Keywords: nerve regeneration, traditional Chinese medicine, ferment, Shuan-Tong-Ling, middle cerebral artery occlusion, cerebral ischemia/reperfusion, silent information regulator 1, inflammation, apoptosis, tumor necrosis factor-α, interleukin-1β, Bcl-2, Bax, acetylated-protein 53, neural regeneration
Abstract
The fermented Chinese formula Shuan-Tong-Ling is composed of radix puerariae (Gegen), salvia miltiorrhiza (Danshen), radix curcuma (Jianghuang), hawthorn (Shanzha), salvia chinensis (Shijianchuan), sinapis alba (Baijiezi), astragalus (Huangqi), panax japonicas (Zhujieshen), atractylodes macrocephala koidz (Baizhu), radix paeoniae alba (Baishao), bupleurum (Chaihu), chrysanthemum (Juhua), rhizoma cyperi (Xiangfu) and gastrodin (Tianma), whose aqueous extract was fermented with lactobacillus, bacillus aceticus and saccharomycetes. Shuan-Tong-Ling is a formula used to treat brain diseases including ischemic stroke, migraine, and vascular dementia. Shuan-Tong-Ling attenuated H2O2-induced oxidative stress in rat microvascular endothelial cells. However, the potential mechanism involved in these effects is poorly understood. Rats were intragastrically treated with 5.7 or 17.2 mL/kg Shuan-Tong-Ling for 7 days before middle cerebral artery occlusion was induced. The results indicated Shuan-Tong-Ling had a cerebral protective effect by reducing infarct volume and increasing neurological scores. Shuan-Tong-Ling also decreased tumor necrosis factor-α and interleukin-1β levels in the hippocampus on the ischemic side. In addition, Shuan-Tong-Ling upregulated the expression of SIRT1 and Bcl-2 and downregulated the expression of acetylated-protein 53 and Bax. Injection of 5 mg/kg silent information regulator 1 (SIRT1) inhibitor EX527 into the subarachnoid space once every 2 days, four times, reversed the above changes. These results demonstrate that Shuan-Tong-Ling might benefit cerebral ischemia/reperfusion injury by reducing inflammation and apoptosis through activation of the SIRT1 signaling pathway.
Introduction
Ischemic stroke is the major cause of mortality and disability worldwide, especially in developing countries (Hankey, 2012). It results from a transient or permanent reduction in cerebral blood flow restricted to major brain arteries. At present, the most effective treatment strategy is to restore cerebral blood flow immediately. However, reperfusion may injure the brain when the blood supply is restored leading to poor clinical outcomes and a series of pathophysiologic events, such as inflammation, apoptosis, oxidative stress, glutamate excitotoxicity, calcium overload and mitochondrial dysfunction (Lanzillotta et al., 2013; Thompson et al., 2015; Yang et al., 2015; Han et al., 2016). Although thrombolytic agents are used to treat ischemic stroke in the clinic, their narrow therapeutic window and other safety problems limit their application (Yepes et al., 2009). Therefore, it is vital to explore new targets for the prevention and treatment of ischemic stroke.
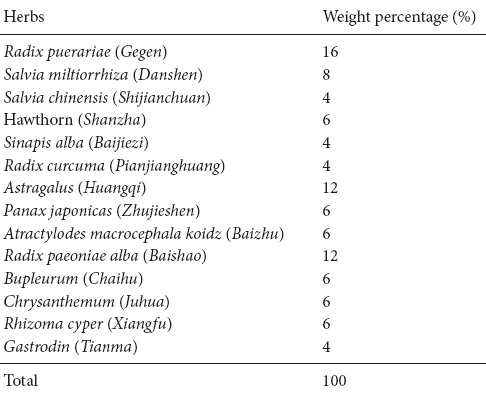
Traditional Chinese medicine has positive effects on ischemic stroke (Wu et al., 2011; Hao et al., 2012; Zhang et al., 2014). Shuan-Tong-Ling (STL) is a novel fermented Chinese formula adapted from a classical prescription for Sanpian Decoction, which is a famous traditional Chinese formula for curing vascular migraine and stroke, invented by Shiduo Chen, a great doctor in the Ming Dynasty (Liang et al., 2011; Li et al., 2012a). STL comprises 14 herbs, whose aqueous extract is fermented with lactobacillus, bacillus aceticus and saccharomycetes. Based on the theory of traditional Chinese medicine (Chen et al., 2012; Law et al., 2016), the herbs in STL are classified into three types. Radix puerariae (Gegen), salvia miltiorrhiza (Danshen), radix curcuma (Jianghuang), hawthorn (Shanzha), salvia chinensis (Shijianchuan), and sinapis alba (Baijiezi) promote qi and enhance blood circulation. Astragalus (Huangqi), panax japonicas (Zhujieshen), and atractylodes macrocephala koidz (Baizhu) are used to invigorate qi and enrich the blood. Radix paeoniae alba (Baishao), bupleurum (Chaihu), chrysanthemum (Juhua), rhizoma cyperi (Xiangfu) and gastrodin (Tianma) are used to modulate abnormal liquid metabolism and disperse stagnated liver qi. In traditional Chinese medicine, qi is an energy that invigorates the body and enhances the blood circulation and meridian circulation (Ni, 1995; Zhao et al., 2012; Li et al., 2014). Ischemic stroke is mainly caused by blood stasis, qi deficiency and stagnation. Based on the normal dosage used in the clinic, we used four times the weight of Radix puerariae, astragalus and radix paeoniae alba as a “monarch medicine” to enhance its efficacy to invigorate qi and blood circulation. Other herbs were present at their normal dosage and played a role of “minister medicine” or “assistant medicine”. The fermented STL tasted better than the common decoction of STL and it was accepted more easily by patients and healthy people. Based on this novel formulation and its pharmacological effects, we have applied for a Chinese patent (CN105833184A).
