Abstract
Background: Labeling prohibits delivery of insulin at the site of subcutaneous continuous glucose monitoring (CGM). Integration of the sensing and insulin delivery functions into a single device would likely increase the usage of CGM in persons with type 1 diabetes.
Methods: To understand the nature of such interference, we measured glucose at the site of bolus insulin delivery in swine using a flexible electrode strip that was laminated to the outer wall of an insulin delivery cannula. In terms of sensing design, we compared H2O2-measuring sensors biased at 600 mV with redox mediator-type sensors biased at 175 mV.
Results: In H2O2-measuring sensors, but not in sensors with redox-mediated chemistry, a spurious rise in current was seen after insulin lis-pro boluses. This prolonged artifact was accompanied by electrode poisoning. In redox-mediated sensors, the patterns of sensor signals acquired during delivery of saline and without any liquid delivery were similar to those acquired during insulin delivery.
Conclusion: Considering in vitro and in vivo findings together, it became clear that the mechanism of interference is the oxidation, at high bias potentials, of phenolic preservatives present in insulin formulations. This effect can be avoided by the use of redox mediator chemistry using a low bias potential.
Keywords: : Continuous glucose monitoring, Type 1 diabetes, Insulin delivery, Continuous subcutaneous insulin infusion, Insulin delivery cannula
Introduction
In subjects with type 1 diabetes (T1D), the combination of continuous glucose monitoring (CGM) with continuous subcutaneous insulin infusion therapy led to significantly greater reductions in hemoglobin A1C than insulin injections, without increasing hypoglycemia.1 Other CGM studies found a reduced incidence of hypoglycemia.2,3 However, despite the effectiveness of insulin pump usage and CGM, only a small minority of patients with T1D use both technologies concurrently on a regular basis.4
Due to presumed interference, labeling of medical devices prohibits delivery of insulin at or near the site of subcutaneous glucose monitoring. Since the mechanism of such interference is not clear, we performed experiments in which insulin was delivered through a cannula whose outer wall served as an amperometric glucose sensor.
Since one of insulin's physiologic effects is to promote uptake of glucose into fat cells, it might be assumed that it would not be possible to measure glucose at the site of insulin delivery. In contrast, a series of reports, mostly from Austria, suggested that the glucose measurement error resulting from local insulin delivery might be relatively low.5–8
We performed this study to address several questions: (1) Is insulin-induced interference with glucose measurement a positive- or negative-going artifact? (2) Is the cause of such interference due to a physiological effect of insulin or to an excipient in insulin formulation? (3) Is interference transient or persistent? (4) To what degree does the type of sensing chemistry influence insulin-induced interference?
H2O2-measuring sensors biased at 600 mV and redox mediator-type sensors biased at 175 mV were compared under three conditions. In one, a rapidly acting insulin formulation was given through the sensing cannula; in another, saline was given in a volume identical to the insulin delivery volume; and in the third, nothing was given through the cannula. Inclusion of the final two conditions allowed assessment of sensing artifact that could arise from aqueous liquid delivery.
Materials and Methods
Mechanical and electrical design
In preparation for in vitro and in vivo studies, we created planar arrays of electrodes that were later individualized and laminated to rigid insulin delivery cannulae, followed by application of sensing chemicals and polymers to the curved sensor surface. We used microfabrication techniques to create planar electrode arrays, each of which contained 48 electrode units. Each unit contained one silver electrode and three indicating electrodes (platinum or gold), each of which had a surface area of ∼1 mm2. The silver electrode was later converted to an Ag/AgCl reference electrode by exposing the silver surface to ferric chloride. Using a custom-built sputter chamber, the silver and the indicating electrode metals were sputtered on to titanium foil that had previously been laminated to a polyimide sheet.
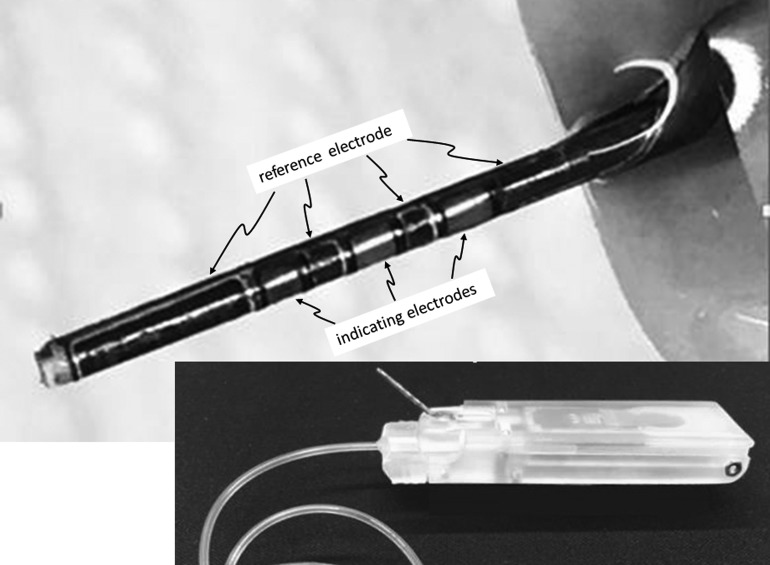
The patterns and shapes of electrodes were created by standard photolithography techniques or by microablation using an infrared wavelength (1064 nm) laser tool. Using an ultraviolet wavelength (355 nm) laser tool, the electrode units were micromachined into single units. To create a device that could be used as a continuous glucose sensor and as a conduit for insulin delivery, each individualized electrode strip was then laminated to the surface of a stainless steel tube using epoxy cement. For these experiments in active pigs, a large diameter robust tube (21 gauge) was used. A photograph of a fully fabricated sensing cannula, after deposition of chemical layers, is shown in the upper part of Figure 1. To create a fluid path for insulin delivery, the proximal portion of the stainless steel tube was connected to a watertight polymeric passage that connected to standard plastic tubing that interfaced with Medtronic or Animas-type insulin pump reservoirs. Each sensing cannula was 12 mm in length and was designed to be inserted at a 40° angle.
FIG. 1.
