Abstract
BACKGROUND:
Obstructive sleep apnea (OSA) is a common disorder worldwide; however, epidemiological studies on its prevalence lack in Saudi Arabia. This study aimed to determine the prevalence and risk factors of OSA in Saudi Arabia.
METHODS:
The study was performed from 2013 to 2015 in two stages. The screening stage was first; a random sample of Saudi employees (n = 2682) 30–60 years of age completed a survey that included the Wisconsin questionnaire. According to these data, the subjects were categorized as habitual, moderate, or nonsnorers (NSs). The confirmatory second stage was a case–control study conducted on 346 individuals selected from each group using polysomnography (PSG).
RESULTS:
In the first stage, the prevalence of habitual snoring was 23.5%, moderate snoring was16.6%, while 59.9% of the sample was NSs. Among the 346 individuals who underwent PSG, a total of 235 (67.9%) subjects had OSA with an apnea-hypopnea index (AHI) of ≥5; 76 (22.0%) had OSA syndrome (OSAS), defined by an AHI of ≥5 plus daytime sleepiness; and 227 (65.6%) had clinically diagnosed OSA syndrome (COSAS), as defined by the American Academy of Sleep Medicine. A conservative estimate of at least 8.8% (12.8% in men and 5.1% in women) was calculated for the overall prevalence of OSA. Similarly, the overall estimated prevalence of OSAS and COSAS was 2.8% (4.0% in men and 1.8% in women) and 8.5% (12.4% in men and 4.8% in women), respectively. A multivariate analysis revealed age, gender, obesity, and hypertension as independent risk factors of OSA.
CONCLUSIONS:
Our study demonstrated that the rate and risk factors of OSA in the Saudi population are similar to those observed in Western studies.
Key words: Daytime sleepiness, obstructive sleep apnea, prevalence, risk factors, syndrome
Obstructive sleep apnea (OSA) refers to recurrent episodes of an absence of or decline in breathing during sleep despite a continuous effort to breathe normally. The condition is characterized clinically by excessive daytime sleepiness (EDS), disruptive snoring, and periodic nocturnal hypoxemia with frequent arousals during sleep.[1,2] OSA is also associated with serious health complications, such as an increased risk of vehicle crashes, occupational accidents, hypertension, cardiovascular and cerebrovascular diseases, glucose intolerance, decreased functional ability, and impotence.[2,3] Furthermore, OSA may also increase all-cause mortality, particularly vascular mortality, which leads to increased utilization of health-care services.[4,5,6] More importantly, treating OSA may reduce not only morbidities and mortalities but also the related economic burden.[2,7,8,9]
In the Western world, the prevalence of OSA syndrome (OSAS), defined as OSA with associated EDS, is 3%–7% in men and 2%–5% in women.[10,11] Despite lower obesity rates, the prevalence is similar in Asia, possibly due to the craniofacial features among Asians.[12] This finding suggests that OSA is an equally common disorder in both the developing and developed world.[13] Nevertheless, few studies from the Middle East have estimated the prevalence of sleep apnea. Furthermore, those studies actually evaluated the risk of OSA using simple validated questionnaires and were not based on the gold standard objective measures, namely, overnight polysomnography (PSG).[14,15,16] Therefore, the aim of this study was to assess the prevalence of OSA based on PSG findings in a random sample from the Saudi population and determine the potential risk factors. Accordingly, this study may lay a foundation for reducing the burden of OSA through early diagnosis, appropriate treatment, and the modification or reduction of predictive risk factors.
Methods
Study population
Our target population comprised Saudi school employees aged 30–60 years, including porters, drivers, teachers, and administrators. Schools were selected using a stratified random sample from two lists of male and female schools. A school-based cross-sectional survey was adopted given that schools are the most feasible source of national employees in Saudi Arabia; the stratified random sample was conducted with equal allocation in gender. The sample excludes older age groups (retired people) and people living in small cities, towns, and villages. Nevertheless, the sample age range represented a wide spectrum of the Saudi population. Furthermore, the total sample size was 3000, which is sufficiently large to allow for generalizing the results to the Saudi population. In total, 129 schools were randomly selected from the 1119 schools in the Jeddah area. Ethical approval was obtained from the Ethical Committee of King Abdulaziz University Hospital, Jeddah.
