Abstract
Background
Preservation of adequate blood flow and exclusion of flow from lesions are key concepts of vascular neurosurgery. Indocyanine green (ICG) fluorescence videoangiography is now widely used for intraoperative assessment of vessel patency.
Objective
Here we present proof of concept investigation of fluorescence angiography with augmented microscopy enhancement (FAAME): real-time overlay of fluorescence videoangiography within the white light field-of-view of conventional operative microscopy.
Methods
The femoral artery was exposed in seven anesthetized rats. The dissection microscope was augmented to integrate real-time electronically processed near-infrared (NIR) filtered images with conventional white light images seen through the standard oculars. This was accomplished using an integrated organic light emitting diode display to yield superimposition of white light and processed NIR images. ICG solution was injected into the jugular vein and fluorescent femoral artery flow was observed.
Results
FAAME was able to detect ICG fluorescence in a small artery of interest. Fluorescence appeared as bright green signal in the ocular overlaid with the anatomical image and limited to the anatomical borders of the femoral artery and its branches. Surrounding anatomical structures were clearly visualized. Observation of ICG within the vessel lumens permitted visualization of the blood flow. Recorded video loops could be reviewed in offline mode for more detailed assessment of the vasculature.
Conclusion
The overlay of fluorescence videoangiography within the field-of-view of the white light operative microscope allows real-time assessment of the blood flow within vessels during simultaneous surgical manipulation. This technique could improve intraoperative decision-making during complex neurovascular procedures.
Keywords: indocyanine green, microscopy, angiography, aneurysm, arteriovenous malformation, fluorescence, intraoperative imaging
INTRODUCTION
Preservation of blood flow in normal vasculature and exclusion of flow from vascular lesions are key concepts of neurosurgery. Even minimal compromise of vessel lumens can cause ischemic changes; conversely, residual flow into a vascular malformation can maintain the catastrophic risk associated with rupture. 1 Several methods exist for intraoperative assessment of blood vessel patency.2–6 Intraoperative digital subtraction angiography (DSA) has been successfully implemented in complex vascular cases. Multiple reports suggest that based on DSA results, 7–34% of cases required additional manipulation to restore optimal blood flow.3, 7–10 While DSA is established as the gold standard for vascular imaging, it requires substantial resources, including additional time and staff to perform the procedure. Intraoperative Doppler ultrasound and flowmetry are effective for detection of major vessel stenosis after aneurysm clip placement. However, these techniques do not have high accuracy and only provide an indirect assessment of vessel lumen compromise.2, 4
Fluorescence angiography with fluorescein sodium and indocyanine green (ICG) was introduced for neurovascular procedures in the 1960s.11 Initially, fluorescence angiography was used for observation of vessels on the brain surface; static images were captured and analyzed after surgery.12 This evolved into modern videoangiography: fluorescence excitation sources and filters integrated into the operative microscope allow acquisition of multiple video loops that can be analyzed intraoperatively.5 Observation of a fluorescent contrast agent introduced into the vasculature allows assessment of local blood flow dynamics.13 Multiple clinical trials have shown the effectiveness of fluorescence angiography in the management of vascular pathologies of the brain and spinal cord. 5, 14–18
Currently, ICG videoangiography is acquired as video loops on an integrated camera after switching the white light optics to a NIR filter set. Light from the field is directed to the camera, and, with the exception of some small background bleed through, the NIR image contains only fluorescence emitted from the ICG while the white light image is recorded independently. We hypothesized that, with sufficient contrast, the angiographic image can be added in real time back to the white light image seen in the oculars. In such fluorescence angiography with augmented microscopy enhancement (FAAME), the relationship between the angiographic data and the rest of the tissue or instrumentation could be directly visualized rather than inferred. Here, we introduce experimental realization of FAAME and present proof of concept investigation of real-time observation of NIR fluorescence angiography augmenting the conventional white light field of view within the oculars of the operating microscope.
METHODS
Microscope design
The augmented microscope integrates in real time the electronically processed near-infrared (NIR) images (synthetic objects), with the visible images (real objects) obtained in the conventional microscope configuration. This is accomplished using the optical see-through display technology, which allows superimposing real and synthetic images. The resultant composite image (augmented reality) is displayed in standard binocular configuration and represents a user-controlled balance of features of interest. The augmented microscope was constructed using a commercial stereomicroscope (Olympus SZX7). Galilean optics employed in this microscope features two (left and right) parallel optical paths, in which modular units can be inserted for desired functionalities. A custom NIR module was developed for simultaneous and independent acquisition of visible and NIR images (Fig 1A).
Figure 1.
