Abstract
BACKGROUND:
We report a meta-analysis of recent studies comparing the diagnostic yields of endobronchial ultrasonography plus fluoroscopically-guided transbronchial biopsy (EBUS + TBB) with that of conventional fluoroscopically-guided TBB for peripheral pulmonary lesions (PPLs).
METHODS:
We searched Medline, the Cochrane Library, PubMed, and Google Scholar through 31 March 2013 using the keywords: lung neoplasm, pulmonary lesions, diagnosis, endobronchial ultrasound, fluoroscopy, and fluoroscopic.
RESULTS:
Four studies were included in the study with a total of 461 patients, 222 in the EBUS + TBB group and 239 in the TBB only group. The meta-analysis revealed that the group with EBUS + TBB was more favored in terms of positive diagnostic yield than the group diagnosed with only conventional TBB (odds ratio [OR] = 2.211, 95% confidence interval [CI] = 1.422–3.438, P < 0.001). Subgroup analysis based on lesion size found that smaller PPLs had higher accuracy (OR = 4.502, 95% CI = 2.002–10.126, P < 0.001) than PPLs of large size (OR = 1.849, 95% CI = 1.033–3.311, P = 0.039).
CONCLUSION:
Obtaining TBB samples for histopathological diagnosis is enhanced by the addition of EBUS to conventional fluoroscopic guidance; this is, especially important for patients with small peripheral lung lesions who benefit greatly from early diagnosis.
Key words: Endobronchial ultrasound, fluoroscopy-guided, lung biopsy, meta-analysis, peripheral pulmonary lesion
Two key factors are important in improving survival rates of lung cancer: Early detection of peripheral pulmonary lesions (PPLs) and histopathologic diagnosis of biopsy samples of the lesions. Diagnostic procedures for highly prevalent lung cancer are crucial to its early detection, which can have a major benefit for patient survival. According to the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute of the USA report, the 5-year-survival rate in patients with early stages of lung cancer is 31%–45% compared with only 1%–5% for later stages.[1] Histopathologic examination of a biopsy sample is also necessary for directing the choice of treatment. Because many of the patients are older and may be debilitated, less-invasive procedures than surgical biopsy are preferred.
Transchronial biopsy (TBB) has been used extensively to localize the lung lesions.[2] Before computer tomography (CT), TBB was typically guided by fluoroscopy. A brush or forceps inserted through the bronchoscope is used to obtain a biopsy sample. The procedure has few complications and is less invasive than transdermal biopsy or surgery, but has lower diagnostic yields.[2] Diagnostic yield is affected by patient movement and locating the lesion in the three-dimensional lung space using fluoroscopy, which gives a two-dimensional image, can be difficult. Published diagnostic yields of TBB for PPLs that are <2 cm in diameter vary from 5% to 75%.[3]
The addition of endobronchial ultrasonography (EBUS) to TBB has shown promise in improving the diagnostic yield of PPLs.[4] EBUS enables clear visualization of a lesion surrounding or adjacent to a bronchus, which simplifies the biopsy procedure. In addition to being more likely to yield a diagnosis, EBUS also enables the lesion to be found and sampled more quickly particularly for PPLs <30 mm, reducing the time that patients and staff are exposed to fluoroscopy radiation, especially evident for PPLs smaller than 30 mm.[5] The use of a sheath to guide the flexible bronchoscope probe is a further refinement to the procedure, and has improved the rate and decreased the time of peripheral lung cancer diagnosis.[6,7]
A limited number of studies have compared the effectiveness of EBUS and traditional fluoroscopic methodology in diagnosing PPLs. The purpose of this meta-analysis was to compare the diagnostic yields of EBUS plus fluoroscopically-guided TBB (defined as EBUS + TBB group) with that of fluoroscopically-guided TBB without EBUS (defined as TBB group) for PPLs obtained via brushes or transbronchial forceps in patients referred for diagnostic bronchoscopy. In this report, a PPL is defined as a pulmonary lesion surrounded by lung parenchyma and not endoscopically visible by bronchoscopy (<30 mm in diameter). The primary outcome was the diagnostic yield for PPLs.