Silent information regulator 1 (SIRT1) deacetylase activity is dependent on nicotinamide adenine dinucleotide (NAD+) levels, which can directly activate SIRT1 as a substrate (Zschoernig and Mahlknecht, 2008; Ma et al., 2015). SIRT1 was implicated as a key molecule that protects against cerebral ischemia/reperfusion (I/R) injury (Paraíso et al., 2013). Cerebral hypoperfusion/ischemia, inflammation and apoptosis induce vascular cognitive impairment (Hattori and Ihara, 2016). SIRT1 can block cerebral hypoperfusion and ischemia through deacetylating anti-inflammatory and anti-apoptotic molecules as well as endothelial nitric oxide synthase to induce arterial dilation (Hernández-Jiménez et al., 2013; Pan et al., 2016). In addition, some components within STL had a protective effect on cerebral ischemia-reperfusion via SIRT1 in other studies. For example, curcumin protected ischemic brains by SIRT1 activation, leading to reduced acetylated-protein 53 (Ac-p53) expression and Bax and the increased expression of Bcl-2. Curcumin also mitigated inflammatory responses and mitochondrial dysfunction in cerebral ischemia (Miao et al., 2016). Salvianolic acid B upregulated SIRT1 and Bcl-2 expression, and downregulated Ac-FOXO1 and Bax expression (Lv et al., 2015). However, the mechanism of STL activation remains unclear. Therefore, this study investigated the mechanism involved in the beneficial effects of STL using in vivo experiments. We hypothesize that STL protects the brain from cerebral I/R injury and attenuates inflammation and apoptosis after I/R by activating SIRT1.
Materials and Methods
STL preparation
Fourteen herbs within STL were purchased from the Yichang Hospital of Traditional Chinese Medicine, Hubei province, China, and were identified by Maohua Chen a pharmacist who is Chief of the Pharmacy Department of Yichang Hospital, China. The weight percentage of each crude herb of STL is listed in Table 1. Combined herbs were minced and the mixture was submerged in water (five times the weight of the herbs) for 30 minutes and then heated for 1 hour at 100°C in marmite. The pH of the filtered STL water decoction was adjusted to 7.0 using 1 M NaOH and autoclaved for 20 minutes at 120°C. For fermentation, the STL water decoction was inoculated (1%, v/v) with three probiotics (saccharomyces, lactobacillus, and bacillus aceticus) for 2 days at 37°C. After sterilizing and pasteurization, the fermented STL liquid was canned and stored at 4°C.
Table 1.
Weight percentage of Shuan-Tong-Ling raw materials (Tan et al., 2016)
Quality control by high performance liquid chromatography (HPLC)
Standard chemicals, including puerarin, paeoniflorin, salvianolic acid B, chikusetsu V, astragaloside IV and curcumin, were purchased from Chengdu Mann Stewart Biological Technology Co., Ltd., China. Briefly, the quality of the fermented STL was determined by HPLC fingerprints. The multiple components of STL were characterized using an Alliance 2695 HPLC system (Waters Corporation, MA, USA). All chromatographic separations were performed on a Welchrom C18 column (4.6 × 250 mm, 5 μm; Welch Materials, Inc., MD, USA) at room temperature. The mobile phase consisted of 0.2% phosphoric acid water (A) and acetonitrile (B). The following gradient program was used: 0–60 minutes, 10–65% B. The flow rate was 1 mL/min, and the ultraviolet detection wavelength was set at 254 nm. The column temperature was 30°C, and the sample injection volume was 10 μL. The chromatographic data were recorded and processed with Empower 3.0 software (Waters Corporation, Milford, MA, USA).
Animals
All protocols were approved by the Experimental Animal Center of China Three Gorges University, China. Specific-pathogen-free male Sprague-Dawley rats aged 8 weeks and weighing 250–280 g were purchased from the Laboratory Animal Center of Three Gorges University, China (license No. SCXK (E) 2011-0061). All rats were kept under a controlled environment with a 12-hour light and dark cycle, with 60 ± 5% humidity and at 22 ± 3°C. They were allowed free access to water and food. Rats were acclimatized for 1 week before experiments. Animal welfare and experimental procedures were carried out strictly in accordance with the Laboratory Animal Management Committee of Three Gorges University and the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All rats were randomly assigned to the following groups (n = 8 per group): sham, I/R, I/R + STL (5.7 mL/kg), I/R + STL (17.2 mL/kg) and I/R + STL (17.2 mL/kg) + EX527.
Rat models of focal cerebral I/R
Cerebral I/R models were established by middle cerebral artery occlusion (MCAO) as previously described (Longa et al., 1989). First, rats were intraperitoneally anesthetized with 10% chloral hydrate (350 mg/kg) (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) before surgery. The rats were placed on a 37°C operating table. The right common carotid artery, the right external carotid artery and the internal carotid artery were exposed and isolated using a midline neck incision. A monofilament nylon suture (4-0) was inserted into the internal carotid artery through an external carotid artery stump and then advanced into the internal carotid artery approximately 18–22 mm from the carotid bifurcation until mild resistance to block the origin of the middle cerebral artery. At 90 minutes after ischemia, the nylon suture was slowly pulled out to allow reperfusion.
Drug administration
As reported previously (Yu et al., 2014; Lv et al., 2015; Hu et al., 2016), the SIRT1 inhibitor EX527 (Selleckchem, Houston, TX, USA) was first dissolved in dimethyl sulfoxide and diluted to a final concentration with normal saline (final dimethyl sulfoxide concentration < 2%). The sham group and the I/R group rats were administered physiological saline (5.7 mL/kg) once daily for 7 days before MCAO. The I/R + STL (5.7 or 17.2 mL/kg) group rats received the intragastric administration of STL at 5.7 or 17.2 mL/kg, once per day for 7 days before MCAO (Tan et al., 2016). The I/R + STL (17.2 mL/kg) + EX527 group rats were intragastrically administrated STL at a dose of 17.2 mL/kg once daily for 7 days and received the subarachnoid administration of the SIRT1 inhibitor EX527 at a dose of 5 mg/kg every 2 days, four times in total, before MCAO.
Evaluation of neurological deficits
Neurological deficits were measured after 24 hours of reperfusion according to the method of Longa et al. (1989). The neurological findings were scored as previously described: normal, 0 = no motor deficits; mild, 1 = forelimb weakness and torso turning to the ipsilateral side when held by tail; moderate, 2 = circling to the contralateral side but normal posture at rest; severe, 3 = unable to bear weight on the affected side at rest; and critical, 4 = no spontaneous locomotor activity or barrel rolling.
Assessment of infarct volume
Infarct volume was assessed by staining with 2,3,5-triphenyltetrazoliumchloride (TTC) at 24 hours after reperfusion. Rats in each group were decapitated immediately following euthanasia using 10% (v/v) chloral hydrate (350 mg/kg) 24 hours after cerebral I/R. The brains were rapidly removed and frozen for 10–15 minutes at −20°C. Five 2-mm-thick sections of each brain were sliced and stained with 2% TTC for 15 minutes at 37°C. The images were photographed and analyzed using Image-pro plus 6.0 software (Media Cybernetics, Rockville, MD, USA). The infarct volume was calculated by using the formula (%) = (area of the contralateral hemisphere − area of the normal part in the ipsilateral hemisphere)/area of the contralateral hemisphere × 100%.