The upper portion shows a glucose-sensing cannula fabricated by laminating a flexible electrode strip to the outer wall of a stainless steel tube. The lower portion shows the entire device, including the electronic housing, used in swine studies. The sensing cannula protrudes at a 40° angle and is attached to housing that also connects to insulin delivery tubing. This housing is attached to an electronic module, which includes a transceiver, a battery, and circuitry that applies a bias potential to the sensor electrodes.
A Bluetooth low-energy radio microcontroller/transceiver (NRF-51822; Nordic, Inc.) designed to provide a continuous bias to the sensors and to acquire and transmit data was placed inside a skin-worn battery-powered electronic module shown in Figure 1, lower portion. Conducting interconnects originating from the indicating and reference electrodes were routed to the microcontroller. For swine studies, sensor data were transmitted from the electronic module to a laptop computer to which a USB Bluetooth dongle was attached.
Fabrication of a glucose sensor based on detection of hydrogen peroxide
For some experiments, glucose concentration in the subcutaneous interstitial fluid of swine was estimated by measuring the oxidation of H2O2 at a platinum indicating electrode. For these devices, the indicating electrodes were continuously biased at a potential of +600 mV. To create these glucose sensors, an aqueous mixture of glucose oxidase (GOX; Amresco, Inc.), bovine serum albumin (Sigma), and glutaraldehyde (Sigma) in a w:w:w ratio of 6:4:1 was applied to the indicating electrodes through a pipette or through a custom-built inkjet printer. A proprietary inner membrane was applied to avoid oxidation of interfering substances. An outer membrane comprising an experimental copolymer of silicone and polyurethane, kindly provided by Lubrizol, Inc., dissolved in a cosolvent of tetrahydrofuran and dimethylacetamide (9:1 v/v), was applied over the entire shaft, including all electrodes and interconnect traces.
Fabrication of a glucose sensor based on use of a redox mediator
To synthesize a redox mediator, a process disclosed in the 1990s was used.9 To make the redox mediator, osmium in the form of ammonium hexachloroosmate (Sigma) was first reacted with the ligand, 4,4′-dimethyl, 2,2′-bipyridine (diMeBPY; Sigma), using a reflux-condensing procedure. The product was then reacted with poly (1-vinyl imidazole) (PVI; BOC Sciences), using reflux condensing, to synthesize osmium diMeBPY PVI. This compound, known as the redox mediator polymer (RMP), was mixed with GOX and glutaraldehyde (glut) in a w:w:w ratio of 40:10:1 (RMP:GOX:glut) and applied to each gold indicating electrode. An overcoat of polyurethane (Pellethane; Lubrizol, Inc.), dissolved in the same cosolvent as mentioned above, was applied over the entire shaft of the sensing cannula, then dried.
In vitro studies
The effect of a rapidly acting insulin formulation on an H2O2-measuring platinum electrode
The electrochemical workstation included a custom-made multichannel transimpedance amplifier board and an electrochemical software package (Signal Express; National Instruments). Bare platinum electrodes biased at 600 mV were stabilized in stirred phosphate-buffered saline (PBS), pH 7.4. When stable, H2O2 was added to create a concentration of 25 μM. At 10-min intervals, insulin aspart (Novo -Nordisk) was added to make concentrations of 1.4, 2.8, and 5.6 units/mL. Because aspart contains m-cresol 1.72 mg/mL and phenol 1.5 mg/mL, the corresponding concentrations of total phenolics (phenol plus m-cresol) were 45, 90, and 180 μg/mL. Similar experiments were carried out with another preparation of insulin that had no phenolic-based excipients (Regular Recombinant Insulin; Gibco, Life Sciences).
The effect of varying bias potentials on the redox response to phenolic preservatives in insulin formulations
These experiments were carried out using bare gold indicating electrodes and bare platinum indicating electrodes. The electrodes were immersed in a solution of phenol 10 mM or m-cresol 10 mM dissolved in PBS. During data collection, the indicating electrodes were poised at differing bias potentials, ranging in 50-mV increments from −450 to +600 mV. Current measurements were taken after a short stabilization period (2 min after insertion). Between measurements, electrode surfaces were quickly cleaned with acetone-deionized water and emery paper. For each reading, a measurement period of 2 min was not exceeded because after this period of time, at some bias potentials, the readings would decline progressively (poisoning).
Animal studies
Study design
The main goal was to compare two sensor chemistry designs in terms of interference from insulin formulations. Before beginning the study, a power analysis was carried out using the G power program.10 To detect a 30% difference with 85% power, the analysis found that 66 total data sets, divided among the insulin and noninsulin groups, would be needed (for the optimal design, redox sensors, we actually collected 64 data sets). No data were classified as outliers. The major endpoint (comparison of the measured blood glucose with the sensor's measurement of the tissue glucose) was determined prospectively.
Animal studies were carried out in the vivarium at Oregon Health and Science University (OHSU) in Portland, OR, after receiving approval from the OHSU Institutional Animal Care and Use Committee. Animal subjects included four intact female Yucatan minipigs that were repeatedly studied. Pigs weighed between 35 and 65 kg and were euthanized only when they became too large to handle. Office of Laboratory Animal Welfare policies were followed.
Sensing cannulae were inserted in the afternoon after preanesthetic sedation with Telazol 2–3 mg/kg IM, followed by administration of isoflurane 2–2.5 L/min delivered by nose cone. The abdomen was shaved and sterilized with povidone-iodine. The sensing cannulae, each attached to an electronic module, were inserted into the abdominal subcutaneous space with the aid of a sharp 28-gauge stainless steel stylet that was temporarily indwelled within the lumen of the sensing cannula. For most experiments, six sensing cannulae were placed so that some could be used for insulin delivery, some could be used for delivery of saline as a control, and some could be used without any fluid delivery (dry). The electronic module was affixed to the skin with cyanoacrylate glue.
After cannula placement, padding was placed over the skin-worn modules and affixed in place with Elastikon tape. Finally, a Lycra body stocking was placed over the animal to minimize the chance of dislodgement. The isoflurane was discontinued and the animals were directly observed until they were walking and eating.