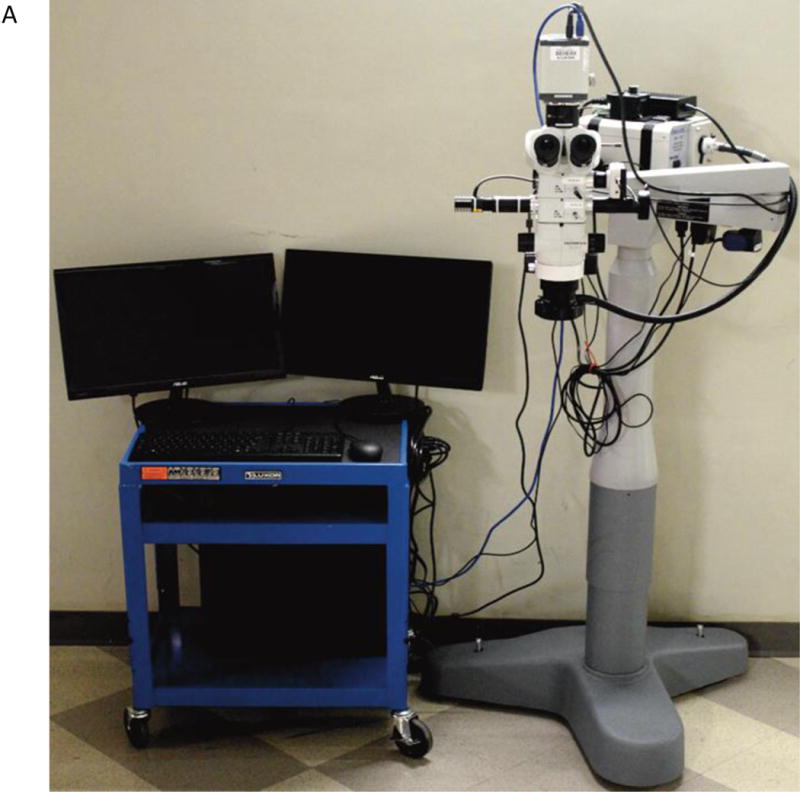
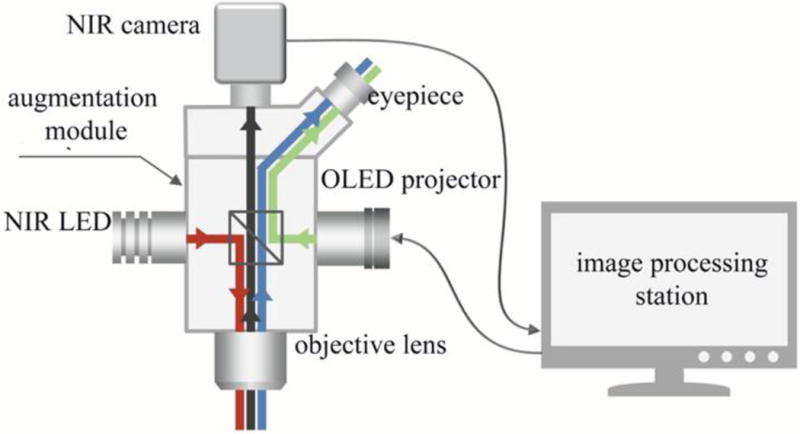
(A) Stereomicroscope modified for FAAME. (B) The optical scheme for the augmented fluorescence microscope. The light from a high power NIR LED is inserted into the microscope optical path using a dichroic mirror (red line). The resulting NIR fluorescence image is separated from the optical path of the microscope by a NIR band pass filter (black line). NIR fluorescence images were captured by the NIR CCD camera and converted into visible green pseudo-color images using the image processing station and a monochromatic OLED projector. The OLED projects the fluorescence image onto a half-mirror placed in the ocular of the microscope, where the processed (synthetic) image overlays with the original (real) visible light image from the objective (green line) to produce augmented, or composite, images. FAAME – fluorescence angiography with augmented microscopy enhancement, NIR – near infrared, LED – light emitting diode, OLED – organic light emitting diode.
In the NIR path, light from a high power LED centered at 780 nm for ICG excitation is inserted into the microscope optical path using a dichroic mirror. The resulting NIR fluorescence image is separated from the optical path of the microscope by a NIR band pass filter matching the emission range of ICG and captured using a complementary metal-oxide-semiconductor (CMOS) sensor with extended sensitivity in near infrared (Orca Flash 4.0, Hamamatsu, Middlesex, NJ). NIR fluorescence images were converted into visible green pseudo-color images using a monochromatic organic light emitting diode (OLED), (eMagin, Bellevue, WA). Light intensity produced by the OLED is representative of fluorescence emission intensity captured by the CMOS. The OLED projects the fluorescence image onto a half-mirror placed in the ocular of the microscope, where the processed (synthetic) image overlays with the original (real) visible light image from the objective. Operation of the NIR path described above retains the original path of the visible image within the microscope, with minimal losses due to the half-mirror. (J. R. Watson et al., Augmented microscopy with near-infrared fluorescence detection, unpublished data, July 2014) (Fig 1B).
In addition, this system provides capability to observe traditional fluorescence images on the monitor screen. Images can be projected onto the monitor in real-time, or retrospectively from the saved video file(s). Images can be projected on the monitor screen in either the visible, NIR, or augmented format. All visual formats are collected simultaneously, but may be visualized separately (visible and/or NIR only) or combined (augmented) as desired. Fluorescence excitation and recording is under user control and can be turned on and off as appropriate.
In Vitro experiments
The first set of experiments was performed using glass tubes (VWR, Radnor, PA), which were filled with 2.3 μg/ml ICG (Akorn, Lake Forest, IL) in 60 g/L albumin solution and sealed. In these experiments the capability of FAAME to produce quality overlay images was assessed, and microscope parameters as well as image capture functions were optimized (Fig 2).
