Abstract
Background
Chronic hepatitis C virus (HCV) infection is an important cause of hepatocellular carcinoma (HCC). Epithelial to mesenchymal transition (EMT) is a key process associated with tumor metastasis and poor prognosis. HCV infection, HCV core and NS5A protein could induce EMT process, but the role of NS4B on EMT remains poorly understood.
Methods
We overexpressed HCV NS4B protein in HepG2 cells or Huh7.5.1 cells infected by HCVcc, the E-cadherin expression, N-cadherin expression and the EMT-associated transcriptional factor Snail were determined. The migration and invasion capabilities of the transfected cells were evaluated using wound-healing assay. Additionally, we used Snail siRNA interference to confirm the relation of HCV NS4B and Snail on EMT promotion.
Results
HCV NS4B increased the expression of EMT related markers and promoted cell migration and invasion. Snail knock-down almost completely eliminated the function of NS4B protein in EMT changes and reversed cell migration capacity to lower level. HCV NS4B protein could reduce the expression of Scribble and Hippo signal pathway were subsequently inactivated, resulting in the activation of PI3K/AKT pathway, which may be the reason for the up-regulation of Snail.
Conclusions
This study demonstrates that HCV NS4B protein induces EMT progression via the upregulation of Snail in HCC, which may be a novel underlying mechanism for HCV-associated HCC development, invasion and metastasis.
Keywords: HCV, Epithelial to mesenchymal (EMT), Cadherin, Snail, Hippo pathway
Background
The epithelial-mesenchymal transition (EMT) is a fundamental biological process by which epithelial cells lose their cell polarity and cell-cell adhesion, undergo a transition from an epithelial phenotype to a mesenchymal phenotype and gain migratory and invasive properties. It can take place in a variety of biological processes, including embryonic development, morphogenesis, tissue repair, carcinogenesis and metastasis [1, 2]. Epithelial cells can adhere to each other by all kinds of cell-to-cell junctions, such as adherens junctions, desmosomes and tight junctions. During EMT procession, cells acquire invasive and metastatic properties and easily invade through the extracellular matrix [3–5]. The underlying mechanism of EMT is associated with some transcription factors, such as Snail, TWIST and ZEB (zinc finger E-box-binding protein) families, and reduced expression of E-cadherin [6]. Zinc-finger protein Snail is a transcriptional factor of EMT that negatively regulates the expression of E-cadherin through binding to the E-box motif on the promoter region of E-cadherin [7]. Snail plays an important role in the progression of hepatocellular carcinoma (HCC) caused by HCV infection, associating with the disruption of adherens junction, invasion, metastasis and poor prognosis. Yang and coworkers reported that the increasing of snail was more frequently observed in HCC related to HCV infection than other chronic liver diseases [8]. EMT is also linked to some proteins which participate in regulation of cytoskeleton and motility, including vimentin, fibroblast-specific protein1 (FSP1), osteopontin, α-smooth muscle actin (α-SMA). Accumulating evidence suggests that hepatocellular EMT plays a pivotal role in the dissemination of malignant hepatocytes during HCC progression [9].
HCV is a member of the Flaviviridae family of viruses in the hepacivirus genus, HCV infection is a major cause of chronic liver diseases, cirrhosis and HCC. End-stage liver disease caused by chronic HCV infection is the leading cause of liver transplantation in developed countries [10]. Nearly over 170 million people are suffering from HCV infection worldwide and 3–4 million people are newly infected each year. There is no effective vaccines to prevent HCV infection, HCV can establish a long-standing persistent infection in hepatocytes [11]. The recurrence rate of HCV related HCC are much higher than other types of hepatitis virus after hepatic resection, suggesting that HCV plays a key role in carcinogenesis and metastasis [12]. HCV core protein can repress the expression of E-cadherin and up-regulate the mesenchymal phenotypic markers, such as fibronectin, vimentin and N-cadherin, driving EMT procession [13]. Moreover, recent evidence indicates HCV core protein can directly inhibit the expression of E-cadherin via hypermethylation of the promoter region of E-cadherin gene by elevating levels of DNA methyltransferase 1 and 3b [14].
HCV NS4B is a 27KD non-structural protein, plays a crucial role in HCV life cycle [15]. It is a hydrophobic, highly conserved protein. NS4B expression causes alteration of endoplasmic reticulum (ER) membrane and formation of membranous web, which can provide a platform for HCV viral replication [16]. HCV NS4B also contributes to carcinogenesis, which activates cancer-related STAT3 pathway and NF-κB transcription factor via the endoplasmic reticulum overload response [17, 18]. Our previous work had shown that HCV NS4B could interact with Scribble to facilitate its degradation via proteasome pathway [19]. Scribble, classified as a LAP protein containing four PDZ domains in its C-terminal, is a consisting member of epithelial cell polarity complex. The formation of epithelial cell polarity consists of three function complex, including the Scribble, Par and Crumbs complex [20, 21]. The Scribble polarity module is composed of three proteins, Scribble, Discs Large (Dlg) and Lethal giant larvae (Lg1), localizing at basolateral membrane domain. The Par complex localizing in tight junctions of epithelia, cooperates with Crumbs complex for establishment and maintenance of apical cell polarity, having a mutually antagonistic relationship with Scribble complex [22]. Loss of Scribble resulting in deregulation of apical-basal cell polarity can promote migration capacity of epithelia cells [23].
