Abstract
Hypothalamic inflammation contributes to metabolic dysregulation and the onset of obesity. Dietary saturated fats activate microglia via a nuclear factor-kappa B (NFκB) mediated pathway to release pro-inflammatory cytokines resulting in dysfunction or death of surrounding neurons. Fatty acid binding proteins (FABPs) are lipid chaperones regulating metabolic and inflammatory pathways in response to fatty acids. Loss of FABP4 in peripheral macrophages via either molecular or pharmacologic mechanisms results in reduced obesity-induced inflammation via a UCP2-redox based mechanism. Despite the widespread appreciation for the role of FABP4 in mediating peripheral inflammation, the expression of FABP4 and a potential FABP4-UCP2 axis regulating microglial inflammatory capacity is largely uncharacterized. To that end, we hypothesized that microglial cells express FABP4 and that inhibition would upregulate UCP2 and attenuate palmitic acid (PA)-induced pro-inflammatory response. Gene expression confirmed expression of FABP4 in brain tissue lysate from C57Bl/6J mice and BV2 microglia. Treatment of microglial cells with an FABP inhibitor (HTS01037) increased expression of Ucp2 and arginase in the presence or absence of PA. Moreover, cells exposed to HTS01037 exhibited attenuated expression of inducible nitric oxide synthase (iNOS) compared to PA alone indicating reduced NFκB signaling. Hypothalamic tissue from mice lacking FABP4 exhibit increased UCP2 expression and reduced iNOS, tumor necrosis factor-alpha (TNF-α), and ionized calcium-binding adapter molecule 1 (Iba1; microglial activation marker) expression compared to wild type mice. Further, this effect is negated in microglia lacking UCP2, indicating the FABP4-UCP2 axis is pivotal in obesity induced neuroinflammation. To our knowledge, this is the first report demonstrating a FABP4-UCP2 axis with the potential to modulate the microglial inflammatory response.
Keywords: Neuroinflammation, reactive oxygen species, palmitic acid, obesity
Graphical abstract
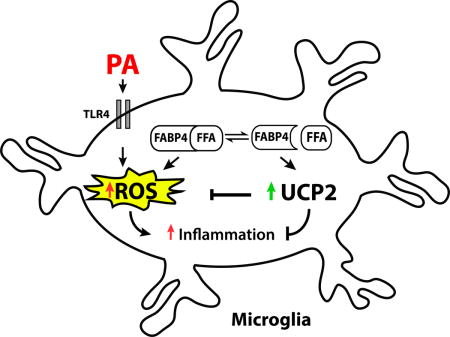
Introduction
Saturated fatty acids (SFAs) such as palmitic acid (PA) contribute to the onset of metabolic inflammatory diseases, including obesity, in part through hypothalamic dysregulation and degeneration (Cai, 2013; Karmi et al., 2010; Thaler et al., 2012; Valdearcos et al., 2014). In the hypothalamus, dietary PA activates microglia (immune cells of the brain) via a nuclear factor kappa B (NFκB)-mediated pathway to release pro-inflammatory cytokines and contribute to damage of neurons responsible for regulating body weight (Thaler et al., 2013; Wang et al., 2012).
Microglia are sensitive and highly dynamic in response to changes in the surrounding microenvironment. As microglia respond to the surrounding environment, they are activated to either a pro-inflammatory (M1) or anti-inflammatory, protective (M2) phenotype, depending on external stimuli. For example, PA activates microglia via a toll like receptor (TLR)-4 and induces the release of pro-inflammatory cytokines and factors such as tumor necrosis factor-alpha (TNF-α) and inducible nitric oxide synthase (iNOS) (Duffy et al., 2015; Valdearcos et al., 2014). Conversely, microglia activated with the anti-inflammatory cytokine interleukin (IL)-4 polarize to an M2 protective state, characterized by the release of anti-inflammatory cytokines and factors such as arginase-1 (Fumagalli et al., 2011). The polarization of microglial cells is a highly energetic process and dependent upon mitochondrial integrity and activation. Uncoupling protein 2 (UCP2) has been implicated in mediating energetic processes of microglial activation states (Emre and Nubel, 2010). Microglia activated to an M1 phenotype have reduced UCP2 activity and expression, resulting in increased production of reactive oxygen species (ROS) (De Simone et al., 2015). Conversely, UCP2 activity is robustly increased following activation of an M2 protective phenotype (De Simone et al., 2015), indicating a potential target to manipulate microglial activation states.
UCP2 activity is regulated by fatty acid binding proteins (FABP), lipid chaperones regulating metabolic and inflammatory pathways in response to fatty acids (Hotamisligil and Bernlohr, 2015). Targeted deletion of the adipocyte FABP (FABP4, also known as aP2) is sufficient to prevent obesity induced insulin resistance, diabetes, atherosclerosis and asthma, (Boord et al., 2004; Furuhashi et al., 2007; Hotamisligil et al., 1996; Shum et al., 2006). Mice lacking FABP4 (also referred to as aP2 deficient mice) have been used to extensively characterize diabetes, atherosclerosis and asthma linking FABP4 signaling to important roles in metabolic homeostasis and immunometabolic diseases, as reviewed in (Hotamisligil and Bernlohr, 2015). In peripheral murine macrophages, the loss of FABP4 protects against the development of atherosclerosis and dyslipidemia (Makowski et al., 2001). The loss of FABP4 in macrophages via either molecular or pharmacologic means results in attenuated obesity-induced inflammation through a UCP2-redox based mechanism (Xu et al., 2016; Xu et al., 2015). While the role of the FABP4-UCP2 axis in peripheral macrophages has been extensively characterized, this axis has not been explored in the brain immune cells such as microglia.