A prolonged two-level glucose clamp experiment was carried out the next morning after fasting overnight. After Telazol preanesthetic, the animal was endotracheally intubated and isoflurane was given through the endotracheal tube at a rate of 2–3 L/min. Accompanying the isoflurane was medical air (not pure oxygen) so that the inhaled gas would be similar to that inspired by free-living humans. To avoid hypothermia, a thermostatically controlled warm air blanket (Bair Hugger) was placed over the animal for the duration of general anesthesia. For administration of dextrose, a Teflon intravenous cannula was placed in an ear vein. To receive transmitted sensor data, a computer was placed within 1–2 m of the animal. Heart rate, heart rhythm, concentration of CO2 in expired air, respiratory rate, tidal volume, and oxygen saturation were continuously monitored.
After a 90-min stabilization period, Humalog (lis-pro) insulin (Lilly) was given through two of the cannulae in a dose of 0.11 units per kilogram per cannula (total 0.22 units/kg). The total dose was typically between 8 and 13 units. Through two other cannulae, PBS was given in a volume equal to that of the delivered insulin volume. Insulin and saline were administered through Medtronic or Animas insulin pumps. Nothing was given through the remaining cannulae.
Throughout the experiment, until minute 200, the euglycemic clamp was maintained to keep blood glucose between 75 and 90 mg/dL. A computerized program11 was used to deliver 20% dextrose IV to maintain euglycemia. Until minute 200, blood obtained from an ear nick was used for glucose measurement every 10 min. Blood glucose was measured either by a HemoCue 201 meter or by a Bayer Contour Next meter.
At minute 200, to assess sensor function, a hyperglycemic clamp was undertaken for 25 min to reach a glucose level of 250–300 mg/dL. The frequency of obtaining blood for glucose measurement was increased to every 5 min for the remainder of the experiment. At minute 225, the IV dextrose was discontinued. As glucose declined, data were obtained for another 35 min until minute 260. The anesthesia was then stopped, the sensing cannulae removed, and the pig extubated. The animal was observed until it was walking and eating. Apart from some minor abrasions from the Elastikon tape and two episodes of transient postprocedure emesis, there were no complications.
Data analysis for animal studies
There were six animal experiments in which H2O2-sensing cannulae were studied and five experiments in which the redox-sensing cannulae were studied. Values of electrical current were received by telemetry and recorded every 2 s. Analysis was performed on data sets that had been decimated down to 1 value per minute. Initially, there were 128 data sets for H2O2 sensors. However, 33 were found to be unevaluable due to an open circuit, typically a result of fracturing an electrode interconnect (after sensor insertion, animals were often observed to traumatize the region into which the sensors had been inserted). Others (14) were unevaluable due to a short circuit (a high railed current) as a result of an abnormal connection that developed between the indicating electrode and the reference electrode. In six cases, there was a telemetry failure. Thus, there were a total of 75 evaluable data sets for platinum-based H2O2-sensing electrodes. In these 75 data sets, there were 2394 data pairs (corresponding capillary blood glucose and sensor glucose values) that were evaluated.
Initially, there were 97 data sets for redox mediator electrodes, 16 of which were open circuits, 7 of which were short circuits, 3 of which were telemetry failures, and 7 of which exceeded the measurable current range. Thus, there were 64 evaluable data sets for redox-type electrodes. In these data sets, there were 2102 matched blood glucose–sensor glucose data pairs.
Although a formal algorithm that converts sensor current to glucose has not yet been developed, a preliminary calibration method, suggested by Lodwig et al. for early stage sensor evaluation, was used.12 Specifically, for each data set, a least squares regression slope and a y-axis intercept were calculated using all blood glucose and all sensor current points. To obtain the most accurate correlation, blood glucose values were shifted forward in time 10 min due to the large accuracy error introduced by rapid intravenous infusion of dextrose. This infusion often led to a rate of change in glucose that was an order of magnitude higher than typical13changes in persons with diabetes. This time shift was used to calculate the regression slope used for calibration and for determination of relative differences and absolute relative differences between blood glucose and sensor glucose. However, a time shift was not used in generating the graphs of blood glucose versus sensor glucose and blood glucose versus sensor current.
In nearly all calculations of the regression slopes between sensor current and blood glucose, there was a close correlation between the two variables that was then used to predict glucose from the sensor current. However, in the condition in which the insulin formulation was given through H2O2-sensing platinum electrodes, there was a very high degree of interference with the sensing process. For this reason, there was no correlation between sensor current and blood glucose; thus, no attempt was made to estimate glucose in that condition. Instead, the graphs of these experiments simply plotted sensor current and blood glucose as a function of time.
To test for differences, two-tailed unpaired t-tests were performed. Since there were multiple statistical comparisons (3), a Bonferroni correction was used to set the significance level at 0.05/3 or 0.0167.
Results
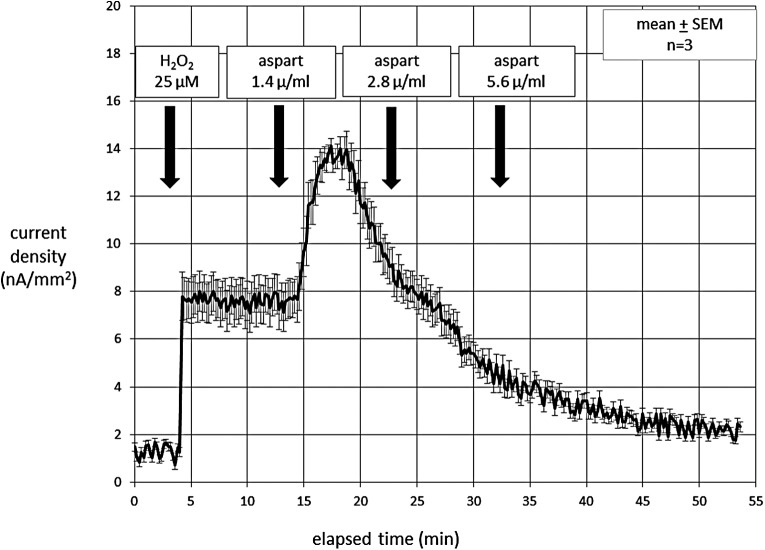
During in vitro experiments with the H2O2 sensor biased at 600 mV, aspart insulin caused a large artifact, as shown in Figure 2. In the beginning of the experiment, the addition of H2O2 gave the expected stable fixed step response. In contrast, when the insulin aspart formulation was added to the PBS solution, the current rose rapidly, then spontaneously fell. Despite additional aspart, the current continued to decline and ultimately fell to below the initial level despite the continued presence of H2O2 in the solution. This behavior strongly suggests irreversible electrode poisoning.