Hippo signaling pathway is associated with organ size, cell proliferation and tumorigenesis [24]. In mammals, Hippo pathway is an evolutionarily conserved protein kinase cascade to regulate transcription coactivator YAP (Yes-associated protein) and its homologs TAZ (transcriptional coactivator with PDZ binding motif). Once Hippo pathway was activated, MST1/2 (mammalian Ste2-like kinases) phosphorylated LATS1/2 (large tumor suppressor kinase 1/2), resulting in LATS1/2-mediated phosphorylation of YAP. Phosphorylated YAP was binding to 14-3-3 protein and was degraded by an ubiquitin-proteasome-dependent manner in cytoplasm. Hippo pathway is a vital mechanism related to the carcinogenesis and development of liver cancer. Impaired Hippo pathway signaling can lead to YAP activation and the downstream cancer-related pathway TGF-β or/and PI3K/AKT pathway are activated [25, 26]. Meanwhile, the Scribble is proved to be an upstream regulator of Hippo signaling pathway [27]. So, a hypothesis is proposed that Hippo pathway may be a bridge connecting Scribble and HCV NS4B protein related EMT procession.
In this study, we demonstrated that HCV NS4B played a critical role in EMT procession by upregulation of Snail. And our study also proved our hypothesis that Scribble-Hippo-PI3K/AKT axis was a crucial molecular mechanism in HCV NS4B caused EMT changes.
Methods
HCV infection system
The plasmid pFL-J6/JFH1 contained HCV J6/JFH-1 cDNA from a Japanese patient with fulminant hepatitis behind a T7 promoter, which was lineared at the 3′ end of the HCV cDNA by XbaI digestion [28]. The linear plasmid DNA was used as a template to create HCV RNA. HCV RNA was transfected into Huh7.5.1 cells. Virus could be harvested from cell culture supernatants, which was filtered through a 0.45 μm pore size membrane. HCV RNA level of the supernatants was quantified by real-time PCR (ABI 7300). The viral titer was determined by the average number of HCV NS5A positive foci at the highest dilutions as described previously [29].
The construction of plasmids
The pEGFPC1 was purchased from Invitrogen (Invitrogen, Carlsbad, CA, USA). Full length of NS4B gene with HCV genotype 1b was generated from Con1 plasmid which was kindly provided by Prof. Ralf Bartenschlager (University of Heidelberg, Germany) by using PCR. Then full length of NS4B was inserted into pEGFPC1 plasmid to construct pEGFPC1-NS4B plasmid.
Transient transfection
The HepG2 cells were maintained in Dulbecco’s modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS) and cultured at 37 °C in a humidified atmosphere with 5% CO2. When cells reached approximately 90% confluence in six well cell culture plate, about 1 × 106 cells were transfected with 3 μg plasmids (1 and 5 μg as gradient contrast) by 5 μl lipofectamine-2000 (Invitrogen, Karlsruhe, Germany) in 150 μl DMEM medium without 10% FBS, according to manufacturers’ instructions. After 48 h, the cells were harvested and washed twice with ice-cold PBS for western blot or other experiments. Snail or control siRNA and plasmids were co-transfected into HepG2 cells at approximately 80% confluence by 5 μl lipofectamine-2000 (Invitrogen, Karlsruhe, Germany) in 150 μl DMEM medium without FBS, according to manufacturers’ instructions.
Western blotting analysis
Transfected or HCVcc infected cells were harvested and washed twice with ice-cold PBS and then lysed in sodium dodecyl sulfate-polyacrylamide gelelectrophoresis (SDS-PAGE) loading buffer (50 mM Tris-HCl, 2% SDS, 0.1% Bromophenol blue, 10% Glycerine, 1% 2-Mercaptoethanol) for 5 min. Lysates were heated at 100 °C for 20 min, then proteins were separated on SDS-PAGE gel and were transferred into polyvinylidene fluoride (PVDF) membrane (Immobion®-P Transfer Membrane). The membrane was blocked with 5% nonfat milk in TBS-tween (TBS-T) solution and incubated with primary antibodies overnight at 4 °C. Then the membrane was washed with TBS-T for three times and incubated with secondary antibodies (KPL) at room temperature for 2 h. After washed three times with TBS, the protein was visualized by SuperSignal® West Pico Chemiluminescent Substrate (Thermo Scientific).