We hypothesized that inhibition of FBAP4 in microglia would attenuate PA-induced pro-inflammatory response through a UCP2 mediated mechanism. Herein we demonstrate that mice lacking FABP4 have increased expression of UCP2 and reduced expression of TNF-α, iNOS, and ionized calcium-binding adapter molecule 1 (Iba1, a marker of microglial activation) in the hypothalamus. Moreover, pharmacological inhibition of FABP increases UCP2 expression and reduces PA-induced pro-inflammatory response and ROS production. Further, this effect is negated in microglia lacking UCP2, indicating the FABP4-UCP2 axis is pivotal in obesity-induced neuroinflammation.
Materials and Methods
Cell culture and reagents
Immortalized murine microglial cells (BV2) and UCP2 knockdown BV2 microglia (UCP2kd) were grown in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad CA USA) supplemented with 10% fetal bovine serum and 1% penicillin, streptomycin, and neomycin and maintained at 37°C with 5% CO2. Pan-FABP inhibitor (HTS01037; Cayman Chemical) was suspended in dimethyl sulfoxide (DMSO; Sigma Aldrich, St. Louis MO USA) and diluted to 30 μM in DMEM. Palmitic acid (PA; Sigma Aldrich) was conjugated to fatty acid free bovine serum (BSA; Sigma Aldrich) and diluted to 0.1 mM in DMEM. Cells were seeded in 6 well plates at 6 × 105 cells per well and grown to ~80% confluency. For gene expression studies, cells were pretreated with HTS01037 or vehicle control for 3 h and challenged with PA (or vehicle) for 12 h.
shRNA knockdown of UCP2 in microglia
BV2 microglia were transduced with a short hairpin RNA (shRNA) lentivirus as previously described (Curtis et al., 2010). Green fluorescent protein (GFP) scrambled and UCP2 targeting sequences were obtained from Open Biosystems (Pittsburgh, PA USA). The following were used UCP2 (GenBank accession number NM_011671) targeting sequence (UCP2 knockdown; UCP2kd) 5′-CCGGTCTCCCAATGTTGCCCGTAATCTCGAGATTACGGGCAACATTGGGAGATTTTTG-3′; scrambled sequence, 5′-AACGTACGCGGAATACTTCGA-3′. UCP2 expression knockdown is approximately 90% (Supplemental Fig 1).
Real-time RT PCR
Whole hypothalamic tissue was dissected from fifteen week old FABP4/aP2 knockout (also referred to as AKO) and wildtype (WT) mice maintained on 60% high fat diet (HFD) for 12 weeks (Hertzel et al., 2006; Kotz et al., 2012). Mice were obtained from our breeding colony and the experimental protocol was approved by the Institutional Animal Care and Use Committee at the University of Minnesota. Total RNA was extracted from microglia and hypothalamic tissue with the aid of Trizol (Invitrogen) (Butterick et al., 2012; Chomczynski, 1993). A final concentration of mRNA was determined spectrophotometerically (Nanodrop ND-8000; ThermoFisher Scientific, Waltham MA USA). Real-time thermal cycling were carried out in a Roche LightCycler (Roche Diagnostics Corporation, Indianapolis, IN USA) by one-step RT-PCR using the general method as previously described (Duffy et al., 2015). Target gene expression (Table 1) was determined using SYBR Green detection normalized to GAPDH using the Δ−ΔCT method (Livak and Schmittgen, 2001).
Table 1.
Real-time qPCR primer sequences
| Target | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| Arginase | TAACCTTGGCTTGCTTCGGAAC | TCTGTCTGCTTTGCTGTGATGC |
| Gapdh | GACATCAAGAAGGTGGTGAAGCAG | AAGGTGGAAGAATGGGAGTTGC |
| Iba1 | GTCCTTGAAGCGAATGCTGG | CATTCTCAAGATGGCAGATC |
| iNos | CCTACCAAAGTGACCTGAAAGAGG | TTCTGGAACATTCTGTGCTGTCCC |
| Tnf-α | AACACAAGATGCTGGGACAGTGAC | TGGAAAGGTCTGAAGGTAGGAAGGC |
| Ucp2 | TCGGAGATACCAGAGCACTGTCG | GCATTTCGGGCAACATTGG |
| Fabp4 | ATGAAATCACCGCAGACGACA | CATAAACTCTTGTGGAAGTCACGCC |
| Fabp7 | TGGCAAGATGGTCGTGACTC | CCAGTGCTTCATTAGCTGGC |
| Fabp5 | TCCCACCATGGCCAGTCTTA | ACCGTGATGTTGTTGCCATC |
Amplification products were separated via electrophoresis on 3% agarose gels stained with SYBR green. qRT-PCR products were purified using a commercially available kit (MinElute PCR Purification, Qiagen Valencia CA USA) and validated using Sanger di-deoxyterminator sequence method at the University of Minnesota Genomics Center.
Reactive oxygen species assay
Intracellular ROS production was determined using Deep Red Fluorescence kit (Abcam, Cambridge GBR) as previously described (Duffy et al., 2016). Briefly, cells were pretreated with HTS01037 or vehicle for 3 h and then challenged with or without PA for 1 h (time points based on (De Simone et al., 2015)). Cells were then exposed to the ROS Deep Red Dye for 1 h in 5% CO2 at 37°C. Intracellular superoxide and hydroxyl radicals react with the deep red dye, producing a fluorescent signal which was measured using a spectrophotometer at 650Ex/675Em (SpectraMax-M5; Molecular Devices, Sunnyvale CA USA). Data are presented as relative fluorescence units.