FIG. 2.
Electrochemical data collected in vitro showing the response of a platinum electrode, biased at 600 mV, to H2O2 added to a PBS solution (mean data are shown in black, error bars shown in gray). After the addition of H2O2, there were three doses of aspart insulin that were sequentially added to the solution. There was a large initial rise in current in response to the aspart, but over time, a pattern of electrode poisoning emerged as the current declined below the H2O2 response baseline.
We suspected that the compounds responsible for this behavior might be the phenolic preservatives present in aspart insulin: phenol (1.5 mg/mL in the formulation) and m-cresol (1.72 mg/mL). For this reason, we carried out the same experiment with regular insulin formulation without these preservative compounds (Gibco, Life Technologies, Inc.). There was no electrochemical response to the Gibco insulin; the step response of the electrode to H2O2 remained stable and fixed (not shown). This finding provided additional evidence that an excipient in the insulin formulation was responsible for the abnormal response.
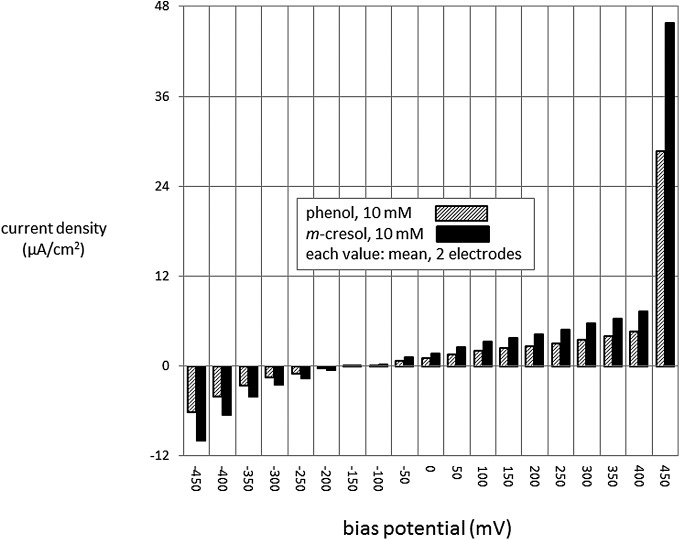
Experiments were carried out to measure the direct electrochemical effects of phenol and m-cresol. The effect of varying bias potentials in gold electrodes during exposure to these compounds is shown in Figure 3. It is clear that the magnitude of the bias is a major determinant of responses to both compounds. There is an especially large response at potentials above 400 mV. Although not shown in the figure, the responses were even higher for potentials over +450 mV. The patterns of responses for the two compounds were quite similar. This experiment was also carried out in platinum electrodes, the pattern of which was very similar to that of gold (not shown).
FIG. 3.
In vitro studies in which the responses of gold electrodes to phenol or m-cresol were measured in aqueous solution at many different bias potentials. There were large oxidative responses to high, but not low, bias potentials. Responses were similar for phenol and cresol. Results obtained using platinum electrodes were quite similar (not shown).
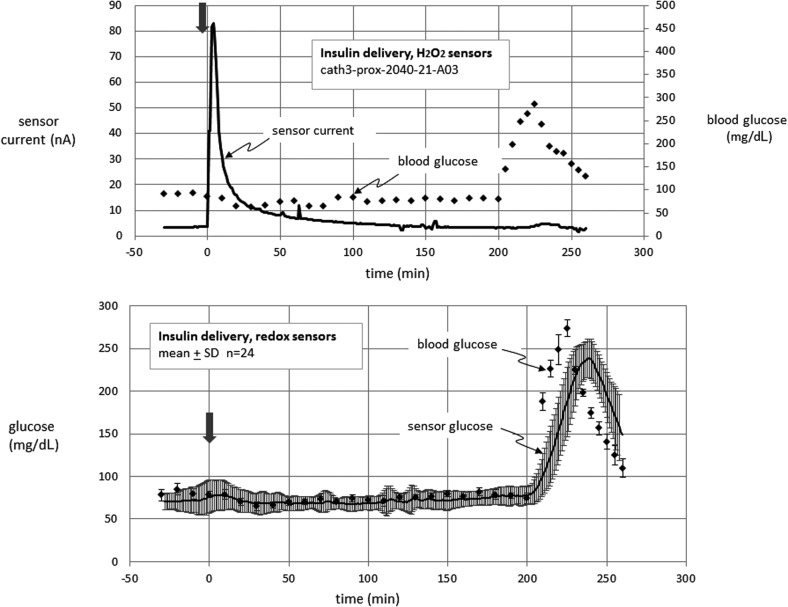
Results of animal studies are shown in Figures 4–6 and in the Supplementary Figures 1–6 (Supplementary Data are available online at www.liebertpub.com/dia). Figure 4 (upper panel) shows an example from an experiment in which lispro insulin was delivered through the cannula of an H2O2 sensor. Note the large rise in current immediately after insulin delivery. The current spontaneously declined after peaking, but remained elevated over baseline for several hours. This example also shows a typical inability of the sensor to respond to hyperglycemia late in the study, a finding reminiscent of the in vitro poisoning mentioned above. This possibility is supported by the finding that in the three platinum electrodes with the highest response to insulin, the response to hyperglycemia (area under the curve [AUC]) for 60 min after beginning hyperglycemia (−10 min baseline) averaged only 9 nA-min. In contrast, in the three platinum electrodes with the lowest response to insulin, the corresponding AUC averaged 467 nA-min, a finding that supports the concept that a large degree of interference from insulin formulation is associated with subsequent electrode poisoning.
FIG. 4.