Detection of inflammatory cytokines
TNF-α and IL-1β enzyme linked immunosorbent assay (ELISA) kits were purchased from NeoBioscience Technology Co., Ltd., Shenzhen, Guangdong Province, China. Twenty-four hours after reperfusion, rats were deeply anesthetized and decapitated. The hippocampus was rapidly removed and grinded until fully homogenized. Saline (500 μL) was added to the samples and these were centrifuged at 10,000 × g for 15 minutes. The supernatant was collected and stored at −20°C. All standards were prepared before starting the assay. The procedure was performed according to the manufacturer's recommendations. The mean optical density value at 450 nm for each set of reference standards and samples was calculated. All data were recorded with a microplate reader (Multiskan MK3, Thermo, MA, USA).
Western blot assay
The hippocampus of the ischemic penumbra was collected and lysed in ice-cold lysis buffer for 10 minutes. The lysates were centrifuged at 12,000 × g for 15 minutes, and the resulting supernatant was collected, boiled and stored at −80°C. Equal amounts of total protein were separated by 10% (w/v) sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then proteins were transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). Membranes were blocked in 5% (w/v) non-fat milk in phosphate buffered saline (PBS) containing 0/1% (v/v) Triton X-100 (PBST) for 1 hour at room temperature. The membranes were incubated overnight with rabbit anti-mouse SIRT1 polyclonal antibody (1:1,000; Abcam, Cambridge, MA, USA), rabbit anti-mouse Ac-p53 polyclonal antibody (1:500; Santa Cruz Biotechnology, Buffalo, CA, USA), anti-mouse Bcl-2 monoclonal antibody (1:500; Santa Cruz Biotechnology), anti-mouse Bax monoclonal antibody (1:500; Santa Cruz Biotechnology) and goat anti-rat β-actin antibody (1:2,000; Boster, Wuhan, Hubei Province, China) at 4°C. Membranes were washed twice with TBST (10 minutes each). The blots were incubated with peroxidase-conjugated goat anti-rabbit or rabbit anti-mouse IgG (1:3,000; Boster) for 1 hour at room temperature. The blots were immersed in Immobilon Western Chemiluminescent Horseradish Peroxidase Substrate (Boster). Protein expression was expressed as the optical density percentage of the target protein to β-actin using Image J Software (Wayne Rasband, National Institutes of Health, USA).
Statistical analysis
HPLC results were directly obtained from the HPLC device. Other data are expressed as the mean ± SEM. All analyses were carried out using SPSS 19.0 (SPSS, Chicago, IL, USA). Analysis of variance was used for multiple comparisons of groups. The least significant difference test was used to compare the means between each of the two groups. A P value < 0.05 was considered statistically significant.
Results
Qualitative analysis of STL components
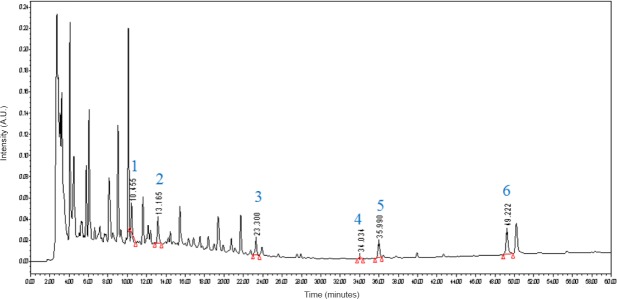
HPLC was used to identify the active ingredients of STL extracts. As shown in Figure 1, by comparing the retention times and the ultraviolet spectra of the referene standards, six compounds, including (1) puerarin, (2) paeoniflorin, (3) salvianolic acid B, (4) chikusetsu V, (5) astragaloside IV and (6) curcumin, were definitively identified in STL.
Figure 1.
High performance liquid chromatography fingerprint chromatogram of fermented Shuan-Tong-Ling at 254 nm.
Shuan-Tong-Ling contained (1) puerarin, (2) paeoniflorin, (3) salvianolic acid B, (4) chikusetsu V, (5) astragaloside IV and (6) curcumin, which were detected at 10.455 minutes, 13.165 minutes, 23.300 minutes, 34.034 minutes, 35.990 minutes and 49.222 minutes, respectively.
STL improved neurological deficits induced by cerebral I/R
At 24 hours after cerebral I/R, rats in the sham group did not present any symptoms of neurobehavioral dysfunction (Longa's score 0), while rats in the I/R group showed significant neurobehavioral dysfunction (P < 0.01, vs. sham group). Pretreatment with different concentrations of STL produced significant improvements in neurological function (P < 0.01, vs. I/R group). However, I/R + STL (17.2 mL/kg) + EX527 treatment did not significantly improve the neurological function of I/R rats compared to STL at 17.2 mL/kg treatment (Figure 2).
Figure 2.
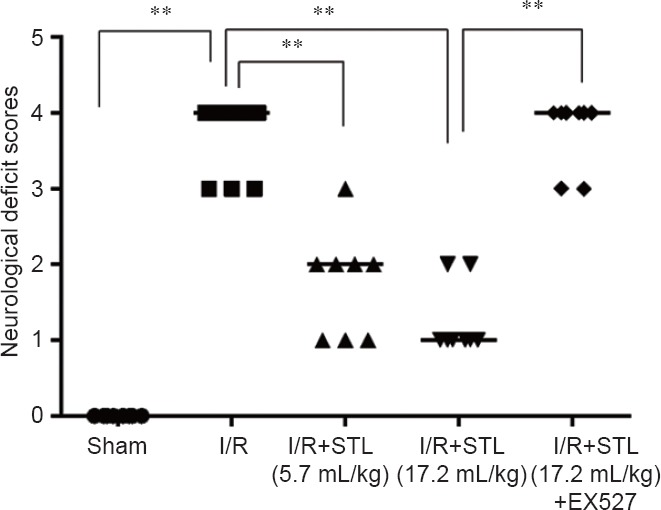
Effects of STL on neurological deficits 24 hours after cerebral I/R injury in rats.
Neurological function was assessed by Longa's score, with higher scores indicating severer neurological deficits. Data are analyzed by analysis of variance followed by the least significant difference test. **P < 0.01. STL: Shuan-Tong-Ling; I/R: ischemia/reperfusion; EX527: Silent information regulator 1 inhibitor.