Statistical analysis
Significant differences were determined by either a one- or two-way ANOVA followed by Holm-Sidak’s correction for multiple comparisons using Graph Pad Prism 6 (GraphPad Software, Inc. La Jolla CA USA). Letters indicate significant differences between treatment groups (e.g. columns with the same letters do not differ from each other, while columns with different letters are significantly different).
Results
Microglial cells express FABP4 and FABP5, but not FABP7
To verify that microglial cells express FABP, real-time PCR products of FABP4 (Fig 1A), FABP5 (Fig 1B), and FABP7 (Fig 1C) were separated via electrophoresis and visualized on an agarose gel. Similar to macrophages, BV2 microglial cells express both FABP4 and FABP5, whereas in total brain lysates, FABP4, FABP5, and FABP7 are expressed.
Figure 1. Microglial cells express FABP4 and FABP5 but not FABP7. A.
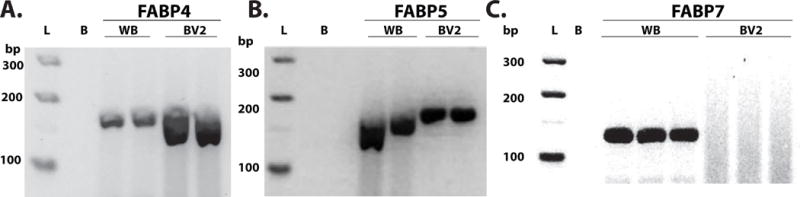
Fatty acid binding protein is expressed in both whole brain (WB) and microglial cells. A visualization of real-time PCR products on agarose gel electrophoresis to evaluate gene expression. Gene product of 174 bp was expected for FABP4. B. FABP5 is expressed in both WB and BV2 microglial cells. Gene product of 168 bp was expected for FABP5. C. FABP7 is expressed in WB but not BV2 microglial cells. Gene product of 131 bp was expected for FABP7. L, ladder, B, water blank.
Hypothalamic pro-inflammatory response is suppressed in mice lacking FABP4
In peripheral macrophages, lack of FABP4 contributes to upregulation of UCP2 (Xu et al., 2015). To determine if the absence of FABP4 leads to upregulated UCP2 in microglial cells, gene expression in hypothalamic tissue from FABP4 knockout (referred to as AKO) and WT mice was assessed. Similar to peripheral tissue, brain tissue from AKO mice have upregulated UCP2 expression (Fig 2A; p<0.05 vs. WT). In peripheral macrophages, loss of FABP4 and upregulation of UCP2 is correlated with decreased NFκB activity, a polarization switch from M1 to M2 and reduced expression of inflammatory markers. Likewise, UCP2 mediates microglial polarization (De Simone et al., 2015). Similar to the results in peripheral macrophages, hypothalamic tissues from wild type and AKO mice exhibited significantly reduced expression of Iba1, TNF-α, and iNOS compared to WT mice (Fig 2B–D; p<0.05).
Figure 2. Hypothalamic gene expression is altered in FABP4/aP2 knockout animals.
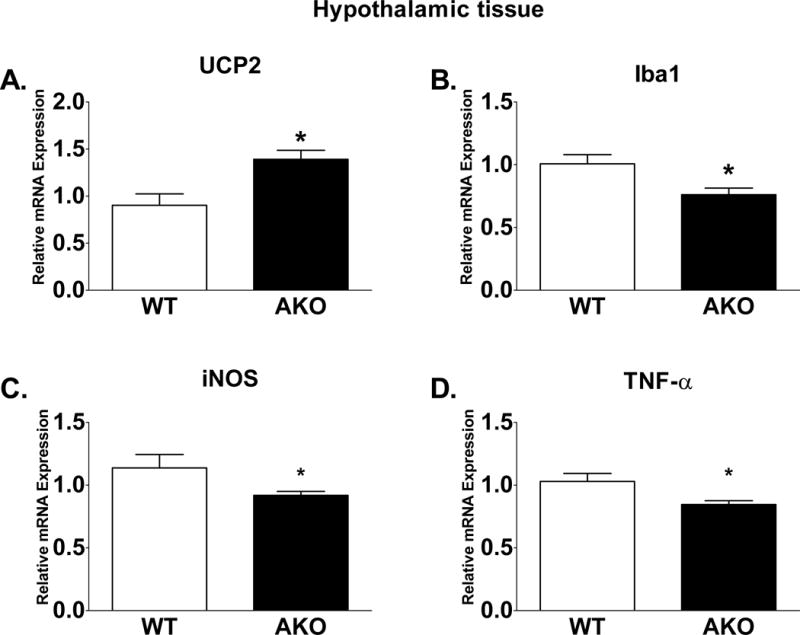
UCP2 and Iba-1 expression is upregulated in FABP4/aP2 knockout mice (A, D; p<0.05 vs. WT). Pro-inflammatory genes iNOS and TNF-α trend to be suppressed in FABP4/aP2 knockout mice (B–C). Letters indicate significant differences between treatment groups.