Figure 2.
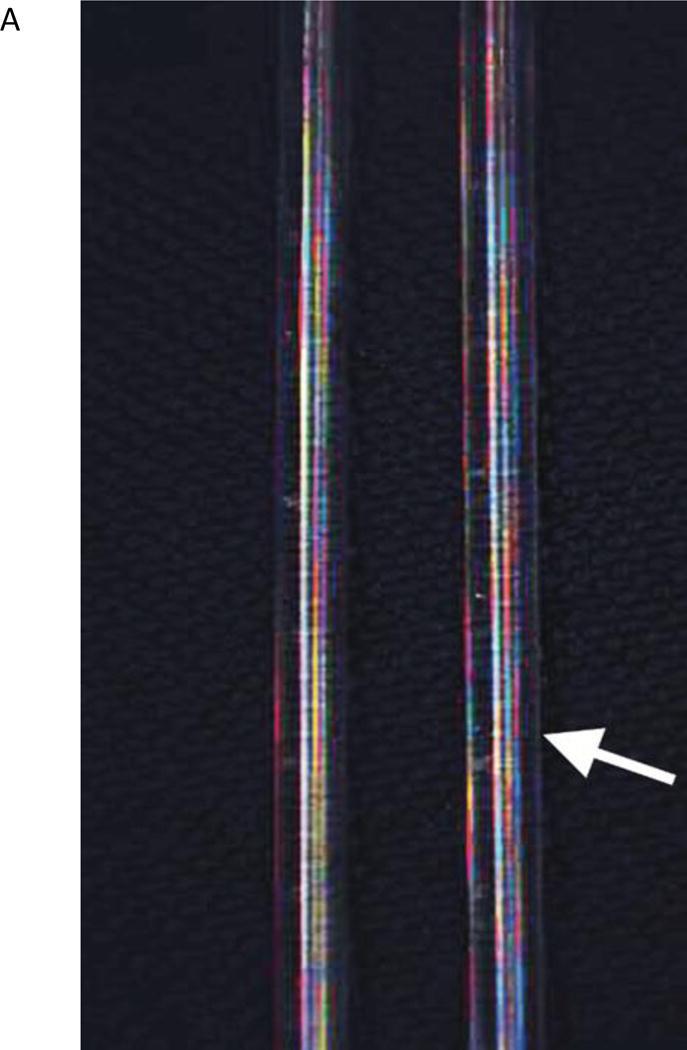
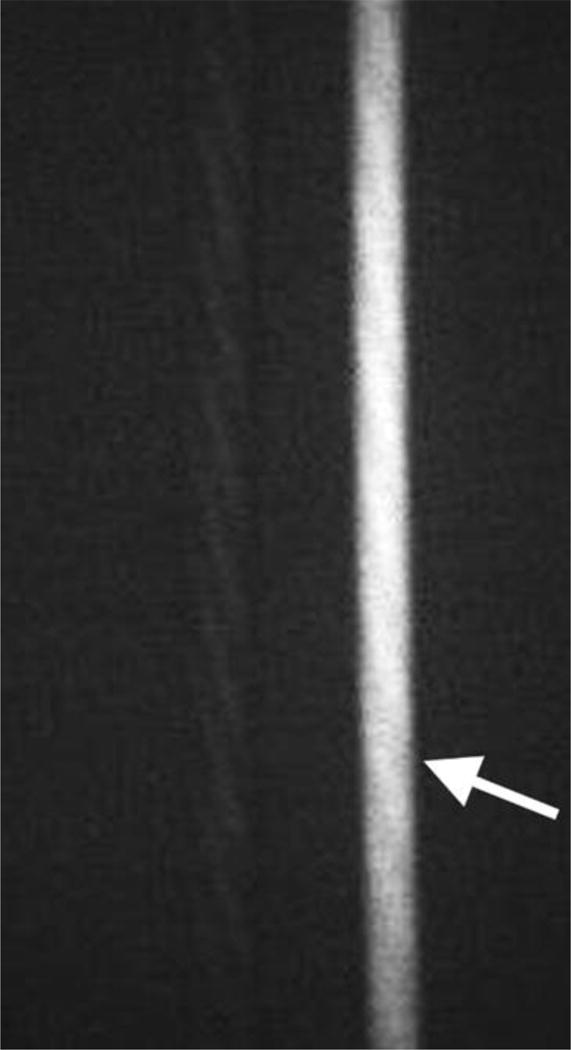
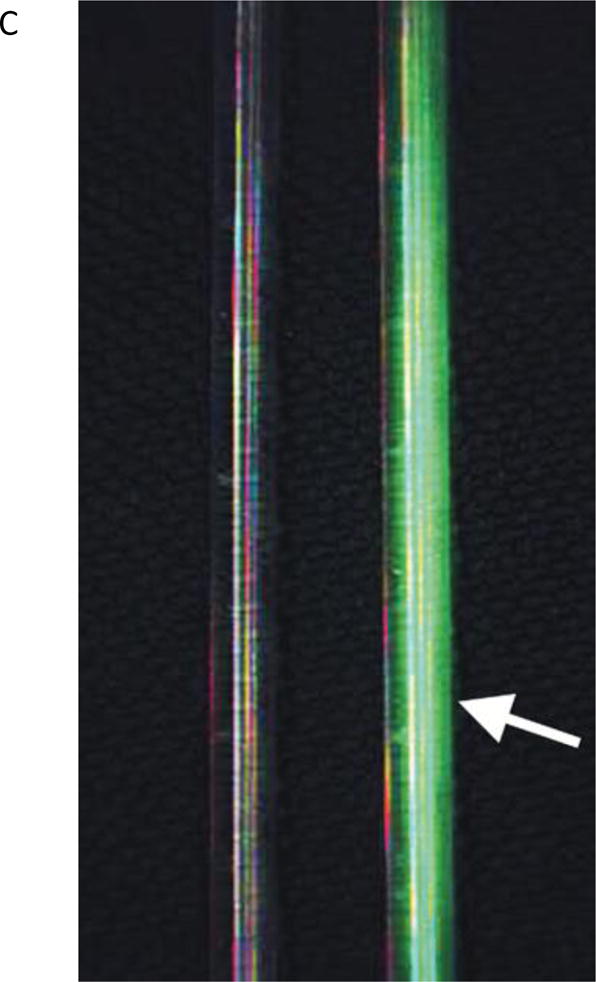
The in vitro images showing two glass tubes, one filled with saline and another with ICG solution (arrow). All images captured using FAAME. (A) Image obtained using visible optical path only. The saline and ICG filled tubes cannot be distinguished. (B) Image obtained using NIR path only. ICG fluorescence pattern delineates the glass tube shape. The saline filled tube cannot be clearly identified. (C) Image obtained by augmentation of the visible light with NIR fluorescence. The saline filled tube appears identical to white light image. Bright ICG fluorescence corresponds to the shape glass tube. NIR – near infrared, ICG – indocyanine green.
Ex Vivo experiments
Once image quality, acquisition, and overlay were optimized, we performed experiments to assess the feasibility of the microscope system to perform ex vivo videoangiography. Turkey wing brachial arteries (n=3) were dissected using microsurgical technique. The proximal ends of the exposed vessels were transected for cannulation. A 16-gauge cannula was inserted into the proximal vessel lumen and