Abstract
Background
Physiological responses to reward and extinction are believed to represent the Behavioral Activation System (BAS) and Behavioral Inhibition System (BIS) constructs of Reinforcement Sensitivity Theory and underlie externalizing behaviors, including substance use. However, little research has examined these relations directly.
Methods
We assessed individuals’ cardiac pre-ejection periods (PEP) and electrodermal responses (EDR) during reward and extinction trials through the “Number Elimination Game” paradigm. Responses represented BAS and BIS, respectively. We then examined whether these responses provided incremental utility in the prediction of future alcohol, marijuana, and cigarette use.
Results
Zero-inflated Poisson (ZIP) regression models were used to examine the predictive utility of physiological BAS and BIS responses above and beyond previous substance use. Physiological responses accounted for incremental variance over previous use. Low BAS responses during reward predicted frequency of alcohol use at year 3. Low BAS responses during reward and extinction and high BIS responses during extinction predicted frequency of marijuana use at year 3. For cigarette use, low BAS response during extinction predicted use at year 3.
Conclusions
These findings suggest that the constructs of Reinforcement Sensitivity Theory, as assessed through physiology, contribute to the longitudinal maintenance of substance use.
Keywords: Physiology, RST, substance use, pre-ejection period, EDR
1. Introduction
Innate motivational systems that govern approach and avoidance behaviors have long been recognized (Gray, 1970). The revised Reinforcement Sensitivity Theory (RST; Gray and McNaughton, 2000) indicates that three interdependent neurobiological systems influence an individual’s responses to reinforcement. The Behavioral Activation System (BAS) guides approach behavior to conditioned and unconditioned reward. In contrast, the Fight-Flight-Freezing system (FFFS) responds to cues for threat and promotes the avoidance of unconditioned aversive stimuli through withdrawal and freezing behavior (Gray and McNaughton, 2000). The Behavioral Inhibition System (BIS) serves to detect competing influences from environmental stimuli and resolve conflict by inhibiting ongoing action (McNaughton and Gray, 2002). Despite interdependence in action, these systems are differentially mediated; the BAS is believed to be mediated by dopaminergic pathways originating in the ventral tegmental area (Matthews and Gilliland, 1999), and the BIS is believed to be mediated by the amygdala and septo-hippocampal systems (McNaughton and Corr, 2004).
Self-report measures of BAS and BIS contribute to meaningful outcomes, such as various forms of psychopathology (Bijttebier et al., 2009; Mellick et al., 2014). Some have argued that abnormally high BAS activation or low BIS activation could contribute to externalizing behavior (Newman and Wallace, 1993; Quay, 1997). Given the importance of motivation for reward in the development of problematic substance use and the shared neural substrates between substance-based reward learning and the BAS (Dawe et al., 2004; Hyman et al., 2006), it stands to reason that individuals with highly active BAS may be at high risk for substance-related problems. Consistent with this notion, there is a large literature showing that people who report high self-reported reinforcement sensitivity (i.e., heightened BAS responding) are more likely to meet criteria for substance use disorder (Hundt et al., 2008; Johnson et al., 2003). Although self-reported BIS has demonstrated relations with outcomes associated with anxiety and other internalizing conditions (Kasch et al., 2002; Mellick et al., 2014), low BIS has demonstrated less consistent relations to substance use (Franken and Muris, 2006; Hundt et al., 2008).
1.1. Measuring RST Constructs via Physiological Responses
There exists a tenuous framework linking autonomic nervous system activity with RST constructs (Beauchaine, 2001; Fowles, 1980; Fowles, 1988; Tomaka and Palacios-Esquivel, 1997). In a recent review of this topic, Beauchaine (2001) asserts that cardiac pre-ejection period (PEP) and electrodermal responding (EDR) represent BAS and BIS, respectively. Shortened PEP is conceptualized as a relatively “pure” measure of sympathetic nervous system excitation that reflects beta-adrenergic influence on the heart. According to Beauchaine (2001), shortened PEP is associated with approach behavior and serves as an index of BAS activity when assessed during the delivery of reward. In contrast, increased EDR reflects cholinergic (vs. adrenergic) pathways to the skin and is unrelated to PEP. EDR is implicated in the affective experience of anxiety (Biederman et al., 1993; Scarpa et al., 1997), a characteristic associated with BIS (Fowles, 1980; 1988). When assessed during exposure to motivational conflict (changes in reward conditions), higher EDR is believed to measure BIS activation (see Beauchaine, 2001).
Despite being a novel understanding of BIS/BAS, there are emerging studies examining the utility of the physiological assessment of RST constructs. Tomaka and Palacios-Esquivel (1997) measured PEP and EDR in groups of individuals participating in a reward/punishment task. Results indicated that, as hypothesized, BAS (PEP) change scores increased and stabilized during the reward condition, but decreased during the punishment condition. While BIS (EDR) change scores were hypothesized to increase in the punishment condition, signaling the motivation to inhibit responses, no significant trend was identified. Beauchaine and colleagues (2001) also explored physiological BIS/BAS responding during a task of reward and extinction. Although it was hypothesized that BAS (PEP) response would increase during reward (as assessed through the shortening of PEP during reward trials), this response was markedly low for children with externalizing disorders. BIS (EDR) responses during extinction were not found to differentiate between children with externalizing disorders and comparison children.