STL reduced infarct volume after cerebral I/R
At 24 hours after cerebral I/R, the infarct volumes were measured by TTC assay. Obvious cerebral infarcts were detected in I/R rats. As shown in Figure 3, pretreatment with STL at 5.7 mL/kg or 17.2 mL/kg significantly reduced the infarct volume of I/R rats (P < 0.05, P < 0.01, vs. I/R group). Furthermore, the protective effect of STL at 17.2 mL/kg was partly blocked by EX527 (Figure 3).
Figure 3.
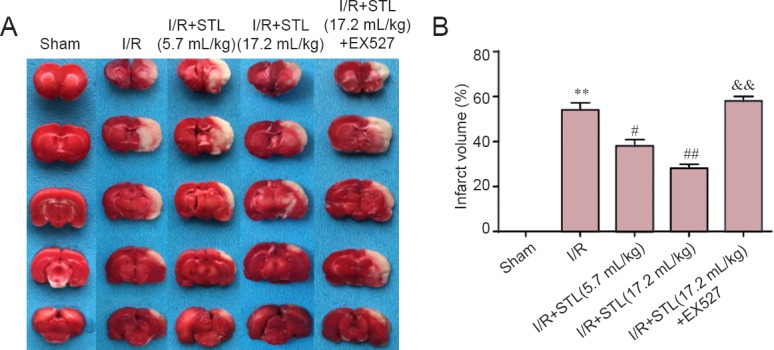
Effect of STL on infarct volume 24 hours after cerebral I/R injury in rats.
Infract volume was assessed using 2,3,-triphenyltetrazolium chloride assay. (A) Infarct picture of staining. Normal areas are red, and ischemic areas are white. (B) Quantitative results of infarct volume. Data are expressed as the mean ± SEM (n = 4 rats per group), and were analyzed by analysis of variance followed by the least significant difference test. **P < 0.01, vs. sham group; #P < 0.05, ##P < 0.01, vs. I/R group; &&P < 0.01, vs. I/R + STL (17.2 mL/kg) group. STL: Shuan-Tong-Ling; I/R: ischemia/reperfusion; EX527: silent information regulator 1 inhibitor.
STL reduced the expression of inflammatory cytokines in ischemic brain after cerebral I/R
To elucidate the effects of STL on inflammation induced by I/R, we evaluated the production of IL-1β and TNF-α in the hippocampus on the ischemic side of the brain by ELISA at 24 hours after cerebral I/R. As shown in Figure 4, the levels of IL-1β and TNF-α were significantly increased after cerebral I/R (P < 0.01, vs. sham group). Pretreatment with STL at 5.7 mL/kg or 17.2 mL/kg remarkably attenuated the increasing levels of IL-1β and TNF-α (P < 0.01, vs. I/R group) and these decreases were significantly suppressed by EX527 treatment (P < 0.01, vs. I/R + STL (17.2 mL/kg) group).
Figure 4.
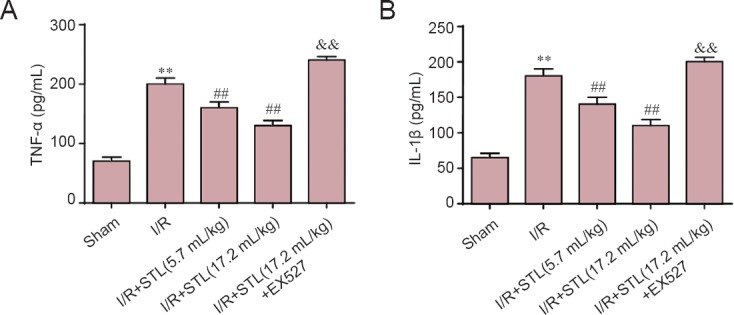
Inhibitory effect of STL on imflammatory cytokines in the brain at 24 hours after cerebral I/R injury.
Quantitative results of (A) TNF-α levels and (B) IL-1β levels. Values are expressed as the mean ± SEM (n = 4 rats per group), and were analyzed by analysis of variance followed by the least significant difference test. **P < 0.01, vs. sham group; ##P < 0.01, vs. I/R group; &&P < 0.01, vs. I/R + STL (17.2 mL/kg) group. STL: Shuan-Tong-Ling; I/R: ischemia/reperfusion; TNF: tumor necrosis factor; IL: interleukin; EX527: silent information regulator 1 inhibitor.
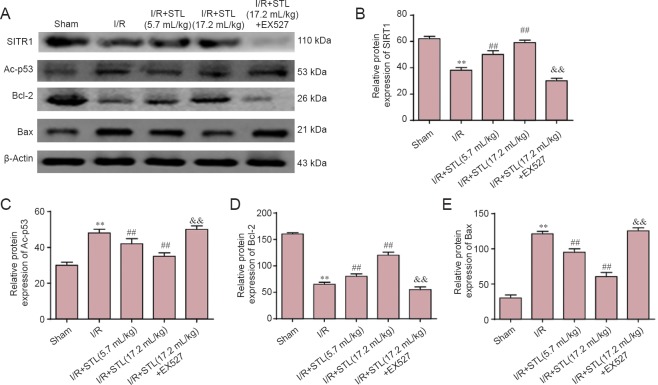
Effects of STL on SIRT1, Ac-p53, Bcl-2, and Bax expression
As shown in Figure 5, low levels of SIRT1 and Bcl-2 protein expression and high levels of Ac-p53 and Bax protein expression were detected in the hippocampus of ischemic brains at 24 hours after cerebral I/R by western blot assay. STL (5.7 and 17.2 mL/kg) treatment markedly augmented SIRT1 and Bcl-2 protein expression compared with the I/R group (P < 0.01). EX527 treatment significantly lowered SIRT1 and Bcl-2 expression in the I/R + STL (17.2 mL/kg) + EX527 group compared with the I/R + STL (17.2 mL/kg) group (P < 0.01). Moreover, STL (5.7 and 17.2 mL/kg) treatment also decreased the expression of Ac-p53 and Bax compared with the I/R group (P < 0.01). EX527 significantly increased Ac-p53 and Bax expression in the I/R + STL (17.2 mL/kg) + EX527 group compared with the I/R + STL (17.2 mL/kg) group (P < 0.01; Figure 5).
Figure 5.
Effects of STL on the protein expression of SIRT1, Ac-p53, Bcl-2 and Bax in the brain 24 hours after cerebral I/R.
