like function of H2AV is required for hematopoiesis. Nevertheless, differentiation within the lymph gland still appeared disrupted and partial loss of the primary lymph gland lobes could be seen. In addition, the expression of Antp was at times seen to expand into the posterior lobes of the lymph gland. This lack of full rescue could be due to a decreased stability of the H2AVCT protein. However, the presence of H2AVCT in an otherwise His2Av wild-type background was sufficient to cause abnormalities of the lymph gland lobes. Furthermore, overexpression of wild-type or of phosphorylation mutants of H2AV also caused hematopoietic abnormalities. Together, these results imply that a precise dosage of H2AV protein is essential for normal hematopoiesis in Drosophila. Similar alterations in differentiation might also occur in other organs and tissues. In this regard, care should be taken when using His2Av-GFP and His2Av-RFP transgenes, which are popular markers in live imaging.
The formation of black melanotic masses in the His2Av810 null mutant establishes larval hemocytes as a useful tool for further studies of H2AV function. Furthermore, given the role that H2AV plays not only in undifferentiated prohemocytes, but also in the germline and cyst stem cells found in the testis (Morillo Prado et al., 2013), it will be interesting to test whether H2AV also regulates stem cells found in other tissues.
MATERIALS AND METHODS
Fly lines and crosses
Mutant or transgenic fly stocks used in these studies included: domeless-GAL4, UAS-GFP (Grigorian et al., 2013; Mandal et al., 2007); Antennapedia-GAL4, UAS-GFP (Grigorian et al., 2013; Mandal et al., 2007); His2Av810/TM3,Sb1 (Bloomington Drosophila Stock Center); His2AvCT (Clarkson et al., 1999); UAS-His2AvWT (Kotova et al., 2011); UAS-His2AvSE (Kotova et al., 2011); UAS-His2AvSA (Kotova et al., 2011); UAS-His2AvHMO5177 RNAi (Bloomington Drosophila Stock Center); and His2Av-RFP (Schuh et al., 2007). Additional stocks with balancer chromosomes expressing dominant larval markers were obtained from the Bloomington Stock Center. All GAL4×UAS-RNAi crosses were carried out at 29°C. All other stocks and crosses were grown at 25°C in standard laboratory conditions.
Antibodies
Primary antibodies were against Antennapedia (mouse; 1:4; Developmental Studies Hybridoma Bank), P1 (mouse; 1:10) (Kurucz et al., 2007), Propo (rabbit; 1:1000) (Muller et al., 1999), L1 (mouse; 1:10) (Kurucz et al., 2003), H2AV (rabbit; 1:1000) (Leach et al., 2000), H2AVph (mouse; 1:25; Developmental Studies Hybridoma Bank) (Lake et al., 2013), Hedgehog (rabbit; 1:200) (Taylor et al., 1993), Wingless (mouse; 1:50; Developmental Studies Hybridoma Bank) and GFP [either rabbit (1:1000; Abcam, ab6556) or mouse (1:250; Abcam, ab1218)]. All fluorescent secondary antibodies were conjugated to Alexa Fluor dyes (Thermo Fisher Scientific; mouse 594, A11032; mouse 488, A11029; rabbit 594, A11037; rabbit 488, A11034) and were used at 1:500. Samples were counterstained with the DNA dye TOTO-3 iodide (Thermo Fisher Scientific) added at 1:250-1:500 to the Vectashield (Vector Labs) in which the samples were mounted.
Larval dissection, fixation and immunostaining
Lymph glands were dissected from wandering third instar larvae. Lymph glands were dissected by pulling out mouth hooks with lymph glands attached. Tissue was fixed for 10 min in a 1:3 solution of 37% formaldehyde: 1×PBS. Immunostaining was carried out as previously described (Grigorian et al., 2011).
Acknowledgements
We are grateful to I. Ando, R. Glaser, S. Heidmann, H. Muller, K. Suyama, A. Tulin, the Bloomington Drosophila Stock Center, FlyBase and the Developmental Studies Hybridoma Bank for providing stocks, database access and antibodies.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
All experiments were performed by M.G. and H.D. Analysis of data, conceptual planning, and preparation of the manuscript were performed by M.G. and J.S.L.