Total RNA extraction and qRT-PCR
Total RNA was extracted from cells with Trizol reagent (TaKaRa) according to the manufacturer’s instructions. Reverse transcription was conducted following the protocol of Prime Script® RT Master Mix Perfect Real Time (Takara) and qPCR was performed as the instruction of SYBR® Premix Ex TaqTM II (Takara) on the ABI 7300 Real Time PCR System (Applied Biosystems). β-actin was used as the internal control. The Primers were designed according to intron-crossing principle to remove the interference of genome DNA (Table 1). Each sample was performed in triplicate and the results represent mean ± SE.
Table 1.
Primers of EMT related genes
| Gene | Primers |
|---|---|
| E-cadherin | Sense: 5′-AACAGGATGGCTGAAGGTGACA-3′ |
| Antisense: 5′- GTAAGCGATGGCGGCATTGTAG-3 | |
| N-cadherin | Sense: 5′- AGAACGCCAGGCCAAACAAC-3′ |
| Antisense: 5′- ATTCGTCGGATTCCCACAGG-3′ | |
| Snail | Sense: 5′- TGAGGCCAAGGATCTCCAGG-3′ |
| Antisense: 5′-AAGGGCTTCTCGCCAGTGTG-3′ | |
| β-actin | Sense: 5′-TACGCCAACACAGTGCTGTCTG-3′ |
| Antisense: 5′-CACATCTGCTGGAAGGTGGACA-3′ |
Immunofluorescence analysis
At 24 h post-transfection, cells were washed twice with ice-cold PBS and fixed in 4% paraformaldehyde for 15 min at room temperature. Cells were permeabilized in 0.1% Triton X-100 in PBS for 3–5 min, blocked with 3% FBS in PBS for 1 h, and then incubated with primary antibody overnight at 4 °C followed by incubated with secondary antibody conjugated to red fluorochromes for 1 h at room temperature. The cell nuclei were stained with 1 μg/ml dihydrochloride (Beyotime, China) for 15 min at room temperature. Cover slips were mounted upside down on slides with suitable volume of ProLong Gold Antifade Reagent (Invitrogen, USA). After that, the cells were examined using an OLYMPUS IX81 fluorescence microscope equipped with the appropriate filter sets and OLYMPUS FluoView Ver.2.0a Viewer software.
Wound-healing assay
Cells grown to 90% confluence in 6-well plates were mechanically injured with a sterile micropipette tip. The photographs were taken at 0, 12, and 24 h and wound healing rate was evaluated by measuring wound closure rate.
Statistical analysis
Experimental data were analyzed using GraphPad Prism 4 (GraphPad Software, La Jolla, CA, USA). The value was presented as means ± S.D. Two-tailed student’s t-test was used for all statistical analysis; a P value of less than 0.05 was considered as statistically significance.
Results
HCVcc-infected cells presented the changes of EMT and upregulation of Snail
Huh7.5.1 cells were infected with HCVcc for 48 h and the cells not infected as control group. Then cells were harvested for western blot assay. In HCVcc infected cells, the expression of epithelial phenotypic markers E-cadherin was decreased whereas mesenchymal phenotypic marker N-cadherin was increased compared with the control group (Fig. 1). Meanwhile, we also observed the upregulation of Snail, one of the most important transcription factors associated with EMT. These results revealed that EMT process really occurred in HCVcc infection cells and the initiation of EMT may be due to upregulation of Snail.
Fig. 1.
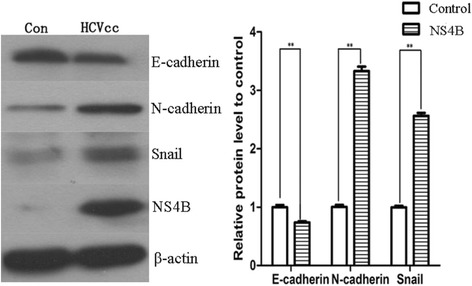
HCVcc-infected cells presented the changes of EMT. The expression of E-cadherin, N-cadherin and Snail in Huh7.5.1 cells (Control group) and HCVcc infected Huh7.5.1 cells (HCVcc group). *P < 0.05, **P < 0.01
HCV NS4B protein induced hepatocellular EMT
To investigate the effect of HCV NS4B protein on hepatocellular EMT, we transfected empty vector or pNS4B plasmids into HepG2 cells, and then we examined the hallmarks of EMT at 48 h. It was found that cells transfected with pNS4B plasmids had lower expression of E-cadherin and higher expression of N-cadherin than the cells transfected with empty vector, as similar to the EMT phenotype in HCVcc infected cells, suggesting that HepG2 cells were in EMT procession (Fig. 2a). Furthermore, we transfected empty vector, pNS4B plasmids (1, 3, 5 μg) in HepG2 cells to investigate whether the EMT process was related to NS4B levels, at 48 h post transfection, we found that the expression of E-cadherin decreased gradually and N-cadherin upregulated, along with the levels of pNS4B plasmids increased. These results indicated that HCV NS4B was the reason for HepG2 cell EMT (Fig. 2b). In addition, the qRT-PCR results showed that E-cadherin mRNA levels were decreased and the expression levels of N-cadherin increased after pNS4B plasmids transfection (Fig. 2c). Immunofluorescence analysis also confirmed the decreased expression of epithelial marker E-cadherin and increased expression of mesenchymal marker N-cadherin (Fig. 2d). These results demonstrated that NS4B contributed to promoting HepG2 cells EMT.