Inhibition of FABP leads to increased UCP2 expression in BV2 microglial cells
Prior reports demonstrate UCP2 expression is increased in macrophages treated with the FABP inhibitor HTS01037 (Xu et al., 2015). We tested whether the same was true in BV2 microglia when treated with HTS01037. As predicted, UCP2 expression was significantly increased following HTS01037 treatment in the presence or absence of PA (Fig 3; p<0.0001 C vs. HTS, p<0.001 C vs. HTS+PA, p<0.001 PA vs. HTS, 0.05 PA vs. HTS+PA). Furthermore, the expression of UCP2 was dependent upon the presence or absence of FABP4, as PA alone did not alter basal UCP2 expression (Fig 3).
Figure 3. FABP abundance regulates UCP2 expression in microglia.
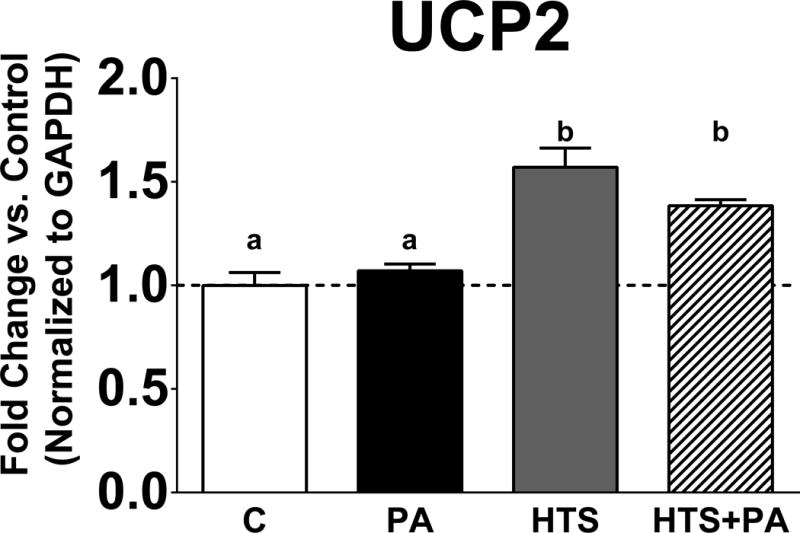
UCP2 expression is significantly upregulated following HTS01037 treatment in the presence or absence of PA in BV2 microglia. (p<0.0001 C vs. HTS, p<0.001 C vs. HTS+PA, p<0.001 PA vs. HTS, 0.05 PA vs. HTS+PA). Letters indicate significant differences between treatment groups.
UCP2 is necessary for microglial inflammatory response
To determine if UCP2 is necessary for mediating PA-induced inflammatory response, we assessed changes in gene expression of iNOS in BV2 versus UCP2kd microglia. Exposure to PA significantly increased iNOS expression in BV2 and UCP2kd microglia (Fig 4; p<0.001 PA vs. C). Inhibition of FABP attenuated PA-induced iNOS expression below basal levels in control cells, but iNOS was significantly increased in UCP2kd cells (Fig 4; p<0.001 PA vs. HTS, HTS+PA). Further, UCP2kd microglia treated with HTS01037 no longer exhibited attenuated PA-induced iNOS (Fig 4; p<0.05 C vs. HTS+PA, p<0.001 C vs. HTS, p<0.0001 C vs. PA, p<0.0001 PA vs. HTS, HTS+PA). To determine if UCP2 is necessary to mediate the anti-inflammatory microglial activation, we evaluated changes in arginase-1 expression in BV2 and UCP2kd microglia. Arginase-1 expression was significantly increased in BV2 microglia exposed to HTS01037 alone or in the presence of PA (Fig 5; p<0.0001 C vs HTS, p<0.0001 PA vs. HTS, p<0.05 PA vs. HTS+PA). Remarkably, arginase-1 expression was significantly reduced in UCP2kd microglia treated with PA alone, HTS01037 only, or HTS01037 plus PA (Fig 5; p<0.0001 C vs. HTS, PA, and HTS+PA).
Figure 4. UCP2 is needed to oppose PA-induced upregulation of pro-inflammatory marker iNOS.
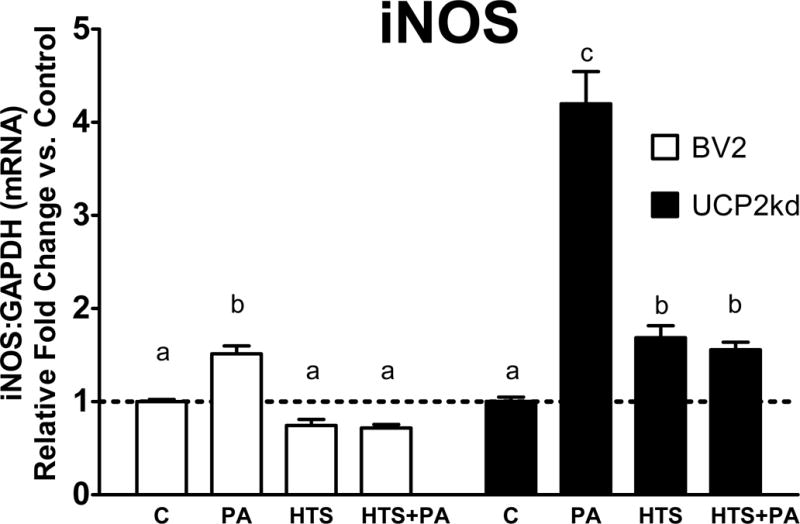
Pretreatment with HTS01037 attenuates PA-induced iNOS expression in BV2 microglia (p<0.001 PA vs. C, HTS, HTS+PA). In UCP2kd microglia, PA increases iNOS expression, however, HTS01037 is no longer able to attenuate PA-induced iNOS upregulation (p<0.05 C vs. HTS+PA, p<0.001 C vs. HTS, p<0.0001 C vs. PA, p<0.0001 PA vs. HTS, HTS+PA). Letters indicate significant differences between treatment groups.
