Keywords: nerve regeneration, peripheral nerve injury, estrogen, 17β-estradiol, N-methyl-D-aspartic acid receptor 1, pain, sciatic nerve, chronic constriction injury, neuropathic pain; D(-)-2-amino-5-phosphonopentanoic acid, dorsal root ganglion, spinal cord, immunoreactivity, western blot assay, neural regeneration
Abstract
Estrogen affects the generation and transmission of neuropathic pain, but the specific regulatory mechanism is still unclear. Activation of the N-methyl-D-aspartate acid receptor 1 (NMDAR1) plays an important role in the production and maintenance of hyperalgesia and allodynia. The present study was conducted to determine whether a relationship exists between estrogen and NMDAR1 in peripheral nerve pain. A chronic sciatic nerve constriction injury model of chronic neuropathic pain was established in rats. These rats were then subcutaneously injected with 17β-estradiol, the NMDAR1 antagonist D(-)-2-amino-5-phosphonopentanoic acid (AP-5), or both once daily for 15 days. Compared with injured drug naïve rats, rats with chronic sciatic nerve injury that were administered estradiol showed a lower paw withdrawal mechanical threshold and a shorter paw withdrawal thermal latency, indicating increased sensitivity to mechanical and thermal pain. Estrogen administration was also associated with increased expression of NMDAR1 immunoreactivity (as assessed by immunohistochemistry) and protein (as determined by western blot assay) in spinal dorsal root ganglia. This 17β-estradiol-induced increase in NMDAR1 expression was blocked by co-administration with AP-5, whereas AP-5 alone did not affect NMDAR1 expression. These results suggest that 17β-estradiol administration significantly reduced mechanical and thermal pain thresholds in rats with chronic constriction of the sciatic nerve, and that the mechanism for this increased sensitivity may be related to the upregulation of NMDAR1 expression in dorsal root ganglia.
Introduction
The results of many previous studies examining the pathogenesis of neuropathic pain as well as its prevention and treatment strategies have suggested that pain threshold is gender specific. Estrogen receptors are distributed in many pain-related regions in the central and peripheral nervous systems, and estrogen can affect the generation and transmission of pain at many levels (Ramírez-Barrantes et al., 2016; Ray et al., 2016). The N-methyl-D-aspartic acid receptor 1 (NMDAR1) is an excitatory neurotransmitter glutamate receptor subtype that exists widely in the nervous system and is involved in the generation, transmission, and maintenance of various types of pain (Bi et al., 2003; Bursztajn et al., 2004; Ultenius et al., 2006; Pedersen and Gjerstad, 2008; Gu et al., 2009; Liu et al., 2013), Moreover, the NMDAR1 plays a primary role in the transmission and regulation of damage information in neuropathic pain (Foy et al., 1999; Charlet et al., 2008; Tang et al., 2008). This study aimed to investigate whether NMDAR1 plays a role in the effects of estrogen on neuropathic pain.
Materials and Methods
Animals
Healthy 3-month-old male Sprague-Dawley rats weighing approximately 250 g [animal license No. SYXK (Xin) 2016-0002] were used in this study. Only those animals with a thermal pain threshold as determined by a paw withdrawal thermal latency (PWTL) greater than 5 seconds and less than 30 seconds were selected for use. The laboratory temperature was 20–22°C, with humidity levels of 50–60%. The lights were on from 08:00–20:00. All rats received the same food and were single-housed in a quiet room for one week to habituate to the environment (Xu et al., 2007; Gardella et al., 2011). All animal procedures were approved by the Animal Care and Use Committee of the First Affiliated Hospital of Medical College of Shihezi University of China (approval No. A2016-117-01) and were in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals formulated by the Ministry of Science and Technology of China.
Chronic constriction injury (CCI) model of chronic neuropathic pain and drug interventions
Fifty rats were equally and randomly divided into the following five groups (n = 10 per group): sham-operated; CCI; estrogen; D(-)-2-amino-5-phosphonopentanoic acid (AP-5), which is an NMDAR1 antagonist; and a combination of estrogen and AP-5.