(A) Western blot assay for protein expression in each group. (B–E) Density analysis of SIRT1 (B), Ac-p53 (C), Bcl-2 (D), and Bax (E) protein expression. β-Actin was used as a loading control. The protein expressions were expressed as the optical density percentage of the target protein to β-actin. Values are expressed as the mean ± SEM (n = 4 rats per group), and were analyzed by analysis of variance followed by the least significant difference test. **P < 0.01, vs. sham group; ##P < 0.01, vs. I/R group; &&P < 0.01, vs. I/R + STL (17.2 mL/kg) group. STL: Shuan-Tong-Ling; I/R: ischemia/reperfusion; Ac-p53: acetylated-protein 53; EX527: SIRT1 inhibitor; SIRT1: silent information regulator 1.
Discussion
Cerebral I/R during ischemic stroke activates many pathophysiological processes, including inflammation, apoptosis and oxidative stress (Chen et al., 2012; Yin et al., 2013; Cao et al., 2015; Xiang et al., 2015; Zhang et al., 2016a). Inflammation and apoptosis have crucial roles in the pathophysiological process of cerebral ischemia, and TNF-α and IL-1β are the key inflammatory cytokines (Kong et al., 2009; Palencia et al., 2015; Ma and Yin, 2016). These cytokines are involved in inflammatory responses after ischemic stroke that induce endothelial cell damage, microglial activation, and neuronal injury (Sharief and Thompson, 1992; Pytel and Alexander, 2009; Li et al., 2015; Zhang et al., 2016b; Zhao et al., 2016). Therapeutic interventions that attenuate inflammatory responses are effective for curing ischemic stroke (Sieber et al., 2011). In addition, during post-ischemia reperfusion, many vulnerable neurons, particularly in the penumbra region, undergo apoptosis (MacManus and Buchan, 2000). Apoptosis can be triggered by intrinsic stimulation through the mitochondrial signaling pathway, or by extrinsic stimulation through cell surface death receptors (Liu et al., 2016). Members of the Bcl-2/Bax family have important roles in modulating apoptotic pathways (Dirnagl et al., 1999).
To maintain health and treat disease, Chinese people have developed traditional Chinese medicine over many years (Lin et al., 2008; Yang et al., 2016). Based on the theory of traditional Chinese medicine, qi is a vital energy that invigorates blood circulation and improves immunocompetence. Ischemic stroke is usually attributed to qi deficiency, qi-stagnancy and blood stasis. For targeted treatment, we designed the prescription of STL to nourish qi, invigorate the blood circulation, and smooth liver qi. In this study, HPLC was used to identify the active ingredients of fermented STL, and puerarin, paeoniflorin, salvianolic acid B, chikusetsu V, astragaloside IV and curcumin were confirmed. We found that puerarin protected against brain injury via counteracting the inflammation after cerebral I/R via the cholinergic anti-inflammatory pathway. Zhang et al. (2015) found that paeoniflorin inhibited the apoptosis of neurons and accumulation of Bcl-2 expression, and reduced Bax expression to protect neurons. Another active ingredient, salvianolic acid B, also exerted anti-inflammatory effects as indicated by the decreased TNF-α and IL-1β levels in brain tissues. Salvianolic acid B upregulated SIRT1 and Bcl-2 expression, and downregulated Ac-FOXO1 and Bax expression (Lv et al., 2015). Furthermore, Duan et al. (2016) reported that chikusetsu V protected cerebral I/R in diabetes through the AMPK-mediated phosphorylation of GSK-3β downstream of the APN-LKB1 pathway. Li et al. (2012b) found that astragaloside IV exerted a protective effect by decreasing the production of intercellular adhesion molecule-1, which was attenuated through the inhibition of TNF-α, IL-1β and NF-κB levels after I/R. In addition, curcumin protected ischemic brains through SIRT1 activation, leading to reduced Ac-p53 and Bax expression and the increased expression of Bcl-2. Curcumin also mitigated inflammatory responses and mitochondrial dysfunction in cerebral ischemia (Miao et al., 2016). Fermentation is a slow decomposition process whereby organic substances are degraded by microorganisms (Talebnia et al., 2010). It significantly ruptures the cells releasing molecules to the menstruum, allowing bacterial enzymes to break down cell membranes to help herb ingredients to leach into the preparation. A recent study showed that fermented traditional Chinese medicine was safe (Park et al., 2014), and can be used as an anti-tumor, anti-fungal (Kuwaki et al., 2002), and anti-oxidative stress agents as well as improve neuroprotection (Yang et al., 2011; Weon et al., 2014). Taken together, the above active constituent mixtures combined with the fermentation process may act together to achieve a beneficial efficacy of fermented STL.
SIRT1 is highly expressed in various brain areas involved in cognitive functions and regulation of gene expression (Paraíso et al., 2013; Yang et al., 2013). Many studies have suggested that SIRT1 might have multiple roles in gene silencing, inflammation, apoptosis, stress, and senescence (Chung et al., 2010; Rahman et al., 2012; Yao and Rahman, 2012), including I/R (Morris et al., 2011) and neurodegeneration (Donmez, 2012). NF-κB acetylation at lysine 310 has a crucial role in inflammatory factor transcription and increased inflammatory reactions were observed after the reduced expression of SIRT1 (Ousman and Kubes, 2012; Pan et al., 2016). SIRT1 physically interacted with RelA/p65, a subunit of NF-κB and de-acetylated the RelA/p65 of lysine 310 residue to inhibit its transcription (Yeung et al., 2004; Shen et al., 2016). A previous study showed that Ac-p53-mediated apoptosis was regulated by promoting SIRT1 translocation from the nucleus to the cytoplasm (Gu et al., 2016). In cerebral I/R, the deacetylase activity of SIRT1 is critical for preventing nerves from brain ischemia by deacetylation and suppressing p53 and NF-κB-induced inflammatory and apoptotic signaling pathways (Hernández-Jiménez et al., 2013). SIRT1 also mediated tolerance of hyperbaric oxygen preconditioning-induced ischemia of the rat brain (Yan et al., 2013). STL intervention markedly increased SIRT1 levels and lowered Ac-p53 levels, as well as increasing anti-apoptotic protein Bcl-2 expression and reducing pro-apoptotic protein Bax expression. In contrast, EX527 administration blocked the above effects. These results suggest that SIRT1 activation might mediate the neuroprotective effects of STL through de-acetylating p53 and extenuating apoptosis of the ischemic brain.
