Abstract
BACKGROUND:
Surgery remains the gold standard for patients with resectable nonsmall cell lung cancer. Current guidance identifies patients with poor pulmonary reserve to fall within a high-risk cohort. The aim of this study was to determine the clinical and quality of life outcomes of anatomical lung resection in patients deemed high risk based on pulmonary function measurements.
METHODS:
A retrospective review of patients undergoing anatomical lung resection for nonsmall cell lung cancer between January 2013 and January 2015 was performed. All patients with limited pulmonary reserve defined as predicted postoperative forced expiratory volume in 1 s or transfer factor of the lung for carbon monoxide of <40% were included in the study. Postoperative complications, admission to the Intensive Care Unit, length of stay, and 30-day in-hospital mortality were recorded. The European Organization for Research and Treatment of Cancer quality of life questionnaire lung cancer 13 questionnaire was used to assess quality of life outcomes.
RESULTS:
Fifty-three patients met the inclusion criteria. There was no in-hospital mortality, and 30-day mortality was 1.8%. No complications were seen in 64% (n = 34), minor complications occurred in 26% (n = 14), while 9% had a major complication (n = 5). Quality of life outcomes were above the reference results for patients with early stage lung cancer.
CONCLUSION:
Anatomical lung resection can be performed safely in selected high-risk patients based on pulmonary function without significant increase in morbidity or mortality and with acceptable quality of life outcomes. Given that complications following lung resection are multifactorial, fitness for surgery should be thoroughly assessed in all patients with resectable disease within a multidisciplinary setting. High operative risk by pulmonary function tests alone should not preclude surgical resection.
Key words: Anatomical lung resection, high-risk patients, nonsmall cell lung cancer
Surgical resection remains the gold standard for management of patients with nonsmall cell lung cancer. Unfortunately, despite recent improvements, reported resection rates in the UK remain low at 12%–25%.[1] The reasons for this are likely multifactorial but can perhaps be simplified into two broad areas. First, does the disease stage allow for curative surgical resection and second, does the patient have the physical reserve to endure the required surgical resection? It is this second aspect of fitness for surgery that we have addressed in this study.
The current British Thoracic Society guidelines for assessment for radical lung cancer surgery use a tripartite model including operative mortality, cardiovascular morbidity, and assessment of lung function. Preoperative lung function has been shown to be an important factor in the prediction of perioperative mortality, morbidity, and postresection dyspnea.[2] Numerous series have demonstrated a correlation between low forced expiratory volume in 1 s (FEV1)/transfer factor of the lung for carbon monoxide (TLCO) and increased complications and mortality following lung surgery.[3,4,5,6] Based on this, an optimum cutoff of 40% for postoperative predicted FEV1 and TLCO is now used to identify higher risk patients. It is important to note here that FEV1 and TLCO have been shown to measure very different aspects of lung function. Moreover, patients with normal spirometry but a predicted postoperative (ppo) TLCO <40% still have an increased risk following lung surgery, demonstrating TLCO to be an independent predictor of postoperative complications.[7] In view of this, all patients should undergo full pulmonary function testing before lung resection, even in the presence of normal spirometry.
Given the ongoing improvements in perioperative techniques and postoperative management, thoracic surgeons have in recent years been able to offer surgical resection to patients with ppo FEV1 or ppo TLCO of <40%. The increased frequency of thoracoscopic lung resection may also have an impact on outcomes in patients with limited cardiopulmonary reserve. Within our institution, we noted that a cohort of patients with poor lung function had undergone surgery for lung cancer. As per the current UK and European guidelines, these patients would be considered moderate to high risk based on pulmonary reserve. The aim of this retrospective review was to assess clinical outcomes for this specific group of patients. Lowering the acceptable threshold for pulmonary function could allow more patients to undergo surgery, but we do not currently have good data as to how this will affect postoperative dyspnea. For this reason, we have also looked at quality of life outcome measures within this cohort.