To study chronic neuropathic pain, an animal model mimicking chronic peripheral nerve injury was established using a unilateral sciatic nerve CCI (Bennett and Xie, 1988). Rats were anesthetized with isoflurane inhalation anesthesia, and the left sciatic nerve was exposed. Proximal to the bifurcation, approximately 7 mm of the sciatic nerve was freed from the surrounding connective tissue and ligated using three 4-0 chromic catgut ligatures at 1-mm intervals so that the nerve was minimally constricted but its blood supply was unimpeded. The muscle and skin layers were individually sutured. Penicillin (400,000 U, Batch number 0203446; North China Pharmaceutical Factory, China) was intramuscularly injected to prevent infection. All surgical procedures were conducted by one person. Each animal was examined to determine the success of the CCI model (Bennett and Xie, 1988).
For the sham-operated group, the left sciatic nerve was separated and exposed without ligation, and a volume of saline equal to that for the experiment drugs used in the operated rats was injected subcutaneously just below the back of the neck. For the estrogen group, rats were subjected to the CCI operation and then subcutaneously injected with 17β-estradiol (2 μL/day, Sigma, St. Louis, MO, USA). For the AP-5 group, rats were subjected to the CCI operation and then subcutaneously injected with AP-5 (100 nmol/day; Sigma). For the combination group, rats were subjected to the CCI operation and then subcutaneously injected with 17β-estradiol (2 μL/day) and AP-5 (100 nmol/day). For the CCI group, rats were subjected to the CCI operation and then subcutaneously injected with a volume of saline equal to that for the two experiment drugs.
Fourteen days after surgery, the rats were deeply anesthetized with isoflurane and then killed. Samples of the left lumbar spinal cord 4–6 (L4–6) dorsal root ganglia (DRG) were taken, and the level of NMDAR1 expression in the DRG was measured using immunohistochemical and western blot assays (Aldrich et al., 2009; Finnerup et al., 2010; Peppin and Webster, 2011).
Paw withdrawal mechanical threshold (PWMT) test
The PWMT was tested 1 day before (T0), and 1 (T1), 3 (T2), 7 (T3), and 14 (T4) days after surgery. To reduce stress from a new environment, the rats were placed in independent transparent boxes made of Plexiglas with a 5 mm × 5 mm bottom plate made of metal mesh for 30 minutes before the test. Von Frey filaments (Stoeling Co., Italy) were used to stimulate the central part of the left hind foot of the rats vertically, with stimulation strength increasing in order from 1, 1.4, 2, 4, 6, 8, and 10 g, to 15 g. To avoid substantial injury to rats, the maximum strength of the stimulation was limited to 15 g, which was achieved when the 15-g filament bent slightly. The stimulation was conducted five times at an interval of more than 30 seconds, and each stimulus lasted for 5 seconds. The PWMT was considered the lowest force (in grams) at which the rat lifted or licked its foot. Five results were obtained for each rat. The average value was calculated by using the results of three measurements, with the highest and lowest values excluded. All tests were conducted from 08:00 to 10:00 (Gandhi et al., 2004).
PWTL test
The PWTL was measured at T0, T1, T2, T3, and T4 using a PL-200 thermal stimulator (Chengdu TME, China). The responses of the rats to thermal stimulation at the center of the left hind foot were measured, and the time needed from the start of thermal stimulation to paw withdrawal responses (raising foot, dodging, or licking foot) was recorded as the PWTL (in seconds). To avoid substantial injury to animals, thermal stimulation was limited to 20 seconds per stimulation, with an interval of 5 minutes between a total of 5 stimulations. Five results were obtained for each rat. The average value was calculated by using the results of three measurements, with the highest and lowest values excluded (Bi et al., 2003; Liu et al., 2013).