Stage 1: Screening procedure
The study was performed in two stages (from 2013 to 2015). Stage 1 was a cross-sectional study of a random sample of the target population. It consisted of two trained teams of male and female interviewers interviewing school employees. A pilot study was conducted involving one school and 21 subjects from both genders. The aim of the pilot study was to assess the implementation of the questionnaire and reliability of the measurements.
Camps for interviews and another for performing measurements were organized in each selected school after obtaining written informed consent. A modified Wisconsin sleep questionnaire [17] that also included questions about demographics, EDS using the Epworth Sleepiness Scale,[18] sleep symptoms, medical history, social class, and consanguinity was used. Biometric data were also collected, including height (H), weight (W), body mass index (BMI), neck circumference (NC), triceps skin fold thickness, waist/hip ratio, and percent predicted neck circumference (PPNC) using the formula PPNC = (1000 × NC)/([0.55 × H] +310).[19,20] Furthermore, blood pressure was measured according to standard methods using a digital sphygmomanometer (OMRON, Kyoto, Japan).
Based on the Wisconsin questionnaire and using a five-point scale (0–4), data from six survey questions were used to categorize subjects based on the frequency of snoring as habitual snorer (HS), moderate snorer (MS), or nonsnorer (NS).[17]
Stage 2: Polysomnography
The second (confirmatory) stage was a case–control study involving the collection of PSG data. After analyzing the stage 1 data, respondents were classified based on snoring status. The initial plan was to obtain PSG on all HS, 50% of MS, and 25% of NS. Polysomnography (SOMNO Medics Plus; SOMNOmedics, Randersacker, Germany) consisted of continuous recordings from surface leads for electroencephalography (EEG), electrooculography, electromyography (submental and bilateral anterior tibialis muscles), electrocardiography, nasal pressure, nasal and oral airflow (thermocouple), chest and abdominal impedance belts for respiratory muscle efforts, pulse oximetry for oxygen saturation and pulse rate, a tracheal microphone for snoring, and body position sensors for sleep position. PSG records were scored manually according to the American Academy of Sleep Medicine (AASM) 2012 scoring.[21] Full PSG was conducted in two different locations, the participants' homes and the Sleep Medicine and Research Center at King Abdulaziz University Hospital, using the same device to increase convenience and participant cooperation.
Abnormal obstructive breathing events during monitored sleep were described according to the latest recommendation of the AASM as a decrease in airflow by 90% or more from baseline for at least 10 s (apnea) and a discernible reduction in airflow of at least 30% of the pre-event baseline using nasal pressure associated with a reduction in oxygen saturation of at least 3% and/or followed by an EEG arousal (hypopnea), despite persistent chest and abdominal muscle efforts to overcome the obstruction.[22] EEG arousal was defined according to the recommendation of the AASM.[22] The average number of these apnea and hypopnea events per hour of sleep (i.e., the apnea-hypopnea index [AHI]) was then calculated.
Subjects with an AHI of ≥5 were categorized as having OSA, whereas those with EDS and an AHI of ≥5 were categorized as having OSAS.[1,10,11] Clinically diagnosed OSA (COSAS) was defined as per the latest AASM recommendations (2014),[23] i.e., A-an AHI of ≥15 determined by PSG or B-an AHI of ≥5 but <15 events, in addition to one of the following: (1) Daytime sleepiness, nonrestorative sleep, fatigue, or insomnia symptoms; (2) incidences of waking up with gasping or choking sensations; (3) reported snoring, breathing interruptions, or both during sleep; or (4) a known history of hypertension, mood or cognitive dysfunction, coronary artery disease, stroke, congestive heart failure, atrial fibrillation, or diabetes mellitus. Three registered polysomnographic technologists were assigned to manually score data from these PSG studies. Quality control of the scoring process was achieved by randomly selecting five cases every month to be scored by all three technologists to audit interobserver reproducibility and accuracy.