Methods
Search strategy
Medline, the Cochrane Library, PubMed, and Google Scholar were searched from inception through 31 March 2016 using the following key words: Lung neoplasm, pulmonary lesions, diagnosis, endobronchial ultrasound, fluoroscopy, and fluoroscopic. Reference lists of relevant studies were hand-searched to identify other potentially pertinent studies not identified in the database search.
Eligibility criteria
Studies were included if they were randomized clinical trials, two-arm prospective studies, or retrospective studies. In included studies, patients had CT-scan evidence of PPLs referred for diagnostic bronchoscopy or had received radial EBUS-guided bronchoscopy with or without a guide sheath (GS), and with or without fluoroscopic guidance. Studies were excluded if they were cohort studies, letters, comments, editorials, case reports, meeting proceedings, or personal communications; if they had no quantitative primary outcome, or if the patients had centrally-located pulmonary lesions.
Study selection and data extraction
Two independent reviewers identified the studies to be included and where there was uncertainty regarding eligibility, a third reviewer was consulted.
Information and data extracted from the studies that met the inclusion criteria included the name of the first author, year of publication, study design, number of participants in each group, participants' age and gender, and outcomes of interest.
Quality assessment
The selected studies were observational. Therefore, we used the Newcastle–Ottawa scale (NOS) for cohort studies to assess the quality of the data.[8] The NOS is a validated tool to evaluate the quality of nonrandomized studies in three broad areas: Enrollment criteria, comparability of study groups, and the assessment of outcomes. The quality of each study was scored on the basis the areas mentioned above, with four of the nine total points indicating the quality of the patient selection; two points indicating the quality of between-group comparability, and three points for the quality of the outcome assessment. A score of nine indicates the highest quality, a score of 0, the lowest. Two independent reviewers utilized the NOS list to assess the quality of the included studies.
Statistical analysis
The primary outcome is the diagnostic yield of PPLs using EBUS + TBB or using TBB only. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to compare EBUS + TBB to TBB quantitatively. An OR >1 indicated that the EBUS + TBB method was favored, whereas an OR <1 implied that TBB was favored. Heterogeneity was assessed using the Cochran Q test and the indicator I2. If the P value of Cochran is <0.1 or I2 is >50%, an obvious heterogeneity between studies exists, and a random-effects model (DerSimonian-Laird method) was used. Otherwise, the fixed-effects model was used (Mantel-Haenszel method). Studies including data on dichotomous size of PPLs (i.e., cut-off between small and large size, 20 or 30 mm depending on the study) were grouped for subgroup analysis, and the same computations of ORs as well as 95% CIs were performed for each group. Sensitivity analysis was performed based on the leave-one-out approach. A two-tailed P < 0.05 was considered statistically significant. The meta-analysis for PPL evaluation was conducted using the Comprehensive Meta-analysis software, version 2.0 (Biostat, Englewood, NJ, USA).
Results
Literature search
As shown in Figure 1, our search found 44 full-text articles that were assessed for eligibility. Of these, 22 were excluded because they were single-arm studies, eleven because they used a different comparator groups, 3 due to not reporting outcomes of interest, two were not relevant to the study, and two were a duplicate. Four studies were included in the qualitative review and three in our quantitative meta-analysis.
Figure 1.