Measurement of NMDARl immunoreactivity in DRG
After determining the pain threshold on T4, five rats were randomly selected from each group. The rats were deeply anesthetized with isoflurane and then secured on their backs. A thoracotomy was rapidly conducted to expose the heart. A cannula was inserted into the ascending aorta through the left ventricle. Then, the right atrium was cut, and a rapid cardiac perfusion was performed using 4°C sterile saline until the outflow was clear. Subsequently, a rapid and then slow perfusion with 4% paraformaldehyde was performed for approximately 30 minutes for fixation. Then, the hair of the left hind leg was removed. The tendon was exposed at the central position of the thigh bone using blunt dissection. The sciatic nerve was traced to find the L4–6 spinal ganglia, which were removed and placed in 10% neutral formalin solution for 48 hours. Paraffin embedding was performed, followed by continuous sectioning to obtain 4-mm sections. The sample was stained according to the manufacturer's instructions provided in the SP two-step immunohistochemical kit (Zhongshan Jinqiao, Beijing, China). Five sections were used for each rat. The main steps were as follows: (1) Endogenous peroxidase was blocked with 3% H2O2. (2) The sample underwent antigen retrieval with citric acid at a high temperature and pressure for 8 minutes and then cooled to room temperature. (3) The sample was rinsed three times (5 minutes/time) using 1× phosphate buffered saline at room temperature. (4) Fifty μL of primary rabbit NMDAR1 polyclonal antibody (1:800, ab59302; Abcam, UK) was dropped on the section on the slide and incubated in the refrigerator at 4°C for 12 hours. (5) Secondary antibody (50 μL; 1:200; goat anti-rabbit IgG; ready-to-use MaxVision KIT 5005; Maixin Biotech, Fuzhou, China) was added to each section at room temperature (20–25°C) for 2 hours. (6) 3,3′-Diaminobenzidine staining was performed, and (7) the sections were counterstained with hematoxylin. (8) The sections were cleared using xylol, and (9) mounted using neutral resin. Cells of the rat L4–6 DRG immunopositive for NMDAR1 had mostly circular or oval cytoplasm, which was stained brown, indicating strong positive expression.
Densitometry that is the measurement of the average optical density of NMDARl immunoreactivity was conducted using a Motic Med 6.0 digital color medical image analysis system (Motic, Beijing, China). The average optical density value was obtained by dividing the integrated optical density value by the selected object area. The average optical density represented the average intensity of all selected objects in the field of view, that is, the positive cells, and the integrated optical density represented the sum of the intensities of reactions for all selected objects in the entire field of view.
Measurement of NMDARl protein expression in DRG using western blot assay
The remaining five rats in each group were deeply anesthetized and decapitated. The L4–6 spinal DRG were removed rapidly and homogenized in an ice bath. The total protein was extracted, and the protein concentration was measured using a bicinchoninic acid assay. Protein samples with a total weight of 40 μg were mixed with loading buffer of the same volume. The mixture was boiled for 8 minutes and then rapidly cooled. After electrophoresis conducted using 8% sodium dodecyl sulfate-polyacrylamide gels, the samples were transferred to 0.25-μm polyvinylidene fluoride membranes. The membranes were then blocked in 5% skim milk powder (1 g of skim milk powder, 20 mL of 1× Tris-buffered saline, 0.5 mL of Tween-20) at room temperature for 1 hour. Rabbit NMDAR1 primary polyclonal antibody (1:200, ab59302; Abcam, UK) and mouse anti-β-actin (1:1,000; ZSGB BIO, China) were added and incubated at 4°C for 12 hours. After six washes with 1× Tris-buffered saline with Tween-20 (5 minutes/time), the secondary antibody (1:4,000, goat anti-rabbit IgG; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was added and incubated at room temperature (25°C) for 2 hours. Antibody detection was performed using an enhanced chemiluminescence kit (Thermo, 34094, China). The grayscale values of the protein bands were analyzed using ImagePro 4.0 software (Bio-Rad, Hercules, CA, USA). Data are presented as the absorbance ratio of NMDAR1/β-actin. β-Actin was used as a loading control.