Calculation of prevalence
Subjects in the PSG group were compared with those in the non-PSG group in terms of the mean age and BMI. If no significant differences were identified, the prevalence of OSA in the PSG group of the HS, MS, or NS groups would be considered representative of the entire screened population.[24] If significant differences were observed between the PSG and non-PSG groups, a conservative estimate was implemented, and the OSA subjects were handled as the only OSA subjects in the entire screened population. The extrapolated prevalence of OSA in the whole cohort would be estimated as follows:
Number of subjects with OSA/total number of questionnaire respondents × 100%.[24,25,26]
Statistical analysis
The data were collected and analyzed using the Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA), version 21. Data were presented using the means ± standard deviations for continuous variables and percent frequency for categorical variables. Comparisons between groups were performed using Student's t-test for continuous variables and a Pearson Chi-square test for discrete variables. Statistical significance was set at an alpha level of 0.05 with two-tailed probability. Multiple logistic regression analysis was used to identify significant independent risk factors of OSA (0 = no, 1 = yes) using the forward conditional stepwise method with 0.05 probabilities for entry and 0.1 for removal. Odds ratios (ORs) and 95% confidence intervals (CIs) were presented.
Results
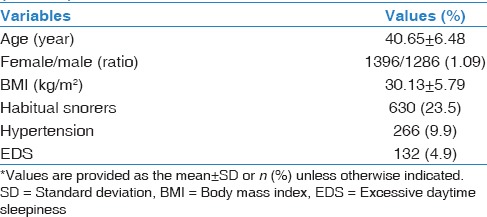
A total of 2682 participants (52.1% females, n = 1397) were recruited, assuming a participation rate of 89.4%. Snoring was reported in 40.1% of the participants (53% of males and 28% of females, P < 0.01). The rate of HS, MS, and NS in the screened population was 23.5%, 16.6%, and 59.9%, respectively.[27] The demographic data of the screened population are summarized in Table 1.
Table 1.
Demographic profile of the study population (n=2682)*
Polysomnography
A descriptive flowchart of the screened population is illustrated in Figure 1.
Figure 1.
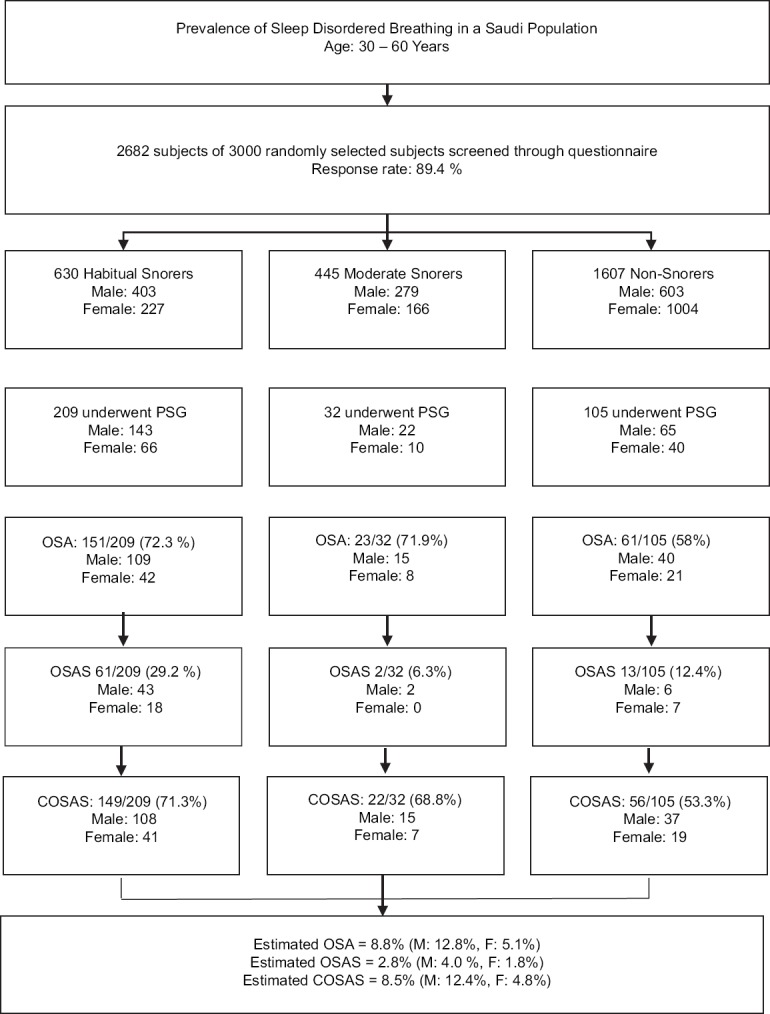
A descriptive flowchart of the screened population. OSA: AHI ≥5. OSAS: AHI ≥5 with EDS. COSAS: AHI ≥5 and any symptoms based on AASM criteria. EDS = Excessive daytime sleepiness, OSA = Obstructive sleep apnea, AHI = Apnea-hypopnea index, OSAS = Obstructive sleep apnea syndrome, COSAS = Clinically diagnosed obstructive sleep apnea syndrome, AASM = American Academy of Sleep Medicine
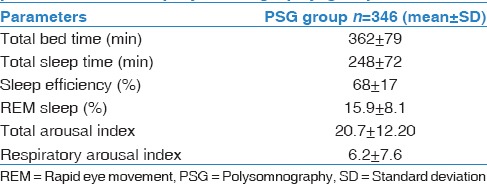
PSG studies were conducted for 375 subjects. Studies were conducted at home for 301 subjects, while 74 studies were performed at the sleep center as per the participants' request. Thirty-five subjects had PSG repeated due to insufficient data in the initial trial. However, 29 of these 35 subjects were ultimately excluded due to persistently poor sleep efficiency. Thus, the final data included that of 346 subjects, which was 12.9% of all questionnaire responders. Table 2 summarizes the PSG parameters.