Preferred Reporting Items for Systematic Reviews and Meta-analyses flow diagram
Study characteristics
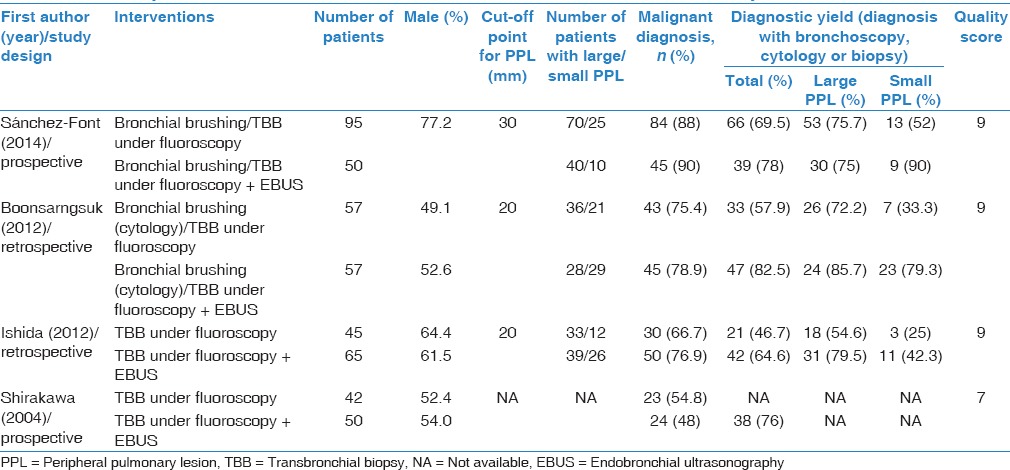
Characteristics of the four studies are summarized in Table 1. Four hundred and sixty-one patients were included, with a range of 42–95 patients the TBB group and a range of 50–65 patients in the EBUS + TBB group. Diagnostic yield rates ranged from 46.7% to 69.5% in the TBB group and from 64.6% to 82.5% in the EBUS + TBB group (P < 0.001).
Table 1.
Summary of characteristics and outcomes of selected studies for meta-analysis
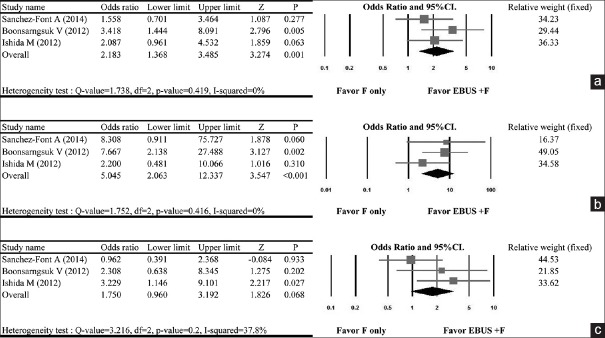
Rate of diagnostic yield
Three studies were included in the meta-analysis for diagnostic yield.[9,10,11] Shirakawa et al., 2004 was not included as the study did not report diagnostic yield. A fixed-effect model was used as the no heterogeneity was observed across the data (Q statistic of fixed-effect model = 1.738; I2 = 0%; P = 0.419). The pooled analysis indicated that the EBUS + TBB was more favored with respect to diagnostic yield than the TBB group (OR = 2.183, 95% CI = 1.368–3.485, P = 0.001) [Figure 2a]. The advantage of EBUS + TBB compared with TBB alone was even more evident in subgroup analysis that evaluated the diagnostic yield of “small” PPLs (i.e., <20 mm) (OR = 5.045, 95% CI = 2.063–12.337, P < 0.001) [Figure 2b]. The odds of achieving a greater diagnostic yield with EBUS + TBB was also apparent in the subgroup analysis of “large” PPLs (i.e., ≥20 mm); although, this did not reach statistical significance (OR = 1.750, 95% CI = 0.960–3.192, P = 0.068) [Figure 2c].
Figure 2.
Forest plot of the diagnostic yield using endobronchial ultrasound plus fluoroscopy-guided bronchoscopy compared with fluoroscopy guidance only on peripheral pulmonary lesions based on including (a) all peripheral pulmonary lesions, (b) only small size peripheral pulmonary lesions, and (c) only large size peripheral pulmonary lesions. CI = confidence interval; EBUS + F = endobronchial ultrasound plus fluoroscopy, F = fluoroscopy only
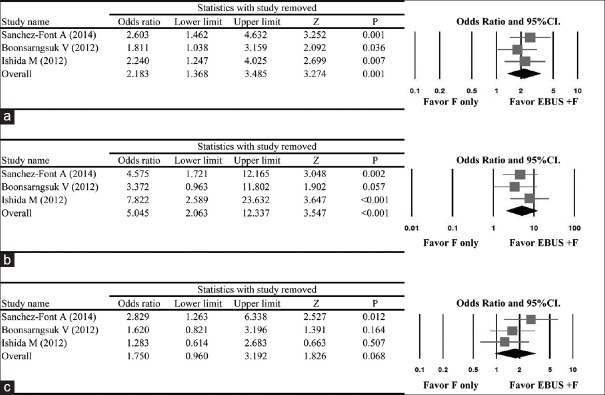
Sensitivity analysis
The results of the sensitivity analysis in which the studies were omitted one at a time indicated that removal of any one study did not significantly change the direction or magnitude of the pooled ORs for the overall data [Figure 3a] or for the subgroup analysis which evaluated diagnostic yield by size of PPL [Figure 3b and c].