Funding
This investigation was supported by U.S. Public Health Service National Institutes of Health grants R01 CA128836 (J.S.L.), T32 CA09151 (M.G.) and T32 HG000044 (H.D.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.142729.supplemental
References
- Badenhorst P. (2014). What can we learn from flies: epigenetic mechanisms regulating blood cell development in Drosophila. In Transcriptional and Epigenetic Mechanisms Regulating Normal and Aberrant Blood Cell Development (ed. Bonifer C. and Cockerill P.), pp. 15-47. Berlin: Springer. [Google Scholar]
- Baek Y.-S., Haas S., Hackstein H., Bein G., Hernandez-Santana M., Lehrach H., Sauer S. and Seitz H. (2009). Identification of novel transcriptional regulators involved in macrophage differentiation and activation in U937 cells. BMC Immunol. 10, 18 10.1186/1471-2172-10-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi S. and Becker P. B. (2013). The variant histone H2A.V of Drosophila–three roles, two guises. Chromosoma 122, 245-258. 10.1007/s00412-013-0409-x [DOI] [PubMed] [Google Scholar]
- Billon P. and Côté J. (2013). Precise deposition of histone H2A.Z in chromatin for genome expression and maintenance. Biochim. Biophys. Acta 1819, 290-302. 10.1016/j.bbagrm.2011.10.004 [DOI] [PubMed] [Google Scholar]
- Braun A., Lemaitre B., Lanot R., Zachary D. and Meister M. (1997). Drosophila immunity: analysis of larval hemocytes by P-element-mediated enhancer trap. Genetics 147, 623-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson M. J., Wells J. R. E., Gibson F., Saint R. and Tremethick D. J. (1999). Regions of variant histone His2AvD required for Drosophila development. Nature 399, 694-697. 10.1038/21436 [DOI] [PubMed] [Google Scholar]
- Cook R. K., Christensen S. J., Deal J. A., Coburn R. A., Deal M. E., Gresens J. M., Kaufman T. C. and Cook K. R. (2012). The generation of chromosomal deletions to provide extensive coverage and subdivision of the Drosophila melanogaster genome. Genome Biol. 13, R21 10.1186/gb-2012-13-3-r21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creyghton M. P., Markoulaki S., Levine S. S., Hanna J., Lodato M. A., Sha K., Young R. A., Jaenisch R. and Boyer L. A. (2008). H2AZ is enriched at polycomb complex target genes in ES cells and is necessary for lineage commitment. Cell 135, 649-661. 10.1016/j.cell.2008.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C. J., Hartenstein V. and Banerjee U. (2003). Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev. Cell 5, 673-690. 10.1016/S1534-5807(03)00335-6 [DOI] [PubMed] [Google Scholar]
- Evans C. J., Liu T. and Banerjee U. (2014). Drosophila hematopoiesis: markers and methods for molecular genetic analysis. Methods 68, 242-251. 10.1016/j.ymeth.2014.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T., Ueda T., Nagata S. and Fukunaga R. (2010). Essential role of p400/mDomino chromatin-remodeling ATPase in bone marrow hematopoiesis and cell-cycle progression. J. Biol. Chem. 285, 30214-30223. 10.1074/jbc.M110.104513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorian M., Mandal L. and Hartenstein V. (2011). Hematopoiesis at the onset of metamorphosis: terminal differentiation and dissociation of the Drosophila lymph gland. Dev. Genes Evol. 221, 121-131. 10.1007/s00427-011-0364-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorian M., Liu T., Banerjee U. and Hartenstein V. (2013). The proteoglycan Trol controls the architecture of the extracellular matrix and balances proliferation and differentiation of blood progenitors in the Drosophila lymph gland. Dev. Biol. 384, 301-312. 10.1016/j.ydbio.2013.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanratty W. P. and Dearolf C. R. (1993). The Drosophila Tumorous-lethal hematopoietic oncogene is a dominant mutation in the hopscotch locus. Mol. Gen. Genet. 238, 33-37. [DOI] [PubMed] [Google Scholar]
