Abstract
Palmitic acid (PA), an abundant dietary saturated fatty acid, contributes to obesity and hypothalamic dysregulation in part through increase in oxidative stress, insulin resistance, and neuroinflammation. Increased production of reactive oxygen species (ROS) as a result of PA exposure contributes to the onset of neuronal apoptosis. Additionally, high fat diets lead to changes in hypothalamic gene expression profiles including suppression of the anti-apoptotic protein B cell lymphoma 2 (Bcl-2) and upregulation of the pro-apoptotic protein B cell lymphoma 2 associated X protein (Bax). Orexin A (OXA), a hypothalamic peptide important in obesity resistance, also contributes to neuroprotection. Prior studies have demonstrated that OXA attenuates oxidative stress induced cell death. We hypothesized that OXA would be neuroprotective against PA induced cell death. To test this, we treated an immortalized hypothalamic cell line (designated mHypoA-1/2) with OXA and PA. We demonstrate that OXA attenuates PA-induced hypothalamic cell death via reduced caspase-3/7 apoptosis, stabilization of Bcl-2 gene expression, and reduced Bax/Bcl-2 gene expression ratio. We also found that OXA inhibits ROS production after PA exposure. Finally, we show that PA exposure in mHypoA-1/2 cells significantly reduces basal respiration, maximum respiration, ATP production, and reserve capacity. However, OXA treatment reverses PA-induced changes in intracellular metabolism, increasing basal respiration, maximum respiration, ATP production, and reserve capacity. Collectively, these results support that OXA protects against PA-induced hypothalamic dysregulation, and may represent one mechanism through which OXA can ameliorate effects of obesogenic diet on brain health.
Keywords: Neurodegeneration, Neuroprotection, Palmitic acid, Hypocretin, Apoptosis, Reactive oxygen species
Graphical abstract
Introduction
Diets rich in the saturated fatty acid palmitic acid (PA) promote hypothalamic dysregulation and contribute to obesity through disruption of appetite regulating signals (Benoit et al., 2009; Kleinridders et al., 2009). Mechanisms triggered by PA exposure include increased oxidative stress, insulin resistance, release of pro-inflammatory cytokines, and apoptosis (Benoit et al., 2009; Moraes et al., 2009; Thaler et al., 2012; Zhang et al., 2008). In the hypothalamus, diet-induced obesity also alters genes regulating the apoptotic pathway. For example, hypothalamic expression of anti-apoptotic protein B cell lymphoma 2 (Bcl-2) is decreased, while expression of the pro-apoptotic protein B cell lymphoma 2 associated X protein (Bax) is upregulated (Moraes et al., 2009). These gene expression changes are linked to obesity pathogenesis. High fat diets (HFD)also contribute to the overproduction of reactive oxygen species (ROS) in the brain, resulting in increased oxidative stress and cell damage (Pipatpiboon et al., 2013; Pipatpiboon et al., 2012; Zhang et al., 2005). Because production of ROS is a crucial signal resulting in cell death, oxidative stress and apoptosis are closely related pathways. Further, ROS suppresses neuronal anti-apoptotic proteins including Bcl-2 (Pugazhenthi et al., 2003).
Because dietary components such as PA can directly and negatively affect brain health, it is likely that protection against obesity involves neural mechanisms capable of ameliorating diet effects on central nervous system signaling. One potential candidate are the orexins (hypocretins), hypothalamic peptides important in maintaining energy metabolism, sleep/wake cycles, and promoting obesity resistance (Butterick et al., 2013; Kotz et al., 2012). Orexin A (OXA) and orexin B (OXB) act through two G-protein coupled receptors (orexin receptors 1 and 2; OX1R and OX2R, respectively) to alter intracellular metabolic functions (Harada et al., 2011; Sellayah et al., 2011; Sikder and Kodadek, 2007) and promote cell survival against oxidative stress and ischemic events (Butterick et al., 2012; Yuan et al., 2011) in part through activation of Akt (protein kinase B) (Sokolowska et al., 2014). Here we focus on elucidating neuroprotective pathways through which orexin alters brain response to PA, a major component of obesogenic diets.