secured with a silk tie. The cannula was connected to an infusion system with a flow regulator and the infusion bag was filled with the ICG solution. The augmented microscope was positioned above the vessel and FAAME was then initiated. The infusion of the ICG was initiated after a five-second delay. Using FAAME, the green image representing fluorescence emission was observed as dynamic flow overlaid with the visible vessel. FAAME was also recorded conventionally into video loops and analyzed separately (Fig 3).
Figure 3.
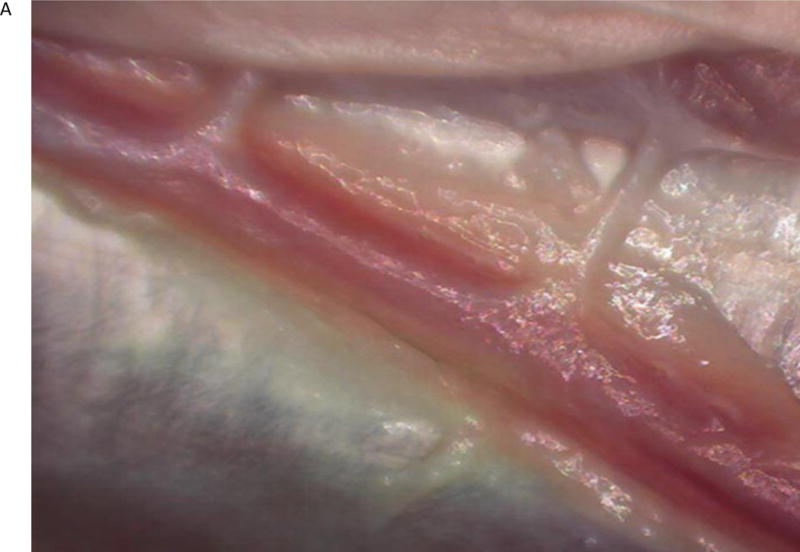

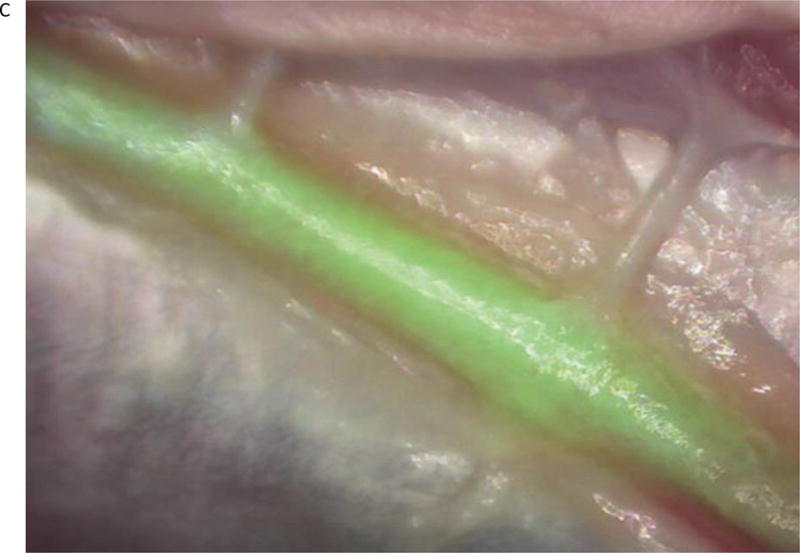
The ex vivo FAAME images of the turkey wing after ICG injection into brachial artery. (A) Image obtained using white light path only. Dissected brachial artery is seen within field of view. (B) Image obtained using NIR path only. ICG fluorescence pattern delineates anatomical boundaries of the brachial artery. The surrounding anatomical structures are not visible. (C) Image obtained by augmentation of the visible light with NIR fluorescence. The ICG fluorescence pattern and surrounding anatomical structures are seen within the field of view. NIR – near infrared, ICG – indocyanine green.