Methods
A retrospective review of patients who had undergone lobectomy, bilobectomy, or pneumonectomy for nonsmall cell lung cancer between January 2013 and January 2015 under two of the consultants at Guy's Hospital, London, was performed. The segment counting method was used to estimate ppo FEV1 and ppo TLCO where ppo FEV1 = preoperative FEV1× (remaining segments/19). Preoperative lung function tests were examined to identify all those with a ppo FEV1 or ppo TLCO of <40% for inclusion in the study. Overall surgical risk was estimated using the current British Thoracic Society guidelines.[2]
Data were obtained from patient medical records. Data collected included age, sex, preoperative spirometry and transfer coefficient, procedure, complications, admission to the critical care unit, length of stay, and status at follow-up. Cardiopulmonary testing was only performed in five patients for whom these results were also noted. The decision as to whether cardiopulmonary testing was required was made by the operating consultant surgeon. Postoperative lower respiratory tract infection or pneumonia was recorded in patients with X-ray changes of consolidation, raised inflammatory markers, signs of infection in sputum microbiology, and those treated with respiratory antibiotics. A prolonged air leak was defined as an air leak lasting over 5 days. All complications were graded using the Ottawa Thoracic Morbidity and Mortality classification.[8] Lung function measurements and ppo percentages were summarized as mean ± standard deviation (SD). The Kaplan–Meier method was used to plot overall survival.
The European Organization for Research and Treatment of Cancer quality of life questionnaire – lung cancer 13 is a well-validated quality of life tool specifically designed for patients with lung cancer and following lung cancer treatment.[9] This questionnaire was used to collect quality of life data from our patient group. The questionnaire was sent with an explanatory letter to all surviving patients in July 2016. This was a minimum of 18 months postoperatively and up to a maximum of 42 months. In addition to the standard questions, we also asked “Do you feel you made the correct decision in undergoing surgery.”
Results
During the study period, 53 patients were identified with a ppo FEV1 or TLCO of <40%. Within this cohort, 30 (57%) were female and 23 (43%) were male. The mean ± SD age was 70 ± 8 years (range 39–86). Forty-six patients underwent lobectomy with 52% performed thoracoscopically and the remaining through standard posterolateral thoracotomy incision. Of these resections, three patients required a sleeve resection and one underwent additional chest wall resection. Bilobectomy was performed in three patients and pneumonectomy in four. The majority of patients had adenocarcinoma (53%), followed by squamous cell carcinoma (36%), large cell carcinoma (7%), or undifferentiated nonsmall cell lung carcinoma (4%).
The preoperative lung function tests and ppo FEV1 and TLCO results are demonstrated in Table 1. Absolute values for both FEV1 and TLCO were collected in 94% of patients, and percentage values were obtained for all patients. The lowest ppo FEV1 was 29%, and the lowest ppo TLCO was 21%. Within the cohort, 12 patients were identified with both ppo FEV1 and ppo TLCO <40%. Cardiopulmonary testing was performed in five patients with a mean VO2max of 14.8 mL/kg/min (range 11.8–18.3 mL/kg/min).
Table 1.
Pulmonary function data

There was no in-hospital mortality, and 30-day mortality was 1.8% (one patient). The overall survival data were plotted using Kaplan–Meier method [Figure 1]. The majority of patients made an uneventful recovery following surgery and had no complications (64%). Minor complications occurred in 26% (n = 14) while 9% had a major complication (n = 5) [Table 2]. Within our unit, the standard of care is for postoperative patients to return to a level 0 thoracic ward unless previously arranged due to specific comorbidities or operative concerns. Within this cohort, nine patients required admission to a Level 1 critical care bed with three of these being planned electively. Median length of stay was 7 days overall and 5 days in patients who had undergone a VATS procedure.
Figure 1.
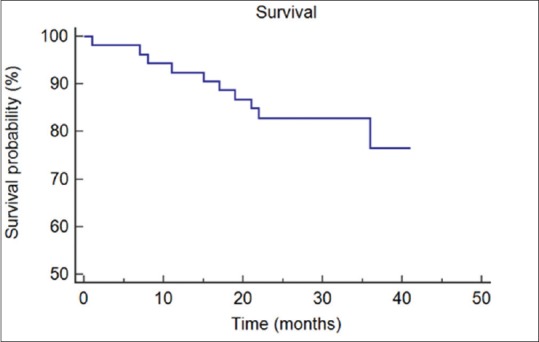
Kaplan–Meier curve for overall survival in months
Table 2.