Work from our lab has demonstrated that OXA attenuates caspase-3/7 mediated apoptosis in response to H2O2, an initiator of oxidative stress (Butterick et al., 2012). Because HFDs are known to induce oxidative stress and neuronal cell death, (Moraes et al., 2009; Wu et al., 2004) we sought to determine whether OXA protects against PA-induced cell death. To test this, we evaluated the response of an immortalized murine hypothalamic cell line (designated mHypoA-1/2) (Belsham et al., 2009) to OXA. We demonstrate here that OXA attenuates PA-induced apoptosis via reducing caspase-3/7 activity, increasing Akt activation, stabilizing expression of the pro-survival gene Bcl-2, and inhibiting ROS production. Finally, we show that OXA alters intracellular metabolic function in mHypoA-1/2 cells in real-time via increased basal respiration, maximum respiration, ATP production, and reserve capacity.
Methods
Cell culture and reagents
Differentiated immortalized adult mouse hypothalamic (mHypoA-1/2, cited elsewhere as CLU172;CELLutions-Cedarlane, Burlington ON CAN) (Belsham et al., 2009; Guo and Feng, 2012) were maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad CA USA) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic cocktail (penicillin, streptomycin and neomycin) at 37°C with 5% CO2. The mHypoA-1/2 cells do not express endogenous orexin (Belsham et al., 2004), independently confirmed by our laboratory (data not shown). Orexin A peptide (ThermoFisher Scientific, Waltham MA USA) was suspended in phosphate buffered saline (PBS; Invitrogen) and diluted to a final concentration of 300 nM in DMEM. Palmitic acid (Sigma-Aldrich, St Louis, MO USA) was suspended in dimethyl sulfoxide (DMSO) and diluted to a final concentration 0.1 mM in DMEM. Dual orexin receptor antagonist (DORA; TCS 1102; Tocris Bioscience, Avonmouth GBR) was reconstituted in DMSO and diluted to a final concentration of 1.16 nM in DMEM (Callander et al., 2013). The dual orexin receptor antagonist used in this study binds similarly to both OX1R and OX2R rapidly with high affinity (Callander et al., 2013).
Treatments
In general for treatment assays, cells underwent two steps: First, a 24 h pretreatment with a test substance (e.g. orexin or vehicle); and second, a 2 to 24 h challenge with palmitic acid or vehicle, with or without the test substance used for 24 h pretreatment. Specifically, for cell viability assays, neurons were treated with DORA or DMSO vehicle for 20 minutes to block orexin receptors, subjected to a 24 h treatment with OXA or PBS vehicle, and finally challenged with vehicle (PBS+DMSO), PA, DORA, or OXA for 24 h. For the caspase-3/7, ROS, and gene expression experiments, cells underwent 24 h pretreatment with OXA or PBS vehicle, followed by 2 h challenge with PA or DMSO vehicle in the presence of OXA or PBS vehicle. For the mitochondrial respiration assays without PA challenge, cells were exposed to vehicle (PBS+DMSO), OXA (50, 150, or 300 nM) or DORA for 2 h. For the mitochondrial assay with PA challenge, cells were first pretreated with OXA (300 nM) or vehicle control (PBS) for 24 h followed by a 6 h challenge with vehicle (PBS+DMSO), PA, or OXA. All concentrations and time points were based on previous studies (Butterick et al., 2014; Butterick et al., 2012; Callander et al., 2013; Xu et al., 2015).
Cell viability assay
Cell survival was determined using a resazurin-based assay (Presto Blue, Invitrogen) as previously described (Butterick et al., 2012). Viable cells produce a fluorescent signal (560EX/590EM) as determined using a spectrophotometer (SpectraMax-M5; Molecular Devices, Sunnyvale CA USA). Data are reported as percent relative fluorescence units (RFU) change vs. control.
