Abstract
Telomerase is an enzyme that maintains telomeres in dividing cells using a template on its inherent RNA component. Additionally, the protein part TERT (Telomerase Reverse Transcriptase) has various non-canonical functions. For example, it can localize to mitochondria under increased stress and protect cells in vitro from oxidative stress, DNA damage and apoptosis. Recently it has been demonstrated that TERT protein persists in adult neurons in the brain and data emerge suggesting that it might have a protective function in these post-mitotic cells as well. We have recently published that TERT protein accumulated in mitochondria from brain tissue of mice that have undergone short-term dietary restriction (DR) and rapamycin treatment. This localization correlated to lower levels of oxidative stress in these brain mitochondria. Since rapamycin treatment decreases mTOR signaling which is also thought to play an important role for the beneficial effects of DR, we conclude that the mTOR pathway might be involved in the TERT localization and its effects in brain mitochondria in vivo. These data are in line with previous findings from our group about increased mitochondrial localization of TERT in Alzheimer's disease (AD) brains and a protective function of TERT protein in neurons in vitro against pathological tau.
Keywords: telomerase, brain, neuron, mitochondria, mTOR, ROS, dietary restriction, rapamycin
Telomerase and Mitochondria
The telomerase complex is composed of telomerase RNA and the catalytic protein subunit, TERT (telomerase reverse transcriptase) as well as various associated proteins. The canonical function of telomerase is to counteract telomere shortening and thus the enzymatic telomerase activity is a predominant feature of dividing cells. However, there are various non-telomeric functions of the TERT protein (for review see Saretzki, 2014). One of these non-canonical functions is the protective role of TERT protein for cells against oxidative stress through its effects on mitochondria by shuttling to and localizing within the organelle from the nucleus. Mitochondria perform vital functions in life such as provision of energy (ATP), generation of iron–sulphur clusters, participating in Ca2+ regulation and apoptosis, and having major roles in fatty acid and amino-acid metabolism. They also produce reactive oxygen species (ROS) as metabolic by-products of oxidative phosphorylation, which can participate in cellular signaling pathways but also damage cellular components via oxidative stress. The TERT protein of higher organisms harbours a mitochondrial localization sequence, and the best known stimulus for the shuttling of TERT protein out of the nucleus and into mitochondria is oxidative stress: either acute or chronic, extrinsic by applying hydrogen peroxide (H2O2), hyperoxia or irradiation (Ahmed et al., 2008; Singhapol et al., 2013) or intrinsic, such as senescence-associated increase in cellular ROS levels (Haendeler et al., 2004). Our group and others have demonstrated that mitochondrial localization of TERT results in decreased cellular oxidative stress, lower nuclear and mitochondrial DNA damage levels and less apoptosis (Ahmed et al., 2008; Haendeler et al., 2009; Sharma et al., 2012; Singhapol et al., 2013). The exact mechanisms for these beneficial effects are not entirely clear, although various functions have been proposed, such as binding of TERT to mtDNA, increasing mitochondrial complex I activity and reverse transcriptase activity of TERT by complexing with mitochondrial RNAs (Haendeler et al., 2009; Sharma et al., 2012). However, no direct causality between these different mitochondrial functions of TERT and its protective function within mitochondria has yet been demonstrated and most of these functions were investigated in cellular systems in vitro using overexpression of various TERT protein versions. Thus, most protective functions of mitochondrial TERT have been initially demonstrated in cultivated cells, rather than neurons.
Telomerase in Brain
Various groups including ours have shown that TERT protein seems to have a relevance in brain and specifically neurons. Mark Mattson's group pioneered this research more than a decade ago. They showed a strong protective effect of telomerase in primary cultivated hippocampal embryonic mouse neurons and its significance in mediating the effect of growth factors and neurodegenerative agents such as β-amyloid (Fu et al., 2002). This data corresponds well to our recent findings in cultivated primary embryonic forebrain mouse neurons where the presence of TERT protein protects them from pathological tau and decreases oxidative stress and cellular damage compared to neurons lacking TERT (Spilsbury et al., 2015).