In conclusion, the fermented Chinese formula STL protected against ischemic stroke by activating SIRT1 signaling pathways, leading to decreased Ac-p53 and Bax expression and increased Bcl-2 expression. Activated SIRT1 attenuated inflammation by decreasing TNF-α and IL-1β levels in experimental stroke rats. We plan to offer STL as a recovery therapy for cerebral ischemia in stroke patients and as an effective health-care food for clinical targets suffering from ischemic stroke or other vascular encephalopathies. However, the specific mechanism involved remains to be determined. We will use sham + EX527, I/R + EX527, and EX527 alone groups to derive further information. Recently, endothelial progenitor cells were associated with the outcome of ischemic stroke (Yang et al., 2015). Therefore, we will investigate whether STL mitigates cerebral I/R injury via affecting circulating endothelial progenitor cells in the future.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81202625; and Open Fund of Key Laboratory of Cardiovascular and Cerebrovascular Diseases Translational Medicine of China Three Gorges University of China, No. 2016xnxg101.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using CrossCheck to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Pack M, Yu J, Li CH, Qiu Y, Song LP, Zhao M
References
- Cao YL, Chen CF, Wang AW, Feng YB, Cheng HX, Zhang WW, Xin W. Changes of peripheral-type benzodiazepine receptors in the penumbra area after cerebral ischemia-reperfusion injury and effects of astragaloside IV on rats. Genet Mol Res. 2015;14:277–285. doi: 10.4238/2015.January.23.1. [DOI] [PubMed] [Google Scholar]
- Chen L, Wang L, Zhang X, Cui L, Xing Y, Dong L, Liu Z, Li Y, Zhang X, Wang C, Bai X, Zhang J, Zhang L, Zhao X. The protection by octreotide against experimental ischemic stroke: up-regulated transcription factor Nrf2, HO-1 and down-regulated NF-κB expression. Brain Res. 2012;1475:80–87. doi: 10.1016/j.brainres.2012.07.052. [DOI] [PubMed] [Google Scholar]
- Chung S, Yao H, Caito S, Hwang JW, Arunachalam G, Rahman I. Regulation of SIRT1 in cellular functions: role of polyphenols. Arch Biochem Biophys. 2010;501:79–90. doi: 10.1016/j.abb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Donmez G. The neurobiology of sirtuins and their role in neurodegeneration. Trends Pharmacol Sci. 2012;33:494–501. doi: 10.1016/j.tips.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Duan J, Yin Y, Cui J, Yan J, Zhu Y, Guan Y, Wei G, Weng Y, Wu X, Guo C, Wang Y, Xi M, Wen A. Chikusetsu saponin iva ameliorates cerebral ischemia reperfusion injury in diabetic mice via adiponectin-mediated AMPK/GSK-3β pathway in vivo and in vitro. Mol Neurobiol. 2016;53:728–743. doi: 10.1007/s12035-014-9033-x. [DOI] [PubMed] [Google Scholar]
- Gu X, Gu B, Lv X, Yu Z, Wang R, Zhou X, Qiao W, Mao Z, Zuo G, Li Q, Miao D, Jin J. 1,25-Dihydroxy-vitamin D3 with tumor necrosis factor-alpha protects against rheumatoid arthritis by promoting p53 acetylation-mediated apoptosis via Sirt1 in synoviocytes. Cell Death Dis. 2016;7:e2423. doi: 10.1038/cddis.2016.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JQ, Liu CL, Wang ZY, Liu L, Cheng L, Fan YD. Anti-inflammatory properties of lipoxin A4 protect against diabetes mellitus complicated by focal cerebral ischemia/reperfusion injury. Neural Regen Res. 2016;11:636–640. doi: 10.4103/1673-5374.180750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankey GJ. Anticoagulant therapy for patients with ischaemic stroke. Nat Rev Neurol. 2012;8:319–328. doi: 10.1038/nrneurol.2012.77. [DOI] [PubMed] [Google Scholar]
- Hao CZ, Wu F, Shen J, Lu L, Fu DL, Liao WJ, Zheng GQ. Clinical efficacy and safety of buyang huanwu decoction for acute ischemic stroke: a systematic review and meta-analysis of 19 randomized controlled trials. Evid Based Complement Alternat Med 2012. 2012:630124. doi: 10.1155/2012/630124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y, Ihara M. SIRT1. Nihon Rinsho. 2016;74:589–594. [PubMed] [Google Scholar]
- Hernández-Jiménez M, Hurtado O, Cuartero MI, Ballesteros I, Moraga A, Pradillo JM, McBurney MW, Lizasoain I, Moro MA. Silent information regulator 1 protects the brain against cerebral ischemic damage. Stroke. 2013;44:2333–2337. doi: 10.1161/STROKEAHA.113.001715. [DOI] [PubMed] [Google Scholar]
- Hu MZ, Zhou B, Mao HY, Sheng Q, Du B, Chen JL, Pang QF, Ji Y. Exogenous hydrogen sulfide postconditioning protects isolated rat hearts from ischemia/reperfusion injury through Sirt1/PGC-1α signaling pathway. Int Heart J. 2016;57:477–482. doi: 10.1536/ihj.15-506. [DOI] [PubMed] [Google Scholar]
- Kong XX, Wang R, Liu XJ, Zhu LL, Shao D, Chang YS, Fang FD. Function of SIRT1 in physiology. Biochemistry (Mosc) 2009;74:703–708. doi: 10.1134/s0006297909070013. [DOI] [PubMed] [Google Scholar]
- Kuwaki S, Ohhira I, Takahata M, Murata Y, Tada M. Antifungal activity of the fermentation product of herbs by lactic acid bacteria against tinea. J Biosci Bioeng. 2002;94:401–405. [PubMed] [Google Scholar]
- Lanzillotta A, Pignataro G, Branca C, Cuomo O, Sarnico I, Benarese M, Annunziato L, Spano P, Pizzi M. Targeted acetylation of NF-kappaB/RelA and histones by epigenetic drugs reduces post-ischemic brain injury in mice with an extended therapeutic window. Neurobiol Dis. 2013;49:177–189. doi: 10.1016/j.nbd.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Law YB, Mok WS, Wu GA, Lam WC, Yu XM, Wong KV. New potential pharmacological functions of chinese herbal medicines via regulation of autophagy. Molecules. 2016;21:359. doi: 10.3390/molecules21030359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Li RJ, Li F. Modified Sanpian decoction in treating migraine randomized controlled clinical study. Shiyong Zhongyi Neike Zazhi. 2012a;26:12–13. [Google Scholar]