Caspase Activity
Caspase 3/7 activity was determined as previously described (Butterick et al., 2014; Butterick et al., 2012). Briefly, caspase-3/7 activity was determined by the addition of a luminogenic caspase substrate DEVD based assay (Caspase-Glo 3/7, Promega, Madison WI USA). Changes in relative luminance units (RLU; 650 nm) were analyzed using a microplate spectrometer reader (SpectraMax-M5). Data are presented as fold increase in caspase activity normalized to cell number.
Reactive Oxygen Species assay
Intracellular ROS (superoxide and hydroxyl radical) production was determined using a commercially available deep red fluorescence kit following manufacturer's protocol (Abcam, Cambridge GBR). Cells were pretreated with OXA (or vehicle) for 24 h,then challenged with PA (or vehicle) plus OXA (or vehicle) for 2 h. During the last hour of challenge, the cell-permeable deep red dye was added to cells and allowed to incubate at 37°C and 5% CO2. Intracellular superoxide and hydroxyl radicals oxidize the deep red dye producing a fluorescent signal (Gliyazova et al., 2013). Fluorescence was measured at 650EX/675EM using a spectrophotometer (SpectraMax-M5). Data are presented as fold change vs. control.
Real time RT-PCR
Total RNA was extracted from cultured mHypoA-1/2 cells with the aid of Trizol reagent (Invitrogen). A final concentration of mRNA (100 ng/μl) was determined using 260 and 280 nm readings on a spectrophotometer (Nanodrop ND-8000; ThermoFisher). Primers were designed using MacVector 12 (MacVector Inc, Cary NC USA) and sequences are listed in table 1. Relative expression of target genes was determined with SYBR Green using the 2-ΔΔCT method, normalized to GAPDH (Livak and Schmittgen, 2001).
Table 1. Real-time qPCR primer sequences.
| Target and accession number | Forward primer (5ʹ to 3ʹ) | Reverse primer (5ʹ to 3ʹ) |
|---|---|---|
| Bcl-2 (NM_177410.2) | CACCGCGAGGGGACGCTTTG | AGGTCGCATGCTGGGGCCATA |
| Bax(NM_007527.3) | GCTGAGCGAGTGTCTCCGGC | ACGCGGCCCCAGTTGAAGTT |
| GAPDH (NM_017008) | GACATCAAGAAGGTGGTGAAGCAG | AAGGTGGAAGAGTGGGAGTTGC |
Cell-based enzyme-linked immunosorbent assay (ELISA)
Changes in Akt activation were determined using a commercially available kit (R&D Systems, Minneapolis, MN). Briefly, cells were pretreated with OXA (or vehicle) for 24 h and challenged with PA (or vehicle) and an additional dose of OXA (or vehicle) for 1 h. Time point was based on Sokolowska et al 2014 (Sokolowska et al., 2014). Cells were fixed with 4% formaldehyde, blocked, and incubated with primary antibodies (anti-phospho-Akt (S473) and anti-total Akt) overnight. Unbound primary antibodies were washed away and secondary antibodies (HRP-conjugated or AP-conjugated) were added. Unbound secondary antibodies were washed away and fluorogenic substrates were added. Fluorescence was measured on a spectrophotometer (540EX/600EM and 360EX/450EM). Data are reported as the ratio of phospho-Akt normalized to total Akt in each well.
Mitochondrial respiration assay
Mitochondrial function was assessed using either the XF24 or XFe96 extracellular flux analyzer (Seahorse Biosciences, North Billerica MA USA). Cell number and inhibitor titration experiments were performed for optimal response based on manufacturer instructions. For dose response assay, mHypoA-1/2 cells were plated on V7 microplates (Seahorse Biosciences) at 2×104 cells per well in DMEM supplemented with 10% FBS and 1% PSN and incubated overnight at 37°C in 5% CO2. Cells were treated with increasing concentrations of OXA (50, 150, 300 nM) or vehicle for 2 h (n=4-5/treatment group). Following incubation, DMEM was removed and replaced with XF Assay media (Seahorse Biosciences, supplemented with pyruvate (1 mM), L-glutamine (2 mM), and glucose (10 mM) containing respective treatment groups described above (Yao et al., 2009). During the assay, cells were treated with compounds in the following order: oligomycin (2 μM; Sigma) to determine mitochondrial ATP production, an uncoupler FCCP (carbonyl cyanide-4-phenylhydrazone; 1.2 μM; Sigma) to determine the maximal respiration, and antimycin A (4 μM; Sigma) to determine the spare capacity.