Glucose-sensing cannula results obtained in anesthetized nondiabetic swine. An example data set for an H2O2-sensing electrode during lis-pro insulin delivery is shown in the upper panel. Note very high spurious response to the insulin formulation. All individual data sets for this condition are shown in Supplementary Figures 1–6 (available at www.liebertonline.com/dia). The lower panel shows mean data for redox sensor cannulae during lis-pro insulin delivery—no such spurious rise in current was noted in these devices. In these experiments, glucose was clamped at euglycemia until minute 200, at which time hyperglycemia was induced by dextrose infusion. The infusion was then stopped to monitor the fall of the sensor signal. The arrow indicates the insulin bolus.
FIG. 5.
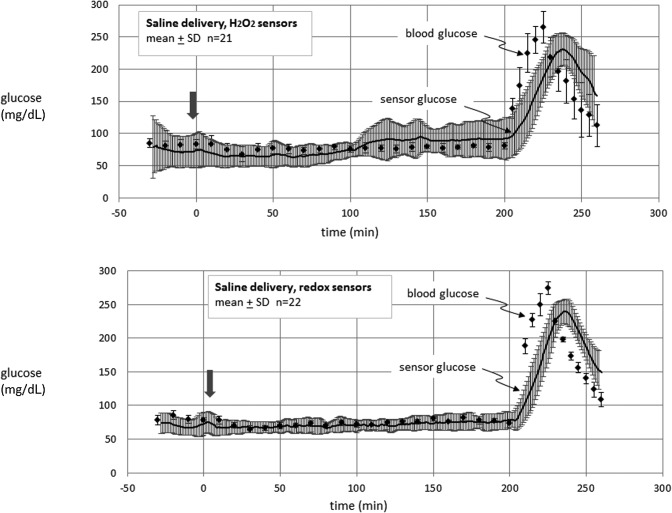
Glucose-sensing cannula results in swine. Data are shown for H2O2-sensing electrodes (upper panel) and on redox sensor electrodes (lower panel) through which PBS was given in volumes that were identical to those for insulin delivery. The arrow indicates the timing of the saline bolus.
FIG. 6.
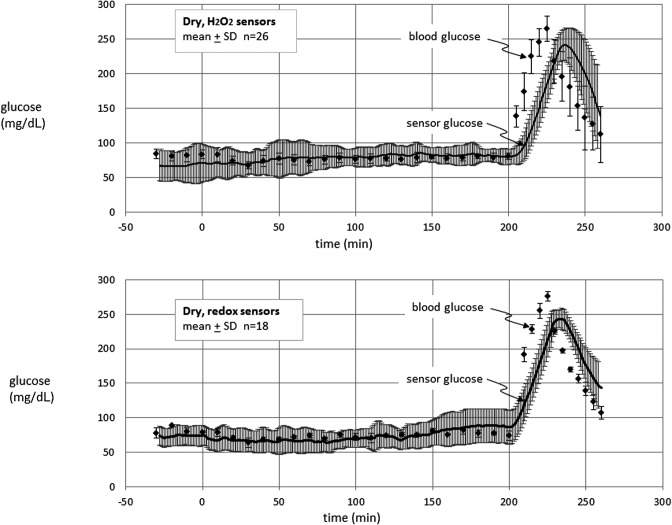
Glucose-sensing cannula results in swine. Data are shown for electrodes on H2O2-sensing cannulae (upper panel) and on redox sensor cannulae (lower panel) through which no fluid was delivered.
Figure 4 shows sensor data from a typical example of insulin delivery through the cannula of an H2O2 sensor. Since the results for this condition were variable, we chose to show all of the data sets rather than the mean values. The results of all of the individual results for this condition are shown in Supplementary Figures S1–S6. Examination of these figures shows that the rise in current after insulin delivery in the H2O2 sensors was present in about 60% of experiments (see Discussion section).
Figure 5 shows the mean results for all experiments in which saline was delivered in the vicinity of H2O2 sensors (upper) versus redox sensors (lower). A slight rise in current was sometimes seen in both types of sensors immediately after saline delivery, but typically declined to baseline values after about 10 min. Apart from this small response, the sensor outputs generally remained constant after fluid delivery during the euglycemic phase and rose appropriately to hyperglycemia toward the end of the experiments.
Figure 6 shows the mean results in the dry condition for H2O2 sensors and redox sensors. As was the case for saline, the responses of both types of sensors remained steady during euglycemia and responded briskly to the hyperglycemic phase.
Figure 7 shows a statistical analysis of the responses to insulin delivery (vs. noninsulin controls) in both types of sensors. Due to the fact that sensor estimates of glucose were not available for insulin delivery in H2O2 sensors, this analysis was performed on electrical current responses. Since these responses were not different between saline delivery and the dry condition, the two are considered together. The AUC was calculated as the area over 60 min above the 10 min predelivery baseline. Of note is the finding that the high AUC in the H2O2-sensing electrodes after insulin was significantly greater than the AUC in the comparable noninsulin conditions and was also greater than the AUC after insulin in the redox mediator-type sensors. The P-values for these comparisons were significant, that is, they fell beneath the value of alpha using the correction for multiple comparisons.
FIG. 7.