Summary of complications (Ottawa Thoracic Morbidity and Mortality classification) and number of patients affected
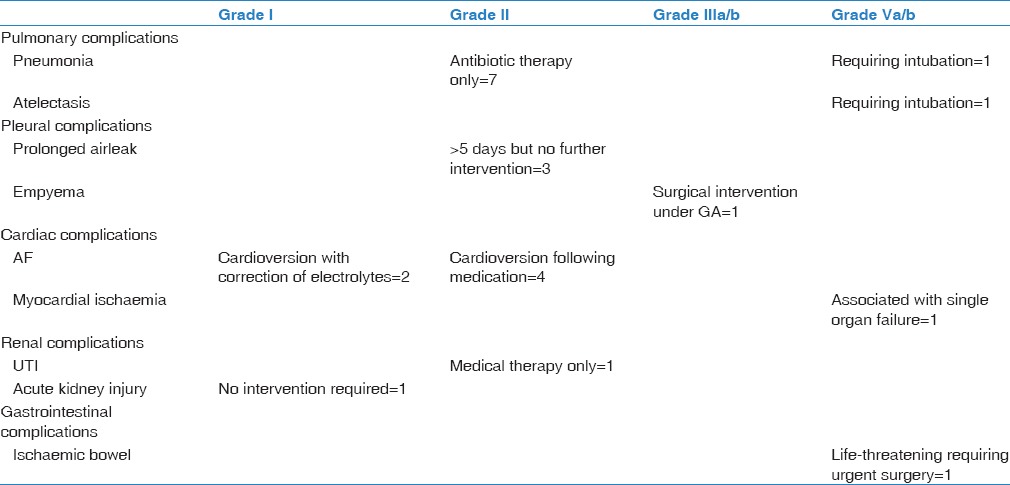
When considering the subset of 12 patients with both ppo FEV1 and ppo TLCO of <40%, there were 4 minor postoperative complications including lower respiratory tract infection, atrial fibrillation, and prolonged air leak. The median length of stay was 6.5 days (range 4–9 days).
Questionnaires were sent to all surviving patients. A total of 27 replies were received within the 3-month period given for responses. This amounted to a 66% response rate. All but one patient answered that they felt they had made the correct decision to undergo surgery. When considering questions which focused on activities of daily living such as self-care and walking short distances, 80%–90% answered that they had no or little difficulty. These outcomes are more positive than the reference results for patients with early stage lung cancer.[10] For long walks and strenuous activity, 30%–50% reported no or little difficulty which is in keeping with the reference values.
When asked specifically about shortness of breath during the past week, 52% responded not at all, 30% reported quite a bit, and 18% answered very much. Despite this, however, the global quality of life and overall health outcomes were better than expected with an average answer of 5 when asked to rate their experiences on a scale of 1–7 (1 being very poor and 7 excellent).
Conclusion
Surgical resection remains the curative management option of choice in patients with resectable nonsmall cell lung cancer. Assessment of the risk of lung surgery remains a difficult judgment with a wide variation in opinion. One reason for this may be that there is currently still no consensus regarding the use of a scoring system to estimate operative mortality in the UK population. Thoracoscore and the European Society Objective Score (ESOS.01) are two systems that have been evaluated but both were found to overestimate mortality within the UK.[11] The Thoracoscore was also found to be a poor discriminative tool for predicting postoperative pulmonary complications.[12] More recently, the Liverpool group has put forward an alternative risk assessment tool based on a single center experience.[13] They reported that this system was more accurate than both the thoracoscore and ESOS.01 in predicting in-hospital mortality within their patient cohort. Although promising this scoring system has yet to be widely validated or introduced in other units.
A number of studies have been carried out to try to establish specific cutoff values in terms of ppo FEV1 and ppo TLCO to allow for better risk stratification.[3,4,5] The value often quoted in the guidelines of <40% is, however, based on research performed in the 1980s and 1990s. Recent advances in perioperative and postoperative care could, therefore, allow patients with more limited cardiopulmonary reserve to undergo surgery without a significant increase in risk.
More recently, authors have reported reasonable outcomes following lung resection in patients with ppo FEV1 or TLCO of <40%.[14,15] This may in part be due to a lung volume reduction effect in patients with poor spirometry due to chronic obstructive pulmonary disease (COPD). If the primary tumor is present in a lobe with significant emphysematous changes, then the actual loss of functioning lung is likely to be minimal, and there may even be improvement in symptoms following resection. For this reason, the European guidelines recommend the cutoff for high-risk patients based on pulmonary function should be lowered to a ppo FEV1/TLCO of 30%.[16] The British Thoracic Guidelines, however, still recommend the higher value of 40%; these differences reflect the ongoing issue that the lower limit of surgical tolerance remains elusive.
In the present study, we have shown that anatomical lung resection can be carried out safely in selected patients with a ppo FEV1 or ppo TLCO of <40%. The majority of patients made an uneventful recovery following surgery. The overall complication rate was 36% (9% major), which is in keeping with rates reported in a general population undergoing thoracic surgery.[17] Mortality was also below that seen in the UK in patients undergoing anatomical lung resection with no in-hospital deaths and a 30-day mortality of only 1.8%. When considering the subgroup of patients with both ppo FEV1 and ppo TLCO of <40%, there were only four minor complications identified.