Fig. 2.

HCV NS4B induced hepatocellular EMT. a HepG2 cells were transfected with pEGFPC1 plasmid (control group) and pEGFPC1-NS4B plasmid (NS4B group), 48 h later, the expression of E-cadherin and N-cadherin in control group and NS4B group. b HepG2 cells were transfected with pEGFPC1 plasmid (control group), 1, 3, 5 μg pEGFPC1-NS4B plasmid respectively, 48 h later, the expression of E-cadherin and N-cadherin in each group. c qRT-PCR analysis the mRNA expression of E-cadherin and N-cadherin in control group and NS4B group. d Immunofluorescence analysis of E-cadherin and N-cadherin in control group and NS4B group. *P < 0.05, **P < 0.01
Upregulation of Snail was coupled with the process of HCV NS4B promotion-EMT
It was identified that upregulation of Snail could induce EMT changes [30]. We transfected empty vector, pNS4B plasmids (1, 3 and 5 μg) into HepG2 cells, we observed the expression of Snail was increased compared with the empty vector in the HepG2 cells after HCV NS4B-transfected for 48 h (Fig. 3a), meanwhile, we also observed a positive correlation between the upregulation of Snail levels and pNS4B plasmids levels (Fig. 3b). But the levels of Snail mRNA that we measured after pNS4B plasmids transfected for 48 h had no significant difference with the empty vector (Fig. 3c), suggesting the upregulation of Snail may rely on a translation-dependent manner. In order to confirm the effect of snail on EMT process, we co-transfected Snail siRNA and NS4B into HepG2 cells, the expression level of Snail in HepG2 cells decreased after Snail siRNA transfection, and we found the phenomenons that downregulation of E-cadherin and upregulation of N-cadherin reversed while knockdown of snail. These results provided a powerful evidence that snail played a vital role in NS4B-induced EMT changes (Fig. 3d).
Fig. 3.
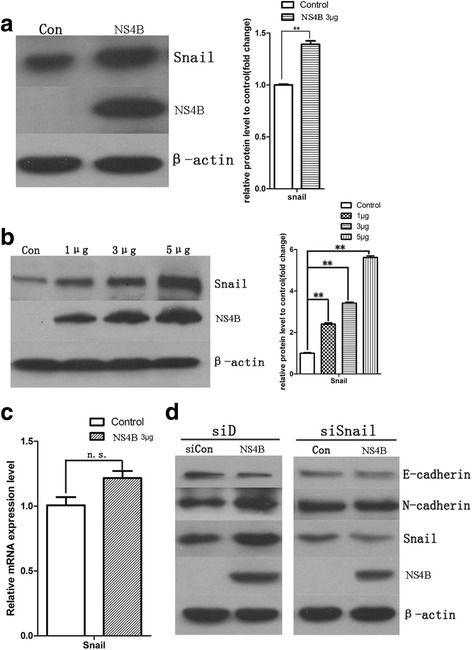
HCV NS4B promoted EMT due to upregulation of Snail. a HepG2 cells were transfected with pEGFPC1 plasmid (control group) and pEGFPC1-NS4B plasmid (NS4B group), 48 h later, the expression of Snail in control group and NS4B group. b HepG2 cells were transfected with pEGFPC1 plasmid (control group), 1, 3, 5 μg pEGFPC1-NS4B plasmid respectively, 48 h later, the expression of Snail in each group. c qRT-PCR analysis the mRNA expression of Snail in control group and NS4B group, The HepG2 cells were transfected with 3 μg pEGFPC1-NS4B plasmids in the qRT-PCR assay. d Western blotting analysis the expression of E-cadherin, N-cadherin, snail and HCV-NS4B in plasmids transfected cells, Snail siRNA for knockdown Snail. Co-transfected Snail siRNA and 3 μg pEGFPC1-NS4B plasmids into HepG2 cells, the expression level of Snail in HepG2 cells decreased after Snail siRNA transfection, and the phenomenons that downregulation of E-cadherin and upregulation of N-cadherin reversed while knockdown of snail. Con ~ for control group, n.s. ~ for no significant different, *P < 0.05, **P < 0.01
HCV NS4B protein promoted hepatoma cell migration and invasion
The wound healing assay was used to examine the migration and invasion capabilities of the HepG2 cells transfected with HCV NS4B. In the same period of time, the HepG2 cells with HCV NS4B transfected expansion to fill the gap further, indicated that HCV NS4B promoted HepG2 cell motility and invasiveness. To test whether HCV NS4B transfected cells acquired migration and invasion capabilities through upregulation of Snail, we cotransfected snail or control siRNA and NS4B plasmid into HepG2 cells. We found that the increased migration in NS4B transfected cells was reversed by snail siRNA treatment, cells cotransfected with Snail siRNA showed slower healing of the wound in comparison to the cells treated with control siRNA, which meant that snail played an important role in HCV NS4B induced hepatoma cell migration and invasion (Fig. 4).