In Vivo experiments
Animal Care
All procedures concerning animal use in this study were approved by the local Institutional Animal Care and Use Committee.
Three-month-old female Sprague Dawley rats (n=7) weighing an average body weight of 350 grams were anesthetized by an intramuscular injection of Ketamine/Xylazine/Acepromazine cocktail. The animals were placed supine and the femoral arteries and jugular veins were exposed using standard microsurgical technique under the augmented microscope. The microscope was positioned to image the femoral artery and FAAME was then initiated. Using a 1 cc syringe and a 30 gauge needle, 0.3 ml of 0.3 mg/ml ICG solution (a 0.3 mg/kg dose) was injected under direct visualization into the jugular vein. High contrast fluorescent signal was visible in the oculars presenting a dynamic pattern of real-time flow into the femoral artery and its branches. Video loops were recorded and replayed for further analysis. After completion of experiments, animals were euthanized according to institutional guidelines (Fig 4, Video).
Figure 4.
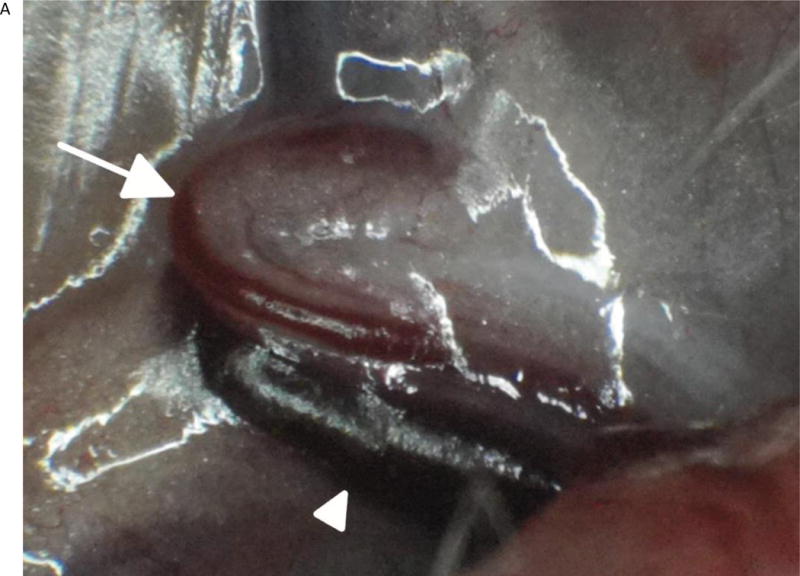

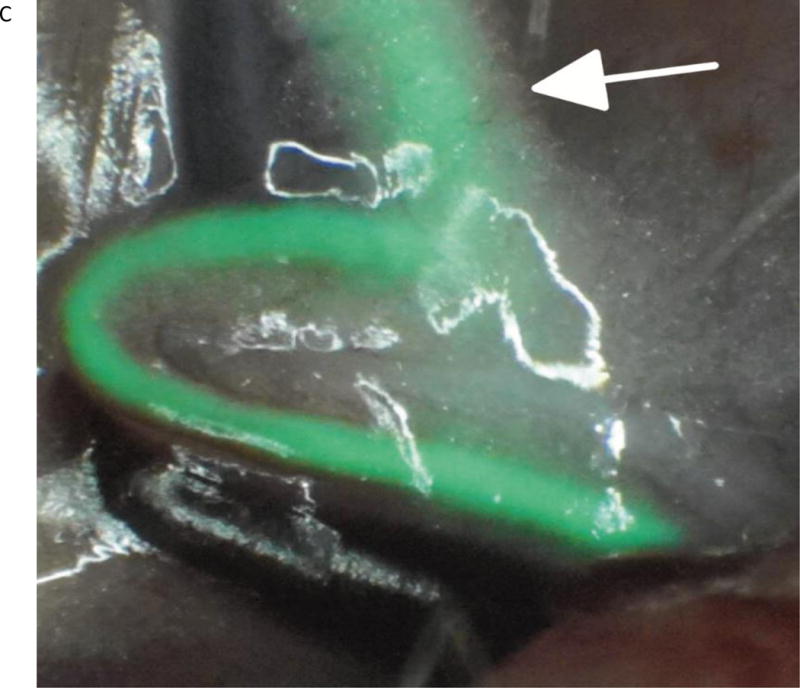
The in vivo images using FAAME of the femoral artery of the rat after ICG injection into jugular vein. (A) Image obtained using white light path only. Exposed femoral artery (arrow) and femoral vein (arrowhead) are visible within the field of view. (B) Image obtained using NIR path only. ICG fluorescence is observed within anatomical borders of femoral artery. The surrounding anatomical structures cannot be identified. (C) Image obtained by augmentation of the visible light with NIR fluorescence. The anatomical structures within the field of view can be identified. The ICG fluorescence appears as a bright green signal within anatomical borders of the femoral artery. The segment of the femoral artery covered by fat and connective tissue is clearly delineated (arrow). NIR – near infrared, ICG – indocyanine green.