Figure 3.
Results of sensitivity analysis to examine the influence of individual studies on pooled estimates as determined using the leave-one-out approach for (a) all studies included, (b) studies including small size peripheral pulmonary lesions, and (c) studies including large size peripheral pulmonary lesions. CI = confidence interval; EBUS + F = endobronchial ultrasound plus fluoroscopy; F = fluoroscopy only
Quality assessment
Table 1 shows the quality score (range 0–9; higher scores indicate better quality) for the four studies included in the analysis. Across the studies, the scores ranged from;[7,8,9] therefore the studies are of adequate quality. Only one study Shirakawa et al.,[6] did not score on the comparability section due to the lack of adjusted variable controls.
Discussion
PPLs are difficult to evaluate using bronchial biopsies due to limited access. Conventional methods for diagnosing PPLs use fluoroscopy as guidance during TBB. However, the method is hindered by shifting of the marked location during the biopsy procedure. Some studies suggest that the addition of EBUS to TBB may improve biopsy results. The purpose of this study was to compare the effectiveness of EBUS + TBB to TBB alone in diagnosing PPLs. Four studies were included in the analysis. The results showed that fluoroscopy combined with EBUS had a higher diagnostic yield in PPL biopsies compared with TBB. Subgroup analysis indicated that the increased yield is particularly apparent when lesions were small (<30 mm in diameter). These findings suggest that EBUS + TBB improves diagnostic yield for PPL biopsies overall, and especially smaller lesions. This is of clinical importance as smaller PPLs are difficult to locate with fluoroscopy. The ability to collect biopsy samples of smaller lesions that yield a histopathology diagnosis allows earlier treatment and increases the probability of survival. It also reduces the number of patients who need a second procedure to obtain a suitable biopsy sample.
Two of the four included studies reported complication rate/adverse events. The study of Ishida et al. reported one patient in each group with pneumothorax, and the study of Sánchez-Font et al. reported nine patients with minor bleeding; however, they did not specify the incidence of bleeding per treatment arm. Although the data are limited, these findings suggest that EBUS + TBB are well tolerated. The four studies did not describe the type of anesthesia used. It is possible that the choice of anesthesia could impact complication rate and length of time required for the procedure.
Our findings suggest that adding EBUS to TBB increases diagnostic yield of PPLs. Prior studies have indicated the importance of probe position with respect to diagnostic yield and that PPL size can influence diagnostic yield.[12] Chen et al.[12] summarized their 5-year experience for diagnosis of PPLs. Using EBUS as the only guide, they found the nodule in 446 of 467 (96%) of cases. When the radial probe position was within the target lesion, the diagnostic yield was 84% compared with 48% when the probe was positioned adjacent to the lesion. Overall, they obtained a diagnostic biopsy sample in 321 of 467 (69%) patients. Their diagnostic yield was related to the size of the PPL, ranging from 58% for nodules 1–2 cm in diameter to 88% for nodules larger than 5.1 cm.
In addition to being more likely to yield a diagnosis, especially with smaller lesions, adding EBUS to TBB enables the lesion to be found and sampled more quickly, reducing the time that patients and staff are exposed to fluoroscopy radiation.[7] Adding a sheath to guide the flexible bronchoscope probe is a further refinement to the procedure that improves the diagnostic yield and reduced the time even further.[7,12] Shinagawa et al. found that adding a GS also improved the diagnostic yield for nonmalignant lesions.[13] We did not evaluate the relative time for EBUS + TBB and TBB as the included studies did not evaluate this outcome.