Table 2.
Descriptive statistics of polysomnography parameters in the polysomnography group
Unfortunately, we were unable to perform PSG on the target participants as initially planned despite the capacity to do so within the time limit. This was mainly due to the poor response rate from participants in the second stage despite all efforts. Consequently, all subjects in the screened population were eventually approached to participate in stage 2. Hence, the demographic data of the PSG group were not similar to the non-PSG group. The mean age was 42.86 ± 6.64 and 40.31 ± 6.38 years in the PSG and non-PSG groups, respectively, with P < 0.05; however, the mean BMI was 30.11 ± 5.52 and 30.14 ± 5.82 kg/m2, respectively, with no significant differences (P > 0.05).
Prevalence of obstructive sleep apnea
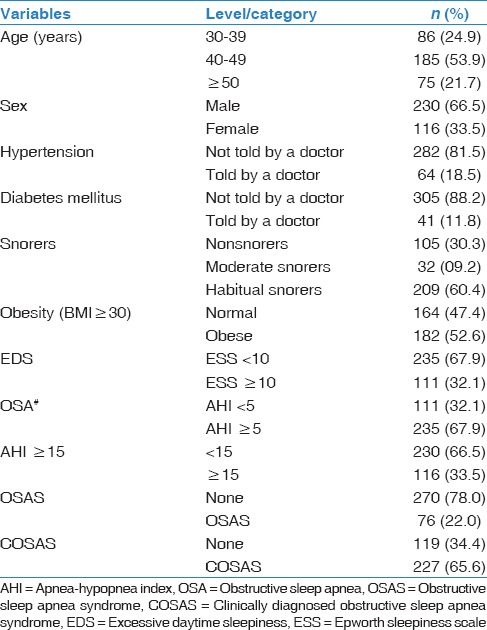
Among the 346 subjects who underwent PSG, 235 had OSA, and 151, 23, and 61 subjects were categorized as HS, MS, and NS. The prevalence of OSA was 72.3% in HS, 71.9% in MS, and 58% in NS. The characteristics of the PSG group are summarized in Table 3.
Table 3.
Characteristics of the polysomnography group (n=346)
Considering that significant differences were noted in the mean age between the PSG and non-PSG groups, a conservative estimate was implemented, and the documented OSA subjects were considered the only subjects with OSA in the screened population. Accordingly, the overall prevalence of OSA and OSAS in the screened population was estimated to be at least 8.8% and 2.8%, respectively, affecting 12.8% and 4.0% of men and 5.1% and 1.8% of women, respectively. Furthermore, when COSAS was taken into consideration, its prevalence among the PSG group as recommended by the AASM was 65.6% (227). Hence, the extrapolated prevalence among the screened population was 8.5% (12.4% in males and 4.8% in females).