Statistical analysis
Statistical analyses were performed using SPSS 13.0 software (SPSS, Chicago, IL, USA). Data are expressed as the mean ± SD. Multi-group comparisons were performed by one-way analysis of variance and intergroup differences were compared using Student-Newman-Keuls tests. Values of P less than 0.05 were considered statistically significant.
Results
Estrogen affected CCI-induced rat behavior
Following the operation to induce CCI, no rat developed a wound infection, and all rats sought food without difficulty. The rats in the sham-operated group behaved normally. The rats in the CCI, estrogen, AP-5, and combination groups demonstrated closed toes, eversion of toes on the operated side in some cases, and limping. When standing, they lifted their injured hind leg and supported their bodies by using their healthy legs. Foot licking behavior was observed in these groups, but not self-biting. The symptoms observed for rats in the CCI, estrogen, AP-5, and combination groups were most apparent at T3, indicating that the CCI model of chronic neuropathic pain was successful in all groups.
Estrogen affected PWMT and PWTL in rats with CCI
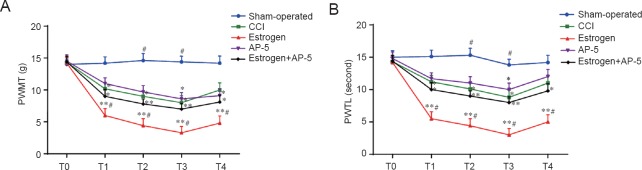
Compared with rats in the sham-operated group, the rats in the CCI and the estrogen groups showed a lower PWMT and a shorter PWTL at all times examined after the operation (all P < 0.05). Compared with rats in the CCI group, the rats in the estrogen group showed a lower PWMT and a shorter PWTL at all times examined after the operation (all P < 0.05) (Figure 1).
Figure 1.
Effects of estrogen on the CCI-induced changes in PWMT and PWTL.
PWMT (A) and PWTL (B) of rats in the five treatment groups at various times after nerve injury. Data are expressed as the mean ± SD from 10 rats in each group (one-way analysis of variance and Student-Newman-Keuls test). Data are calculated by using the results of three measurements. *P < 0.05, **P < 0.01, vs. sham-operated group; #P < 0.05, vs. CCI group. Sham-operated group: Exposed sciatic nerve without ligation. Estrogen group: CCI surgery + 2 μL/day 17β-estradiol injected subcutaneously. AP-5 group: CCI surgery + 100 nmol/day AP-5 injected subcutaneously. Estrogen + AP-5 group: CCI model + 2 μL/day 17β-estradiol + 100 nmol/day AP-5 injected subcutaneously. T0–T4: 1 day before surgery and 1, 3, 7, 14 days after surgery, respectively. CCI: Chronic constriction injury; AP-5: D(-)-2-amino-5-phosphonopentanoic acid; PWMT: paw withdrawal mechanical threshold; PWTL: paw withdrawal thermal latency.
Effects of estrogen on NMDAR1 immunoreactivity in L4–6 DRG neurons
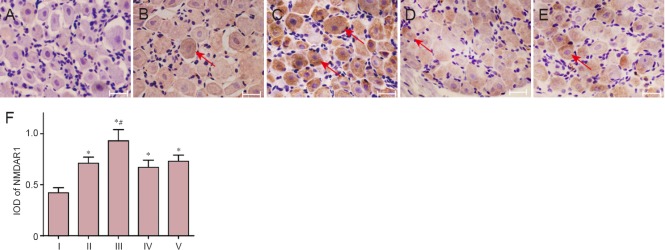
The average optical density for NMDAR1 immunoreactivity was highest in the estrogen group (0.93 ± 0.11) and lowest in the sham-operated group (0.42 ± 0.05). Compared with that in the sham-operated group, the average optical densities were significantly higher in the CCI (0.71 ± 0.06), estrogen, AP-5 (0.65 ± 0.15) and estrogen + AP-5 (0.78 ± 0.11) groups (P < 0.05). The average optical density in the estrogen group was significantly higher than that in the CCI group (P < 0.05). However, the average optical density was not significantly different in the AP-5 and estrogen + AP-5 groups compared with that in the CCI group (Figure 2).