This meta-analysis of four recent studies (2012–2014) updates and supports previous meta-analyses which evaluated EBUS in diagnosis of PPLs.[14,15] Wang Memoli et al., (2012) assessed a wide variety of approaches for locating and obtaining a PPL biopsy samples. Thirty-nine studies were included with a total of 3052 lesions. The pooled diagnostic yield for EBUS with or without GS was 73.2% (95% CI = 64.4%–81.9%) and 71.1% (95% CI = 66.5%–75.7%), respectively. The pooled data also showed that the yield depended on the size of the lesion, in agreement with the report by Ostendorf et al.[16] The diagnostic yield was significantly higher in lesions >20 mm in size (82.5%; 95% CI = 78.6%–86.4%) than for lesions ≤20 mm (60.9%; 95% CI = 54%–67.7%).[15]
The meta-analysis of Steinfort et al.[14] included 16 studies with 1420 patients that underwent EBUS (with or without fluoroscopy) for the diagnosis of PPLs. The pooled sensitivity was 73% (95% CI = 70%–76%). Similar to Wang Memoli et al., Steinfort et al. found diagnostic yield depended on the size of the lesions, with lesions >20 mm in size being associated with greater diagnostic yield (77.7%; 95% CI = 73%–82%) than lesions ≤20 mm in size (56.3%; 95% CI = 51%–61%).
A variety of methods for locating PPLs and obtaining biopsy samples using EBUS are have been evaluated since the addition of EBUS to TBB was first described by Kikuchi et al.[17] and Kurimoto et al.[7] Several groups have reported using EBUS without fluoroscopic support, with an average diagnostic yield of 64.1%,[3,18,19,20,21] whereas others have reported using EBUS with fluoroscopy guidance.[2] The combination of EBUS with virtual bronchoscopic navigation has been reported to be between 63.3% and 84.4%.[22] The presence of the bronchus sign on CT, that is, a bronchus containing air adjacent to the PPL, helps guide the bronchoscope to the lesion and increases the diagnostic yield.[23] Another refinement in place of the GS is to measure the distance from the opening of the bronchus to the lesion as a guide for inserting the biopsy forceps.[24]
Factors in addition to lesion size that affect diagnostic yield include location of the lesions (improved diagnostic yield is associated with lesions close to the hilum), visualization on fluoroscopy, malignant versus benign disease, and combination of diagnostic tools.[19,25,26,27,28] Although, the diagnostic yield for guided bronchoscopic techniques is lower than that reported for transthoracic needle aspiration, bronchoscopic techniques are associated with significantly fewer adverse event.[16] Most studies have evaluated the use of EBUS in solid lung masses, less is known regarding its performance in subsolid lesions and pure ground glass opacities.[2]
Ost et al. measured and identified factors that impact diagnostic yield for bronchoscopy in patients with PPLs.[29] They included data from patients with EBUS, electromagnetic navigation, and peripheral transbronchial needle aspiration and concluded that the diagnostic yield of EBUS and electromagnetic navigation are overestimated in comparison to peripheral transbronchial needle aspiration for the diagnosis of PPLs. The findings of Ost et al., are in contradiction to other studies that indicate that diagnostic yield increases with better image-assisted technology. Additional studies are necessary to further evaluate the capabilities of the different methodologies.