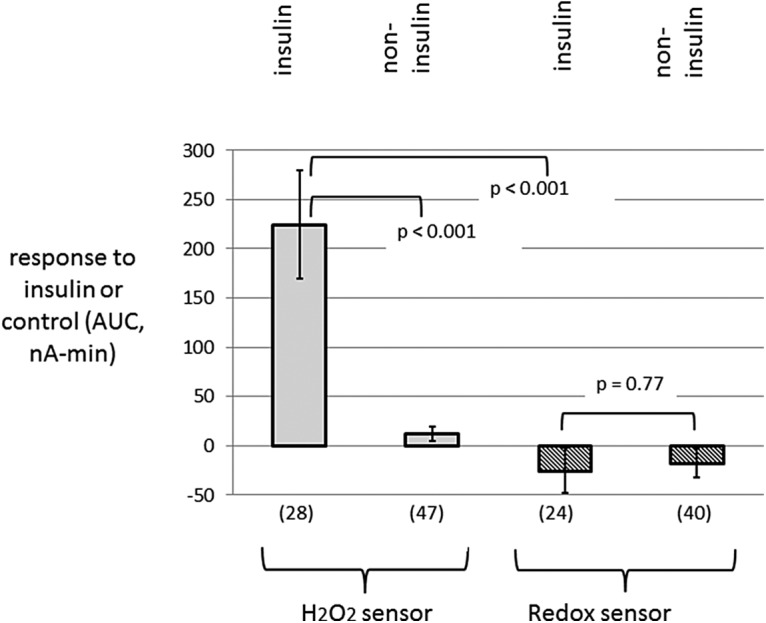
Summary data in which the response to insulin (or control) boluses were measured by calculating the AUC for 60 min (minus a 10 min baseline). The numbers of electrodes are enclosed in parentheses. Note a large AUC after the insulin formulation was given through H2O2-sensing cannulae. In contrast, there was little to no AUC in the noninsulin condition (combined saline and dry conditions) in H2O2 electrodes and in both insulin and noninsulin conditions in redox mediator electrodes. AUC, area under the curve.
Figure 7 shows mean values. Since there was some tendency for the AUC values to be skewed, we also report here the median values, which, like the mean values, were very different for insulin versus noninsulin condition in H2O2-sensing electrodes (86.3 vs. 6.4 nA-min) and were quite similar for insulin versus noninsulin conditions in redox mediator-type electrodes (0.8 vs. −1.8 nA-min).
To estimate glucose in these amperometric electrodes, we obtained a regression slope and y-intercept by comparing sensor output with blood glucose in each experiment. Since no human data have been obtained, we acknowledge that this calibration method is unsophisticated. Nonetheless, by use of this method, preliminary accuracy metrics are provided in Table 1. The absolute relative (percentage) difference provides an overall measure of inaccuracy, which is independent of sign. The relative difference, also known as percentage bias, is a signed value used to detect systematic bias. The finding that each mean relative difference is near zero suggests the absence of consistent overestimation or underestimation.
Table 1.
Summary of Accuracy Metrics Obtained During Studies in Pigs
| Absolute relative difference | Relative difference | ||||
|---|---|---|---|---|---|
| Mean, % | Median, % | Mean, % | Median, % | n (data pairs) | |
| Redox-mediated sensor | |||||
| Saline delivery | 13.5 | 10.3 | −2.2 | −2.6 | 724 |
| No fluid delivery | 17.5 | 11.8 | 0.5 | −3.1 | 586 |
| Insulin delivery | 14.1 | 9.8 | −1.8 | −3.4 | 792 |
| Hydrogen peroxide sensor | |||||
| Saline delivery | 21.1 | 14.2 | 1.6 | −2.0 | 648 |
| No fluid delivery | 17.2 | 12.5 | 1.9 | 0.0 | 830 |
| Insulin delivery | a | a | a | a | 916 |
Due to interference from the insulin formulation, there was no correlation between capillary blood glucose and sensor current; therefore, glucose could not be predicted in this condition. For each experiment, a least squares regression slope and a y-axis intercept were calculated retrospectively using all blood glucose and all sensor current points as discussed in the text. This slope and y-intercept were used to estimate interstitial glucose. These estimates were compared with blood glucose values to obtain absolute values for relative (percentage) differences and the simple (signed) relative differences. The latter metric tests for bias.
The major result of the animal study was that in contrast to the H2O2-sensing electrodes, the redox-type electrodes were not susceptible to interference from the insulin formulation. In addition, the response to hyperglycemia tended to be lower in the H2O2-sensing electrodes exposed to insulin than in other conditions, especially in those in which the insulin caused a large artifact.
Discussion
Despite the demonstrated effectiveness of insulin pump usage and CGM,1,3,14 only a small minority of patients with T1D use both technologies concurrently on a regular basis.4 We carried out this study to determine if CGM functionality could be successfully incorporated into a hollow tube used as a conduit for insulin delivery. To fabricate this device, the electrodes and chemicals necessary for CGM were disposed on to a flexible electrode strip that was laminated to the outer wall of a stainless steel cannula.
In vitro and in vivo studies examined the response of the sensor to insulin formulations that included standard excipients and to the preservatives contained in those formulations, notably phenol and its methylated relative, m-cresol.
Our major finding was that administration of lis-pro insulin near a platinum indicating electrode that was biased at 600 mV caused a large protracted positive-going artifact. When the in vitro and in vivo results are taken together, including those in which insulin without preservatives was studied, it is clear that this large oxidative current was due not to insulin per se, but to oxidation of the phenolic preservatives in insulin. Lis-pro insulin contains m-cresol in a concentration of about 3 mg/mL, a concentration nearly as great as the concentration of the insulin analog itself. All commonly sold insulin preparations contain m-cresol alone or the combination of m-cresol and phenol such that the total concentration of phenolic compounds is about 3 mg/mL. Since the electrochemical response to the two compounds is so similar, we believe that the interference from phenolic preservatives is largely independent of the specific type of insulin formulation.
The magnitude of the artifact observed after delivery of insulin formulation through H2O2-sensing cannulae was variable; some underwent a very high response to insulin formulation and others did not. A plausible explanation of this difference is that after delivery into subcutaneous tissue, the amount of insulin that refluxes back on to the cannula shaft is variable. In some cases, it is likely that much of the formulation travels away from the cannula. At any rate, when taken together, the rise in current found in the H2O2-sensing electrodes was much greater than the change in H2O2-sensing electrodes not associated with insulin delivery and much greater than redox-type electrodes, irrespective of insulin or noninsulin condition (P < 0.001 in all cases).