Both the European and British guidelines discuss the use of cardiopulmonary testing to further evaluate patients with borderline pulmonary function tests.[2,16] Meta-analysis has confirmed that exercise capacity expressed as VO2max is lower in patients who develop complications following curative lung resection.[16] No single value, however, has been used to describe prohibitive risk for surgery. In general, patients with a VO2max >20 mL/kg/min are considered low risk for surgery, while for those with a VO2max <10 mL/kg/min, surgery would be contraindicated. Patients with a value of 10–15 mL/kg/min would be considered very high risk.[18] Despite this, there is again evidence that even in patients with poor cardiopulmonary reserve surgery can be undertaken with acceptable risk. The Cancer and Leukemia Group B (CALBG) reported outcomes for 68 patients with a VO2max <15 mL/kg/min undergoing lung resection with an operative mortality of 4% and no increase in postoperative complications.[19] Within our study, only five patients underwent cardiopulmonary testing as we did not think the information would add to the overall risk assessment in the majority of cases. All patients who were assessed had a value of >10 ml/kg/min, and the test did not, therefore, discriminate any patient with “prohibitive risk.”
Quality of life and postoperative dyspnea are often not specifically considered when looking at outcomes in high-risk patient groups. In the present study, we have included these outcomes as they are vital when contemplating whether it is acceptable to lower the threshold for surgical fitness. Overall quality of life outcomes within this cohort were better than expected and above the reference values for this group.[10] Of particular note, 80%–90% reported no or little difficulty in performing day-to-day activities.
The current study has a number of limitations. Firstly, we have considered risk based on pulmonary function tests alone. As highlighted by the numerous scoring systems to estimate mortality, risk in thoracic surgery is multifactorial. Other comorbidities such as cardiac disease, diabetes, and previous stroke are all significant risk factors. Performance status was also not considered here although FEV1 has been suggested as a surrogate for performance status in predicting perioperative mortality.[20] We have, however, looked at postoperative dyspnea and quality of life which are likely to be closely linked to pulmonary function and to be the most important outcomes for patients with limited pulmonary reserve. Secondly, as previously discussed, the lung volume reduction effect is likely to be significant in these patients with severe COPD. Although data regarding the surgical procedure and lobe was collected, we have not looked at whether the tumor was present within a more diseased section of lung and cannot, therefore, assess the contribution of this effect. This aspect could perhaps be addressed in future by performing a quantitative ventilation–perfusion scan in borderline patients. Finally, we recognize that the number of patients within the present study is relatively small.
Despite this, our findings add to the growing body of evidence to support lung resection in selected patients with poor pulmonary function.[14,15,19] We have demonstrated an acceptable risk in patients with a ppo FEV1 or ppo TLCO of <40% without increased postoperative complications. More widespread use of surgical techniques such as a video-assisted or robotic approach is likely to allow surgeons to continue to push these boundaries. We must not, however, lose sight of important outcomes such as unacceptable postoperative dyspnea when assessing patient fitness for surgery. Although we have not yet established a firm cutoff for pulmonary reserve or an accurate scoring system for surgical risk, when taken together the current investigations can allow for well-informed patient consent. High operative risk based on pulmonary function tests alone should not preclude curative surgical resection in patients with nonsmall cell lung cancer.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.National Lung Cancer Audit Annual Report 2015 (For the Audit Period 2014) London: Royal College of Physicians; 2015. Royal College of Physicians. [Google Scholar]
- 2.Lim E, Baldwin D, Beckles M, Duffy J, Entwisle J, Faivre-Finn C, et al. Guidelines on the radical management of patients with lung cancer. Thora×. 2010;65(Suppl 3):iii1–27. doi: 10.1136/thx.2010.145938. [DOI] [PubMed] [Google Scholar]