A number of advanced bronchoscopic modalities are currently available, in addition to EBUS and TBB, for diagnosing PPLs including image-guided percutaneous needle biopsy, electromagnetic navigational bronchoscopy, virtual navigational bronchoscopy, thin and ultrathin bronchoscopy, and bronschoscopic transparenchymal nodule access.[2] The pooled diagnostic yield and sensitivity of electromagnetic navigational bronchoscopy has been reported to be 65% and 71%, respectively.[30]
A limitation of our analysis is the small sample size, in part reflecting that fact only studies with ≥2 arms were included. Hence, the current study may potentially be over-estimating the effect of EBUS + TBB in diagnosing PPLs. Further large scale studies are warranted to provide better evidentiary support, and perhaps establish protocols for biopsy PPLs of smaller sizes. Only three of the four studies were included in the quantitative analysis due to the lack of reported data for diagnostic yield in one study (i.e., Shirakawa et al., 2004). We did not exclude this study as it provided some insight with respect to diagnostic yield in studies performed over a decade ago. In addition, definition of small and large lesions varied across studies, which may have confounded our findings. For example, Sánchez-Font et al. used <30 mm as the cut-off, and Boonsarngsuk et al. and Ishida et al. used <20 mm cut-off. Due to limited number of studies included in our analysis, we were unable to perform subgroup analysis for the diagnostic yield of EBUS with or without guided sheath. Several single arm studies suggest EBUS with a GS can further increase the rate of diagnostic yield. However, no trials have directly compared the use of EBUS plus guided sheath, EBUS, and fluoroscopy. It would be of clinical interest to perform another meta-analysis to evaluate such biopsy methods so as to gain insight into the optimum choice of technology for bronchoscopy for PPLs. Other technologies such as electromagnetic navigation and virtual guidance system, based on reconstructed CT images, to guide the bronchoscope to the target lesion should also be compared.
The results of this study provide strong evidence that there is clinical benefit in using EBUS + TBB, as the combined approach had higher diagnostic yield compared with TBB alone. This is of important for diagnosis of both malignant and benign pulmonary lesions since accurate diagnosis allows for early treatment intervention and may reduce the need for a patient to undergo a second biopsy. The use of EBUS + TBB is more accurate and may reduce the time a patient is exposed to radiation caused by conventional fluoroscopy. In addition, EBUS is noninvasive, and the use of a guided sheet may increase the accuracy of locating the lesion. Finally, the studies included in this analysis are recent (2012–2014) suggesting the methods evaluated are current.
Conclusion
Overall EBUS + TBB has higher diagnostic yield in bronchial biopsies for PPLs compared with conventional fluoroscopy and is the improvement in diagnostic yield is particularly great for small lesions under 30 mm. With the continuing development of new technologies, future studies are required to further investigate the optimum choice of biopsy equipment for PPL biopsy.
Financial support and sponsorship
This study was supported by the Medical Science and Technology Project Foundation of Zhejiang Province (project number: 2015KYB288) and by the Science and Technology Development Project of Hangzhou (project number: 20130633B06).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Survival Rates for Non-small Cell Lung Cancer, by Stage. Reported on the World Wide Web. [Last updated on 2014 Aug 15]. Available from: http://www.cancer.org/cancer/lungcancer-non-smallcell/detailedguide/non-smallcell-lung-cancer-survival-rates .
- 2.Mudambi L, Ost DE. Advanced bronchoscopic techniques for the diagnosis of peripheral pulmonary lesions. Curr Opin Pulm Med. 2016;22:309–18. doi: 10.1097/MCP.0000000000000284. [DOI] [PubMed] [Google Scholar]
- 3.Herth FJ, Ernst A, Becker HD. Endobronchial ultrasound-guided transbronchial lung biopsy in solitary pulmonary nodules and peripheral lesions. Eur Respir J. 2002;20:972–4. doi: 10.1183/09031936.02.00032001. [DOI] [PubMed] [Google Scholar]
- 4.Hürter T, Hanrath P. Endobronchial sonography in the diagnosis of pulmonary and mediastinal tumors. Dtsch Med Wochenschr. 1990;115:1899–905. doi: 10.1055/s-2008-1065241. [DOI] [PubMed] [Google Scholar]
- 5.Fujita Y, Seki N, Kurimoto N, Inoue K, Miyazawa T, Abe T, et al. Introduction of endobronchial ultrasonography (EBUS) in bronchoscopy clearly reduces fluoroscopy time: Comparison of 147 cases in groups before and after EBUS introduction. Jpn J Clin Oncol. 2011;41:1177–81. doi: 10.1093/jjco/hyr122. [DOI] [PubMed] [Google Scholar]
- 6.Shirakawa T, Imamura F, Hamamoto J, Honda I, Fukushima K, Sugimoto M, et al. Usefulness of endobronchial ultrasonography for transbronchial lung biopsies of peripheral lung lesions. Respiration. 2004;71:260–8. doi: 10.1159/000077424. [DOI] [PubMed] [Google Scholar]
- 7.Kurimoto N, Miyazawa T, Okimasa S, Maeda A, Oiwa H, Miyazu Y, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest. 2004;126:959–65. doi: 10.1378/chest.126.3.959. [DOI] [PubMed] [Google Scholar]
- 8.Wells GA, Shea B, O'Connell D, Peterson J, Welch V. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Metaanalyses. [Last accessed 2016 Aug]. Available from: http://www.medicine.mcgill.ca/rtamblyn/Readings/The%20Newcastle%20.%20Scale%20for%20assessing%20the%20quality%20of%20 .