For DORA studies, cells were seeded at a density of 1×104 cells/well on XF96 microwell plates and incubated overnight at 37°C in 5% CO2. Cells were treated with either vehicle control (PBS and DMSO), OXA only (300 nM), DORA only (1.16 nM), or DORA plus OXA for 2 h (n=10-12/treatment group). Following incubation, DMEM was removed and replaced with XF Assay media (supplemented with pyruvate, L-glutamine, and glucose) containing respective treatment groups described above. During the assay, cells were treated with oligomycin (1 μM; Seahorse Biosciences), FCCP (0.5 μM; Seahorse Biosciences), and antimycin A (4 μM; Seahorse Biosciences).
For OXA and PA studies, cells were seeded at a density of 2×103 cells/wellon XF96 microwell plates and incubated overnight at 37°C in 5% CO2. Cells were then pretreated with vehicle (PBS) or OXA (300 nM) for 24 h and challenged with PA (or vehicle) and an additional dose of OXA (or vehicle) for 6 h (n=10-12/treatment). Time points based were on (Xu et al., 2016). Following incubation, DMEM was removed and replaced with XF Assay media as described above. During the assay cells were exposed to compounds in the following order oligomycin (1 μM; Seahorse Biosciences), FCCP (0.5 μM; Seahorse Biosciences), and antimycin A (4 μM; Seahorse Biosciences).
The oxygen consumption rate (OCR) was automatically calculated and recorded by the Seahorse XFe software, respiration rates were manually calculated and normalized to cell number as described by Brand and Nicholls (Brand and Nicholls, 2011). The non-mitochondrial respiration rate was calculated as the minimum rate following antimycin A injection. Basal respiration was determined as the last rate measurement before oligomycin injection minus the non-mitochondrial respiration rate. ATP turnover was calculated as the last rate measurement before oligomycin injection minus the minimum rate measurement after oligomycin injection. Maximum respiration was determined by subtracting non-mitochondrial respiration from maximum rate measurement following FCCP injection. The reserve capacity was determined by subtracting the basal respiration from the maximal respiration.
Statistical methods
Statistical differences were determined using a one-way ANOVA followed by Tukey's post hoc test, using GraphPad Prism 5 (GraphPad Software, San Diego CA USA). Letters indicate significant differences between treatment groups (e.g. columns with the same letters do not differ from each other, while columns with different letters are significantly different).
Results
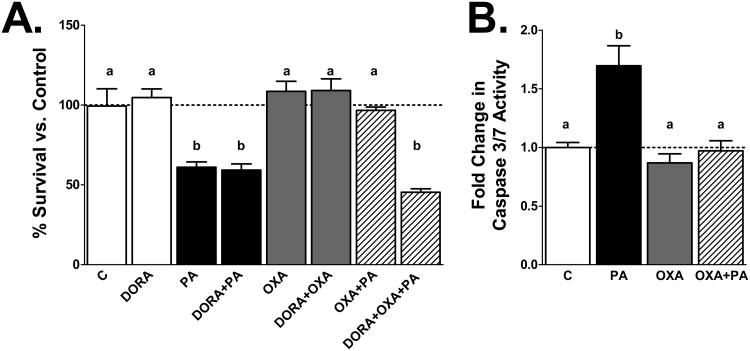
Figure 1: OXA attenuates PA induced cell death
Figure 1. Orexin A attenuates PA-induced hypothalamic cell death.