To our knowledge, only one study has explored the physiological assessment of RST constructs and substance use. This is important, given that the physiological assessment of BIS/BAS may provide an important link between a useful theoretical approach and biological disorder. Incorporating prior work, Brenner and Beauchaine (2011) revised predictions to suggest that BAS responding during reward should be lowered in children and adolescents with externalizing problems including substance use. Findings indicated that low PEP response during reward trials was indeed identified as a predictor in multilevel modeling analyses of alcohol use initiation. Responding during extinction trials was not explored.
This omission of responses during extinction in Brenner and Beauchaine (2011) could be quite important. Although results from prior work have evidenced links between these RST systems and externalizing behaviors, as noted by Carver (2006), this work may oversimplify the role of the BIS/BAS in reinforcement learning. Carver has argued that responses during frustrative non-reward may be more heavily influenced by the BAS than by the BIS. This hypothesis is based on a series of studies showing that under conditions of frustrative non-reward (i.e., participants were led to believe they could earn a reward but then failed to do so), negative affective responding (i.e., frustration, sadness) was correlated with the strength of the BAS but not the BIS (Carver, 2004). Therefore, BAS response under conditions of both reward and the withdrawal of reward may be important in understanding how people cope with non-reward when reward is expected.
1.2. Current Study
Despite known relations between self-reported RST constructs and substance use, the physiological representation of BAS and BIS can provide incremental utility in understanding how this theory lends to our understanding of biological disorder. The current study examines the utility of physiological responses during reward and frustrative non-reward (hereafter described as extinction) stimuli in the prediction of later substance use. Substance use and physiological data were collected from 230 college freshmen at year 1, and substance use data was collected again at follow-up (year 3). Physiological measures of BIS/BAS collected during a reward and extinction task were used as predictors of future substance use at year 3, controlling for substance use at year 1. With only one existing study contributing to this literature (Brenner and Beauchaine, 2011), we sought to explore relations between physiological assessment of RST constructs and later substance use.
Although Beauchaine and colleagues’ work with children and young adolescents has shown associations between low BAS (lengthened PEP) responding during reward and childhood externalizing disorders (2001), we did not believe that this finding will replicate in an older, non-clinical sample given other previous work (Tomaka and Palacios-Esquivel, 1997). We hypothesized that shortened PEP responses during reward trials would indicate a stronger dopaminergic response to reward and would therefore be significant predictors of future alcohol, marijuana, and cigarette use. Due to emerging work targeting the role of BAS in frustrative non-reward responding (Carver, 2004; 2006), we hypothesized that shortened PEP response during extinction would be an equally significant predictor of later substance use outcomes. Although EDR fluctuations during extinction are believed to represent BIS functioning (Beauchaine, 2001), the lack of evidence associating BIS with substance use outcomes (Franken and Muris, 2006; Hundt et al., 2008) led us to hypothesize that EDR responses during extinction trials not be a predictor of later substance use.
2. Method
2.1. Participants
Participants (N = 230) were assessed yearly starting freshman year of college. The average age of participants at assessment was 18.49 years (SD = .72), and most were under 21 years of age (99%). Participants were recruited from introductory psychology courses and received course credit and monetary incentives for participation. Approximately 79% of participants identified as Caucasian, 13% African-American, and 8% as other.
“High risk” participants were over-recruited to ensure sufficient variability in substance use, and made up 26% of the sample. Students in introductory psychology courses were administered a screening questionnaire during a mass testing during the first two weeks of the semester. The screening measure assessed conduct problem behaviors that occurred prior to age 18 (e.g., stealing, lying, fighting). A composite was used to determine the distribution of scores for predicted substance use risk (calculated separately by gender). Those whose scores fell within the top 25% for their gender were specifically invited to participate through email.
2.2 Procedure and Attrition
Participants completed a substance use assessment and a physiological reward and extinction task at year 1. Substance use was assessed again at year 3. Of the original 230 individuals in the study, 218 had useable substance use data at year 1, 204 had usable physiological data at year 1, and 105 had useable substance use data at year 3. Across all assessments, 95 had complete data to be used in individual analyses (53% female). Independent t-tests conducted for group demonstrated that the non-completers did not differ on any study variables from year 1 assessments (t scores ranged from .01 - 1.98; all p’s > .05).
2.3. Measures
2.3.1. Substance use
The Life History Calendar is a retrospective, computer-assisted interview method for collecting data on life events and behaviors (Caspi et al., 1996). Information was obtained regarding alcohol, marijuana, and cigarette use. Participants selected from six choices describing the frequency with which they used during each month of the past year (0 = did not use, 1 = once a month or less, 2 = once a week, 3 = 2 or 3 times a week, 4 = 4 or 5 times a week, 5 = every day). Data from year 1 represented our control variable in analyses (concurrent with the physiological assessment), and data from year 3 represented future substance use.
2.3.2. Number Elimination Game task
All physiological responses were collected individually during the Number Elimination Game (NEG) task (see Beauchaine et al., 2001; Brenner and Beauchaine, 2011). Participants were seated in front of a computer in a temperature and humidity controlled testing room. Physiological signals were first collected during a 5-minute baseline where participants were asked to relax and visually fixate on a plus sign on the computer screen. Next, physiological responses continued to be collected during the NEG, a computerized repetitive response task, which included conditions of reward and extinction. During the task, single-digit, odd numbers (1, 3, 5, 7, or 9) were randomly presented on a computer screen. Participants were required to press the matching number on the keyboard to advance to the next stimuli. Incorrect responses were not recorded and did not result in penalties other than delay of advancing to the next number. The task was performed across five, 2-minute blocks, each separated by a 75 second intertrial baseline.