RESULTS
As a result of in vitro experiments, the process of device calibration was simplified and image acquisition parameters were optimized. In order to calibrate the augmented microscope, we ensured proper co-registration of the on lay fluorescence image with the real-time white light image to reproduce shapes and sizes of test objects. The proper co-registration of visible (real) and near-infrared (synthetic) images is accomplished before use with a calibration phantom. Using computer controls, the synthetic image projected by OLED is repositioned until it is aligned with the real image, as monitored within the eyepieces of the microscope. Since the visible and NIR images share the same optical pathway, once aligned, the microscope maintains proper co-registration throughout working conditions. Therefore, visible/NIR co-registration is maintained through the full range of optical zoom setting (0.8–5.6×) and positions of the articulated arm, with no need to re-adjust (Fig 2). We found alignment drift to be minimal on our system and only performed routine re-calibration after physically moving the scope to different locations.
FAAME produced high contrast pseudo-colored real-time green angiograms of ex vivo and in vivo microvasculature samples. The overlaid ICG angiograms were not disruptive to the white light images and could be adjusted or eliminated on demand. They appeared as a bright green signal within anatomic boundaries of the vessel wall. The direction of flow of the angiogram confined within the vessels was accurate. Adjacent anatomical structures, e.g., skin, muscles, were also visible as with conventional surgical microscope. The segment of the artery covered by fat and connective tissue could be identified as well. During FAAME, at no point did the surgeon need to look away from the oculars or lose track of the view of the operative field. The ICG solution concentration and diameter of the vessels visualized (1.5 mm ± 0.3 mm) were consistent with common operative neurovascular conditions. The optics and electronics used are all amenable to integration with operating room microscopes (Figs 3, 4, Video).
DISCUSSION
FAAME maintains full direct stereoscopic visualization of white light anatomy in conjunction with the ICG angiogram viewed as a pseudo-colored overlay. Using a modified commercial stereomicroscope and a live rat model with small vessels, this study demonstrates the feasibility of FAAME for use in vascular neurosurgical procedures (Figs 2, 3, 4, Video).
ICG injection
We used 2.3 μg/ml ICG in 60 g/L albumin solution for the ex vivo experiments. ICG exhibits fluorescence when dissolved in blood/albumin. This concentration does not necessarily reflect amount of ICG used in patients. However, it provided a grossly equivalent intensity of fluorescence for in vitro/ex vivo experiments where flow and metabolism are not present. The goal of in vitro/ex vivo experiments was to optimize parameters for static and dynamic image acquisition. Final compatibility tests to emulate clinical settings were in vivo experiments in rats. The usual dose for ICG videoangiography in clinical settings ranges from 0.2 to 0.5 mg/kg, and the total daily dose should not exceed 5 mg/kg.5 Thus, FAAME allowed sufficient and useful visualization of ICG fluorescence in vivo at an ICG concentration routinely used in clinical practice (0.3mg/kg).
ICG videoangiography
ICG videoangiography has become a widely disseminated tool in the vascular neurosurgical armamentarium. Reviewing the isolated black and white angiography video loops provides comprehensive information about aneurysm and parent vessel patency, vascular architecture within surgical field, obliteration status of arteriovenous pathology, direction of blood flow, and assessment of bypass grafts and collateral blood flow.14 Raabe et al. introduced micro videoangiography to the neurosurgical audience in 2003 using a system separate from the operative microscope.5 Neurosurgeons and microscope manufactures quickly recognized the merit of this technology and integrated it into the surgical microscope.6, 18, 19 Since then, software-based analysis of ICG angiographic video has emerged as a method of quantitative analysis of blood-flow.13 FAAME merges existing imaging technologies to simultaneously present information available from an intraoperative angiogram while maintaining all the current capabilities of microscope-integrated ICG angiography.
Advantages of FAAME
Currently, ICG NIR fluorescence is captured before or after manipulation and analyzed on a video monitor. The surgeon may simultaneously perform manipulations while observing fluorescent angiography on the screen or, in some cases, as a video signal injected directly into the oculars; however, the angiogram remains separate from the white light image. The system introduced here allows flow assessment and manipulation of tissue and vasculature during microangiography with instant and accurate feedback regarding how such manipulation alters the blood flow. We also noted that this technique helped identify small vessels buried in a thin covering of the connective tissue that became more obvious with the direct anatomical correlates than they were on the ICG only vascular map. The ability to see and manipulate small vessels covered with a thin tissue using a strong contrast agent like ICG could be helpful in a number of instances including dissection of the distal superficial temporal artery for bypass procedures.