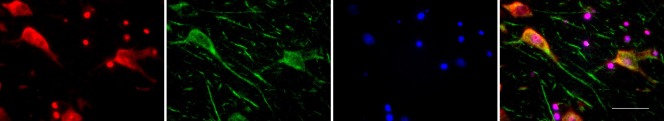
TERT protein persists in adult brain while telomerase activity is downregulated very early during gestation in human brain (Ulaner et al., 1998; Ishaq et al., 2016), and in mouse brain the activity is found to be switched off early postnatally (Klapper et al., 2001). A possible mechanism of this decrease could be the strong downregulation of the telomerase RNA component, as we demonstrated recently (Ishaq et al, 2016). The telomerase protein TERT persists in adult mammalian brain specifically in neurons (Iannilli et al., 2013; Spilsbury et al., 2015; Eitan et al., 2016; Figure 1), while astrocytes have no telomerase activity beyond embryonic stages. There is also some TERT protein in activated microglia cells, as expected, since they are immune cells which are telomerase competent (Spilsbury et al., 2015). Eitan et al. (2016) found that TERT protein persists in Purkinje neurons of adult mice and the same authors described an increase of TERT protein in brain tissue using a synthetic telomerase activator which seems to prevent NMDA toxicity and delays disease symptoms in a mouse model of amyotrophic lateral sclerosis (ALS) (Eitan et al., 2012).
Figure 1.
TERT localizes to neurons in the human hippocampus (CA4 region of a control Braak 0 brain).
Neurotrace staining (magenta, left image), TERT staining (green, 2nd from left, Epitomics antibody), DAPI (2nd from right) and merged image (right). DAPI: 4′,6-Diamidino-2-phenylindole; TERT: telomerase reverse transcriptase. The scale bar shown in the merged image is 50 μm.
In mice, TERT gene expression and protein levels seem to decrease with age in brain tissue (Miwa et al., 2016), however we have not found any strong evidence for a decline in TERT levels during human brain ageing or due to neurodegeneration (Spilsbury et al., 2015; Ishaq et al., 2016). Iannilli et al. (2013) found that TERT forms a complex with RNA stress particles and the cell cycle inhibitor p15, which can be released when stress is applied to neurons. Thus, there is strong evidence for the presence of TERT protein in adult mammalian brain, specifically in neurons. So the questions are: does TERT also have a function in neuronal mitochondria, and if so, is it similar to that described in non-neuronal cell types?
Telomerase in Brain Mitochondria
Brain is very a energy demanding organ and known to consume 20% of the oxygen we breathe, which is mostly utilised by mitochondria. There is a close association between mitochondrial dysfunction and neurodegenerative diseases, and neurons are very sensitive to mitochondrial defects and oxidative stress (Gandhi and Abramov, 2012).
We recently demonstrated that there is a higher mitochondrial localization of TERT protein in hippocampal neurons of Alzheimer's brains compared to aged-matched healthy controls (Spilsbury et al., 2015). It is important to emphasize that in neurons TERT protein does not seem to localize to nuclei, corresponding to a lack of cell division and telomerase activity in postmitotic neurons (Figures 1 and 2A). This finding corresponds well to that of Iannilli et al. (2013) who also found TERT protein exclusively in the cytoplasm of adult neurons while other authors also describe a certain nuclear TERT localization in neurons and brain tissue (Eitan et al., 2012, 2016). Thus, although still slightly controversial, it seems that neuronal TERT is bound into cytoplasmic complexes but can be released and is free to localize to mitochondria upon stress or other physiologically relevant stimuli (Iannilli et al., 2013).
Figure 2.