Figure 2.
Estrogen effects on NMDAR1 immunoreactivity as assessed by immunohistochemistry in rat lumbar spine L4–6 dorsal root ganglia 14 days after CCI surgery.
(A–E) NMDAR1 immunoreactivity in L4–6 dorsal root ganglia derived from the sham-operated, CCI, estrogen, AP-5, and estrogen + AP-5 groups, respectively (immunohistochemical staining, light microscope, × 200). Scale bars: 50 μm. Arrows show NMDAR1 immunoreactive cells. (F) Quantification (integrated optical density; IOD) of NMDAR1 immunoreactive cells. Data are expressed as the mean ± SD from five rats in each group (one-way analysis of variance and Student-Newman-Keuls test). *P < 0.05, vs. sham-operated group; #P < 0.05, vs. with CCI group. See Figure 1 legend for group definitions. NMDAR1: N-methyl-D-aspartic acid receptor 1; CCI: chronic constriction injury; AP-5: D(-)-2-amino-5-phosphonopentanoic acid. I: Sham-operated; II: CCI; III: Estrogen; IV: AP-5: IV: Estrogen + AP-5.
Effects of estrogen on NMDAR1 protein expression in L4–6 DRG
The results of the western blot assays showed that NMDARl protein expression in the CCI, estrogen, AP-5, and estrogen + AP-5 groups was higher than that in the sham-operated group. Moreover, the NMDARl protein expression in L4–6 DRG in the estrogen group was significantly higher than that in the sham-operated, CCI, AP-5, and estrogen + AP-5 groups (P < 0.05; Figure 3).
Figure 3.
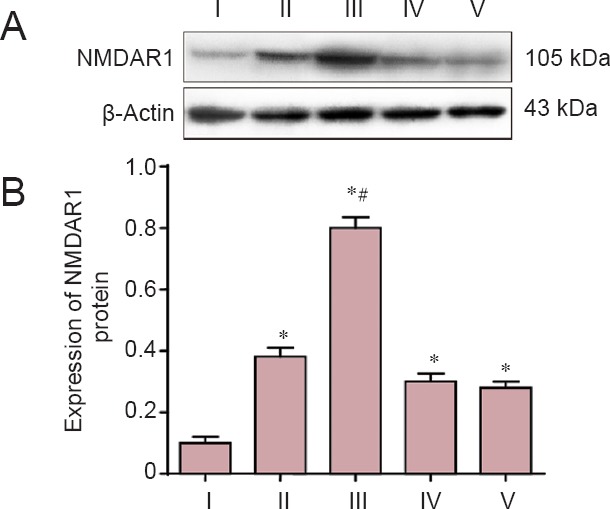
Estrogen effects on NMDAR1 protein expression as assessed by western blot assay in lumbar spine L4–6 dorsal root ganglia of rats with CCI.
(A) NMDAR1 protein expression in rat L4–6 spinal dorsal root ganglia examined 14 days after nerve injury. (B) Quantification (absorbance ratio) of NMDAR1 protein expression in each group. Data are expressed as the mean ± SD from five rats in each group (one-way analysis of variance and Student-Newman-Keuls test). *P < 0.05, vs. sham-operated group; #P < 0.05. vs. CCI group. See Figure 1 legend for group definitions. NMDAR1: N-methyl-D-aspartic acid receptor 1; CCI: chronic constriction injury; AP-5, D(-)-2-amino-5-phosphonopentanoic acid. I: Sham-operated; II: CCI; III: estrogen; IV: AP-5: IV: estrogen + AP-5.
Discussion
Although researchers in various countries and fields have studied the occurrence, mechanism of excitation and transmission, anatomical and histological changes, molecular biology, and electrophysiology of neuropathic pain, current understanding of the disease remains limited (Singh et al., 2013; Grasso et al., 2014).