Risk factors associated with obstructive sleep apnea
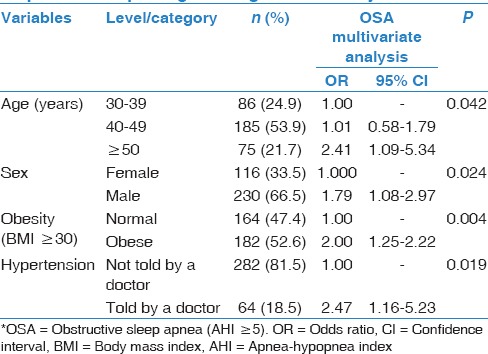
The univariate analysis results are presented in Table 4. However, the multiple logistic regression analysis only identified the male gender, age ≥50 years, obesity as defined by a BMI ≥30 kg/m2, and a history of hypertension as significant risk factors associated with OSA [Table 5].
Table 4.
Sociodemographic, anthropometric, and comorbidity profiles in obstructive sleep apnea and nonobstructive sleep apnea subjects
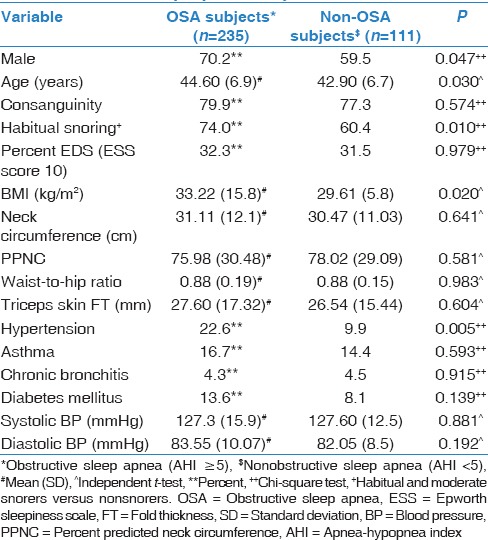
Table 5.
Predictors of OSA* status identified by a stepwise multiple logistic regression analysis
Discussion
The present study is the first population-based survey of OSA from Saudi Arabia. The estimated prevalence of OSA and OSAS in the screened population was 8.8% and 2.8%, respectively, and the prevalence of OSAS in men and women was 4.0% and 1.8%, respectively. However, the overall prevalence of COSAS was 8.5%, affecting 12.4% of men and 4.8% of women. The multivariate analysis revealed that age, male gender, obesity, and hypertension were independent predictive risk factors of OSA.
Prevalence of sleep apnea
As far as OSAS is concerned, the observed outcomes fall within the range of most reported rates. Young et al. established the first recognition of OSA as a public health problem in 1993.[1] In the Wisconsin Sleep Cohort Study, Young et al. stated that the prevalence of sleep apnea was 24% and 9% in middle-aged men and women, respectively.[1] However, only 4% of men and 2% of women met the diagnostic criteria for OSAS.[1] Subsequently, in a large-scale population-based study, Bixler et al. confirmed that 3.9% and 1.2% of men and women, respectively, suffer from OSAS.[28,29] In Spain, a population-based study focusing on both genders between 30 and 70 years reported that OSAS (AHI of ≥10 plus EDS) was identified in 3.4% of men and 3% of women.[30] As such, our findings are in agreement with those of studies conducted in the Western world.
On the other hand, studies have also reported rates similar to our findings in China, Korea, and India. Ip et al. assessed OSA in middle-aged Chinese men. In total, 784 subjects were recruited, and full PSG was performed on 150 of these patients.[25] The prevalence of OSA and OSAS was confirmed to be 8.8% and 4.1%, respectively.[25] In a similar study, PSG was performed in 106 out of 854 female Chinese respondents.[26] OSA and OSAS were identified in 3.7 and 2.1%, respectively.[26] In contrast, Kim et al. performed PSG on 457 Koreans randomly allocated from an initial cohort of 5020 participants between 40 and 69 years of age. The prevalence of OSA was 27% in males and 16% in females; however, the prevalence of OSAS was 4.5% in males and 3.2% in females.[31] A similar epidemiological study was conducted in a semi-urban Indian population aged 30–60 years. OSA was identified in 13.7% of patients, and OSAS was identified in 3.6%.[20]
As reported by Sharma et al.[20] and demonstrated in our current study, it is interesting that the prevalence of OSA is comparable among Chinese, Korean, Indian, American, and Saudi Arabian populations, despite disparate ethnic backgrounds, cephalic features, and obesity rates. This finding may be difficult to explain but warrants further investigation in the future.