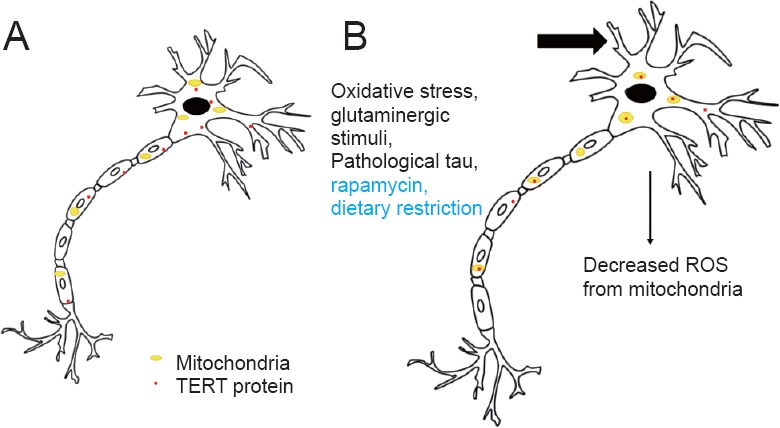
Scheme of known stimuli for TERT mitochondrial localization in brain neurons.
(A) Telomerase reverse transcriptase (TERT) protein mainly in cytoplasm. (B) Known (oxidative stress and pathological tau, Spilsbury et al., 2015; glutaminergic stimuli, Eitan et al., 2016) and novel (rapamycin and dietary restriction, Miwa et al., 2016) stimuli result in increased mitochondrial TERT localization with lower reactive oxygen species (ROS) generation as a consequence.
We also found that in cultivated primary mouse embryonic neurons TERT protein localizes to mitochondria upon increased oxidative stress correlating to decreased ROS (Spilsbury et al., 2015). In mouse Purkinje neurons, increased TERT localization to mitochondria has also been found after brain specific signalling events, for example due to glutaminergic stimuli (Eitan et al., 2016).
Our group recently identified physiologically relevant, previously unrecognized stimuli for TERT localization to mitochondria in brain: dietary restriction (DR) and rapamycin treatment (Miwa et al., 2016). We found that after 3–6 months of short term DR as well as 4 months of rapamycin treatment in mice, the proportion of TERT protein in brain mitochondria was higher and corresponded to lower mitochondrial ROS release than in controls. Seemingly paradoxical, both DR and rapamycin treatment are well-known experimental interventions which result in decreased oxidative stress with lower production of mitochondrial ROS than controls (Miwa et al., 2014). DR can robustly increase mean and maximum lifespan and decrease the incidence of many age-related diseases in a variety of model organisms, and rapamycin treatment seems to have similar properties (Wilkinson et al., 2012). Mitochondrially localized TERT protein might play a specific role in brain, as we did not find the increase in mitochondrial TERT after DR in other tissues such as liver which also expresses substantial amounts of telomerase activity (Miwa et al., 2016). Likewise, Haendeler and co-authors demonstrated that lack of TERT decreased state 3 respiration rates in heart tissue from TERT knock-out mice compared to telomerase positive wild type mice, but not in other organs such as liver (Haendeler et al., 2009). Thus, we speculate that mitochondrial effects of TERT protein might be more pronounced and contribute more specifically to a decrease in mitochondrial ROS in postmitotic and highly oxidative tissues such as brain and heart.
In summary, there seems to be a novel role of the TERT protein in neurons in brain tissue where it can localize to mitochondria and decrease oxidative stress, with several lines of evidence: in cultivated embryonic mouse neurons after H2O2 treatment (Spilsbury et al., 2015), and in brain mitochondria from mice that underwent DR and rapamycin treatment (Miwa et al., 2016).
Roles of mTOR Signalling in the Brain
mTOR is a conserved serine/threonine kinase that is known to consist of two different protein complexes: mTORC1 and mTORC2. mTOR is a crucial nutrient sensor that plays a critical role in cellular metabolism, growth, proliferation and apoptosis as well as the cellular response to oxidative stress. ROS have both activating and inhibitory effects on mTORC1, depending on cellular context, persistence, and strength of the oxidative stress.
