Abstract
Capturing both dynamic changes (‘state’) and persistent signatures (‘trait’) directly associated with disease at the molecular level is crucial in modern medicine. The olfactory neural epithelium, easily accessible in clinical settings, is a promising surrogate model in translational brain medicine, complementing the limitations in current engineered cell models.
Keywords: Olfactory neural epithelium, biomarkers, ‘state’ changes, cell models
Investigating molecular biomarkers and mechanisms of brain disorders has been a significant challenge due to the difficulty in obtaining tissues and cells from the central nervous system (CNS) of living patients. As a result, research for brain disorders has used a wide range of models including animal models, postmortem brains, and peripheral cells derived from living patients to understand the pathological mechanisms of disease. Blood samples may be useful as high-throughput resources, but they may not represent neuron-associated molecular signatures. Postmortem brains are valuable resources for studying molecular signatures within the complex neural architectures, but do not reliably provide any molecular information associated with the onset or the course of functional impairments, including the dynamic ‘state’ changes in living patients. Hence, although such samples have contributed significantly to studies of geriatric neurodegenerative disorders, their contribution to an understanding of neurodevelopmental diseases has been more limited.
Recently, cell modeling utilizing reprogramming procedures has been developed to culture human neural cells from living patients in vitro, including induced neuronal cells (iN) and induced pluripotent stem cells (iPSCs)-derived neurons. These engineered cell models may reflect more accurate neural ‘traits’ of brain cells, but molecular and cellular signatures at the time of a biopsy (‘state’ changes) may be lost in the process. Despite being highly informative, these models harbor some limitations that hamper the study of brain disorder pathophysiology [1].
The potential of using the olfactory neural epithelium (OE) (see Box 1) as a surrogate model to study the CNS was examined decades ago [2]. However, the purity of neural cells from biopsied tissues was suboptimal. Although the procedure of nasal biopsy is relatively non-invasive and almost equivalent to a skin-punch biopsy, the efforts to make this process more efficient and even less invasive are emerging.
Box 1. The Olfactory Neural Epithelium (OE).
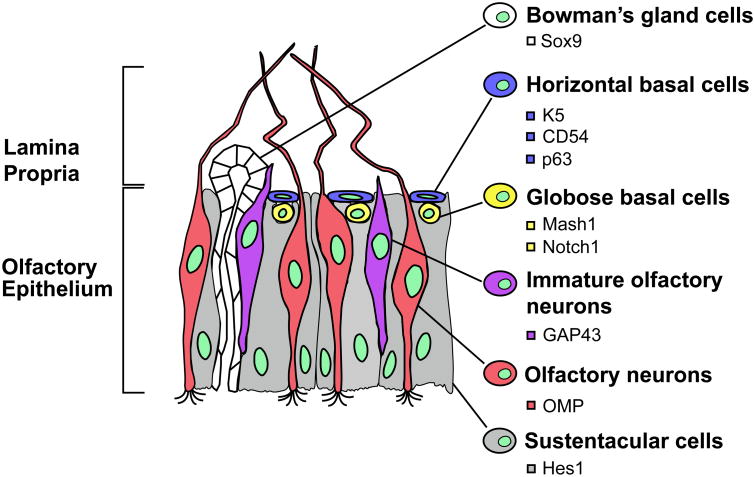
The OE is located in the most superficial layer of the olfactory mucosa containing different cell types, including olfactory neurons, olfactory ensheating cells, sustentacular cells, as well as the horizontal and globose basal cells. Basal cells are considered to be multipotent and/or neural precursor cells that can proliferate and differentiate into either neural or non-neural cells in both humans and rodents. Mature and immature (transitional) olfactory neurons are located in the intermediate layer of the OE, while the apical layer contains sustentacular cells and sensory cilia that are projected from the dendrites of olfactory neurons. The histological scheme is shown in Figure I.
Olfactory neurons are continuously replaced by neurogenesis in the OE throughout adult life. This process is regulated by growth factors that also control neurogenesis in the CNS. An extensive resource about the anatomy and the cellular markers of the olfactory biopsied tissue has been previously published [15].