Moreover, we used another clinical definition of OSAS (COSAS) in our study based on the AASM diagnostic criteria for sleep apnea.[23] Accordingly, the estimated overall prevalence of COSAS was 8.5% (male: 12.4%, female: 4.8%). To the best of our knowledge, no such data of COSAS have been reported in previous epidemiological studies. We feel that the prevalence of COSAS is likely more practically meaningful, with significant clinical implications, than OSA (≥5) and OSAS (≥5 and EDS).
Few studies attempting to address the prevalence of OSA in the Middle East have been reported in the literature, and these studies were solely based on validated questionnaires with no PSG. Therefore, it is not surprising that these studies tended to report a significantly increased prevalence of OSA compared with our study (up to 40%).[15,16,32,33,34] To the best of our knowledge, our study is the first in the region to use objective tests, such as PSG, to determine the prevalence of OSA.
Risk factors of obstructive sleep apnea
OSA is more common in men than women.[2,10,11] Our survey again confirmed the findings of previous epidemiological studies regarding this male predominance, with this condition occurring in 70.2% of males versus 29.8% of females [Figure 1]. This male predominance may be related to several factors, including hormonal effects in the muscles of the upper airways, gender-based differences in the distribution of adipose tissue, variances in pharyngeal shape, size, and collapsibility, and differences in ventilation control.[35] In our study, more males than females participated in stage 2; this difference may have at least partly contributed to the increased male preponderance in the current study. Furthermore, a recent study among the Swedish population reported a high prevalence of OSA among females and no significant association with EDS.[36] This finding raises the possibility that OSA is underestimated in females simply because they have a more diverse clinical presentation of OSA than males.[11]
The prevalence of OSA increases with age independent of other confounders.[28,36,37,38] This finding was confirmed in our survey for OSA [Table 5]. Subjects 50 years or older had a 2.4-fold increased risk of OSA compared to subjects 30–39 years old [Table 5]. Bixler et al. described such an increase in OSA among individuals older than 65 years but a decline in OSAS.[28] Moreover, several studies have reported little if any association between OSA and serious medical consequences at older ages, suggesting that sleep apnea in middle-aged adults, as in our population, is more serious than in seniors.[39]
Obesity is a major predictive factor for OSA, with a majority of patients being overweight.[2,10,11] On the other hand, losing weight either conventionally or through bariatric surgery reduces the severity of sleep apnea.[38,40,41,42,43] In our study, obesity, as assessed by BMI (OR: 2.0; 95% CI: 1.251–2.22; P = 0.004), but not other biometric data, was significantly increased in OSA patients compared with non-OSA patients [Table 5].
Several population-based epidemiological studies have confirmed an independent association between sleep apnea and hypertension.[30,36,44,45,46,47] In our survey, a history of hypertension was significantly increased among OSA patients compared with non-OSA patients, and it was confirmed as an independent risk factor of OSA in the multivariate regression analysis (OR: 2.47; 95% CI: 1.16–5.23; P = 0.019) [Table 5].
Limitations of the study
One of the limitations of this study is our target population, i.e., school employees, which may not be representative of the whole Saudi population. However, our sample population comprised randomly selected Saudi school employees, including porters, teachers, and administrators. Furthermore, this wide spectrum of sociodemographic classes of workers in the Ministry of Education closely resembles the sociodemographic classes of the society as a whole. In addition, our large sample size of 2682 increases the validity of the results in representing the whole Saudi population.
A disappointing limitation of our study was the inability to perform PSG on the snorer and NS participants as initially planned, despite the capacity to do so within the provided time limit. This was mainly due to the poor acceptance rate of participants in the second stage of the study, despite our best efforts.
Conclusion
Similar to other populations, sleep apnea syndrome is a common disorder among Saudis as it is noted in at least 4.0% and 1.8% of males and females, respectively. COSAS is likely a more practically applied definition; its estimated overall prevalence was 8.5%, affecting 12.4% of males and 4.8% of females. The male gender, age, obesity, and hypertension were independent predictive risk factors of OSA based on a multivariate regression analysis.