The commonly used animal models of pain caused by nerve injury consist of spinal cord injury, spared nerve injury, CCI, partial sciatic nerve ligation, spinal nerve ligation, and chronic compression of DRG. The present study used a CCI model because as compared with the other models, the controllability of the degree of damage is easier, the change in pain is more stable, and repeated operations result in similar findings (Ossipov et al., 1999; Amandusson and Blomqvist, 2010; Devall et al., 2011). The behavioral results are consistent with the clinical symptoms of neuropathic pain, which is an ideal model of pain caused by pathological nerve injury.
The results of our behavioral measures indicated that rats with increased estrogen levels were more sensitive to pain. Potential mechanisms that are speculated for this effect include a role for estrogen in the nervous system in pathological conditions, in pain transmission, and in various neurobehavioral aspects. Estrogen may increase limb sensitivity in the CCI rat model.
The pain thresholds measured in this study included both mechanical and thermal (heat), which are two commonly examined forms of pain thresholds in rats (Castillo-Gomez et al., 2016). Alterations in a rat's reaction reflects a change in the mechanical threshold of the pain signal mediated by myelinated nerves, whereas a change in the heat pain threshold reflects the pain signal mediated by unmyelinated C fibers. Therefore, the reason the pain threshold for rats in the estrogen group was reduced to produce hyperalgesia and allodynia may be due to estrogen amplification of the pain signal through both myelinated and unmyelinated C fibers.
NMDAR1, a receptor for the excitatory neurotransmitter glutamate, is present in the nervous system and participates in the generation, propagation and maintenance of pain (Wang et al., 2015a, b). An imbalance in NMDAR1 activity regulation is strongly associated with the pathogenesis of nervous system diseases. The NMDAR1 performs differently in various neurological diseases. Previous studies examining the relationship between NMDAR1 protein and nervous system disease have focused on the central nervous system, that is, the brain and spinal cord, whereas the present study investigated that relationship in L4–6 spinal ganglia, spinal nerves containing pseudounipolar neurons, which are primary sensory neurons with pain afferent fibers. The results of our study showed that estrogen significantly increased NMDAR1 immunoreactivity and protein in DRG and pain hypersensitivity in rats. The distribution of estrogen receptors and NMDAR1s overlap in DRG neurons, and previous studies have shown that estrogen has a direct effect on NMDAR1 (Muniz and Isokawa, 2015; Tatard-Leitman et al., 2015). Thus, an estrogen-mediated increase in NMDAR1 expression could lead to increased opening of the channel and enhanced transmission of nociceptive stimulation of DRG.
Much experimental evidence indicates gender differences for neuropathic pain in human and animals. Studies examining the relationship between estrogen and NMDAR1 suggest that estrogen regulates NMDAR1 activity in certain brain regions and in neurons of the spinal cord by regulating the expression of glutamate receptors or through post-translational modifications. Estrogen has also been shown to increase NMDAR1-mediated activation of hypothalamic neurons (Nordman et al., 2014; Sun et al., 2015). Our study examined NMDAR1 expression in spinal DRG after nerve injury, and we obtained results consistent with these previous studies. Thus, the NMDA receptor NMDAR1 subunit occupies a key position in the development of neuropathic pain (Yao et al., 2015), and administration of the NMDAR1 specific antagonist AP-5 could decrease estrogen-induced NMDAR1 expression upregulation to significantly improve the pain threshold in rats.
In conclusion, during chronic neuropathic pain, estrogen may increase the body's sensitivity to mechanical and thermal stimulation through increased NMDAR1 expression.