- Li JH, Liu AJ, Li HQ, Wang Y, Shang HC, Zheng GQ. Buyang huanwu decoction for healthcare: evidence-based theoretical interpretations of treating different diseases with the same method and target of vascularity. Evid Based Complement Alternat Med 2014. 2014:506783. doi: 10.1155/2014/506783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Qu YZ, Zhao ZW, Wu SX, Liu YY, Wei XY, Gao L, Gao GD. Astragaloside IV protects against focal cerebral ischemia/reperfusion injury correlating to suppression of neutrophils adhesion-related molecules. Neurochem Int. 2012b;60:458–465. doi: 10.1016/j.neuint.2012.01.026. [DOI] [PubMed] [Google Scholar]
- Li ZX, Lv JL, Chen P, Zhao RL. Effects of ischemic postconditioning on related pathways and interleukin 8 expression in rat models of focal cerebral ischemia/reperfusion. Zhongguo Zuzhi Gongcheng Yanjiu. 2015;19:7938–7944. [Google Scholar]
- Liang ZK, Yao GZ, Liang XC. Analysis on Therapeutic effect of migraine treated by Sanpian decoction combined with electroacupuncture. Shiyong Zhongyiyao Zazhi. 2011;27:366–368. [Google Scholar]
- Lin Z, Zhu D, Yan Y, Yu B. Herbal formula FBD extracts prevented brain injury and inflammation induced by cerebral ischemia–reperfusion. J Ethnopharmacol. 2008;118:140–147. doi: 10.1016/j.jep.2008.03.023. [DOI] [PubMed] [Google Scholar]
- Liu Cl, Zhang K, Chen G. Hydrogen therapy: from mechanism to cerebral diseases. Med Gas Res. 2016;6:48–54. doi: 10.4103/2045-9912.179346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Lv H, Wang L, Shen J, Hao S, Ming A, Wang X, Su F, Zhang Z. Salvianolic acid B attenuates apoptosis and inflammation via SIRT1 activation in experimental stroke rats. Brain Res Bull. 2015;115:30–36. doi: 10.1016/j.brainresbull.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Ma CY, Yin L. Neuroprotective effect of angiotensin II type 2 receptor during cerebral ischemia/reperfusion. Neural Regen Res. 2016;11:1102–1107. doi: 10.4103/1673-5374.187044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Nie H, Chen H, Li J, Hong Y, Wang B, Wang C, Zhang J, Cao W, Zhang M, Xu Y, Ding X, Yin SK, Qu X, Ying W. NAD+/NADH metabolism and NAD+-dependent enzymes in cell death and ischemic brain injury: current advances and therapeutic implications. Curr Med Chem. 2015;22:1239–1247. doi: 10.2174/0929867322666150209154420. [DOI] [PubMed] [Google Scholar]
- MacManus JP, Buchan AM. Apoptosis after experimental stroke: fact or fashion? J Neurotrauma. 2000;17:899–914. doi: 10.1089/neu.2000.17.899. [DOI] [PubMed] [Google Scholar]
- Miao Y, Zhao S, Gao Y, Wang R, Wu Q, Wu H, Luo T. Curcumin pretreatment attenuates inflammation and mitochondrial dysfunction in experimental stroke: The possible role of Sirt1 signaling. Brain Res Bull. 2016;121:9–15. doi: 10.1016/j.brainresbull.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Morris KC, Lin HW, Thompson JW, Perez-Pinzon MA. Pathways for ischemic cytoprotection: role of sirtuins in caloric restriction, resveratrol, and ischemic preconditioning. J Cereb Blood Flow Metab. 2011;31:1003–1019. doi: 10.1038/jcbfm.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M. The Yellow Emperor's Classic of Medicine: A New Translation of the Neijing Suwen with Commentary. Boulder, CO, USA: Shambhala Publications; 1995. [Google Scholar]
- Ousman SS, Kubes P. Immune surveillance in the central nervous system. Nat Neurosci. 2012;15:1096–1101. doi: 10.1038/nn.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palencia G, Medrano Jà, Ortiz-Plata A, Farfán DJ, Sotelo J, Sánchez A, Trejo-Solís C. Anti-apoptotic, anti-oxidant, and anti-inflammatory effects of thalidomide on cerebral ischemia/reperfusion injury in rats. J Neurol Sci. 2015;351:78–87. doi: 10.1016/j.jns.2015.02.043. [DOI] [PubMed] [Google Scholar]
- Pan W, Yu H, Huang S, Zhu P. Resveratrol protects against tnf-α-induced injury in human umbilical endothelial cells through promoting sirtuin-1-induced repression of NF-KB and p38 MAPK. PLoS One. 2016;11:e0147034. doi: 10.1371/journal.pone.0147034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraíso AF, Mendes KL, Santos SH. Brain activation of SIRT1: role in neuropathology. Mol Neurobiol. 2013;48:681–689. doi: 10.1007/s12035-013-8459-x. [DOI] [PubMed] [Google Scholar]
- Park H, Hwang YH, Yang HJ, Kim HK, Song KS, Ma JY. Acute toxicity and genotoxicity study of fermented traditional herb formula Guibi-tang. J Ethnopharmacol. 2014;156:182–189. doi: 10.1016/j.jep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Pytel P, Alexander JJ. Pathogenesis of septic encephalopathy. Curr Opin Neurol. 2009;22:283–287. doi: 10.1097/WCO.0b013e32832b3101. [DOI] [PubMed] [Google Scholar]
- Rahman I, Kinnula VL, Gorbunova V, Yao H. SIRT1 as a therapeutic target in inflammaging of the pulmonary disease. Prev Med. 2012;54(Suppl):S20–S28. doi: 10.1016/j.ypmed.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharief MK, Thompson EJ. In vivo relationship of tumor necrosis factor-alpha to blood-brain barrier damage in patients with active multiple sclerosis. J Neuroimmunol. 1992;38:27–33. doi: 10.1016/0165-5728(92)90087-2. [DOI] [PubMed] [Google Scholar]
- Shen J, Fang J, Hao J, Zhong X, Wang D, Ren H, Hu Z. SIRT1 inhibits the catabolic effect of il-1β through TLR2/SIRT1/NF-κB pathway in human degenerative nucleus pulposus cells. Pain Physician. 2016;19:E215–226. [PubMed] [Google Scholar]
- Sieber MW, Claus RA, Witte OW, Frahm C. Attenuated inflammatory response in aged mice brains following stroke. PLoS One. 2011;6:e26288. doi: 10.1371/journal.pone.0026288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talebnia F, Karakashev D, Angelidaki I. Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation. Bioresour Technol. 2010;101:4744–4753. doi: 10.1016/j.biortech.2009.11.080. [DOI] [PubMed] [Google Scholar]