- Harrison D. A., Binari R., Nahreini T. S., Gilman M. and Perrimon N. (1995). Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J. 14, 2857-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S.-H., Evans C. J., Uemura C. and Banerjee U. (2005). The Drosophila lymph gland as a developmental model of hematopoiesis. Development 132, 2521-2533. 10.1242/dev.01837 [DOI] [PubMed] [Google Scholar]
- Kotova E., Lodhi N., Jarnik M., Pinnola A. D., Ji Y. and Tulin A. V. (2011). Drosophila histone H2A variant (H2Av) controls poly(ADP-ribose) polymerase 1 (PARP1) activation in chromatin. Proc. Natl. Acad. Sci. USA 108, 6205-6210. 10.1073/pnas.1019644108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzemien J., Dubois L., Makki R., Meister M., Vincent A. and Crozatier M. (2007). Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature 446, 325-328. 10.1038/nature05650 [DOI] [PubMed] [Google Scholar]
- Kurucz E., Zettervall C.-J., Sinka R., Vilmos P., Pivarcsi A., Ekengren S., Hegedus Z., Ando I. and Hultmark D. (2003). Hemese, a hemocyte-specific transmembrane protein, affects the cellular immune response in Drosophila. Proc. Natl. Acad. Sci. USA 100, 2622-2627. 10.1073/pnas.0436940100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurucz E., Vaczi B., Markus R., Laurinyecz B., Vilmos P., Zsamboki J., Csorba K., Gateff E., Hultmark D. and Ando I. (2007). Definition of Drosophila hemocyte subsets by cell-type specific antigens. Acta Biol. Hung. 58 Suppl., 95-111. 10.1556/ABiol.58.2007.Suppl.8 [DOI] [PubMed] [Google Scholar]
- Kusch T., Florens L., Macdonald W. H., Swanson S. K., Glaser R. L., Yates J. R. III, Abmayr S. M., Washburn M. P. and Workman J. L. (2004). Acetylation by Tip60 is required for selective histone variant exchange at DNA lesions. Science 306, 2084-2087. 10.1126/science.1103455 [DOI] [PubMed] [Google Scholar]
- Kwon S. Y., Xiao H., Glover B. P., Tjian R., Wu C. and Badenhorst P. (2008). The nucleosome remodeling factor (NURF) regulates genes involved in Drosophila innate immunity. Dev. Biol. 316, 538-547. 10.1016/j.ydbio.2008.01.033 [DOI] [PubMed] [Google Scholar]
- Lake C. M., Holsclaw J. K., Bellendir S. P., Sekelsky J. and Hawley R. S. (2013). The development of a monoclonal antibody recognizing the Drosophila melanogaster phosphorylated histone H2A variant (gamma-H2AV). G3 (Bethesda) 3, 1539-1543. 10.1534/g3.113.006833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach T. J., Mazzeo M., Chotkowski H. L., Madigan J. P., Wotring M. G. and Glaser R. L. (2000). Histone H2A.Z is widely but nonrandomly distributed in chromosomes of Drosophila melanogaster. J. Biol. Chem. 275, 23267-23272. 10.1074/jbc.M910206199 [DOI] [PubMed] [Google Scholar]
- Lemaitre B., Meister M., Govind S., Georgel P., Steward R., Reichhart J. M. and Hoffmann J. A. (1995). Functional analysis and regulation of nuclear import of dorsal during the immune response in Drosophila. EMBO J. 14, 536-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorch Y. and Kornberg R. D. (2015). Chromatin-remodeling and the initiation of transcription. Q. Rev. Biophys. 48, 465-470. 10.1017/S0033583515000116 [DOI] [PubMed] [Google Scholar]
- Luger K., Mader A. W., Richmond R. K., Sargent D. F. and Richmond T. J. (1997). Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 389, 251-260. 10.1038/38444 [DOI] [PubMed] [Google Scholar]
- Madigan J. P., Chotkowski H. L. and Glaser R. L. (2002). DNA double-strand break-induced phosphorylation of Drosophila histone variant H2Av helps prevent radiation-induced apoptosis. Nucleic Acids Res. 30, 3698-3705. 10.1093/nar/gkf496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik H. S. and Henikoff S. (2003). Phylogenomics of the nucleosome. Nat. Struct. Biol. 10, 882-891. 10.1038/nsb996 [DOI] [PubMed] [Google Scholar]