Figure I. The Human Olfactory Epithelium with Specific Markers for Different Cell Types.
The olfactory neural epithelium is composed of different cell types including Bowman's gland cells, horizontal basal cells, globose basal cells, olfactory neurons (mature and immature), and sustentacular cells. These cell types express specific markers of differentiation, as represented in the anatomical scheme.
Abbreviations: CD54, Cluster of Differentiation 54; GAP43, Growth Associated Protein 43; Hes1, Hairy and Enhancer of Split-1; K5, Keratin 5; Mash1, Mammalian Achaete Scute Homolog-1; Notch1, Neurogenic Locus Notch Homolog Protein 1; OMP, Olfactory Marker Protein; p63, Tumor Protein p63; Sox9, Sry-Related HMG box 9. Adapted from Sawa A and Cascella NG, Am J Psychiatry, 2009, 166(2):137-139.
With recent technical advances overcoming past drawbacks, here, we reintroduce the discussion of using the OE as a useful surrogate model to investigate brain disorders, complementing iPSCs approaches. We argue that using a combination of these models may represent an important strategy to advance our understanding of brain diseases.
Advances in Sample Preparation
Major advances in the use of the OE for translational research include the exploration of less invasive approaches to obtain tissue biopsies as well as increased efforts to enrich and purify neural cells from biopsied tissues.
A Less Invasive Biopsy Approach: The Brush Swab
OE tissue was conventionally obtained by punch biopsy through nasal endoscopy [3]. In contrast, a newly developed brushing technique allows a quicker and less invasive biopsy minimizing or avoiding the use of local anesthesia. A cytology brush is placed in the nasal cavity, and gently rotated to collect epithelial cells. Specimens can be immediately placed in culture media and processed. The exfoliated cells in culture can be propagated to establish neural precursor banks exhibiting cytoskeletal phenotypes of developing neurons [4].
Enriching Neural Cells: Laser-Captured Microdissection and Olfactory Neurospheres
Recently, two major efforts in enriching neural cells from biopsied OE have been undertaken. First, by combining nasal biopsies with laser-captured microdissection (LCM), the neural layer can be selectively isolated from the OE. The use of this technique dramatically increases the relative expression of olfactory marker protein (OMP) - a marker for mature neurons. In contrast, the relative level of aldehyde dehydrogenase 1A3, a nasal submucosal marker, is negligible in microdissected tissue relative to undissected one [5]. Given that one of the major challenges in neuroscience is identifying ‘state’-dependent neural molecular changes, this protocol provides an opportunity to capture molecular snapshots of neural tissue at the time of biopsy. In addition, fresh cells collected from OE by brush swab can be utilized in the future as resources for studying ‘state’ molecular alterations associated with brain disorders in combination with single-cell profiling technologies (Table 1).
Table 1.
Research Utility of OE Tissue and Stem Cell Approaches in Living Humans.
| Olfactory epithelium (OE) | Current stem cell approach | |
|---|---|---|
| ‘Trait’ |
|
|
| ‘State’ changes |
|
Not applicable |
Second, an advantage of using human OE is that it may be possible to obtain stem cells from the olfactory mucosa, propagated as progenitor cells in olfactory neurospheres (ONS) [6]. These cells can be maintained in culture and differentiated into several types of cells such as neurons and glia. While significant disease-specific alterations in the genome and proteome have been reported in ONS-derived cells of patients with schizophrenia, fibroblasts from these patients have failed to show any disease-specific alterations [6], supporting the utility of OE cells in disease models.
Furthermore, OE tissues are currently being processed in other methodologies for translational efforts such as the investigation of ‘trait’ signatures through biochemical and molecular studies. Cultured OE cells have revealed similar gene expression profiles than stem cells and brain tissues, but not blood cells [7]. OE can also be sectioned, fixed and used for histochemistry purposes; indeed, similar histochemical signatures have been reported between olfactory neurons from biopsied OE and autopsied cerebral tissues from Alzheimer's disease (AD) patients [8].
Pros and Cons: What Is Needed in the System?