Fig. 4.
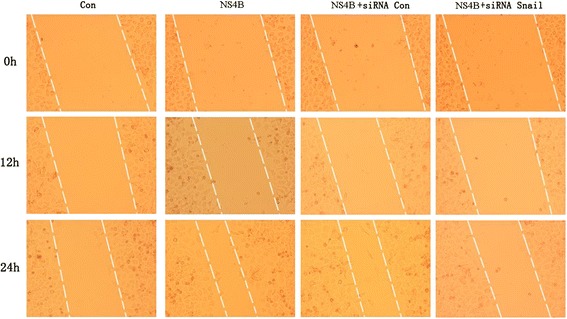
HCV NS4B promoted hepatoma cell migration and invasion. Would healing assay were used to examine the migration and invasion capabilities of the HepG2 cells were transfected with HCV NS4B, cotransfected snail or control siRNA and NS4B. Microscopic observation of cell migration and recorded at 0, 12 and 24 h after scratching
Upregulation of Snail may be caused by Scribble-Hippo-PI3K/AKT pathway
The PI3K/AKT pathway can enhance the stability of Snail via phosphorylation at its two serine residues, and then upregulate its activity by post-translational modification [31]. Therefore, we investigated whether upregulation of Snail by HCV NS4B expression was related to Scribble-Hippo-PI3K/AKT pathway. The key elements in this pathway including Scribble, p-Yap, total-Yap, p-Akt and total-Akt were examined by Western blot. In the HepG2 cells tranfected by HCV NS4B for 24 and 48 h, the expression of Scribble and p-Yap was decreased, but the total Yap had no significant difference. So the ratio of P-Yap/total Yap was decreased, these results suggested NS4B expression in HepG2 cells inhibited the activity of Hippo signal pathway, additionally the down-regulation of Scribble and p-Yap were in a time dependent manner (Fig. 5a). Then we further used HepG2 cells transfected with pNS4B plasmids (1, 3 μg) for 48 h to evaluated the expression of p-Akt and total-Akt, the results showed that p-Akt was increased and total-Akt was decreased. Taken together, these results demonstrated the activation of PI3K/AKT pathway in HepG2 cells with NS4B expression (Fig. 5b).
Fig. 5.
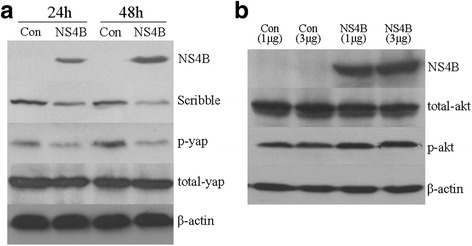
Western blotting analysis the key elements of Scribbe-Hippo-PI3K/AKT pathway in the HepG2 cells with HCV NS4B transfected. a HepG2 cells were transfected with pEGFPC1 plasmid (control group) and pEGFPC1-NS4B plasmid (NS4B group), 24 and 48 h later, the expression of Scribble, p-Yap and total Yap in control group and NS4B group. b HepG2 cells were transfected with 1, 3 μg pEGFPC1 plasmid and 1, 3 μg pEGFPC1-NS4B plasmid, 48 h later, the expression of p-akt, total-akt in each group
Discussion
EMT is a key event in carcinogenesis and EMT has been confirmed to play an important role during HCC progression, whereby epithelial cells lose apical-basal polarity and cell junctions and acquire a mesenchymal phenotype with invasion and metastasis properties. EMT of malignant hepatocytes correlate well with malignancy and poor prognosis [32, 33]. HCV infection was identified to induce EMT in primary hepatocytes and cell lines [13]. Many studies had proved that HCV core or NS5A protein could induce EMT process in hepatoma cells by different kinds of manner [34–36]. Our study firstly observed that NS4B was also involved in EMT of hepatocytes, the underlying molecular mechanism remains elusive and needs to be fully understood in HCC progression.
E-cadherin is a phenotypic membranous adhesion molecule on epithelial cells. It has been proved to be involved in proliferation, migration and the process of EMT, keeping cells in a quiescent, non-motile state. N-cadherin is a molecular marker of acquisition of a motile mesenchymal phenotype and involves in the EMT and metastasis of cancer cells. The loss of E-cadherin and gain of N-cadherin expression is considered as the most common hallmark of EMT. It was reported that the expression of E-cadherin was reduced significant in HCV-caused HCC compared to normal tissues [37]. In the present study, HCV infection or ectopic expression of HCV NS4B in HepG2 cells reduced E-cadherin expression and increased N-cadherin expression. Furthermore, HCV NS4B expression contributed to migration and invasion abilities in hepatoma cells. This study showed high levels of Snail expression in HCV NS4B-transfected HepG2 cells and HCVcc-infected Huh7.5.1 cells. When the snail knockdown by siRNA, the increased migration in NS4B transfected cells was reversed accordingly, moreover, the degree of downregulation of E-cadherin and upregulation of N-cadherin attenuated, which meant that Snail played an important role in HCV NS4B induced EMT.