Footnotes
Funding: This research was supported by the Youth Shihezi University Applied Basic Research Project of China, No. 2015ZRKYQ-LH19.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using CrossCheck to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Smith T, Frenchman B, Wang J, Li CH, Qiu Y, Song LP, Zhao M
References
- Aldrich BT, Frakes EP, Kasuya J, Hammond DL, Kitamoto T. Changes in expression of sensory organ-specific microRNAs in rat dorsal root ganglia in association with mechanical hypersensitivity induced by spinal nerve ligation. Neuroscience. 2009;164:711–723. doi: 10.1016/j.neuroscience.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amandusson A, Blomqvist A. Estrogen receptor-alpha expression in nociceptive-responsive neurons in the medullary dorsal horn of the female rat. Eur J Pain. 2010;14:245–248. doi: 10.1016/j.ejpain.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Bi R, Foy MR, Thompson RF, Baudry M. Effects of estrogen, age, and calpain on MAP kinase and NMDA receptors in female rat brain. Neurobiol Aging. 2003;24:977–983. doi: 10.1016/s0197-4580(03)00012-5. [DOI] [PubMed] [Google Scholar]
- Bursztajn S, Rutkowski MD, Deleo JA. The role of the N-methyl-D-aspartate receptor NR1 subunit in peripheral nerve injury-induced mechanical allodynia, glial activation and chemokine expression in the mouse. Neuroscience. 2004;125:269–275. doi: 10.1016/j.neuroscience.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Castillo-Gomez E, Oliveira B, Tapken D, Bertrand S, Klein-Schmidt C, Pan H, Zafeiriou P, Steiner J, Jurek B, Trippe R, Pruss H, Zimmermann WH, Bertrand D, Ehrenreich H, Hollmann M. All naturally occurring autoantibodies against the NMDA receptor subunit NR1 have pathogenic potential irrespective of epitope and immunoglobulin class. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.125. doi: 10.1038/mp.2016.125. [DOI] [PubMed] [Google Scholar]
- Charlet A, Lasbennes F, Darbon P, Poisbeau P. Fast non-genomic effects of progesterone-derived neurosteroids on nociceptive thresholds and pain symptoms. Pain. 2008;139:603–609. doi: 10.1016/j.pain.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Devall AJ, Santos JM, Lovick TA. Estrous cycle stage influences on neuronal responsiveness to repeated anxiogenic stress in female rats. Behav Brain Res. 2011;225:334–340. doi: 10.1016/j.bbr.2011.07.038. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150:573–581. doi: 10.1016/j.pain.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Gandhi R, Ryals JM, Wright DE. Neurotrophin-3 reverses chronic mechanical hyperalgesia induced by intramuscular acid injection. J Neurosci. 2004;24:9405–9413. doi: 10.1523/JNEUROSCI.0899-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso G, Passalacqua M, Giambartino F, Cacciola F, Caruso G, Tomasello F. Typical trigeminal neuralgia by an atypical compression: case report and review of the literature. Turk Neurosurg. 2014;24:82–85. doi: 10.5137/1019-5149.JTN.7048-12.0. [DOI] [PubMed] [Google Scholar]
- Gu X, Wu X, Liu Y, Cui S, Ma Z. Tyrosine phosphorylation of the N-methyl-D-aspartate receptor 2B subunit in spinal cord contributes to remifentanil-induced postoperative hyperalgesia: the preventive effect of ketamine. Mol Pain. 2009;5:76. doi: 10.1186/1744-8069-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Jiang CY, Fujita T, Luo SW, Kumamoto E. Enhancement by interleukin-1beta of AMPA and NMDA receptor-mediated currents in adult rat spinal superficial dorsal horn neurons. Mol Pain. 2013;9:16. doi: 10.1186/1744-8069-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniz BG, Isokawa M. Ghrelin receptor activity amplifies hippocampal N-methyl-D-aspartate receptor-mediated postsynaptic currents and increases phosphorylation of the GluN1 subunit at Ser896 and Ser897. Eur J Neurosci. 2015;42:3045–3053. doi: 10.1111/ejn.13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordman JC, Phillips WS, Kodama N, Clark SG, Del Negro CA, Kabbani N. Axon targeting of the alpha 7 nicotinic receptor in developing hippocampal neurons by Gprin1 regulates growth. J Neurochem. 2014;129:649–662. doi: 10.1111/jnc.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipov MH, Bian D, Malan TP, Jr, Lai J, Porreca F. Lack of involvement of capsaicin-sensitive primary afferents in nerve-ligation injury induced tactile allodynia in rats. Pain. 1999;79:127–133. doi: 10.1016/s0304-3959(98)00187-0. [DOI] [PubMed] [Google Scholar]