By properly addressing unanswered questions on this methodology, it may be eventually possible to control its limitations and fine-tune appropriate experimental designs combining different cell models.
Capturing ‘State’ Changes Directly
By using whole OE tissue, LCM-enriched neural tissue, and the brush-swabbed cells, it is possible to investigate ‘state’ markers at the time of biopsy. This may represent an important advantage relative to other models such as iPSCs-derived neurons, where such ‘state’ markers are likely to be ‘erased’ during the course of cell reprogramming and maintenance. For example, OE biopsies combined with LCM have been used to establish a platform to detect neural molecular changes before and after lithium treatment in patients with bipolar disorder [9]. This suggests that, by using this system, it might be possible to investigate molecular ‘state’ changes in response to specific pharmacological treatments, and/or directly associated with brain disease.
No Genetic Reprogramming
A valuable advantage of using OE-derived cells is that they are free from epigenetic changes occurring following a reprogramming procedure - a valid concern in various engineered cell models. It is known that the reprogramming procedure to produce iPSCs leaves some residual epigenetic markers in the resulting iPSCs genome, which possibly interfere with the investigation of differential gene expression and epigenetic processes in brain disorders [10]. In addition, the procedures involved in generating iPSCs and deriving neurons from these are very time-consuming, costly and are not high-throughput. By using OE-derived neurons, confounding events during genetic reprogramming can be bypassed. Thus, we suggest that combining findings from both OE-derived neurons and iPSCs-derived neurons might constitute a powerful complementary strategy to better understand the pathophysiology of certain brain disorders.
Molecular Detection in Clinical Trials
OE tissue appears to be a promising tool in pharmacological research. The OE model was used to measure molecular changes elicited by a drug acting on the CNS since multiple nasal biopsies from the same subject can be performed [11]; the study examined the biological influence of thiamphenicol on the gene expression of excitatory amino acid transporter 2 (EAAT2), in an amyotrophic lateral sclerosis (ALS) clinical trial, as EAAT2 is a putative drug target to treat ALS [12]. Importantly, there are no conventional peripheral tissues that sufficiently express this molecule except for the OE. Consequently, further pharmacological studies should be performed to validate the use of this model as a rapid and accessible tool in drug discovery and clinical trials.
OE as a Drug Route
The use of the olfactory route has become an interesting and promising strategy to deliver some types of drugs into the CNS to treat various brain diseases by bypassing the blood-brain barrier [13]. Considering its safety and non-invasiveness, the direct nose-to-brain route could represent an exciting mode of drug delivery.
Reflection on CNS physiology
As a clear caveat, it is still unclear whether and how cellular and molecular signatures in olfactory receptor neurons accurately reflect those of CNS neurons in living humans. However, molecular profiles of olfactory cells have been shown to be similar to those of mesenchymal stem cells, which can be induced into neuron-like cells or astrocyte-like cells [7]. Human olfactory ecto-mesenchymal stem cells can induce neurogenesis and contribute to the restoration of hippocampal neural networks in mice [14]. This suggests that any ‘Cons’ with regard to the identity of OE cells may not constitute a major concern in translational applications. Nevertheless, a comparison of the molecular profiles obtained from OE-derived cells with those from other experimental resources and models (e.g., iPSCs) from the same individuals will be an important next step. By better understanding the differential gene and pathway expressions, as well as the epigenetic changes between all derived neural models, the experimental challenges will no longer be considered as fatal weaknesses, but rather, as controllable limitations that can be complemented by using different cell models from human subjects.
Various molecular and cellular findings have been documented using OE tissues in psychiatric and neurological conditions including schizophrenia, bipolar disorder, autism, Rett syndrome, fragile X syndrome, AD and Parkinson's disease [15]. Consequently, we posit that OE tissues collected longitudinally and in a relatively non-invasive manner, might serve as a reliable surrogate model to study brain disorders. The possibility to study both ‘state’ and ‘trait’ putative markers of diseases represents an unprecedented advantage in cell modeling. With recent advances in cell engineering technologies and OE tissue collection, we suggest that combining several methodological approaches will undoubtedly enhance our understanding of such diseases.