The in vitro and in vivo findings strongly suggest that use of a low bias potential (175 mV) avoids the high phenolic oxidation currents observed at the higher bias potentials used in the H2O2-sensing electrodes. Since H2O2 is not oxidized to any appreciable extent under 400–500 mV, use of these lower potentials precludes use of H2O2 oxidation as the endpoint of the electrochemical glucose assay. In contrast, when osmium compounds are coordinated with certain electron-donating ligands, they are able to transfer electrons obtained from glucose and GOX to a gold electrode at low bias potentials. In the chemistry that we used, osmium was coordinated with 4,4′-dimethyl, 2,2′-bipyridine and bound to poly (1-vinyl imidazole). The presence of methyl, methoxy, or amino groups on the pyridine moieties allows electron donation to the electrode at low bias potentials. Other workers have created osmium-based sensing chemistries that are capable of transferring electrons at even lower bias potentials that those used in this report.15,16
By including conditions in which saline was delivered, and in which no fluid was delivered, we were able to gain information on the effect of aqueous fluid delivery at the sensing site. In some cases, there was a tendency for saline administration to cause a small positive-going current that lasted for about 10 min. This finding was somewhat surprising since we expected to see a dilution effect in which current would be lower due to reduction of glucose concentration, as discussed by other workers.5–7 Our best explanation is that the delivery of saline at the sensing site creates a stirring effect, that is, a rise in current noted in vitro when stirring is initiated or increased.
It is also interesting to examine the sensor response to hyperglycemia in the different electrode designs and conditions. During in vivo experiments with H2O2-sensing electrodes, we found that those electrodes that underwent the greatest positive-going artifact from the insulin formulations subsequently had the lowest responses to the hyperglycemic phase. In fact, the three electrodes that had the greatest response to insulin were found to have a near-complete loss of responsiveness to hyperglycemia created by rapid glucose infusion.
This finding is best explained by examining the in vitro responses to phenolics. We observed that in the case of exposure to phenolic compounds or to insulin formulations containing phenolics, there was a gradual reduction of the oxidative current over time despite continued presence of the phenolic compounds in the aqueous media. This reduction in current over time is typical of electropolymerization, in which oxidation of some compounds leads to formation of a polymer on the electrode, which leads to the typical exponential decline of signal.17
The tendency of phenol to undergo electropolymerization is known and has been used to prevent interfering compounds such as acetaminophen from reaching peroxide-sensing indicating electrode.18,19 Our results in pigs suggest that m-cresol undergoes a similar process of electropolymerization. We found that after exposure of gold and platinum electrodes to phenol or cresol, there is a reduced response to many analytes, including glucose, H2O2, and even the phenolic compounds themselves. Although a degree of electropolymerization can be quite beneficial in terms of excluding interfering compounds, it also appears that when exposed to high concentrations for a prolonged period at a high bias potential, the response to many analytes (including glucose) becomes markedly reduced. This phenomenon has been described as electrode poisoning.20 The phenomenon of electrode poisoning makes it imperative for designers of amperometric glucose sensors intended for use at the site of insulin delivery to avoid the oxidative effect of insulin preservatives.
We did not observe a major effect of insulin to cause a decline of glucose-induced current at the site of bolus insulin delivery, although we acknowledge that a modest effect could have been missed. Insulin is well known to cause uptake of glucose into fat cells and one might expect local glucose values at the site of insulin delivery to be lower than whole-body values. However, our review of literature on this topic found that the magnitude of uptake into fat cells is almost certainly much less than the magnitude of insulin-induced uptake into muscle cells.21 The recent reports of several workers have been very instructive in understanding the quantitative aspects of this issue. These investigators, during their evaluation of the effect of local insulin on glucose measurement, found that this effect on glucose uptake was transient and minimal, no greater than about 15%.5–8 Our data on this issue in swine must be repeated in humans to understand the relevance of this phenomenon in T1D.
Regarding the interference of phenolic compounds in glucose sensing, one might think that an alternative to modifying sensing chemistry is to simply remove the preservatives from the insulin formulation. However, the preservatives have several important roles. First, phenol and cresol have strong antibacterial and antiseptic roles, dating back more than a century to the early use of phenol (carbolic acid) by Lister to sterilize operating theaters.22 Many patients use improper sterile technique in drawing up insulin from vials and the preservatives minimize the possibility of bacterial contamination. In addition, these compounds appear to provide a biochemical-stabilizing effect on insulin formulation. Teska et al. found that both phenol and m-cresol help minimize degradation of insulin hexamers in lis-pro and aspart insulin formulations.23
With regard to weaknesses of the current study, we concede that we only studied this sensor over a very short period of time in vivo, 24 h. Longer duration studies will be undertaken. In addition, the method that we used to estimate interstitial glucose from sensor current is preliminary and was carried out retrospectively. We do not anticipate being able to develop a sophisticated algorithm using prospective calibration without first carrying out human studies. Another weakness is that in the pigs, we used only one type of insulin formulation, lis-pro, which contains only m-cresol. Although we could have used more than one type of insulin formulation, such a design would have required a greater number of experiments to avoid reducing statistical power. On the bench, the responses to phenol and m-cresol are quite similar. We also concede that this study used only one insulin bolus dose and that responses to other doses or larger doses could be different. Finally, we acknowledge that ideally, a study such as the one reported here would have been conducted in humans, not swine. Human studies will be forthcoming soon.
In summary, we addressed the issue of interference in subcutaneous glucose monitoring at the site of insulin delivery. Two chemical-sensing schemes were compared on the bench and in swine experiments, during which glucose was measured in an electrode strip that was laminated to the walls of a rigid insulin delivery cannula. The results of primary mechanism of interference appear to be the oxidation at high bias potentials of phenolic preservatives present in insulin formulations, accompanied by deposition of polymerized forms of these compounds, which passivate the electrode surface. These effects can be minimized or avoided by the use of a low bias potential applied to gold indicating electrodes on which GOX and redox mediator chemicals have been deposited.
Supplementary Material
Acknowledgments
The authors thank the comparative medicine research staff at OHSU, including Tracy Schaller and Tom Chatkupt. They thank Dr. Erik Sanchez, PhD, of Portland State University for assistance in building metal sputtering chambers to apply electrode materials. The authors thank the funding agencies that sponsored this research, including the Leona M. and Harry B. Helmsley Charitable Trust (Grant No. 2015PG-T1D046), the Juvenile Diabetes Research Foundation (JDRF-PDT IDDP), and the National Institutes of Health (NIDDK), Grant No. 1R43DK100996-01.