Figure 5. UCP2 mediates anti-inflammatory marker arginase-1.
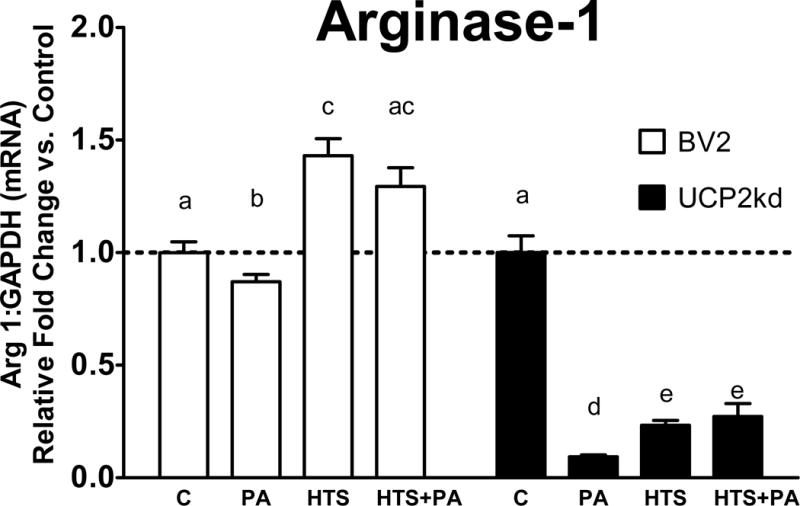
Pretreatment with HTS01037 in the presence or absence of PA robustly increases Arginase expression (p<0.0001 C vs HTS, p<0.0001 PA vs. HTS, p<0.05 PA vs. HTS+PA). In UCP2kd cells arginase-1 is no longer upregulated following HTS01037 treatment (p<0.0001 C vs. HTS, PA, and HTS+PA). Letters indicate significant differences between treatment groups.
UCP2 effect of ROS production in microglia
Next, we analyzed changes in production of ROS in BV2 and UCP2kd microglia. As predicted, we found that PA increased ROS production in BV2 cells (Fig 6; p<0.0001 PA vs. C), and that PA-induced ROS production was attenuated in BV2 cells when pretreated with the FABP inhibitor HTS01037 (Fig 6; p<0.001 vs. HTS and HTS+PA). In UCP2kd microglia, HTS01037 had no effect on ROS production, as PA significantly increased ROS production in all treatment conditions (Fig 6; p<0.0001 PA vs. C, p<0.001 HTS+PA vs. C).
Figure 6. UCP2 effect on ROS production in microglia.
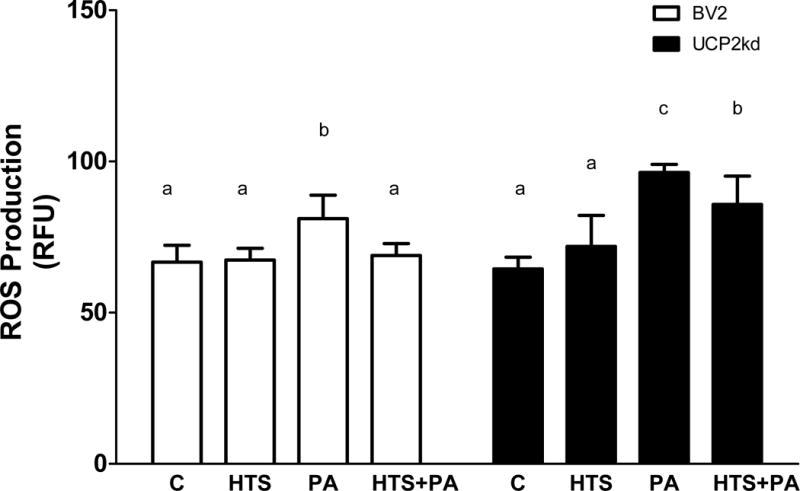
BV2 cells exposed to PA significantly increases ROS production (p<0.0001 PA vs. C), however treatment with HTS01037 attenuates PA induced ROS production (p<0.001 vs. HTS and HTS+PA). In UCP2kd cells, PA increases ROS in the presence or absence of HTS01037 (p<0.0001 PA vs. C, p<0.001 HTS+PA vs. C). Letters indicate significant differences between treatment groups.
Discussion
Obesity is often coupled with peripheral and central chronic low-grade inflammation (Businaro et al., 2012; Uranga et al., 2010). Prolonged overnutrition and excess intake of SFA increase the amount of fatty acids in the brain and induce neuroinflammation (Cai, 2013; Cai and Liu, 2011; Karmi et al., 2010; Thaler et al., 2012; Valdearcos et al., 2014). The onset of chronic neuroinflammation has identified as a major contributor to HFD-induced hypothalamic dysregulation (Valdearcos et al., 2014). In addition to influencing obesity risk, dietary fats, such as palmitic acid, have been linked to development of cognitive disorders such as Alzheimer’s disease (AD) through increase in neuroinflammation (Anstey et al., 2011; Businaro et al., 2012; Xu et al., 2011).