The first two blocks were reward conditions. During these trials, each correct response (pressing the key that matched the stimulus on the screen) resulted in an audio and visual reward signal, a reward of $0.02, and a presentation of a running total of money earned. Audio and visual reward cues were omitted for incorrect responses. The third block included 60 seconds of reward and 60 seconds of nonreward (extinction), during which monetary incentives and audio signals were omitted following responses. The fourth block returned to the full 2 minutes of reward cues. The fifth block began with 60 seconds of extinction followed by 60 seconds of reward. Prior to the task, participants were informed that they could earn more money the faster they played and that they needed to continue responding to advance to the next reward stimuli even if the task no longer reacted to their responses.
2.3.3. Autonomic Measures
Autonomic activity (PEP, EDR) was measured using a BioNex system from Mindware Technologies (Gahanna, OH) and amplified using the appropriate module (BioNex Impedance Cardiograph and GSC, Model 50-371100-00). All acquired channels were sampled at a rate of 1000 Hz. Autonomic data were analyzed according to accepted scoring parameters using Mindware IMP (version 2.6) and EDA software (version 2.6) (Mindware Technologies, Gahanna, OH), which included visual inspection and editing of artifacts.
Cardiac pre-ejection period (PEP), derived from electrocardiogram (ECG) and impedance cardiography (ICG), was assessed using standard lead II and tetrapolar placement of electrodes, respectively (Sherwood et al., 1990). PEP was quantified as the period of time in milliseconds between the onset of ventricular depolarization (Q-wave of ECG) and the opening of the aortic valve (B point of dZ/dt waveform from ICG; Lozano et al., 2007). PEP reflects myocardial contractility and is commonly used as an index of sympathetic cardiac control (Berntson et al. 2004). Lower PEP values (i.e., shorter cardiac latencies) indicate higher cardiac sympathetic activity.
Electrodermal response (EDR) data were collected using two disposable Ag–AgCl electrodes (Mindware Technologies, Model 93-0102-00) placed on the distal phalanges of the index and middle fingers of participants’ non-dominant hand. EDR was scored as the number of non-specific fluctuations in skin conductance exceeding 0.05 μS. Higher EDR values indicate greater sympathetic activity.
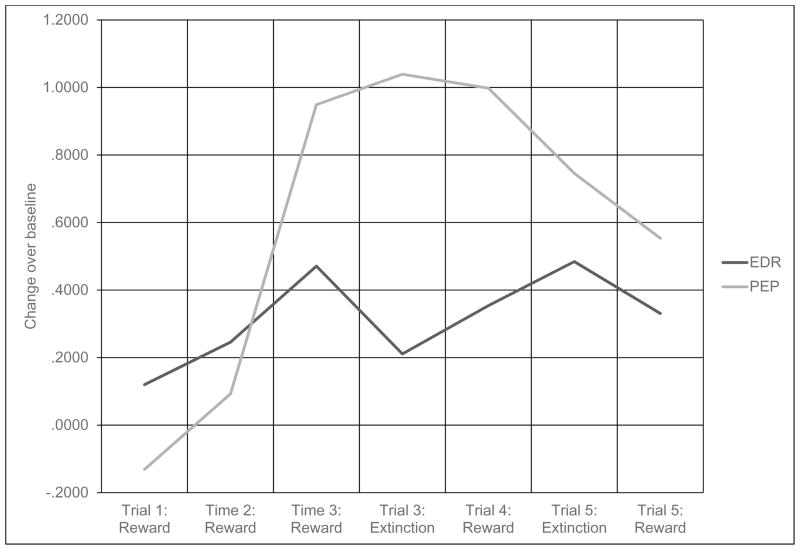
Mean PEP and EDR values were calculated by ensemble-averaging data in 30sec epochs for the following periods: Initial Baseline (final 2 minutes of an initial 5-minute baseline recording), Intertrial Baseline (75 second baseline period between trial blocks), and reward or extinction trials. To assess autonomic response during reward and extinction, change scores were calculated as difference scores between the averaged active trial responses and the Intertrial Baseline value immediately preceding. This yielded five reward response scores (one for each reward block), and two extinction response scores (see Figure 1).
Figure 1.
Physiological change scores across reward and extinction trials.
Note. Scores represent response change over immediately preceding baseline score. All scores are standardized. PEP = Cardiac Pre-Ejection Period. PEP values represent changes in milliseconds. EDR = Electrodermal Response. EDR values represent non-specific fluctuations per minute.
3. Results
3.1. Preliminary Data Screening and Analyses
Table 1 provides raw scores across trials, in the order they were presented during the task, for PEP and EDR. As can be seen from Table 1, average PEP scores demonstrated a subtle rising trend during acclimation to the task, then varied responses above preceding baselines as competing contingencies of reward and extinction were introduced. Average raw EDR scores demonstrated a generally rising trend across the task, with little notable response during competing contingency trials. Graphical representations of these data are available in supplementary materials.
Table 1.