The precise molecular mechanism of the Snail regulation is still unclear, previously, our teams have proved that HCV NS4B targets Scribble for proteasome-mediated degradation to facilitate cell transformation [19]. Scribble is an epithelial polarity protein plays a critical role in establishing and maintaining epithelial cell adhesion, polarity and proliferation, which serves as a tumor suppressor [38]. Loss of Scribble can lead to EMT. Yamben and coworkers reported that deletion Scrib (a mouse homolog of Scribble) in the lens resulted in downregulation of E-cadherin, apical polarity protein ZO-1 (Zonula occludens-1) and upregulation of Snail [39]. In this study, Scribble was decreased in HCV NS4B transfected HepG2 cells and impaired Hippo pathway signaling, resulting in the downregulation of the ratio of p-Yap/total-Yap. Hippo pathway played an important role in the development of HCC. High-level expression of Yes-associated protein (YAP) was more frequently observed in malignant liver tissues than normal liver tissues [40, 41]. Scribble acted as an upstream regulator of Hippo signal pathway and caused deregulation of the Hippo tumor suppressor pathway. Verghese and colleagues reported that Scribble interacted with Expanded and Dachs to regulate Warts level and stability in Drosophila, and then placing Scribble in Hippo pathway network [27]. The nuclear effector of the Hippo Pathway YAP and transcription enhancer factor TEAD acted as a conserved enhancer directly targeting the first intron of PIK3CB (Phosphoinositide 3-Kinase Catalytic Beta Polypeptide), encoding the catalytic subunit p110β of human phosphatidylinositol 3-kinase (PI3K) to activate the PI3K/AKT pathway [26]. In present study, we demonstrated that HCV NS4B suppressed Hippo pathway and activated the PI3K/AKT pathway. Hippo pathway acted as a bridge between the downregulation of Scribble and activation of PI3K/AKT pathway. It had already been proved that PI3K/AKT pathway regulated the stability of Snail to promote EMT. These results suggested that Scribble-Hippo-PI3K/AKT pathway maybe involved in upregulation of Snail.
Conclusions
In summary, our study identified that overexpression of HCV NS4B in HepG2 cells could lead to EMT-like changes, which enhanced migration and invasion of hepatoma cells. The mechanism underlying EMT caused by HCV NS4B were due to upregulation of Snail. Snail knockdown could reverse the procession of EMT and inhibited the migration of HepG2 cells. The regulation of Snail would probably be mediated through Scribble-Hippo-PI3k/AKT pathway (Fig. 6). Our work revealed a potential molecular mechanism underlying EMT caused by HCV NS4B. It may provide novel insight into potential new therapeutic approaches for patients with HCV infection.
Fig. 6.

The delineation of a potential molecular mechanism underlying EMT caused by HCV NS4B
Acknowledgments
This work was supported by Natural Science Foundation of Guangdong Province (No. 2014A030313726 to Yi Zheng) and Open Research Fund Program of the State Key Laboratory of Virology of China (No. 2013006 to Yi Zheng); National Natural Science Foundation of China (No. 81072123 and No. 30571955 to Xinxing Wu).
Authors’ contributions
BH carried out the cell culture and the manuscript preparation. SX participated in the cell culture and carried out the Wound-healing assay. YH, WC and XC participated in the Western blotting analysis and qRT-PCR. YZ carried out the Immunofluorescence analysis and participated in the design of the study. XW conceived of the study and participated in its design and coordination. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The collection of material used in this study complied with the Helsinki Declaration.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AKT
Protein kinase B
- Dlg
Discs Large
- DMEM
Dulbecco’s modified Eagle medium
- EMT
Epithelial to mesenchymal transition
- ER
Endoplasmic reticulum
- FBS
Fetal bovine serum
- FSP
Fibroblast-specific protein
- HCC
Hepatocellular carcinoma
- HCV
Hepatitis C virus
- LAP
Leucine-rich repeats and PDZ domains
- LATS1/2
Large tumor suppressor kinase 1/2
- Lg1
Lethal giant larvae
- MST1/2
Mammalian Ste2-like kinases
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NS4B
Non-structural protein 4B
- PDZ
PSD-95/Dlg-A/ZO-1
- PI3K
Phosphoinositide 3-Kinase
- PIK3CB
Phosphoinositide 3-Kinase Catalytic Beta Polypeptide
- PVDF
Polyvinylidene fluoride
- SDS-PAGE
Sodium dodecyl sulfate-polyacrylamide gelelectrophoresis
- SMA
Smooth muscle actin
- STAT
Signal transducer and activator of transcription
- TAZ
Transcriptional coactivator with PDZ binding motif
- TEAD
Transcriptional enhancer factor domain family member
- TGF-β
Transforming growth factor-β
- YAP
Yes-associated protein
- ZEB
Zinc finger E-box-binding protein
- ZO-1
Zonula occludens-1
Contributor Information
Yi Zheng, Phone: 86-755-27128196, Email: zhengyi1205@126.com.