Acknowledgments
We thank Yukiko L. Lema and Nao J. Gamo for organizing the manuscript and useful discussion. This work was supported by the National Institute of Mental Health MH-084018, MH-094268 Silvio O. Conte center (A.S.), MH-092443 (A.S.), MH-105660 (A.S. and K.I.), as well as foundation grants from Stanley (A.S.), S-R/RUSK (A.S.), NARSAD (A.S. and K.I.), Maryland Stem Cell Research Fund (A.S. and K.I.) and the Canadian Institutes of Health Research (J.L.).
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gamo NJ, Sawa A. Human stem cells and surrogate tissues for basic and translational study of mental disorders. Biol Psychiatry. 2014;75(12):918–9. doi: 10.1016/j.biopsych.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talamo BR, et al. Pathological changes in olfactory neurons in patients with Alzheimer's disease. Nature. 1989;337(6209):736–9. doi: 10.1038/337736a0. [DOI] [PubMed] [Google Scholar]
- 3.Wrobel BB, et al. Assessing the efficacy of endoscopic office olfactory biopsy sites to produce neural progenitor cell cultures for the study of neuropsychiatric disorders. Int Forum Allergy Rhinol. 2013;3(2):133–8. doi: 10.1002/alr.21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benitez-King G, et al. A non-invasive method to isolate the neuronal linage from the nasal epithelium from schizophrenic and bipolar diseases. J Neurosci Methods. 2011;201(1):35–45. doi: 10.1016/j.jneumeth.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Tajinda K, et al. Neuronal biomarkers from patients with mental illnesses: a novel method through nasal biopsy combined with laser-captured microdissection. Mol Psychiatry. 2010;15(3):231–2. doi: 10.1038/mp.2009.73. [DOI] [PubMed] [Google Scholar]
- 6.Matigian N, et al. Disease-specific, neurosphere-derived cells as models for brain disorders. Dis Model Mech. 2010;3(11-12):785–98. doi: 10.1242/dmm.005447. [DOI] [PubMed] [Google Scholar]
- 7.Horiuchi Y, et al. Olfactory cells via nasal biopsy reflect the developing brain in gene expression profiles: utility and limitation of the surrogate tissues in research for brain disorders. Neurosci Res. 2013;77(4):247–50. doi: 10.1016/j.neures.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tabaton M, et al. Abnormal tau-reactive filaments in olfactory mucosa in biopsy specimens of patients with probable Alzheimer's disease. Neurology. 1991;41(3):391–4. doi: 10.1212/wnl.41.3.391. [DOI] [PubMed] [Google Scholar]
- 9.Narayan S, et al. Olfactory Neurons Obtained through Nasal Biopsy Combined with Laser-Capture Microdissection: A Potential Approach to Study Treatment Response in Mental Disorders. J Vis Exp. 2014;(94) doi: 10.3791/51853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tobin SC, Kim K. Generating pluripotent stem cells: differential epigenetic changes during cellular reprogramming. FEBS Lett. 2012;586(18):2874–81. doi: 10.1016/j.febslet.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanza DC, et al. The effect of human olfactory biopsy on olfaction: a preliminary report. Laryngoscope. 1994;104(7):837–40. doi: 10.1288/00005537-199407000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Sattler R, et al. Human nasal olfactory epithelium as a dynamic marker for CNS therapy development. Exp Neurol. 2011;232(2):203–11. doi: 10.1016/j.expneurol.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen MM. Olfactory targeting through intranasal delivery of biopharmaceutical drugs to the brain: current development. Discov Med. 2011;11(61):497–503. [PubMed] [Google Scholar]
- 14.Nivet E, et al. Engraftment of human nasal olfactory stem cells restores neuroplasticity in mice with hippocampal lesions. J Clin Invest. 2011;121(7):2808–20. doi: 10.1172/JCI44489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borgmann-Winter K, et al. Translational potential of olfactory mucosa for the study of neuropsychiatric illness. Transl Psychiatry. 2015;5:e527. doi: 10.1038/tp.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]