Raw physiological scores across trials.
| PEP | PEP SD | EDR | EDR SD | |
|---|---|---|---|---|
| Baseline 1 | 120.43 | 10.10 | 4.53 | 3.83 |
| Trial 1: Reward | 120.24 | 10.21 | 5.10 | 3.96 |
| Baseline 2 | 120.49 | 10.21 | 5.00 | 3.93 |
| Trial 2: Reward | 120.76 | 9.74 | 5.08 | 3.94 |
| Baseline 3 | 120.68 | 10.07 | 5.16 | 4.20 |
| Trial 3: Reward | 121.12 | 10.41 | 5.52 | 4.18 |
| Trial 3: Extinction | 121.47 | 9.90 | 5.51 | 4.13 |
| Baseline 4 | 120.32 | 10.11 | 5.65 | 4.28 |
| Trial 4: Reward | 121.19 | 9.82 | 5.95 | 4.23 |
| Baseline 5 | 120.34 | 10.06 | 5.81 | 4.48 |
| Trial 5: Extinction | 120.43 | 9.89 | 6.29 | 4.44 |
| Trial 5: Reward | 121.58 | 10.61 | 6.31 | 4.45 |
Note. PEP = Cardiac pre-ejection period, quantified as the period of time in milliseconds between the onset of ventricular depolarization (Q-wave) and the opening of the aortic valve (B point of dZ/dt waveform). EDR = Electrodermal response, scored as the number of non-specific fluctuations in skin conductance exceeding 0.05 μS. SD = standard deviation.
Figure 1 represents average changes scores for PEP and EDR across the course of the task. Change scores represent changes over baselines preceding the trials. Consistent with prior work (Tomaka and Palacios-Esquivel, 1997), PEP change scores demonstrated a rising trend during acclimation to reward, then a falling trend when competing contingencies were introduced. EDR change scores did not demonstrate a consistent trend.
Because lower PEP scores represent sympathetic excitation, PEP responses (change scores) were reverse coded for interpretability in all analyses (see Tables 2–6). All subsequent positive values for PEP and EDR represent high activation, and negative values represent low activation of the BAS and BIS systems, respectively. Descriptive statistics are presented in Table 2. Consistent with the general population, substance use scores were positively skewed and exhibited more zeroes than would be compatible with normal regression models. Further tests revealed that the outcome distributions were Poisson-distributed (i.e., the mean was roughly equal to the standard deviation). Therefore, zero-inflated Poisson (ZIP) regression models were used in multivariate analyses.
Table 2.
Means and Standard Deviations for study variables (N = 95).
| Variable | Mean | SD | % of sample |
|---|---|---|---|
| Age Year 1 | 18.92 | .45 | |
| Female | 53.2% | ||
| PEP Response to Reward | −.09 | 2.97 | |
| PEP Response to Extinction | −.34 | 2.89 | |
| EDR Response to Extinction | .34 | .47 | |
| Frequency of Alcohol Use Year 1 | .82 | .98 | |
| Frequency of Alcohol Use Year 3 | .97 | .94 | |
| Alcohol abstinence rate year 1 | 20.2 | ||
| Alcohol abstinence rate year 3 | 11.6 | ||
| Frequency of Marijuana Use Year 1 | .42 | .82 | |
| Frequency of Marijuana Use Year 3 | .54 | 1.21 | |
| Marijuana abstinence rate year 1 | 60.0 | ||
| Marijuana abstinence rate year 3 | 60.0 | ||
| Frequency of Cigarette Use Year 1 | .57 | 1.40 | |
| Frequency of Cigarette Use Year 3 | .61 | 1.53 | |
| Cigarette abstinence rate year 1 | 82.1 | ||
| Cigarette abstinence rate year 3 | 83.2 |
Note. PEP = Cardiac pre-ejection period response (change from baseline scores), reverse-coded such that higher numbers indicate greater sympathetic activity. EDR = Electrodermal response (change from baseline scores). SD = standard deviation. Substance Use variables represent average frequency of use. “Frequency” ranges from 0–5 and indicates the frequency with which participants used during each month of the past year (0 = did not use, 1 = once a month or less, 2 = once a week, 3 = 2 or 3 times a week, 4 = 4 or 5 times a week, 5 = every day).
Table 6.
Predictors of year 3 cigarette use.
| Standardized B | SEB | |
|---|---|---|
| Model 1: Prediction of Year 3 Cigarette Use from Year 1 Cigarette Use and PEP Reward (N=95) | ||
| Zero-Inflated: Contribution to Zero-Inflation/Abstinence | ||
| Intercept | 0.99*** | 0.16 |
| Year 1 Cigarette Use | −0.74*** | 0.08 |
| PEP Reward | 0.26 | 0.11 |
| Continuous: Frequency of Use | ||
| Intercept | 13.69*** | 3.28 |
| Year 1 Cigarette Use | 0.88*** | 0.14 |
| PEP Reward | −0.32 | 0.27 |
| Model 2: Prediction of Year 3 Cigarette Use from Year 1 Cigarette Use and PEP Extinction (N=95) | ||
| Zero-Inflated: Contribution to Zero-Inflation/Abstinence | ||
| Intercept | 1.04*** | 0.15 |
| Year 1 Cigarette Use | −0.71*** | 0.08 |
| PEP Extinction | 0.38** | 0.12 |
| Continuous: Frequency of Use | ||
| Intercept | 14.78*** | 3.48 |
| Year 1 Cigarette Use | 0.96*** | 0.09 |
| PEP Extinction | −0.12 | 0.28 |
| Model 3: Prediction of Year 3 Cigarette Use from Year 1 Cigarette Use and EDR Extinction (N=90) | ||
| Zero-Inflated: Contribution to Zero-Inflation/Abstinence | ||
| Intercept | 1.01*** | 0.19 |
| Year 1 Cigarette Use | −0.79*** | 0.08 |
| EDR Extinction | −0.05 | 0.14 |
| Continuous: Frequency of Use | ||
| Intercept | 15.05*** | 3.01 |
| Year 1 Cigarette Use | 1.00*** | 0.02 |
| EDR Extinction | −0.13 | 0.26 |
Note. PEP = Cardiac pre-ejection period response (change from baseline scores), reverse-coded such that higher numbers indicate greater sympathetic activity. EDR = Electrodermal response (change from baseline scores). Significant coefficients presented in bold type.
p < .01;
p < .001.