- 3.Markos J, Mullan BP, Hillman DR, Musk AW, Antico VF, Lovegrove FT, et al. Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am Rev Respir Dis. 1989;139:902–10. doi: 10.1164/ajrccm/139.4.902. [DOI] [PubMed] [Google Scholar]
- 4.Bolliger CT, Jordan P, Solèr M, Stulz P, Grädel E, Skarvan K, et al. Exercise capacity as a predictor of postoperative complications in lung resection candidates. Am J Respir Crit Care Med. 1995;151:1472–80. doi: 10.1164/ajrccm.151.5.7735602. [DOI] [PubMed] [Google Scholar]
- 5.Pierce RJ, Copland JM, Sharpe K, Barter CE. Preoperative risk evaluation for lung cancer resection: Predicted postoperative product as a predictor of surgical mortality. Am J Respir Crit Care Med. 1994;150:947–55. doi: 10.1164/ajrccm.150.4.7921468. [DOI] [PubMed] [Google Scholar]
- 6.Holden DA, Rice TW, Stelmach K, Meeker DP. Exercise testing, 6-min walk, and stair climb in the evaluation of patients at high risk for pulmonary resection. Chest. 1992;102:1774–9. doi: 10.1378/chest.102.6.1774. [DOI] [PubMed] [Google Scholar]
- 7.Brunelli A, Refai MA, Salati M, Sabbatini A, Morgan-Hughes NJ, Rocco G. Carbon monoxide lung diffusion capacity improves risk stratification in patients without airflow limitation: Evidence for systematic measurement before lung resection. Eur J Cardiothorac Surg. 2006;29:567–70. doi: 10.1016/j.ejcts.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Seely AJ, Ivanovic J, Threader J, Al-Hussaini A, Al-Shehab D, Ramsay T, et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg. 2010;90:936–42. doi: 10.1016/j.athoracsur.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European organization for research and treatment of cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 10.Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A, et al. EORTC QLQ-C30 Scoring Manual. 3rd ed. Brussels: EORTC; 2001. On behalf of the EORTC Quality of Life Group. [Google Scholar]
- 11.Sharkey A, Ariyaratnam P, Anikin V, Belcher E, Kendall S, Lim E, et al. Thoracoscore and European society objective score fail to predict mortality in the UK. World J Oncol. 2015;6:270–5. doi: 10.14740/wjon897w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradley A, Marshall A, Abdelaziz M, Hussain K, Agostini P, Bishay E, et al. Thoracoscore fails to predict complications following elective lung resection. Eur Respir J. 2012;40:1496–501. doi: 10.1183/09031936.00218111. [DOI] [PubMed] [Google Scholar]
- 13.Poullis M, McShane J, Shaw M, Woolley S, Shackcloth M, Page R, et al. Prediction of in-hospital mortality following pulmonary resections: Improving on current risk models. Eur J Cardiothorac Surg. 2013;44:238–42. doi: 10.1093/ejcts/ezs658. [DOI] [PubMed] [Google Scholar]
- 14.Brunelli A, Al Refai M, Monteverde M, Sabbatini A, Xiumé F, Fianchini A. Predictors of early morbidity after major lung resection in patients with and without airflow limitation. Ann Thorac Surg. 2002;74:999–1003. doi: 10.1016/s0003-4975(02)03852-3. [DOI] [PubMed] [Google Scholar]
- 15.Kachare S, Dexter EU, Nwogu C, Demmy TL, Yendamuri S. Perioperative outcomes of thoracoscopic anatomic resections in patients with limited pulmonary reserve. J Thorac Cardiovasc Surg. 2011;141:459–62. doi: 10.1016/j.jtcvs.2010.05.051. [DOI] [PubMed] [Google Scholar]
- 16.Brunelli A, Charloux A, Bolliger CT, Rocco G, Sculier JP, Varela G, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy) Eur Respir J. 2009;34:17–41. doi: 10.1183/09031936.00184308. [DOI] [PubMed] [Google Scholar]
- 17.Agostini P, Cieslik H, Rathinam S, Bishay E, Kalkat MS, Rajesh PB, et al. Postoperative pulmonary complications following thoracic surgery: Are there any modifiable risk factors? Thorax. 2010;65:815–8. doi: 10.1136/thx.2009.123083. [DOI] [PubMed] [Google Scholar]
- 18.Benzo R, Kelley GA, Recchi L, Hofman A, Sciurba F. Complications of lung resection and exercise capacity: A meta-analysis. Respir Med. 2007;101:1790–7. doi: 10.1016/j.rmed.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loewen GM, Watson D, Kohman L, Herndon JE, 2nd, Shennib H, Kernstine K, et al. Preoperative exercise VO2 measurement for lung resection candidates: Results of cancer and leukemia group B protocol 9238. J Thorac Oncol. 2007;2:619–25. doi: 10.1097/JTO.0b013e318074bba7. [DOI] [PubMed] [Google Scholar]
- 20.Falcoz PE, Conti M, Brouchet L, Chocron S, Puyraveau M, Mercier M, et al. The Thoracic surgery scoring system (thoracoscore): Risk model for in-hospital death in 15,183 patients requiring thoracic surgery. J Thorac Cardiovasc Surg. 2007;133:325–32. doi: 10.1016/j.jtcvs.2006.09.020. [DOI] [PubMed] [Google Scholar]