- Tan L, Zhang X, Mei Z, Wang J, Li X, Huang W, Yang S. Fermented chinese formula Shuan-Tong-Ling protects brain microvascular endothelial cells against oxidative stress injury. Evid Based Complement Alternat Med. 2016 doi: 10.1155/2016/5154290. doi: 10.1155/2016/5154290:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JW, Narayanan SV, Koronowski KB, Morris-Blanco K, Dave KR, Perez-Pinzon MA. Signaling pathways leading to ischemic mitochondrial neuroprotection. J Bioenerg Biomembr. 2015;47:101–110. doi: 10.1007/s10863-014-9574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weon JB, Lee B, Yun BR, Lee J, Ma JY, Ma CJ. Neuroprotective and cognitive enhancing activity of the fermented Bozhougyiqi-Tang. Pharmacogn Mag. 2014;10:S249–S255. doi: 10.4103/0973-1296.133266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CJ, Chen JT, Yen TL, Jayakumar T, Chou DS, Hsiao G, Sheu JR. Neuroprotection by the traditional Chinese medicine, Tao-Hong-Si-Wu-Tang, against middle cerebral artery occlusion-induced cerebral ischemia in rats. Evid Based Complement Alternat Med 2011. 2011:803015. doi: 10.1155/2011/803015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang YX, Wang WX, Xue Z, Zhu L, Wang SB, Sun ZH. Electrical stimulation of the vagus nerve protects against cerebral ischemic injury through an anti-infammatory mechanism. Neural Regen Res. 2015;10:576–582. doi: 10.4103/1673-5374.155430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Fang Z, Yang Q, Dong H, Lu Y, Lei C, Xiong L. SirT1 mediates hyperbaric oxygen preconditioning-induced ischemic tolerance in rat brain. J Cereb Blood Flow Metab. 2013;33:396–406. doi: 10.1038/jcbfm.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Ma S, Liu Y, Li Y, Wu W, Han E, Jia G, Wang C. Poor outcome of experimental ischemic stroke in type 2 diabetic rats: impaired circulating endothelial progenitor cells mobilization. J Stroke Cerebrovasc Dis. 2015;24:980–987. doi: 10.1016/j.jstrokecerebrovasdis.2014.12.022. [DOI] [PubMed] [Google Scholar]
- Yang H, Li L, Zhou K, Wang Y, Guan T, Chai C, Kou J, Yu B, Yan Y. Shengmai injection attenuates the cerebral ischemia/reperfusion induced autophagy via modulation of the AMPK, mTOR and JNK pathways. Pharm Biol. 2016;54:2288–2297. doi: 10.3109/13880209.2016.1155625. [DOI] [PubMed] [Google Scholar]
- Yang HJ, Weon JB, Lee B, Ma CJ. The alteration of components in the fermented Hwangryunhaedok-tang and its neuroprotective activity. Pharmacogn Mag. 2011;7:207–212. doi: 10.4103/0973-1296.84234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Duan W, Li Y, Yan J, Yi W, Liang Z, Wang N, Yi D, Jin Z. New role of silent information regulator 1 in cerebral ischemia. Neurobiol Aging. 2013;34:2879–2888. doi: 10.1016/j.neurobiolaging.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Yao H, Rahman I. Perspectives on translational and therapeutic aspects of SIRT1 in inflammaging and senescence. Biochem Pharmacol. 2012;84:1332–1339. doi: 10.1016/j.bcp.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepes M, Roussel BD, Ali C, Vivien D. Tissue-type plasminogen activator in the ischemic brain: more than a thrombolytic. Trends Neurosci. 2009;32:48–55. doi: 10.1016/j.tins.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Tu C, Zhao J, Ou D, Chen G, Liu Y, Xiao X. Exogenous hydrogen sulfide protects against global cerebral ischemia/reperfusion injury via its anti-oxidative, anti-inflammatory and anti-apoptotic effects in rats. Brain Res. 2013;1491:188–196. doi: 10.1016/j.brainres.2012.10.046. [DOI] [PubMed] [Google Scholar]
- Yu L, Sun Y, Cheng L, Jin Z, Yang Y, Zhai M, Pei H, Wang X, Zhang H, Meng Q, Zhang Y, Yu S, Duan W. Melatonin receptor-mediated protection against myocardial ischemia/reperfusion injury: role of SIRT1. J Pineal Res. 2014;57:228–238. doi: 10.1111/jpi.12161. [DOI] [PubMed] [Google Scholar]
- Zhang P, Guo ZF, Xu YM, Li YS, Song JG. N-Butylphthalide (NBP) ameliorated cerebral ischemia reperfusion-induced brain injury via HGF-regulated TLR4/NF-κB signaling pathway. Biomed Pharmacother. 2016a;83:658–666. doi: 10.1016/j.biopha.2016.07.040. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Bian H, Li Y, Guo L, Tang Y, Zhu H. Preconditioning with the traditional Chinese medicine Huang-Lian-Jie-Du-Tang initiates HIF-1α-dependent neuroprotection against cerebral ischemia in rats. J Ethnopharmacol. 2014;154:443–452. doi: 10.1016/j.jep.2014.04.022. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Wang J, Zhang C, Liao S, Li P, Xu D, Lv Y, Yang M, Kong L. The components of Huang-Lian-Jie-Du-Decoction act synergistically to exert protective effects in a rat ischemic stroke model. Oncotarget. 2016b;7:80872–80887. doi: 10.18632/oncotarget.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li H, Huang M, Huang M, Chu K, Xu W, Zhang S, Que J, Chen L. Paeoniflorin, a monoterpene glycoside, protects the brain from cerebral ischemic injury via inhibition of apoptosis. Am J Chin Med. 2015;43:543–557. doi: 10.1142/S0192415X15500342. [DOI] [PubMed] [Google Scholar]
- Zhao LD, Wang JH, Jin GR, Zhao Y, Zhang HJ. Neuroprotective effect of Buyang Huanwu decoction against focal cerebral ischemia/reperfusion injury in rats-time window and mechanism. J Ethnopharmacol. 2012;140:339–344. doi: 10.1016/j.jep.2012.01.026. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Fang Y, Li J, Duan Y, Zhao H, Gao L, Luo Y. Neuroprotective effects of Chrysophanol against inflammation in middle cerebral artery occlusion mice. Neurosci Lett. 2016;630:16–22. doi: 10.1016/j.neulet.2016.07.036. [DOI] [PubMed] [Google Scholar]
- Zschoernig B, Mahlknecht U. SIRTUIN 1: Regulating the regulator. Biochem Biophys Res Commun. 2008;376:251–255. doi: 10.1016/j.bbrc.2008.08.137. [DOI] [PubMed] [Google Scholar]