Studies indicate that changes in mTOR signalling in the brain affect multiple pathways including energy production, mitochondrial function, cell growth and autophagy. In addition, mTOR is present during early neural development and promotes extension of dendrites and axons during neuronal differentiation. It also controls synaptic plasticity and processes underlying memory and learning in adult brain (Garza-Lombó and Gonsebatt, 2016).
Evidence exists for mTOR activation contributing to Alzheimer's disease (AD) progression and being closely associated with the presence of β-amyloid plaques and neurofibrillary tangles as well as cognitive impairment (Cai et al., 2015).
Autophagy, a cellular recycling process, is one of the important functions regulated by mTOR, and has been implicated in neurodegeneration (Fivenson et al., 2017). For example, it has been suggested that reduced autophagy in AD brains results in accumulation of protein aggregates via the hyperactivation of the PI3K/Akt/mTOR axis (Perluigi et al., 2015). Autophagy also contributes greatly to mitochondrial turn-over and ensures a pool of healthy mitochondria (MacVicar, 2013). Thus, the mTOR pathway is increasingly recognised as an important signalling mechanism in brain development, degeneration and in mitochondrial quality control via mitochondrial autophagy (mitophagy).
TERT Mitochondrial Localization, Dietary Restriction and mTOR Signalling
Many of the beneficial effects of DR can be mimicked by decreasing mTOR signalling by rapamycin (Wilkinson et al., 2012), an inhibitor of mTOR signalling which has also been shown to increase lifespan and improve mitochondrial function (Miwa et al., 2014). We were interested to examine whether decreasing mTOR signalling with rapamycin in mice in vivo might also be able to drive the TERT protein into brain mitochondria as seen under short-term DR (Miwa et al., 2016). Indeed, we found that rapamycin feeding of mice for 4 months resulted in a higher proportion of mitochondrially localized TERT, corresponding to a lower mitochondrial ROS release (Miwa et al., 2016). Critically, mitochondrial ROS release did not change in TERT knock out mice after rapamycin feeding, strongly implicating the involvement of mTOR signalling in mediating TERT localisation to mitochondria to reduce mitochondrial ROS release. This observation in brain was confirmed mechanistically in vitro using non-neuronal cells: rapamycin treatment induced nuclear exclusion of TERT protein and decreased ROS levels in MCF-7 cells. We found the same ROS decrease in primary mouse ear fibroblasts from wild type mice under rapamycin treatment while fibroblasts from TERT knock-out mice did not display this effect (Miwa et al., 2016). Using the Src kinase inhibitor bosutinib, TERT exclusion could be blocked and there was no decrease in ROS under this condition in mouse and human cells. Thus, TERT exclusion from the nucleus in response to rapamycin treatment seems to employ the same molecular exclusion mechanism as that induced by oxidative stress requiring Scr kinase dependent phosphorylation of Tyrosine 707 (Haendeler et al., 2003). The amount of TERT shuttling (proportion of cytoplasmically localized TERT) after rapamycin treatment in cells was rather low and comparable to the slow exclusion kinetics of mild chronic oxidative stress induced by 40% oxygen atmosphere in a cell culture incubator which takes several weeks to exclude TERT from the nucleus (Ahmed et al., 2008), but lower than with an acute oxidative stress treatment such as H2O2 which excludes around 50% of TERT protein within a few hours (Ahmed et al., 2008; Singhapol et al., 2013). While rapamycin treatment decreased ROS after 24 hours (unpublished data) we have not performed longer than 3 days rapamycin treatment in the cell culture model with human or mouse cells. Thus, the exact exclusion kinetics of TERT after decreasing mTOR signalling has not yet been analysed in detail.
It is intriguing that the TERT protein can form part of a larger complex including mTOR, in cancer and transformed immune cells (Kawauchi et al., 2005; Sundin et al. 2012). However, whether such a complex also exists in neurons and what functional implications it might have is yet to be determined.