Although EDR responses during reward trials were recorded and averaged as available material for figures, the lack of theory supporting the use of EDR during reward trials did not support its use as a representative construct of Reinforcement Sensitivity Theory in analyses (Beauchaine, 2001). Confirmation analyses were performed to ensure that EDR response during reward did not serve as a viable correlate or predictor of substance use. EDR response during reward did not emerge as a significant correlate or predictor in any of the analyses, and was therefore not included in the reporting of results.
Zero-order correlations between the study variables are presented in Table 3. To control for multiple analyses, probability was set at p < .01. There was a high, positive correlation between PEP responses during reward and extinction stimuli. All substance use variables at year 1 correlated with year 3 of the same substance.
Table 3.
Relations between study variables (N = 95).
| PEP Reward | PEP Extinction | EDR Extinction | Y1 Alcohol | Y3 Alcohol | Y1 Marijuana | Y3 Marijuana | Y1 Cigarettes | |
|---|---|---|---|---|---|---|---|---|
| PEP Extinction | .71*** | -- | ||||||
| EDR Extinction | −.21 | −.21 | -- | |||||
| Year 1 Alcohol Use | −.10 | −.15 | −.06 | -- | ||||
| Year 3 Alcohol Use | −.18 | −.15 | −.06 | .52*** | -- | |||
| Year 1 Marijuana Use | −.16 | −.28** | −.01 | .34** | .17 | -- | ||
| Year 3 Marijuana Use | −.20 | −.34** | .11 | .24** | .20 | .64*** | -- | |
| Year 1 Cigarette Use | −.21 | −.21 | .13 | .23 | .11 | .36*** | .21 | -- |
| Year 3 Cigarette Use | −.26 | −.26 | .12 | .20 | .18 | .45*** | .42*** | .85*** |
Note. PEP = Cardiac pre-ejection period response (change from baseline scores), reverse-coded such that higher numbers indicate greater sympathetic activity. EDR = Electrodermal response (change from baseline scores). Substance use variables represent average frequency of use. Bivariate correlations.
p < .01,
p < .001.
3.2. Planned Hypothesis Tests
Nine zero-inflated Poisson (ZIP) regression models were fit in Mplus 7.2, regressing each of the year 3 substance use variables (alcohol, marijuana, and cigarettes) onto three sets of predictors: (1) substance use at year 1 and PEP response during reward (BAS activity), (2) substance use at year 1 and PEP response during extinction (possible BAS activity), and (3) substance use at year 1 and EDR response during extinction (BIS activity). Each of these models simultaneously estimates a logistic regression of the excessively zero-inflated part of the outcome variable on the predictors (the prediction of degree of abstention) and a regression of the continuous component of the outcome variable on the predictors (frequency of use). The intercept in all models represents estimated outcomes when all predictor values have a value of 0. In the logistic component of the model, this value represents the extent to which zero-inflation is affected (e.g., degree of abstention). All regression coefficients are presented as standardized values.
3.3. Alcohol Use
Results predicting year 3 alcohol use appear in Table 4. In each of the logistic model components, more frequent alcohol use at year 1 predicted a lower probability of contributing to the zero-inflation at year 3 (i.e., lesser degree of abstention). In continuous models, more frequent alcohol use at year 1 predicted more frequent alcohol use at year 3. Among the physiological predictors, low BAS activation during reward significantly predicted higher frequency of alcohol use at year 3. BAS responses and BIS responses during extinction did not predict future alcohol use.
Table 4.
Predictors of year 3 alcohol use.
| Standardized B | SEB | |
|---|---|---|
| Model 1: Prediction of Year 3 Alcohol Use from Year 1 Alcohol Use and PEP Reward (N=94) | ||
| Zero-Inflated: Contribution to Zero-Inflation/Abstinence | ||
| Intercept | −0.15 | 0.13 |
| Year 1 Alcohol Use | −0.85*** | 0.09 |
| PEP - Reward | 0.04 | 0.08 |
| Continuous: Frequency of Use | ||
| Intercept | 6.80*** | 0.89 |
| Year 1 Alcohol Use | 0.90*** | 0.05 |
| PEP - Reward | −0.34** | 0.10 |
| Model 2: Prediction of Year 3 Alcohol Use from Year 1 Alcohol Use and PEP Extinction (N=94) | ||
| Zero-Inflated: Contribution to Zero-Inflation/Abstinence | ||
| Intercept | −0.15 | 0.13 |
| Year 1 Alcohol Use | −0.85*** | 0.08 |
| PEP – Extinction | 0.06 | 0.08 |
| Continuous: Frequency of Use | ||
| Intercept | 7.27*** | 0.96 |
| Year 1 Alcohol Use | 0.96*** | 0.03 |
| PEP - Extinction | −0.15 | 0.10 |
| Model 3: Prediction of Year 3 Alcohol Use from Year 1 Alcohol Use and EDR Extinction (N=89) | ||
| Zero-Inflated: Contribution to Zero-Inflation/Abstinence | ||
| Intercept | −0.18 | 0.14 |
| Year 1 Alcohol Use | −0.09*** | 0.01 |
| EDR – Extinction | 0.19 | 0.15 |
| Continuous: Frequency of Use | ||
| Intercept | 7.72*** | 1.04 |
| Year 1 Alcohol Use | 0.10*** | 0.01 |
| EDR - Extinction | −0.19 | 0.24 |
Note. PEP = Cardiac pre-ejection period response (change from baseline scores), reverse-coded such that higher numbers indicate greater sympathetic activity. EDR = Electrodermal response (change from baseline scores). Significant coefficients presented in bold type.
p < .01;
p < .001.