Microglia promote neuronal health in part via maintaining a favorable microenvironment throughout the central nervous system (Tremblay et al., 2010; Wake et al., 2009). Microglia, once thought to be passive support cells, are now appreciated to maintain neuronal-glial circuitry though removing damaged synapses and altering plasticity (Davalos et al., 2005; Nimmerjahn et al., 2005). As observed in obesity, microglial activation state shifts towards a chronic pro-inflammatory phenotype (Miller and Spencer, 2014; Tang et al., 2015; Valdearcos et al., 2014). In peripheral macrophages, FABP dynamics alters macrophage polarization states (Hotamisligil and Bernlohr, 2015; Xu et al., 2015). Here, we demonstrate microglia express FABP4 and FABP5 but not FABP7 (Fig 1), similar to macrophages. Likewise, we show that mice lacking FABP4 have attenuated hypothalamic HFD-induced pro-inflammatory response (Fig 2). These findings are comparable to other published reports of diet induced neuroinflammation (Thaler et al., 2012; Valdearcos et al., 2014). Others have demonstrated HFD induces M1 microglial activation, leading to impaired phagocytosis and contributing to worsening pathology of neurodegenerative diseases including AD (Graham et al., 2016). Although not tested in this study, it is likely that the microglia in the FABP4/aP2 knockout animals have increased phagocytic function and reduced neuronal stress. Future studies will examine the role of microglia and cognitive function in FABP4 knockout animals.
In peripheral macrophages, the loss or inhibition of FABP4 results in attenuated obesity-induced inflammatory response via a FABP4-UCP2 mediated axis (Xu et al., 2016; Xu et al., 2015). Here we demonstrate the novel FABP4-UCP2 axis in microglia mediating PA-induced inflammation. We show UCP2 expression is increased following inhibition of FABP4 (Fig 3). Additionally, we demonstrate that inhibition of FABP4 upregulates the anti-inflammatory marker arginase and attenuates PA-induced increase in iNOS (Fig 4). Notably, we demonstrate that when UCP2 is knocked down, inhibition of FABP4 no longer suppresses PA-induced iNOS expression and significantly reduces arginase expression (Fig 5). These findings demonstrate that UCP2 is necessary for mediating microglial polarization. The mechanistic relationship between iNOS and arginase 1 exists functionally at the substrate level, as both enzymes utilize L-arginine (Cherry et al., 2014; Rath et al., 2014). However, arginase 1 can effectively outcompete iNOS to downregulate production of nitric oxide, thus suppressing iNOS activity (Rath et al., 2014). Interestingly, L-arginine metabolism is also known to regulate inflammatory function of macrophages however this is unknown in microglia. In macrophages, increased iNOS function is associated with the pro-inflammatory M1 phenotype and is thought to be a part of the metabolic adaptive component of immune cell polarization critical to immunity and inflammation. Although this has been defined in macrophages, it remains unexplored in microglia.
While evidence indicates UCP2 has a role in neuroprotection and in the inflammatory response, the underlying mechanisms remain unresolved. Our data support that promotion of the M2 microglial activation phenotype could represent one such mechanism, resulting in reduced ROS production and inhibition of the toxic M1 phenotype. We demonstrate here that treatment with HTS01037 attenuates PA-induced ROS production in microglia, and this effect is negated when UCP2, a known suppressor of ROS production, is knocked down (Fig 6). To the best of our knowledge, we are the first to describe a FABP4-UCP2 axis regulating ROS and inflammation in microglia. Mitochondrial ROS production is a key driver in the pro-inflammatory response (Naik and Dixit, 2011). When UCP2 deficient macrophages are stimulated with lipopolysaccharide (LPS), ROS production and pro-inflammatory cytokine release is upregulated (Bai et al., 2005; Emre et al., 2007). In microglia, LPS induces the reduction of UCP2, causing mitochondrial depolarization and increased ROS production (De Simone et al., 2015). Moreover, UCP2-silenced microglia fail to transition to an M2 activation state (De Simone et al., 2015). Collectively these findings support that UCP2 is necessary for activation of the M2 protective microglial phenotype. These observations demonstrate UCP2 is an important regulator between the FABP-free fatty acids (FFA) equilibrium and inflammation, which to our knowledge has not been previously described.
FABP4 is expressed in multiple tissues including heart and kidney but the protein itself is only detectable in adipose tissue, peripheral macrophages and microglia (Furuhashi and Hotamisligil, 2008; Hotamisligil and Bernlohr, 2015). Besides FABP4, other FABPs are also expressed in the brain. FABP7 is necessary for brain development, and FABP5 (mal1) is essential in transporting fatty acids across the blood brain barrier (Furuhashi and Hotamisligil, 2008; Pan et al., 2015). Although knocking out FABP5 peripherally results in attenuated diet-induced obesity, the effects are not as robust as in FABP4 knockout models (Hotamisligil and Bernlohr, 2015). Despite the role of other FABPs in the brain, peripheral evidence indicates the lack of FABP4 alters the pool of free fatty acids, specifically, monounsaturated fatty acids (Xu et al., 2015). Monounsaturated fatty acids are ligands for receptors including peroxisome proliferator-activated receptor gamma (PPARγ), which opposes NFκB activity. Interestingly, PPARγ antagonists are neuroprotective in models of neurodegenerative diseases such as AD and PD, through a microglial-mediated mechanism (Garrido-Gil et al., 2012; Yamanaka et al., 2012). Although not tested in the present study, upregulation of PPARγ could be a potential pathway though which the FABP-FFA equilibrium regulates the anti-inflammatory state of microglia. In summary, these data support a role for FABP4-UCP2 axis as a regulator of microglial activation states. Understanding the role of FABP and UCP2 in modulating microglial activation state may provide therapeutic targets for prevention or treatment of neurodegenerative diseases including obesity.