Given the well-known role of decreased mTOR signaling in activating autophagy we speculate that decreased mTOR signaling may improve mitochondrial function both by improved mitochondrial quality control through mitophagy and by reducing ROS via increased mitochondrial TERT localization.
Summary
It is well recognized that mitochondrial dysfunction and increased oxidative stress play important roles in brain aging and the development of neurodegenerative diseases. Our recent findings that i) decreased mTOR signalling and dietary restriction are novel stimuli for mitochondrial localization of TERT protein, and ii) the role of the telomerase protein TERT in brain on lowering mitochondrial ROS in an mTOR signalling dependent manner (Miwa et al., 2016), therefore identify new players and connections in an ever bigger network of signaling events in the brain. Consequently, mTOR signalling and TERT protein could be potential therapeutic targets for neuropathological conditions via improvement of mitochondrial quality and decreased oxidative stress.
Acknowledgments
We thank Dr. Alison Spilsbury for the image in Figure 1 and Dr. Glyn Nelson (Newcastle University Bioimaging unit) for critical comments on the manuscript.
Footnotes
Conflicts of interest: The authors declare no conflict of interest.
References
- Ahmed S, Passos JF, Birket MJ, Beckmann T, Brings S, Peters H, Birch-Machin MA, von Zglinicki T, Saretzki G. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J Cell Sci. 2008;121:1046–1053. doi: 10.1242/jcs.019372. [DOI] [PubMed] [Google Scholar]
- Cai Z, Chen G, He W, Xiao M, Yan LJ. Activation of mTOR: a culprit of Alzheimer's disease? Neuropsychiatr Dis Treat. 2015;11:1015–1030. doi: 10.2147/NDT.S75717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitan E, Tichon A, Gazit A, Gitler D, Slavin S, Priel E. Novel telomerase-increasing compound in mouse brain delays the onset of amyotrophic lateral sclerosis. EMBO Mol Med. 2012;4:313–329. doi: 10.1002/emmm.201200212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitan E, Braverman C, Tichon A, Gitler D, Hutchison ER, Mattson MP, Priel E. Excitotoxic and radiation stress increase TERT levels in the mitochondria and cytosol of cerebellar purkinje neurons. Cerebellum. 2016;15:509–517. doi: 10.1007/s12311-015-0720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fivenson EM, Lautrup S, Sun N, Scheibye-Knudsen M, Stevnsner TV, Nilsen H, Bohr VA, Fang EF. Mitophagy in neurodegeneration and aging. Neurochem Int. 2017 doi: 10.1016/j.neuint.2017.02.007. doi:10.1016/j.neuint.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W, Lu C, Mattson MP. Telomerase mediates the cell survival-promoting actions of brain-derived neurotrophic factor and secreted amyloid precursor protein in developing hippocampal neurons. J Neurosci. 2002;22:10710–10719. doi: 10.1523/JNEUROSCI.22-24-10710.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S, Abramov AY. Mechanism of oxidative stress in neurodegeneration. Oxid Med Cell Longev 2012. 2012:428010. doi: 10.1155/2012/428010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza-Lombó C, Gonsebatt ME. Mammalian target of rapamycin: its role in early neural development and in adult and aged brain function. Front Cell Neurosci. 2016;10:157. doi: 10.3389/fncel.2016.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haendeler J, Hoffmann J, Brandes RP, Zeiher AM, Dimmeler S. Hydrogen peroxide triggers nuclear export of telomerase reverse transcriptase via Src kinase family-dependent phosphorylation of tyrosine 707. Mol Cell Biol. 2003;23:4598–4610. doi: 10.1128/MCB.23.13.4598-4610.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haendeler J, Hoffmann J, Diehl JF, Vasa M, Spyridopoulos I, Zeiher AM, Dimmeler S. Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circ Res. 2004;94:768–775. doi: 10.1161/01.RES.0000121104.05977.F3. [DOI] [PubMed] [Google Scholar]