3.4. Marijuana Use
Results predicting year 3 marijuana use appear in Table 5. Greater frequency of marijuana use at year 1 was associated with a lower probability of contributing to the zero-inflation at year 3 (i.e., had a lesser degree of abstention) and greater frequency of marijuana smoking at year 3. Low BAS activation during both reward and extinction significantly predicted higher frequency of marijuana use at year 3. In addition, high BIS activation during extinction significantly predicted more frequent marijuana use at year 3.
Table 5.
Predictors of year 3 marijuana use.
| Standardized B | SEB | |
|---|---|---|
| Model 1: Prediction of Year 3 Marijuana Use from Year 1 Marijuana Use and PEP Reward (N=94) | ||
| Zero-Inflated: Contribution to Zero-Inflation/Abstinence | ||
| Intercept | 0.43*** | 0.09 |
| Year 1 Marijuana Use | −0.67*** | 0.11 |
| PEP - Reward | 0.08 | 0.10 |
| Continuous: Frequency of Use | ||
| Intercept | 5.26*** | 0.71 |
| Year 1 Marijuana Use | 0.46*** | 0.10 |
| PEP - Reward | −0.54*** | 0.12 |
| Model 2: Prediction of Year 3 Marijuana Use from Year 1 Marijuana Use and PEP Extinction (N=94) | ||
| Zero-Inflated: Contribution to Zero-Inflation/Abstinence | ||
| Intercept | 0.43*** | 0.09 |
| Year 1 Marijuana Use | −0.64*** | 0.12 |
| PEP – Extinction | 0.11 | 0.10 |
| Continuous: Frequency of Use | ||
| Intercept | 2.81*** | 0.40 |
| Year 1 Marijuana Use | 0.38*** | 0.06 |
| PEP - Extinction | −0.82*** | 0.04 |
| Model 3: Prediction of Year 3 Marijuana Use from Year 1 Marijuana Use and EDR Extinction (N=89) | ||
| Zero-Inflated: Contribution to Zero-Inflation/Abstinence | ||
| Intercept | 4.92*** | 0.62 |
| Year 1 Marijuana Use | −0.68*** | 0.11 |
| EDR – Extinction | −0.02 | 0.10 |
| Continuous: Frequency of Use | ||
| Intercept | 0.45*** | 0.12 |
| Year 1 Marijuana Use | 0.90*** | 0.05 |
| EDR - Extinction | 0.44*** | 0.08 |
Note. PEP = Cardiac pre-ejection period response (change from baseline scores), reverse-coded such that higher numbers indicate greater sympathetic activity. EDR = Electrodermal response (change from baseline scores). Significant coefficients presented in bold type.
p < .01;
p < .001.
3.5. Cigarette Use
Results predicting year 3 cigarette use appear in Table 6. Greater frequency of cigarette use at year 1 was associated with a lower probability of contributing to the zero-inflation at year 3 (i.e., had a lesser degree of abstention) and greater frequency of cigarette smoking at year 3. Consistent with alcohol and marijuana findings, results indicated that low BAS activation during extinction was associated with a lower likelihood of abstinence at year 3. BAS responses during reward and BIS responses during extinction did not predict future cigarette use.
4. Discussion
This study explored the possible contributions of physiologically assessed BAS and BIS to later alcohol, marijuana, and cigarette use. Results suggested relations opposite to those predicted for this non-clinical sample. Consistent with Brenner and Beauchaine (2011), individuals with low BAS during reward had significantly higher average frequency of use of alcohol and marijuana two years later. To test Carver’s (2006) hypothesis that BAS response during withdrawal of reward may represent an exaggerated BAS response, we explored PEP responses during extinction trials. Results indicated that low BAS responses during extinction trials were related to frequency of future marijuana use and a higher probability of cigarette use in general. Thus, BAS assessed during extinction may represent a similar pathway to that of responses during reward, and together, these constructs appear to characterize an individual who is under-reactive to the anticipation of reinforcement. Although not predicted, greater BIS response predicted future marijuana use. It appears that those sensitive to changing reward circumstances may be prone to more frequent marijuana use.
Based upon the current findings, multiple systems may be at work. First, these findings support the notion that those whose approach systems are under-responsive to reinforcement (and non-reward) use substances due to boredom-proneness or excitement seeking characteristics (Brenner and Beauchaine, 2011). This “reward deficit syndrome” has been posited as a risk factor in the development of substance use disorders (see Dawe and Loxton, 2004). In fact, those prone to substance abuse have been found to have lower levels of dopamine concentration in neural pathways than comparison individuals, suggesting that they may be more receptive to the reinforcing effects of drugs and alcohol (Blum et al., 2000). Further, as noted by Volkow and colleagues (2004), substance use itself may undermine the typical thresholds required for environmental events to activate dopamine release, thereby causing those with addiction to need more salient cues to provoke dopaminergic activity. In this study, many participants were already using substances at year 1. The low BAS activity recorded may be indicative of dopaminergic response already depleted by substance abuse.