Xinxing Wu, Phone: 86-27-68758898, Email: wuxinxing9755@163.com.
References
- 1.Panebianco C, Saracino C, Pazienza V. Epithelial-mesenchymal transition: molecular pathways of hepatitis viruses-induced hepatocellular carcinoma progression. Tumour Biol. 2014;35:7307–7315. doi: 10.1007/s13277-014-2075-x. [DOI] [PubMed] [Google Scholar]
- 2.Kwon YC, Ray RB, Ray R. Hepatitis C virus infection: establishment of chronicity and liver disease progression. EXCLI J. 2014;13:977–996. [PMC free article] [PubMed] [Google Scholar]
- 3.Ye X, Weinberg RA. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol. 2015;25:675–686. doi: 10.1016/j.tcb.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Firrincieli D, Boissan M, Chignard N. Epithelial-mesenchymal transition in the liver. Gastroenterol Clin Biol. 2010;34:523–528. doi: 10.1016/j.gcb.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Lin CW, Kao SH, Yang PC. The miRNAs and epithelial-mesenchymal transition in cancers. Curr Pharm Des. 2014;20:5309–5318. doi: 10.2174/1381612820666140128204508. [DOI] [PubMed] [Google Scholar]
- 6.Tania M, Khan MA, Fu J. Epithelial to mesenchymal transition inducing transcription factors and metastatic cancer. Tumour Biol. 2014;35:7335–7342. doi: 10.1007/s13277-014-2163-y. [DOI] [PubMed] [Google Scholar]
- 7.Muqbil I, Wu J, Aboukameel A, Mohammad RM, Azmi AS. Snail nuclear transport: The gateways regulating epithelial-to-mesenchymal transition. Semin Cancer Biol. 2014;27:39–45. doi: 10.1016/j.semcancer.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang MH, Chen CL, Chau GY, Chiou SH, Su CW, Chou TY, et al. Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology. 2009;50:1464–1474. doi: 10.1002/hep.23221. [DOI] [PubMed] [Google Scholar]
- 9.Jou J, Diehl AM. Epithelial-mesenchymal transitions and hepatocarcinogenesis. J Clin Investig. 2010;120:1031–1034. doi: 10.1172/JCI42615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baldo V, Baldovin T, Trivello R, Floreani A. Epidemiology of HCV infection. Curr Pharm Des. 2008;14:1646–1654. doi: 10.2174/138161208784746770. [DOI] [PubMed] [Google Scholar]
- 11.WHO . Hepatitis C, Rep 164, WHO. Geneva: World Health Organization; 2011. [Google Scholar]
- 12.Huang YH, Wu JC, Chen CH, Chang TT, Lee PC, Chau GY, et al. Comparison of recurrence after hepatic resection in patients with hepatitis B vs. hepatitis C‐related small hepatocellular carcinoma in hepatitis B virus endemic area. Liver Int. 2005;25:236–241. doi: 10.1111/j.1478-3231.2005.01081.x. [DOI] [PubMed] [Google Scholar]
- 13.Bose SK, Meyer K, Di Bisceglie AM, Ray RB, Ray R. Hepatitis C virus induces epithelial-mesenchymal transition in primary human hepatocytes. J Virol. 2012;86:13621–13628. doi: 10.1128/JVI.02016-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jungmi P, Kyung LJ. Hepatitis C virus represses E-cadherin expression via DNA methylation to induce epithelial to mesenchymal transition in human hepatocytes. Biochem Biophys Res Commun. 2014;446:561–567. doi: 10.1016/j.bbrc.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Yu X, Guo Y, Kong L. Interaction networks of hepatitis C virus NS4B: implications for antiviral therapy. Cell Microbiol. 2012;14:994–1002. doi: 10.1111/j.1462-5822.2012.01773.x. [DOI] [PubMed] [Google Scholar]
- 16.Elazar M, Liu P, Rice CM, Glenn JS. An N-terminal amphipathic helix in hepatitis C virus (HCV) NS4B mediates membrane association, correct localization of replication complex proteins, and HCV RNA replication. J Virol. 2004;78:11393–11400. doi: 10.1128/JVI.78.20.11393-11400.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li S, Ye L, Yu X, Xu B, Li K, Zhu X, et al. Hepatitis C virus NS4B induces unfolded protein response and endoplasmic reticulum overload response-dependent NF-κB activation. Virology. 2009;391:257–264. doi: 10.1016/j.virol.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 18.Kong L, Li S, Yu X, Fang X, Xu A, Huang M, et al. Hepatitis C virus and its protein NS4B activate the cancer-related STAT3 pathway via the endoplasmic reticulum overload response. Arch Virol. 2016;161:2149–2159. doi: 10.1007/s00705-016-2892-x. [DOI] [PubMed] [Google Scholar]