- Haendeler J, Dröse S, Büchner N, Jakob S, Altschmied J, Goy C, Spyridopoulos I, Zeiher AM, Brandt U, Dimmeler S. Mitochondrial telomerase reverse transcriptase binds to and protects mitochondrial DNA and function from damage. Arterioscler Thromb Vasc Biol. 2009;29:929–935. doi: 10.1161/ATVBAHA.109.185546. [DOI] [PubMed] [Google Scholar]
- Iannilli F, Zalfa F, Gartner A, Bagni C, Dotti CG. Cytoplasmic TERT associates to RNA granules in fully mature neurons: role in the translational control of the cell cycle inhibitor p15INK4B. PLoS One. 2013;8:e66602. doi: 10.1371/journal.pone.0066602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishaq A, Hanson PS, Morris CM, Saretzki G. Telomerase activity is downregulated early during human brain development. Genes (Basel) 2016;7:pii–E27. doi: 10.3390/genes7060027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi K, Ihjima K, Yamada O. IL-2 increases human telomerase reverse transcriptase activity transcriptionally and posttranslationally through phosphatidylinositol, 3’-kinase/Akt, heat shock protein 90, and mammalian target of rapamycin in transformed NK cells. J Immunol. 2005;174:5261–5269. doi: 10.4049/jimmunol.174.9.5261. [DOI] [PubMed] [Google Scholar]
- Klapper W, Shin T, Mattson MP. Differential regulation of telomerase activity and TERT expression during brain development in mice. J Neurosci Res. 2001;64:252–260. doi: 10.1002/jnr.1073. [DOI] [PubMed] [Google Scholar]
- MacVicar T. Mitophagy. Essays Biochem. 2013;55:93–104. doi: 10.1042/bse0550093. [DOI] [PubMed] [Google Scholar]
- Miwa S, Jow H, Baty K, Johnson A, Czapiewski R, Saretzki G, Treumann A, von Zglinicki T. Low abundance of the matrix arm of complex I in mitochondria predicts longevity in mice. Nat Commun. 2014;5:3837. doi: 10.1038/ncomms4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa S, Czapiewski R, Wan T, Bell A, Hill KN, von Zglinicki T, Saretzki G. Decreased mTOR signalling reduces mitochondrial ROS in brain via accumulation of the telomerase protein TERT within mitochondria. Aging. 2016;8:2551–2567. doi: 10.18632/aging.101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perluigi M, Di Domenico F, Butterfield DA. mTOR signaling in aging and neurodegeneration: At the crossroad between metabolism dysfunction and impairment of autophagy. Neurobiol Dis. 2015;84:39–49. doi: 10.1016/j.nbd.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Saretzki G. Extra-telomeric functions of human telomerase: cancer, mitochondria and oxidative stress. Curr Pharm Des. 2014;20:6386–6403. doi: 10.2174/1381612820666140630095606. [DOI] [PubMed] [Google Scholar]
- Sharma NK, Reyes A, Green P, Caron MJ, Bonini MG, Gordon DM, Holt IJ, Santos JH. Human telomerase acts as a hTR-independent reverse transcriptase in mitochondria. Nucleic Acids Res. 2012;40:712–725. doi: 10.1093/nar/gkr758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilsbury A, Miwa S, Attems J, Saretzki G. The role of telomerase protein TERT in Alzheimer's disease and in tau-related pathology in vitro. J Neurosci. 2015;35:1659–1674. doi: 10.1523/JNEUROSCI.2925-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundin T, Hentosh P. In TERTesting association between telomerase, mTOR and phytochemicals. Expert Rev Mol Med. 2012;14:e8. doi: 10.1017/erm.2012.1. [DOI] [PubMed] [Google Scholar]
- Ulaner GA, Hu JF, Vu TH, Giudice LC, Hoffman AR. Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate splicing of hTERT transcripts. Cancer Res. 1998;58:4168–4172. [PubMed] [Google Scholar]
- Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, Woodward MA, Miller RA. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]