During surgical management of arteriovenous malformations, it is crucial to identify feeding and draining vessels. In cases with a large nidus with numerous convoluted vessels, it can be challenging to correlate videoangiography data with the microscopic white light image to properly detect a vessel of interest. This limitation could be overcome by using FAAME which provides an instant merged view of the surgical field and conventional fluorescent angiography. The direction of blood flow can be identified in any vessel visible within the surgical field. Furthermore, the pseudo-color video loop overlay can be replayed long after the fluorescence signal has faded from the field. A static or animated vascular roadmap can be maintained or recalled with ease at any point during the surgery.
FAAME shares many of the advantages of fluorescein microangiography, which has recently been described as a commercially available operative microscope upgrade.20 Fluorescein has an emission spectrum that can be captured by a wide bandpass filter sets. This allows a large amount of multispectral light into the oculars along with the bright yellow-green emission of the fluorophore. Under such conditions, fluorescein emission is easily detectable over the background as it has a peak wavelength that the human fovea is very sensitive to. Nonetheless, as the white light image and fluorescein emission share the spectral bandwidth, fluorescence angiography is compromised by the decreased image intensity and loss of color accuracy. Furthermore, unconjugated fluorescein is a small molecule that tends to stain brain tissue if it comes into direct contact. Disruptions in the blood-brain barrier or even small amounts of bleeding can cause the contrast agent to leak and produce background noise if it is retained in the surrounding tissue. Having a specifically filtered, low background, high contrast ICG angiogram that can be modulated as it is sent back to the oculars allows for fine tuning not permitted by fluorescein microangiography alone.
Conclusion
FAAME technology provides an interactive modular method of visualization of anatomical structures and fluorescent blood flow patterns. We believe that FAAME will improve microvascular neurosurgical techniques and potentially improve patient safety and outcomes. Use of a pseudo-colored overlaid angiogram will be subject to some of the same issues as conventional ICG angiography, such as potential for accumulation of the background signal after multiple doses. The combined white light and pseudo-colored images may lack some of the contrast seen in the conventional video angiogram. However, implementation of FAAME should be completely non-disruptive to existing ICG angiography functionality as this is purely an adjunctive feature. Another avenue being pursued is fully stereoscopic implementation of FAAME such that both the white light and the fluorescence angiogram can be seen simultaneously with true stereoscopy. Integration of FAAME technology into existing operative microscopes will likely be feasible using the augmentation module provided as a modular upgrade. The upgrade path may be significantly simplified in certain surgical microscopes that support image injection into the eyepieces. Further testing is needed to determine the feasibility of FAAME technology in management of neurovascular pathology.
Supplementary Material
Video. The in vivo video loop obtained from the femoral artery of the rat after ICG injection into the jugular vein. The femoral artery and vein are visible within the field of view. Once ICG enters the blood stream it appears as a bright green fluorescence signal confined within the anatomical boundaries of the femoral artery. The direction of the blood flow can be identified. The segment of the femoral artery covered by fat and connective tissue is clearly delineated.
Acknowledgments
Financial support: This work was supported by a National Institutes of Health career development award CA120350 to Marek Romanowski, PhD. Jeffrey Watson was supported by NIH training grant HL007955. Other funding for this project came from the Division of Neurosurgery at the University of Arizona grant to Nikolay L. Martirosyan, MD.
Footnotes
Disclosure: The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
References
- 1.Karhunen PJ. Neurosurgical vascular complications associated with aneurysm clips evaluated by postmortem angiography. Forensic Sci Int. 1991 Oct;51(1):13–22. doi: 10.1016/0379-0738(91)90202-t. [DOI] [PubMed] [Google Scholar]
- 2.Siasios I, Kapsalaki EZ, Fountas KN. The role of intraoperative micro-Doppler ultrasound in verifying proper clip placement in intracranial aneurysm surgery. Neuroradiology. 2012 Oct;54(10):1109–1118. doi: 10.1007/s00234-012-1023-y. [DOI] [PubMed] [Google Scholar]
- 3.Alexander TD, Macdonald RL, Weir B, Kowalczuk A. Intraoperative angiography in cerebral aneurysm surgery: a prospective study of 100 craniotomies. Neurosurgery. 1996 Jul;39(1):10–17. doi: 10.1097/00006123-199607000-00004. discussion 17–18. [DOI] [PubMed] [Google Scholar]