- 9.Boonsarngsuk V, Raweelert P, Juthakarn S. Endobronchial ultrasound plus fluoroscopy versus fluoroscopy-guided bronchoscopy: A comparison of diagnostic yields in peripheral pulmonary lesions. Lung. 2012;190:233–7. doi: 10.1007/s00408-011-9359-3. [DOI] [PubMed] [Google Scholar]
- 10.Ishida M, Suzuki M, Furumoto A, Tsuchihashi Y, Ariyoshi K, Morimoto K. Transbronchial biopsy using endobronchial ultrasonography with a guide sheath increased the diagnostic yield of peripheral pulmonary lesions. Intern Med. 2012;51:455–60. doi: 10.2169/internalmedicine.51.6358. [DOI] [PubMed] [Google Scholar]
- 11.Sánchez-Font A, Giralt L, Vollmer I, Pijuan L, Gea J, Curull V. Endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions. A controlled study with fluoroscopy. Arch Bronconeumol. 2014;50:166–71. doi: 10.1016/j.arbres.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Chen A, Chenna P, Loiselle A, Massoni J, Mayse M, Misselhorn D. Radial probe endobronchial ultrasound for peripheral pulmonary lesions. A 5-year institutional experience. Ann Am Thorac Soc. 2014;11:578–82. doi: 10.1513/AnnalsATS.201311-384OC. [DOI] [PubMed] [Google Scholar]
- 13.Shinagawa N, Nakano K, Asahina H, Kikuchi E, Ito T, Matsuno Y, et al. Endobronchial ultrasonography with a guide sheath in the diagnosis of benign peripheral diseases. Ann Thorac Surg. 2012;93:951–7. doi: 10.1016/j.athoracsur.2011.11.073. [DOI] [PubMed] [Google Scholar]
- 14.Steinfort DP, Khor YH, Manser RL, Irving LB. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: Systematic review and meta-analysis. Eur Respir J. 2011;37:902–10. doi: 10.1183/09031936.00075310. [DOI] [PubMed] [Google Scholar]
- 15.Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest. 2012;142:385–93. doi: 10.1378/chest.11-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostendorf U, Scherff A, Khanavkar B, Ewig S, Hecker E. Diagnosis of peripheral lung lesions by EBUS-guided TBB in routine practice. Pneumologie. 2011;65:730–5. doi: 10.1055/s-0031-1291471. [DOI] [PubMed] [Google Scholar]
- 17.Kikuchi E, Yamazaki K, Sukoh N, Kikuchi J, Asahina H, Imura M, et al. Endobronchial ultrasonography with guide-sheath for peripheral pulmonary lesions. Eur Respir J. 2004;24:533–7. doi: 10.1183/09031936.04.00138603. [DOI] [PubMed] [Google Scholar]
- 18.Casutt A, Prella M, Beigelman-Aubry C, Fitting JW, Nicod L, Koutsokera A, et al. Fluoroscopic-guided radial endobronchial ultrasound without guide sheath for peripheral pulmonary lesions: A safe and efficient combination. Arch Bronconeumol. 2015;51:338–43. doi: 10.1016/j.arbres.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Huang CT, Ho CC, Tsai YJ, Yu CJ, Yang PC. Factors influencing visibility and diagnostic yield of transbronchial biopsy using endobronchial ultrasound in peripheral pulmonary lesions. Respirology. 2009;14:859–64. doi: 10.1111/j.1440-1843.2009.01585.x. [DOI] [PubMed] [Google Scholar]