Authors' Contributions
In terms of roles, W.K.W., J.R.C., and R.S.C. designed the projects and interpreted the data. The authors thank Dr. Joseph El Youssef for his review of the manuscript. M.B. and W.K.W. carried out the animal studies. S.B., M.B., and N.V. measured sensor responses in vitro. J.B. designed and fabricated microfabrication tools. J.K. and S.B. carried out microfabrication and assembly of the sensing electrodes. W.K.W., G.H., and M.V. designed the sensing chemistries. C.K. and S.C. carried out design, fabrication, and assembly of mechanical elements. K.M. and R.S.C. designed and fabricated the electronic/telemetric module. S.M.V. critically reviewed the data and assisted in preparation and review of the manuscript. W.K.W. ensured integrity of the data.
Author Disclosure Statement
All persons except J.R.C. and M.S.V. are employees of Pacific Diabetes Technologies (PDT) and have equity ownership in PDT and, as such, their conflicts are managed by the PDT COI Committee. J.R.C. has ownership in PDT and her conflict is managed by the OHSU COI Committee. M.S.V. is a paid consultant to PDT. In accordance with the PDT COI management and the OHSU COI management, an expert in the field who was not an author and who did not have a conflict reviewed the manuscript and agreed with its methodology and conclusions.
References
- 1.Bergenstal RM, Tamborlane WV, Ahmann A, et al. : Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010;363:311–320 [DOI] [PubMed] [Google Scholar]
- 2.Nakamura S, Hayashi S, Koga K: Effect of periodate oxidation on the structure and properties of glucose oxidase. Biochim Biophys Acta 1976;445:294–308 [DOI] [PubMed] [Google Scholar]
- 3.van Beers CA, DeVries JH: Continuous glucose monitoring: impact on hypoglycemia. J Diabetes Sci Technol 2016;10:1251–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller KM, Foster NC, Beck RW, et al. : Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38:971–978 [DOI] [PubMed] [Google Scholar]
- 5.Hermanides J, Wentholt IM, Hart AA, et al. : No apparent local effect of insulin on microdialysis continuous glucose-monitoring measurements. Diabetes Care 2008;31:1120–1122 [DOI] [PubMed] [Google Scholar]
- 6.Lindpointner S, Korsatko S, Kohler G, et al. : Use of the site of subcutaneous insulin administration for the measurement of glucose in patients with type 1 diabetes. Diabetes Care 2010;33:595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindpointner S, Korsatko S, Kohler G, et al. : Glucose levels at the site of subcutaneous insulin administration and their relationship to plasma levels. Diabetes Care 2010;33:833–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez LT, Friedman KA, Coffman SS, et al. : Effect of the sensor site-insulin injection site distance on the dynamics of local glycemia in the minipig model. Diabetes Technol Ther 2011;13:489–493 [DOI] [PubMed] [Google Scholar]
- 9.Ohara TJ, Rajagopalan R, Heller A: “Wired” enzyme electrodes for amperometric determination of glucose or lactate in the presence of interfering substances. Anal Chem 1994;66:2451–2457 [DOI] [PubMed] [Google Scholar]
- 10.Faul F, Erdfelder E, Buchner A, et al. : Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009;41:1149–1160 [DOI] [PubMed] [Google Scholar]
- 11.Castle JR, El Youssef J, Bakhtiani PA, et al. : Effect of repeated glucagon doses on hepatic glycogen in type 1 diabetes: implications for a bihormonal closed-loop system. Diabetes Care 2015;38:2115–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lodwig V, Heinemann L; Glucose Monitoring Study Group: Continuous glucose monitoring with glucose sensors: calibration and assessment criteria. Diabetes Technol Ther 2003;5:572–586 [DOI] [PubMed] [Google Scholar]
- 13.Dunn TC, Eastman RC, Tamada JA: Rates of glucose change measured by blood glucose meter and the GlucoWatch Biographer during day, night, and around mealtimes. Diabetes Care 2004;27:2161–2165 [DOI] [PubMed] [Google Scholar]
- 14.Nakamura K, Balo A: The accuracy and efficacy of the dexcom G4 platinum continuous glucose monitoring system. J Diabetes Sci Technol 2015;9:1021–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldman B, Brazg R, Schwartz S, et al. : A continuous glucose sensor based on wired enzyme technology—results from a 3-day trial in patients with type 1 diabetes. Diabetes Technol Ther 2003;5:769–779 [DOI] [PubMed] [Google Scholar]
- 16.Kavanagh P, Leech D: Mediated electron transfer in glucose oxidising enzyme electrodes for application to biofuel cells: recent progress and perspectives. Phys Chem Chem Phys 2013;15:4859–4869 [DOI] [PubMed] [Google Scholar]
- 17.Vidal JC, Garcia E, Castillo JR: Electropolymerization of pyrrole and immobilization of glucose oxidase in a flow system: influence of the operating conditions on analytical performance. Biosens Bioelectron 1998;13:371–382 [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Hu Y, Wilson GS: Glucose microbiosensor based on alumina sol-gel matrix/electropolymerized composite membrane. Biosens Bioelectron 2002;17:1005–1013 [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Matsumoto N, Hu Y, et al. : Electrochemically mediated electrodeposition/electropolymerization to yield a glucose microbiosensor with improved characteristics. Anal Chem 2002;74:368–372 [DOI] [PubMed] [Google Scholar]
- 20.Richard KM, Gewirth AA: Observation of electrode poisoning during the electro‐oxidation of aromatic alcohols on (111)Au. J Electrochem Soc 1996;143:2088–2092 [Google Scholar]
- 21.Ward WK, Castle JR, Jacobs PG, et al. : Can glucose be monitored accurately at the site of subcutaneous insulin delivery? J Diabetes Sci Technol 2014;8:568–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toledo-Pereyra LH, Toledo MM. A critical study of Lister's work on antiseptic surgery. Am J Surg 1976;131:736–744 [DOI] [PubMed] [Google Scholar]
- 23.Teska BM, Alarcon J, Pettis RJ, et al. : Effects of phenol and meta-cresol depletion on insulin analog stability at physiological temperature. J Pharm Sci 2014;103:2255–2267 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.