- 4.Amin-Hanjani S, Meglio G, Gatto R, Bauer A, Charbel FT. The utility of intraoperative blood flow measurement during aneurysm surgery using an ultrasonic perivascular flow probe. Neurosurgery. 2008 Jun;62(6 Suppl 3):1346–1353. doi: 10.1227/01.neu.0000333799.44338.19. [DOI] [PubMed] [Google Scholar]
- 5.Raabe A, Beck J, Gerlach R, Zimmermann M, Seifert V. Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery. 2003 Jan;52(1):132–139. doi: 10.1097/00006123-200301000-00017. discussion 139. [DOI] [PubMed] [Google Scholar]
- 6.Bacigaluppi S, Fontanella M, Manninen P, Ducati A, Tredici G, Gentili F. Monitoring techniques for prevention of procedure-related ischemic damage in aneurysm surgery. World Neurosurg. 2012 Sep-Oct;78(3–4):276–288. doi: 10.1016/j.wneu.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 7.Origitano TC, Schwartz K, Anderson D, Azar-Kia B, Reichman OH. Optimal clip application and intraoperative angiography for intracranial aneurysms. Surg Neurol. 1999 Feb;51(2):117–124. doi: 10.1016/s0090-3019(97)00529-6. discussion 124–118. [DOI] [PubMed] [Google Scholar]
- 8.Derdeyn CP, Moran CJ, Cross DT, 3rd, Sherburn EW, Dacey RG., Jr Intracranial aneurysm: anatomic factors that predict the usefulness of intraoperative angiography. Radiology. 1997 Nov;205(2):335–339. doi: 10.1148/radiology.205.2.9356612. [DOI] [PubMed] [Google Scholar]
- 9.Chiang VL, Gailloud P, Murphy KJ, Rigamonti D, Tamargo RJ. Routine intraoperative angiography during aneurysm surgery. J Neurosurg. 2002 Jun;96(6):988–992. doi: 10.3171/jns.2002.96.6.0988. [DOI] [PubMed] [Google Scholar]
- 10.Tang G, Cawley CM, Dion JE, Barrow DL. Intraoperative angiography during aneurysm surgery: a prospective evaluation of efficacy. J Neurosurg. 2002 Jun;96(6):993–999. doi: 10.3171/jns.2002.96.6.0993. [DOI] [PubMed] [Google Scholar]
- 11.Feindel W, Hodge CP, Yamamoto YL. Epicerebral angiography by fluorescein during craniotomy. Prog Brain Res. 1968;30:471–477. doi: 10.1016/S0079-6123(08)61500-9. [DOI] [PubMed] [Google Scholar]
- 12.Feindel W, Yamamoto YL, Hodge CP. Intracarotid fluorescein angiography: a new method for examination of the epicerebral circulation in man. Can Med Assoc J. 1967 Jan 7;96(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuda K, Kataoka H, Nakajima N, Masuoka J, Satow T, Iihara K. Efficacy of FLOW 800 With Indocyanine Green Videoangiography for the Quantitative Assessment of Flow Dynamics in Cerebral Arteriovenous Malformation Surgery. World Neurosurg. 2014 Jul 18; doi: 10.1016/j.wneu.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Raabe A, Nakaji P, Beck J, et al. Prospective evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green videoangiography during aneurysm surgery. J Neurosurg. 2005 Dec;103(6):982–989. doi: 10.3171/jns.2005.103.6.0982. [DOI] [PubMed] [Google Scholar]
- 15.de Oliveira JG, Beck J, Seifert V, Teixeira MJ, Raabe A. Assessment of flow in perforating arteries during intracranial aneurysm surgery using intraoperative near-infrared indocyanine green videoangiography. Neurosurgery. 2007 Sep;61(3 Suppl):63–72. doi: 10.1227/01.neu.0000289715.18297.08. discussion 72–63. [DOI] [PubMed] [Google Scholar]
- 16.Woitzik J, Horn P, Vajkoczy P, Schmiedek P. Intraoperative control of extracranial-intracranial bypass patency by near-infrared indocyanine green videoangiography. J Neurosurg. 2005 Apr;102(4):692–698. doi: 10.3171/jns.2005.102.4.0692. [DOI] [PubMed] [Google Scholar]
- 17.Killory BD, Nakaji P, Gonzales LF, Ponce FA, Wait SD, Spetzler RF. Prospective evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green angiography during cerebral arteriovenous malformation surgery. Neurosurgery. 2009 Sep;65(3):456–462. doi: 10.1227/01.NEU.0000346649.48114.3A. discussion 462. [DOI] [PubMed] [Google Scholar]
- 18.Hanggi D, Etminan N, Steiger HJ. The impact of microscope-integrated intraoperative near-infrared indocyanine green videoangiography on surgery of arteriovenous malformations and dural arteriovenous fistulae. Neurosurgery. 2010 Oct;67(4):1094–1103. doi: 10.1227/NEU.0b013e3181eb5049. discussion 1103–1094. [DOI] [PubMed] [Google Scholar]
- 19.Dashti R, Laakso A, Niemela M, Porras M, Hernesniemi J. Microscope-integrated near-infrared indocyanine green videoangiography during surgery of intracranial aneurysms: the Helsinki experience. Surg Neurol. 2009 May;71(5):543–550. doi: 10.1016/j.surneu.2009.01.027. discussion 550. [DOI] [PubMed] [Google Scholar]
- 20.Rey-Dios R, Cohen-Gadol AA. Technical principles and neurosurgical applications of fluorescein fluorescence using a microscope-integrated fluorescence module. Acta Neurochir (Wien) 2013 Apr;155(4):701–706. doi: 10.1007/s00701-013-1635-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video. The in vivo video loop obtained from the femoral artery of the rat after ICG injection into the jugular vein. The femoral artery and vein are visible within the field of view. Once ICG enters the blood stream it appears as a bright green fluorescence signal confined within the anatomical boundaries of the femoral artery. The direction of the blood flow can be identified. The segment of the femoral artery covered by fat and connective tissue is clearly delineated.