The present results also suggest that different systems are at work during extinction. BIS activation is theorized to be mediated by the amygdala and septo-hippocampal systems (McNaughton and Corr, 2004), producing upsetting emotional states that may prime individuals to engage in unhealthy patterns of substance use (Baker et al., 2004). Although BIS/BAS activation is often explained in terms of negative and positive affect respectively (e.g., Heponiemi et al., 2003), others have argued that the relation between BIS/BAS and affective state is more complex and that activation of either system can result in negative or positive affect depending on input from a reflective feedback process (Carver, 2004; Carver and Scheier, 1998). Based upon dual significant results for marijuana use, it is possible that both dopamine activation deficits and amygdalar-mediated emotional upset during non-reward work together to promote risk. We are not able to draw firm conclusions regarding the role of affect in these findings without direct assessment of affective state; however, it will be important for future research to explore the possible divergence between physiological/emotional responses to reward and extinction utilizing constructs grounded in personality and/or emotion. There was no measure of dopamine included in the current study; therefore, hypotheses regarding systems and possible treatment options are speculative.
The current work also speaks to the potential biological basis of traits (Zuckerman and Kuhlman, 2000) and suggests that intervening at both physiological and behavioral levels may be the most successful route to substance use prevention and treatment. Some have suggested a multiple systems approach that includes pharmacological and behavioral interventions for the treatment of drug addiction. (Volkow et al., 2003; Volkow et al., 2004). Recommended strategies could include the development of medications that either maintain stable concentrations of dopamine in the brain, adequately block dopaminergic receptors to eliminate the reinforcing, acute effects of drug use, or make the reception of drug aversive through sensitizing relevant receptors (Volkow et al., 2004). Some of these strategies have already demonstrated notable benefits (Ebbert et al., 2015; Rösner et al., 2010), but are not yet available across all substances of abuse. Alternatively, treatment may be directed at weakening conditional associations with drug effects or associated through behavioral conditioning and medications that disrupt memory processes in hippocampus and amygdala. As noted by Volkow and colleagues (2004), beta-blockers have been shown to interfere with conditioned responses to environmental reinforcers as well as to aversive stimuli, which is an effect mediated by the amygdala (Miranda et al., 2003). In addition, cognitive behavioral restructuring of thoughts and behavioral associated with drug cues may enhance the beneficial effects of medication.
There are several limitations to the current work, including retrospective substance use collection and the rather normative nature of the sample. While it is possible that different results may be found with more extensive substance use assessment within an exclusively substance abusing population, the current work provides an important start to this line of inquiry and presents clear evidence for the link between physiological responses to reward and extinction and use of multiple substances. In addition, this work examines only one potential part of this relation: A directional link from physiological response to later substance use. Future work would do well to explore these relations in greater depth, including testing possible bi-directional associations between innate responses themselves, and between innate responses and substance use. It is likely that continued substance use reinforces these physiological responses, thereby creating an intractable reward-based physiology that contributes to addiction in indirect ways.
Acknowledgments
This research was supported by a grant from the National Institute on Drug Abuse (P50 DA05312) and by grants from the National Institutes of Mental Health (T32MH093315; T32MH019927). The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health.
References
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Dev Psychopathol. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Katkin ES, Strassberg Z, Snarr J. Disinhibitory psychopathology in male adolescents: Discriminating conduct disorder from attention-deficit/hyperactivity disorder through concurrent assessment of multiple autonomic states. J Abnorm Psychol. 2001;110:610–624. doi: 10.1037//0021-843x.110.4.610. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Lozano DL, Chen YJ, Cacioppo JT. Where to Q in PEP. Psychophysiology. 2004;41:333–337. doi: 10.1111/j.1469-8986.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- Biederman J, Roesenblaum JF, Bolduc-Murphy EA, et al. A 3-year follow-up of children with and without behavioral inhibition. J Am Acad Child Psychiatry. 1993;32:814–821. doi: 10.1097/00004583-199307000-00016. [DOI] [PubMed] [Google Scholar]
- Bijttebier P, Beck I, Claes L, Vandereycken W. Gray’s Reinforcement Sensitivity Theory as a framework for research on personality-psychopathology associations. Clin Psychol Rev. 2009;29:421–430. doi: 10.1016/j.cpr.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, Lubar JO, Chen TJ, Comings DE. Reward deficiency syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs. 2000;32:1–68. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- Brenner SL, Beauchaine TP. Pre-ejection period reactivity and psychiatric comorbidity prospectively predict substance use initiation among middle-schoolers: A pilot study. Psychophysiology. 2011;48:1587–1595. doi: 10.1111/j.1469-8986.2011.01230.x. [DOI] [PubMed] [Google Scholar]