Financial support and sponsorship
This study was supported by King Abdulaziz City for Science and Technology, Grant Number: A-L-12-0867.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors are grateful to King Abdulaziz City for Science and Technology, Saudi Arabia, for the provided financial support. They are extremely appreciative of Dr. Terry Young and Dr. David Gozal for their valuable directions regarding the study methodology. The authors would also like to express special appreciation to Dr. Nadia Omar Thabit, The head of Research & Survey Section, Jeddah General Education Department; Mrs. Walaa Abuzahra for coordinating the data collection and arranging the procedures; Dr. Ibrahim Zakaria for performing an extraordinary job in collecting data; and Dr. Mona Alotaibi for her great contribution in writing the study proposal. Finally, the authors would like to thank all of the Sleep Medicine and Research Center staff, particularly the registered polysomnographic technologists, Mrs. Haneen Mansour and Mrs. Geezette Gulpo for scoring the PSG data.
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of obstructive sleep apnea: A population-based perspective. Expert Rev Respir Med. 2008;2:349–64. doi: 10.1586/17476348.2.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: Rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ. 2009;108:246–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, et al. Sleep disordered breathing and mortality: Eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O'Connor GT, et al. Sleep-disordered breathing and mortality: A prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kryger MH, Roos L, Delaive K, Walld R, Horrocks J. Utilization of health care services in patients with severe obstructive sleep apnea. Sleep. 1996;19(9 Suppl):S111–6. doi: 10.1093/sleep/19.suppl_9.s111. [DOI] [PubMed] [Google Scholar]
- 7.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: A population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 8.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 9.Albarrak M, Banno K, Sabbagh AA, Delaive K, Walld R, Manfreda J, et al. Utilization of healthcare resources in obstructive sleep apnea syndrome: A 5-year follow-up study in men using CPAP. Sleep. 2005;28:1306–11. doi: 10.1093/sleep/28.10.1306. [DOI] [PubMed] [Google Scholar]
- 10.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population – A review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311–22. doi: 10.3978/j.issn.2072-1439.2015.06.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapur VK. Obstructive sleep apnea: Diagnosis, epidemiology, and economics. Respir Care. 2010;55:1155–67. [PubMed] [Google Scholar]
- 14.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 15.Bahammam AS, Alrajeh MS, Al-Jahdali HH, BinSaeed AA. Prevalence of symptoms and risk of sleep apnea in middle-aged Saudi males in primary care. Saudi Med J. 2008;29:423–6. [PubMed] [Google Scholar]
- 16.Bahammam AS, Al-Rajeh MS, Al-Ibrahim FS, Arafah MA, Sharif MM. Prevalence of symptoms and risk of sleep apnea in middle-aged Saudi women in primary care. Saudi Med J. 2009;30:1572–6. [PubMed] [Google Scholar]
- 17.Young T, Shahar E, Nieto FJ, Redline S, Newman AB, Gottlieb DJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: The Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 18.Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 19.Davies RJ, Stradling JR. The relationship between neck circumference, radiographic pharyngeal anatomy, and the obstructive sleep apnoea syndrome. Eur Respir J. 1990;3:509–14. [PubMed] [Google Scholar]
- 20.Sharma SK, Kumpawat S, Banga A, Goel A. Prevalence and risk factors of obstructive sleep apnea syndrome in a population of Delhi, India. Chest. 2006;130:149–56. doi: 10.1378/chest.130.1.149. [DOI] [PubMed] [Google Scholar]
- 21.Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus CL. Vaughn BV for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.0. Darien, Illinois: American Academy of Sleep Medicine; 2012. www.aasmnet.org . [Google Scholar]
- 22.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.International Classification of Sleep Disorders. 3rd ed. Darien: American Academy of Sleep Medicine; 2014. American Academy of Sleep Medicine. [Google Scholar]
- 24.Keenan SP, Ferguson KA, Chan-Yeung M, Fleetham JA. Prevalence of sleep disordered breathing in a population of Canadian grainworkers. Can Respir J. 1998;5:184–90. doi: 10.1155/1998/403649. [DOI] [PubMed] [Google Scholar]