- Pedersen LM, Gjerstad J. Spinal cord long-term potentiation is attenuated by the NMDA-2B receptor antagonist Ro 25-6981. Acta Physiol (Oxford, England) 2008;192:421–427. doi: 10.1111/j.1748-1716.2007.01756.x. [DOI] [PubMed] [Google Scholar]
- Peppin JF, Webster L. Letter to the editor in response to “The evidence for pharmacological treatment of neuropathic pain,” by Finnerup et al. Pain. 2011;152:1440. doi: 10.1016/j.pain.2011.03.031. [DOI] [PubMed] [Google Scholar]
- Ramírez-Barrantes R, Marchant I, Olivero P. TRPV1 may increase the effectiveness of estrogen therapy on neuroprotection and neuroregeneration. Neural Regen Res. 2016;11:1204–1207. doi: 10.4103/1673-5374.189162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SK, Samntaray S, Banik NL. Future directions for using estrogen receptor agonists in the treatment of acute and chronic spinal cord injury. Neural Regen Res. 2016;11:1418–1419. doi: 10.4103/1673-5374.191212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PM, Dehran M, Mohan VK, Trikha A, Kaur M. Analgesic efficacy and safety of medical therapy alone vs combined medical therapy and extraoral glossopharyngeal nerve block in glossopharyngeal neuralgia. Pain Med (Malden, Mass) 2013;14:93–102. doi: 10.1111/pme.12001. [DOI] [PubMed] [Google Scholar]
- Sun X, Pinacho R, Saia G, Punko D, Meana JJ, Ramos B, Gill G. Transcription factor Sp4 regulates expression of nervous wreck 2 to control NMDAR1 levels and dendrite patterning. Dev Neurobiol. 2015;75:93–108. doi: 10.1002/dneu.22212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B, Ji Y, Traub RJ. Estrogen alters spinal NMDA receptor activity via a PKA signaling pathway in a visceral pain model in the rat. Pain. 2008;137:540–549. doi: 10.1016/j.pain.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatard-Leitman VM, Jutzeler CR, Suh J, Saunders JA, Billingslea EN, Morita S, White R, Featherstone RE, Ray R, Ortinski PI, Banerjee A, Gandal MJ, Lin R, Alexandrescu A, Liang Y, Gur RE, Borgmann-Winter KE, Carlson GC, Hahn CG, Siegel SJ. Pyramidal cell selective ablation of N-methyl-D-aspartate receptor 1 causes increase in cellular and network excitability. Biol Psychiatry. 2015;77:556–568. doi: 10.1016/j.biopsych.2014.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ultenius C, Linderoth B, Meyerson BA, Wallin J. Spinal NMDA receptor phosphorylation correlates with the presence of neuropathic signs following peripheral nerve injury in the rat. Neurosci Lett. 2006;399:85–90. doi: 10.1016/j.neulet.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Wang L, Huo S, Wang YF, Zhao L, Liu BF, Ruan CL, Hui XF. The regulating effect of N-methyl-D-aspartate receptor on neural synaptic plasticity in a rat model of chronic cerebral ischemia. Zhongguo Zuzhi Gongcheng Yanjiu. 2015a;19:6498–6503. [Google Scholar]
- Wang LQ, Liu SZ, Wen X, Wu D, Yin L, Fan Y, Wang Y, Chen WR, Chen P, Liu Y, Lu XL, Sun HL, Shou W, Qiao GF, Li BY. Ketamine-mediated afferent-specific presynaptic transmission blocks in low-threshold and sex-specific subpopulation of myelinated Ah-type baroreceptor neurons of rats. Oncotarget. 2015b;6:44108–44122. doi: 10.18632/oncotarget.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao D, Yoshida M, Sessle BJ. Dura-evoked neck muscle activity involves purinergic and N-methyl-D-aspartate receptor mechanisms. Neuroreport. 2015;26:1155–1160. doi: 10.1097/WNR.0000000000000489. [DOI] [PMC free article] [PubMed] [Google Scholar]