- Mandal L., Martinez-Agosto J. A., Evans C. J., Hartenstein V. and Banerjee U. (2007). A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature 446, 320-324. 10.1038/nature05585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakhina S. and Steward R. (2006). Melanotic mutants in Drosophila: pathways and phenotypes. Genetics 174, 253-263. 10.1534/genetics.106.061978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillo Prado J. R., Srinivasan S. and Fuller M. T. (2013). The histone variant His2Av is required for adult stem cell maintenance in the Drosophila testis. PLoS Genet. 9, e1003903 10.1371/journal.pgen.1003903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller H.-M., Dimopoulos G., Blass C. and Kafatos F. C. (1999). A hemocyte-like cell line established from the malaria vector Anopheles gambiae expresses six prophenoloxidase genes. J. Biol. Chem. 274, 11727-11735. 10.1074/jbc.274.17.11727 [DOI] [PubMed] [Google Scholar]
- Rizki T. M. and Rizki R. M. (1980). Developmental analysis of a temperature-sensitive melanotic tumor mutant in Drosophila melanogaster Wilhelm Roux's Arch . 189, 197-206. 10.1007/BF00868678 [DOI] [PubMed] [Google Scholar]
- Rodriguez A., Zhou Z., Tang M. L., Meller S., Chen J., Bellen H. and Kimbrell D. A. (1996). Identification of immune system and response genes, and novel mutations causing melanotic tumor formation in Drosophila melanogaster. Genetics 143, 929-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh M., Lehner C. F. and Heidmann S. (2007). Incorporation of Drosophila CID/CENP-A and CENP-C into centromeres during early embryonic anaphase. Curr. Biol. 17, 237-243. 10.1016/j.cub.2006.11.051 [DOI] [PubMed] [Google Scholar]
- Scully R. and Xie A. (2013). Double strand break repair functions of histone H2AX. Mutat. Res. 750, 5-14. 10.1016/j.mrfmmm.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinenko S. A., Mandal L., Martinez-Agosto J. A. and Banerjee U. (2009). Dual role of wingless signaling in stem-like hematopoietic precursor maintenance in Drosophila. Dev. Cell 16, 756-763. 10.1016/j.devcel.2009.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan J., Baxter E. M. and Corces V. G. (2005). The role of histone H2Av variant replacement and histone H4 acetylation in the establishment of Drosophila heterochromatin. Genes Dev. 19, 65-76. 10.1101/gad.1259105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. M., Nakano Y., Mohler J. and Ingham P. W. (1993). Contrasting distributions of patched and hedgehog proteins in the Drosophila embryo. Mech. Dev. 42, 89-96. 10.1016/0925-4773(93)90101-3 [DOI] [PubMed] [Google Scholar]
- Ueda T., Watanabe-Fukunaga R., Ogawa H., Fukuyama H., Higashi Y., Nagata S. and Fukunaga R. (2007). Critical role of the p400/mDomino chromatin-remodeling ATPase in embryonic hematopoiesis. Genes Cells 12, 581-592. 10.1111/j.1365-2443.2007.01080.x [DOI] [PubMed] [Google Scholar]
- van Daal A. and Elgin S. C. (1992). A histone variant, H2AvD, is essential in Drosophila melanogaster. Mol. Biol. Cell 3, 593-602. 10.1091/mbc.3.6.593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson K. L., Johnson T. K. and Denell R. E. (1991). Lethal(1) aberrant immune response mutations leading to melanotic tumor formation in Drosophila melanogaster. Dev. Genet. 12, 173-187. 10.1002/dvg.1020120302 [DOI] [PubMed] [Google Scholar]
- Zettervall C.-J., Anderl I., Williams M. J., Palmer R., Kurucz E., Ando I. and Hultmark D. (2004). A directed screen for genes involved in Drosophila blood cell activation. Proc. Natl. Acad. Sci. USA 101, 14192-14197. 10.1073/pnas.0403789101 [DOI] [PMC free article] [PubMed] [Google Scholar]