- 20.Hur J, Lee HJ, Byun MK, Nam JE, Moon JW, Kim HS, et al. Computed tomographic fluoroscopy-guided needle aspiration biopsy as a second biopsy technique after indeterminate transbronchial biopsy results for pulmonary lesions: Comparison with second transbronchial biopsy. J Comput Assist Tomogr. 2010;34:290–5. doi: 10.1097/RCT.0b013e3181bc93ef. [DOI] [PubMed] [Google Scholar]
- 21.Hsia DW, Jensen KW, Curran-Everett D, Musani AI. Diagnosis of lung nodules with peripheral/radial endobronchial ultrasound-guided transbronchial biopsy. J Bronchology Interv Pulmonol. 2012;19:5–11. doi: 10.1097/LBR.0b013e31823fcf11. [DOI] [PubMed] [Google Scholar]
- 22.Asano F, Eberhardt R, Herth FJ. Virtual bronchoscopic navigation for peripheral pulmonary lesions. Respiration. 2014;88:430–40. doi: 10.1159/000367900. [DOI] [PubMed] [Google Scholar]
- 23.Eberhardt R, Anantham D, Ernst A, Feller-Kopman D, Herth F. Multimodality bronchoscopic diagnosis of peripheral lung lesions: A randomized controlled trial. Am J Respir Crit Care Med. 2007;176:36–41. doi: 10.1164/rccm.200612-1866OC. [DOI] [PubMed] [Google Scholar]
- 24.Fielding DI, Chia C, Nguyen P, Bashirzadeh F, Hundloe J, Brown IG, et al. Prospective randomised trial of endobronchial ultrasound-guide sheath versus computed tomography-guided percutaneous core biopsies for peripheral lung lesions. Intern Med J. 2012;42:894–900. doi: 10.1111/j.1445-5994.2011.02707.x. [DOI] [PubMed] [Google Scholar]
- 25.Boonsarngsuk V, Kanoksil W, Laungdamerongchai S. Diagnosis of peripheral pulmonary lesions with radial probe endobronchial ultrasound-guided bronchoscopy. Arch Bronconeumol. 2014;50:379–83. doi: 10.1016/j.arbres.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Chavez C, Sasada S, Izumo T, Watanabe J, Katsurada M, Matsumoto Y, et al. Endobronchial ultrasound with a guide sheath for small malignant pulmonary nodules: A retrospective comparison between central and peripheral locations. J Thorac Dis. 2015;7:596–602. doi: 10.3978/j.issn.2072-1439.2015.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada N, Yamazaki K, Kurimoto N, Asahina H, Kikuchi E, Shinagawa N, et al. Factors related to diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in small peripheral pulmonary lesions. Chest. 2007;132:603–8. doi: 10.1378/chest.07-0637. [DOI] [PubMed] [Google Scholar]
- 28.Tay JH, Irving L, Antippa P, Steinfort DP. Radial probe endobronchial ultrasound: Factors influencing visualization yield of peripheral pulmonary lesions. Respirology. 2013;18:185–90. doi: 10.1111/j.1440-1843.2012.02276.x. [DOI] [PubMed] [Google Scholar]
- 29.Ost DE, Ernst A, Lei X, Kovitz KL, Benzaquen S, Diaz-Mendoza J, et al. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions. Results of the AQuIRE registry. Am J Respir Crit Care Med. 2016;193:68–77. doi: 10.1164/rccm.201507-1332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asano F. Advanced bronchoscopy for the diagnosis of peripheral pulmonary lesions. Respir Investig. 2016;54:224–9. doi: 10.1016/j.resinv.2015.11.008. [DOI] [PubMed] [Google Scholar]