Supplementary Material
Highlights.
FABP4 regulates UCP2 expression in microglia.
UCP2 opposes PA-induced upregulation of pro-inflammatory marker iNOS.
UCP2 mediates expression of the anti-inflammatory marker arginase-1.
Lack of hypothalamic FABP4 increases UCP2 and reduces microglial inflammation.
Acknowledgments
This work was funded by the US Department of Veterans Affairs BLR&D IK2 BX001686 (to TAB), the University of Minnesota Healthy Foods, Healthy Lives Institute (to CMD, JPN and TAB), the National Institutes of Health NIH R01 DK053189 (to DAB), and the Minnesota Nutrition and Obesity Center (NIH P30 DK050456). We also thank members of the Bernlohr lab for assistance with tissue collection.
Abbreviations
- (FABP)
Fatty acid binding protein
- (UCP2)
uncoupling protein 2
- (PA)
palmitic acid
- (ROS)
reactive oxygen species
- (iNOS)
inducible nitric oxide synthase
- (TNF-α)
tumor necrosis factor alpha
- (Iba1)
ionized calcium-binding adapter molecule 1
- (SFA)
saturated fatty acids
- (NFκB)
nuclear factor kappa B
References
- Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev. 2011;12:e426–437. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- Bai Y, Onuma H, Bai X, Medvedev AV, Misukonis M, Weinberg JB, Cao W, Robidoux J, Floering LM, Daniel KW, Collins S. Persistent nuclear factor-kappa B activation in Ucp2−/− mice leads to enhanced nitric oxide and inflammatory cytokine production. J Biol Chem. 2005;280:19062–19069. doi: 10.1074/jbc.M500566200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boord JB, Maeda K, Makowski L, Babaev VR, Fazio S, Linton MF, Hotamisligil GS. Combined adipocyte-macrophage fatty acid-binding protein deficiency improves metabolism, atherosclerosis, and survival in apolipoprotein E-deficient mice. Circulation. 2004;110:1492–1498. doi: 10.1161/01.CIR.0000141735.13202.B6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Businaro R, Ippoliti F, Ricci S, Canitano N, Fuso A. Alzheimer’s disease promotion by obesity: induced mechanisms-molecular links and perspectives. Current gerontology and geriatrics research. 2012;2012:986823. doi: 10.1155/2012/986823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterick TA, Nixon JP, Billington CJ, Kotz CM. Orexin A decreases lipid peroxidation and apoptosis in a novel hypothalamic cell model. Neurosci Lett. 2012;524:30–34. doi: 10.1016/j.neulet.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D. Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trends in endocrinology and metabolism: TEM. 2013;24:40–47. doi: 10.1016/j.tem.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Liu T. Hypothalamic inflammation: a double-edged sword to nutritional diseases. Annals of the New York Academy of Sciences. 2011;1243:E1–39. doi: 10.1111/j.1749-6632.2011.06388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JD, Olschowka JA, O’Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation. 2014;11:98. doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;15:532–534. 536–537. [PubMed] [Google Scholar]
- Curtis JM, Grimsrud PA, Wright WS, Xu X, Foncea RE, Graham DW, Brestoff JR, Wiczer BM, Ilkayeva O, Cianflone K, Muoio DE, Arriaga EA, Bernlohr DA. Downregulation of adipose glutathione S-transferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction. Diabetes. 2010;59:1132–1142. doi: 10.2337/db09-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nature neuroscience. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- De Simone R, Ajmone-Cat MA, Pandolfi M, Bernardo A, De Nuccio C, Minghetti L, Visentin S. The mitochondrial uncoupling protein-2 is a master regulator of both M1 and M2 microglial responses. J Neurochem. 2015;135:147–156. doi: 10.1111/jnc.13244. [DOI] [PubMed] [Google Scholar]
- Duffy CM, Nixon JP, Butterick TA. Orexin A attenuates palmitic acid-induced hypothalamic cell death. Mol Cell Neurosci. 2016;75:93–100. doi: 10.1016/j.mcn.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy CM, Yuan C, Wisdorf LE, Billington CJ, Kotz CM, Nixon JP, Butterick TA. Role of orexin A signaling in dietary palmitic acid-activated microglial cells. Neurosci Lett. 2015;606:140–144. doi: 10.1016/j.neulet.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre Y, Hurtaud C, Nubel T, Criscuolo F, Ricquier D, Cassard-Doulcier AM. Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein UCP2 in macrophages. Biochem J. 2007;402:271–278. doi: 10.1042/BJ20061430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre Y, Nubel T. Uncoupling protein UCP2: when mitochondrial activity meets immunity. FEBS Lett. 2010;584:1437–1442. doi: 10.1016/j.febslet.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Fumagalli M, Lecca D, Abbracchio MP. Role of purinergic signalling in neuro-immune cells and adult neural progenitors. Front Biosci (Landmark Ed) 2011;16:2326–2341. doi: 10.2741/3856. [DOI] [PubMed] [Google Scholar]
- Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov. 2008;7:489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi M, Tuncman G, Gorgun CZ, Makowski L, Atsumi G, Vaillancourt E, Kono K, Babaev VR, Fazio S, Linton MF, Sulsky R, Robl JA, Parker RA, Hotamisligil GS. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007;447:959–965. doi: 10.1038/nature05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Gil P, Joglar B, Rodriguez-Perez AI, Guerra MJ, Labandeira-Garcia JL. Involvement of PPAR-gamma in the neuroprotective and anti-inflammatory effects of angiotensin type 1 receptor inhibition: effects of the receptor antagonist telmisartan and receptor deletion in a mouse MPTP model of Parkinson’s disease. J Neuroinflammation. 2012;9:38. doi: 10.1186/1742-2094-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham LC, Harder JM, Soto I, de Vries WN, John SW, Howell GR. Chronic consumption of a western diet induces robust glial activation in aging mice and in a mouse model of Alzheimer’s disease. Sci Rep. 2016;6:21568. doi: 10.1038/srep21568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzel AV, Smith LA, Berg AH, Cline GW, Shulman GI, Scherer PE, Bernlohr DA. Lipid metabolism and adipokine levels in fatty acid-binding protein null and transgenic mice. Am J Physiol Endocrinol Metab. 2006;290:E814–823. doi: 10.1152/ajpendo.00465.2005. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Bernlohr DA. Metabolic functions of FABPs–mechanisms and therapeutic implications. Nat Rev Endocrinol. 2015;11:592–605. doi: 10.1038/nrendo.2015.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Johnson RS, Distel RJ, Ellis R, Papaioannou VE, Spiegelman BM. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science (New York, NY. 1996;274:1377–1379. doi: 10.1126/science.274.5291.1377. [DOI] [PubMed] [Google Scholar]
- Karmi A, Iozzo P, Viljanen A, Hirvonen J, Fielding BA, Virtanen K, Oikonen V, Kemppainen J, Viljanen T, Guiducci L, Haaparanta-Solin M, Nagren K, Solin O, Nuutila P. Increased brain fatty acid uptake in metabolic syndrome. Diabetes. 2010;59:2171–2177. doi: 10.2337/db09-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz C, Nixon J, Butterick T, Perez-Leighton C, Teske J, Billington C. Brain orexin promotes obesity resistance. Annals of the New York Academy of Sciences. 2012;1264:72–86. doi: 10.1111/j.1749-6632.2012.06585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Makowski L, Boord JB, Maeda K, Babaev VR, Uysal KT, Morgan MA, Parker RA, Suttles J, Fazio S, Hotamisligil GS, Linton MF. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med. 2001;7:699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AA, Spencer SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun. 2014;42:10–21. doi: 10.1016/j.bbi.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. The Journal of experimental medicine. 2011;208:417–420. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science (New York, NY) 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Pan Y, Scanlon MJ, Owada Y, Yamamoto Y, Porter CJ, Nicolazzo JA. Fatty Acid-Binding Protein 5 Facilitates the Blood-Brain Barrier Transport of Docosahexaenoic Acid. Mol Pharm. 2015;12:4375–4385. doi: 10.1021/acs.molpharmaceut.5b00580. [DOI] [PubMed] [Google Scholar]
- Rath M, Muller I, Kropf P, Closs EI, Munder M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front Immunol. 2014;5:532. doi: 10.3389/fimmu.2014.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum BO, Mackay CR, Gorgun CZ, Frost MJ, Kumar RK, Hotamisligil GS, Rolph MS. The adipocyte fatty acid-binding protein aP2 is required in allergic airway inflammation. The Journal of clinical investigation. 2006;116:2183–2192. doi: 10.1172/JCI24767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Purkayastha S, Cai D. Hypothalamic microinflammation: a common basis of metabolic syndrome and aging. Trends Neurosci. 2015;38:36–44. doi: 10.1016/j.tins.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JP, Guyenet SJ, Dorfman MD, Wisse BE, Schwartz MW. Hypothalamic inflammation: marker or mechanism of obesity pathogenesis? Diabetes. 2013;62:2629–2634. doi: 10.2337/db12-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschop MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. The Journal of clinical investigation. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS biology. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uranga RM, Bruce-Keller AJ, Morrison CD, Fernandez-Kim SO, Ebenezer PJ, Zhang L, Dasuri K, Keller JN. Intersection between metabolic dysfunction, high fat diet consumption, and brain aging. J Neurochem. 2010;114:344–361. doi: 10.1111/j.1471-4159.2010.06803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell reports. 2014;9:2124–2138. doi: 10.1016/j.celrep.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Liu D, Wang F, Liu S, Zhao S, Ling EA, Hao A. Saturated fatty acids activate microglia via Toll-like receptor 4/NF-kappaB signalling. Br J Nutr. 2012;107:229–241. doi: 10.1017/S0007114511002868. [DOI] [PubMed] [Google Scholar]
- Xu H, Hertzel AV, Steen KA, Bernlohr DA. Loss of Fatty Acid Binding Protein 4/aP2 Reduces Macrophage Inflammation Through Activation of SIRT3. Molecular endocrinology. 2016;30:325–334. doi: 10.1210/me.2015-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Hertzel AV, Steen KA, Wang Q, Suttles J, Bernlohr DA. Uncoupling lipid metabolism from inflammation through fatty acid binding protein-dependent expression of UCP2. Molecular and cellular biology. 2015;35:1055–1065. doi: 10.1128/MCB.01122-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology. 2011;76:1568–1574. doi: 10.1212/WNL.0b013e3182190d09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka M, Ishikawa T, Griep A, Axt D, Kummer MP, Heneka MT. PPARgamma/RXRalpha-induced and CD36-mediated microglial amyloid-beta phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. J Neurosci. 2012;32:17321–17331. doi: 10.1523/JNEUROSCI.1569-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