- 25.Ip MS, Lam B, Lauder IJ, Tsang KW, Chung KF, Mok YW, et al. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong. Chest. 2001;119:62–9. doi: 10.1378/chest.119.1.62. [DOI] [PubMed] [Google Scholar]
- 26.Ip MS, Lam B, Tang LC, Lauder IJ, Ip TY, Lam WK. A community study of sleep-disordered breathing in middle-aged Chinese women in Hong Kong: Prevalence and gender differences. Chest. 2004;125:127–34. doi: 10.1378/chest.125.1.127. [DOI] [PubMed] [Google Scholar]
- 27.Wali SO, Abaalkhail BA. Prevalence and predictors of habitual snoring in a sample of Saudi middle-aged adults. Saudi Med J. 2015;36:920–7. doi: 10.15537/smj.2015.8.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–8. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 29.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, et al. Prevalence of sleep-disordered breathing in women: Effects of gender. Am J Respir Crit Care Med. 2001;163(3 Pt 1):608–13. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 30.Durán J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163(3 Pt 1):685–9. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, In K, Kim J, You S, Kang K, Shim J, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004;170:1108–13. doi: 10.1164/rccm.200404-519OC. [DOI] [PubMed] [Google Scholar]
- 32.Ozdemir L, Akkurt I, Sümer H, Cetinkaya S, Gönlügür U, Ozsahin SL, et al. The prevalence of sleep related disorders in Sivas, Turkey. Tuberk Toraks. 2005;53:20–7. [PubMed] [Google Scholar]
- 33.Khassawneh B, Ghazzawi M, Khader Y, Alomari M, Amarin Z, Shahrour B, et al. Symptoms and risk of obstructive sleep apnea in primary care patients in Jordan. Sleep Breath. 2009;13:227–32. doi: 10.1007/s11325-008-0240-4. [DOI] [PubMed] [Google Scholar]
- 34.Amra B, Farajzadegan Z, Golshan M, Fietze I, Penzel T. Prevalence of sleep apnea-related symptoms in a Persian population. Sleep Breath. 2011;15:425–9. doi: 10.1007/s11325-010-0353-4. [DOI] [PubMed] [Google Scholar]
- 35.Kapsimalis F, Kryger MH. Gender and obstructive sleep apnea syndrome, Part 2: Mechanisms. Sleep. 2002;25:499–506. [PubMed] [Google Scholar]
- 36.Franklin KA, Sahlin C, Stenlund H, Lindberg E. Sleep apnoea is a common occurrence in females. Eur Respir J. 2013;41:610–5. doi: 10.1183/09031936.00212711. [DOI] [PubMed] [Google Scholar]
- 37.Lindberg E, Elmasry A, Gislason T, Janson C, Bengtsson H, Hetta J, et al. Evolution of sleep apnea syndrome in sleepy snorers: A population-based prospective study. Am J Respir Crit Care Med. 1999;159:2024–7. doi: 10.1164/ajrccm.159.6.9805070. [DOI] [PubMed] [Google Scholar]
- 38.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–21. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 39.Launois SH, Pépin JL, Lévy P. Sleep apnea in the elderly: A specific entity? Sleep Med Rev. 2007;11:87–97. doi: 10.1016/j.smrv.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Barvaux VA, Aubert G, Rodenstein DO. Weight loss as a treatment for obstructive sleep apnoea. Sleep Med Rev. 2000;4:435–52. doi: 10.1053/smrv.2000.0114. [DOI] [PubMed] [Google Scholar]
- 41.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–9. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 42.Grunstein RR, Stenlöf K, Hedner JA, Peltonen M, Karason K, Sjöström L. Two year reduction in sleep apnea symptoms and associated diabetes incidence after weight loss in severe obesity. Sleep. 2007;30:703–10. doi: 10.1093/sleep/30.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenburg DL, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: A meta-analysis. Am J Med. 2009;122:535–42. doi: 10.1016/j.amjmed.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 44.Young T, Peppard P, Palta M, Hla KM, Finn L, Morgan B, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–52. [PubMed] [Google Scholar]
- 45.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 46.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Leiby BE, Vela-Bueno A, et al. Association of hypertension and sleep-disordered breathing. Arch Intern Med. 2000;160:2289–95. doi: 10.1001/archinte.160.15.2289. [DOI] [PubMed] [Google Scholar]
- 47.Haas DC, Foster GL, Nieto FJ, Redline S, Resnick HE, Robbins JA, et al. Age-dependent associations between sleep-disordered breathing and hypertension: Importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation. 2005;111:614–21. doi: 10.1161/01.CIR.0000154540.62381.CF. [DOI] [PubMed] [Google Scholar]