- 19.Hu B, Li S, Zhang Z, Xie S, Hu Y, Huang X, et al. HCV NS4B targets Scribble for proteasome-mediated degradation to facilitate cell transformation. Tumor Biol. 2016;37:12387–12396. doi: 10.1007/s13277-016-5100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humbert P, Russell S, Richardson H. Dlg, Scribble and Lgl in cell polarity, cell proliferation and cancer. Bioessays. 2003;25:542–553. doi: 10.1002/bies.10286. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J Cell Sci. 2006;119:979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- 22.Li P, Mao X, Ren Y, Liu P. Epithelial cell polarity determinant CRB3 in cancer development. Int J Biol Sci. 2015;11:31–37. doi: 10.7150/ijbs.10615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289:113–116. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- 24.Mo JS, Park HW, Guan KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014;15:642–656. doi: 10.15252/embr.201438638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varelas X, Sakuma R, Samavarchi-Tehrani P, Peerani R, Rao BM, Dembowy J, et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol. 2008;10:837–848. doi: 10.1038/ncb1748. [DOI] [PubMed] [Google Scholar]
- 26.Lin Z, Zhou P, von Gise A, Gu F, Ma Q, Chen J, et al. Pi3kcb links Hippo-YAP and PI3K-AKT signaling pathways to promote cardiomyocyte proliferation and survival. Circ Res. 2015;116:35–45. doi: 10.1161/CIRCRESAHA.115.304457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verghese S, Waghmare I, Kwon H, Hanes K, Kango-Singh M. Scribble Acts in the Drosophila Fat-Hippo Pathway to Regulate Warts Activity. PloS One. 2012;7:e47173. doi: 10.1371/journal.pone.0047173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato T, Date T, Miyamoto M, Furusaka A, Tokushige K, Mizokami M, et al. Efficient replication of the genotype 2a hepatitis C virus subgenomic replicon. Gastroenterology. 2003;125:1808–1817. doi: 10.1053/j.gastro.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 29.Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton DR, et al. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci. 2005;102:9294–9299. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng H, Kang Y. Multilayer control of the EMT master regulators. Oncogene. 2014;33:1755–1763. doi: 10.1038/onc.2013.128. [DOI] [PubMed] [Google Scholar]
- 31.Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- 32.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Investig. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasquier J, Abu-Kaoud N, Al Thani H, Rafii A. Epithelial to mesenchymal transition in a clinical perspective. J Oncol. 2015;2015:792182. doi: 10.1155/2015/792182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quan H, Zhou F, Nie D, Chen Q, Cai X, Shan X, et al. Hepatitis C virus core protein epigenetically silences SFRP1 and enhances HCC aggressiveness by inducing epithelial-mesenchymal transition. Oncogene. 2014;33:2826–2835. doi: 10.1038/onc.2013.225. [DOI] [PubMed] [Google Scholar]
- 35.Tiwari I, Yoon MH, Park BJ, Jang KL. Hepatitis C virus core protein induces epithelial-mesenchymal transition in human hepatocytes by upregulating E12/E47 levels. Cancer Lett. 2015;362:131–138. doi: 10.1016/j.canlet.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 36.Akkari L, Grégoire D, Floc’H N, Moreau M, Hernandez C, Simonin Y, et al. Hepatitis C viral protein NS5A induces EMT and participates in oncogenic transformation of primary hepatocyte precursors. J Hepatol. 2012;57:1021–1028. doi: 10.1016/j.jhep.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 37.Llovet JM, Chen Y, Wurmbach E, Roayaie S, Fiel MI, Schwartz M, et al. A Molecular Signature to Discriminate Dysplastic Nodules From Early Hepatocellular Carcinoma in HCV Cirrhosis. Gastroenterology. 2006;131:1758–1767. doi: 10.1053/j.gastro.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 38.Enomoto M, Igaki T. Deciphering tumor-suppressor signaling in flies: Genetic link between Scribble/Dlg/Lgl and the Hippo pathways. J Genet Genomics. 2011;38:461–470. doi: 10.1016/j.jgg.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Yamben IF, Rachel RA, Shatadal S, Copeland NG, Jenkins NA, Warming S, et al. Scrib, is required for epithelial cell identity and prevents epithelial to mesenchymal transition in the mouse. Dev Biol. 2013;384:41–52. doi: 10.1016/j.ydbio.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinhardt AA, Gayyed MF, Klein AP, Dong J, Maitra A, Pan D, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jie L, Fan W, Weiqi D, Yingqun Z, Ling X, Miao S, et al. The hippo-yes association protein pathway in liver cancer. Gastroenterol Res Pract. 2013;2013:187070. doi: 10.1155/2013/187070. [DOI] [PMC free article] [PubMed] [Google Scholar]