- Carver CS, Scheier MF. On the self-regulation of behavior. New York: Cambridge University Press; 1998. [Google Scholar]
- Carver CS. Negative affects deriving from the behavioral approach system. Emotion. 2004;4:3–22. doi: 10.1037/1528-3542.4.1.3. [DOI] [PubMed] [Google Scholar]
- Carver CS. Approach, avoidance, and the self-regulation of affect and action. Motiv Emotion. 2006;30:105–110. [Google Scholar]
- Caspi A, Moffitt TE, Thornton A, Freedman D, Amell JW, Harrington H, Smeijers J, Silva PA. The life history calendar: A research and clinical assessment method for collecting retrospective event-history data. International J Methods Psychiatric Research. 1996;6(2):101–114. [Google Scholar]
- Dawe S, Gullo MJ, Loxton NJ. Reward drive and rash impulsiveness as dimensions of impulsivity: Implications for substance misuse. Addict Behav. 2004;29:1389–1405. doi: 10.1016/j.addbeh.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Dawe S, Loxton NJ. The role of impulsivity in the development of substance use and eating disorders. Neurosci Biobehav Rev. 2004;28:343–351. doi: 10.1016/j.neubiorev.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Ebbert JO, Hughes JR, West RJ, Rennard SI, Russ C, McRae TD, Treadow J, Yu CY, Dutro MP, Park PW. effect of varenicline on smoking cessation through smoking reductiona randomized clinical trial. JAMA. 2015;313(7):687–694. doi: 10.1001/jama.2015.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles DC. The three arousal model: Implications of Gray’s two-factor learning theory for heart rate, electrodermal activity, and psychopathy. Psychophysiology. 1980;17:87–104. doi: 10.1111/j.1469-8986.1980.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Fowles DC. Psychophysiology and psychopathology - a motivational approach. Psychophysiology. 1988;25:373–391. doi: 10.1111/j.1469-8986.1988.tb01873.x. [DOI] [PubMed] [Google Scholar]
- Franken IHA, Muris P. Gray’s impulsivity dimension: a distinction between reward sensitivity versus rash impulsiveness. Pers Individ Dif. 2006;40:1337–1347. [Google Scholar]
- Gray JA. Psychophysiological basis of introversion-extraversion. Behav Res Ther. 1970;8:249–266. doi: 10.1016/0005-7967(70)90069-0. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system. 2. Oxford: Oxford University Press; 2000. [Google Scholar]
- Heponiemi T, Keltikangas-Jarvinen L, Puttonen S, Ravaja N. BIS/BAS sensitivity and self-rated affects during experimentally induced stress. Pers Individ Dif. 2003;34:943–957. [Google Scholar]
- Hundt NE, Kimbrel NA, Mitchell JT, Nelson-Gray RO. High BAS, but not low BIS, predicts externalizing symptoms in adults. Pers Individ Dif. 2008;44:565–575. [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Turner RJ, Iwata N. BIS/BAS levels and psychiatric disorder: An epidemiological study. J Psychopathol Behav. 2003;25:25–36. [Google Scholar]
- Kasch KL, Rottenberg J, Arnow BA, Gotlib IH. Behavioral activation and inhibition systems and the severity and course of depression. J Abnorm Psychol. 2002;111:589–597. doi: 10.1037//0021-843x.111.4.589. [DOI] [PubMed] [Google Scholar]
- Matthews G, Gilliland K. The personality theories of H. J. Eysenck and J. A. Gray: a comparative review. Pers Individ Dif. 1999;26:583–626. [Google Scholar]
- Mellick W, Sharp C, Alfano C. The role of BIS/BAS in the vulnerability for depression in adolescent girls. Pers Individ Dif. 2014;69:17–21. [Google Scholar]
- Miranda MI, LaLumiere RT, Buen TV, Bermudez-Rattoni F, McGaugh JL. Blockade of noradrenergic receptors in the basolateral amygdala impairs taste memory. Eur J Neurosci. 2003;18:2605–2610. doi: 10.1046/j.1460-9568.2003.03008.x. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci Biobehav R. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Gray JA. The neuropsychology of anxiety” as it really is: A response to O’ Mara (2001) Neuropsychol Rehabil. 2002;12(4):363–367. [Google Scholar]
- Newman JP, Wallace JF. Diverse pathways to deficient self-regulation - Implications for disinhibitory psychopathology in children. Clin Psychol Rev. 1993;13:699–720. [Google Scholar]
- Quay HC. Inhibition and attention deficit hyperactivity disorder. J Abnorm Child Psych. 1997;25:7–13. doi: 10.1023/a:1025799122529. [DOI] [PubMed] [Google Scholar]
- Rösner S, Hackl-Herrwerth A, Leucht S, Vecchi S, Srisurapanont M, Soyka M. Opioid antagonists for alcohol dependence. Cochrane Database Systematic Reviews. 2010;8(12):CD001867. doi: 10.1002/14651858.CD001867.pub3. [DOI] [PubMed] [Google Scholar]
- Scarpa A, Raine A, Sarnoff MA, Venables PH. Heart rate and skin conductance in behaviorally inhibited Mauritian children. J Abnorm Psychol. 1997;106:182–190. doi: 10.1037//0021-843x.106.2.182. [DOI] [PubMed] [Google Scholar]
- Sherwood A, Allen MT, Fahrenbert J, Kelsey RM, Lovallo WR, van Doornen LJP. Committee report: Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27:1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Tomaka J, Palacios-Esquivel RL. Motivational systems and stress-related cardiovascular reactivity. Motiv Emotion. 1997;21(4):275–296. [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J Clin Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatr. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Kuhlman DM. Personality and risk-taking: Common bisocial factors. J Pers. 2000;68:999–1029. doi: 10.1111/1467-6494.00124. [DOI] [PubMed] [Google Scholar]