A) mHypoA-1/2 cells were pretreated with OXA for 24 h and then incubated with PA in the presence or absence of OXA or DORA for an additional 24 h. PA significantly reduces cell viability (p<0.001 vs. vehicle control; C). OXA attenuates PA induced cell death (p<0.05 vs. OXA only and OXA+PA). DORA negates OXA induced cell survival (p<0.001 vs. OXA only and OXA plus PA). B) PA significantly increases caspase-3/7 activity (p<0.0001 vs. C). OXA reduces caspase 3/7 induced apoptosis (p<0.0001 vs. OXA only, p<0.001 vs. OXA+PA).
To determine whether OXA inhibits PA-induced neuronal cell death, mHypoA-1/2 cells were pretreated with or without OXA (300 nM) for 24 h, and then exposed to PA in the presence or absence of OXA for an additional 24 h. As expected, PA significantly reduced cell survival (Fig 1A; p<0.001 vs. vehicle control; C). Conversely, OXA pretreatment attenuated PA-induced cell death (Fig 1A; p<0.05 vs. OXA only and OXA+PA). Further, treating the cells in the presence of DORA negates the effects of OXA, preventing attenuation of PA-induced cell death (Fig 1A; p<0.001 vs. OXA only and OXA+PA). To determine if cell death occurred via apoptosis, caspase-3/7 activity was measured. Hypothalamic neurons were pretreated with or without OXA for 24 h, and then challenged with PA in the presence or absence of OXA for an additional 2 h. As predicted, PA significantly increased caspase-3/7 activity (Fig 1B; p<0.0001 vs. C). However, OXA attenuated PA-induced caspase-3/7 activity (Fig 1B; p<0.0001 vs. OXA only, p<0.001 vs. OXA+PA).
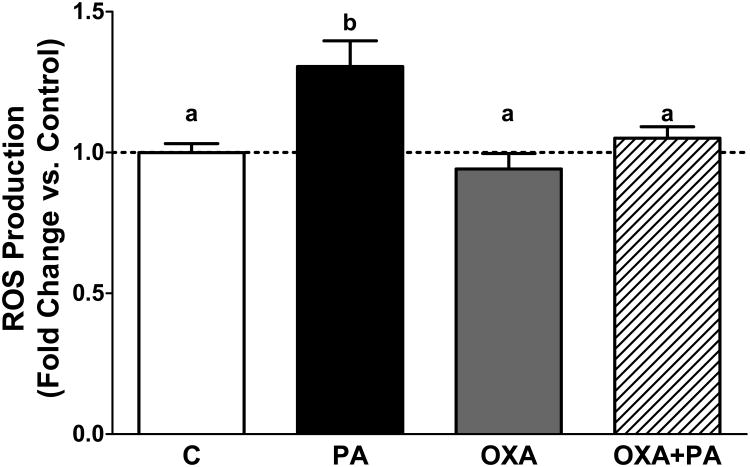
Figure 2. Orexin A reduces reactive oxygen species in mHypoA-1/2 cells
Figure 2. Orexin A reduces reactive oxygen species in mHypoA-1/2 cells.
mHypoA-1/2 cells were pretreated with OXA for 24 h and then incubated with PA in the presence or absence of OXA for an additional 2 h. ROS is significantly increased following 2 h PA exposure (p<0.05 vs. C). OXA treatment attenuates PA-induced ROS production (p<0.001 vs. OXA only, p<0.05 vs. OXA+PA).
Prior data indicate OXA is protective against oxidative stress (Bulbul et al., 2008; Butterick et al., 2012; Sokolowska et al., 2014). To determine if OXA protects against PA-induced ROS production, mHypoA-1/2 cells were pretreated with OXA for 24 h, and then challenged with or without PA for an additional 2 h. As expected, PA significantly increased ROS production compared to control (Fig 2; p<0.05). Further, in the presence of OXA, PA-induced ROS production was attenuated (Fig 2; p<0.05 PA vs. OXA+PA).
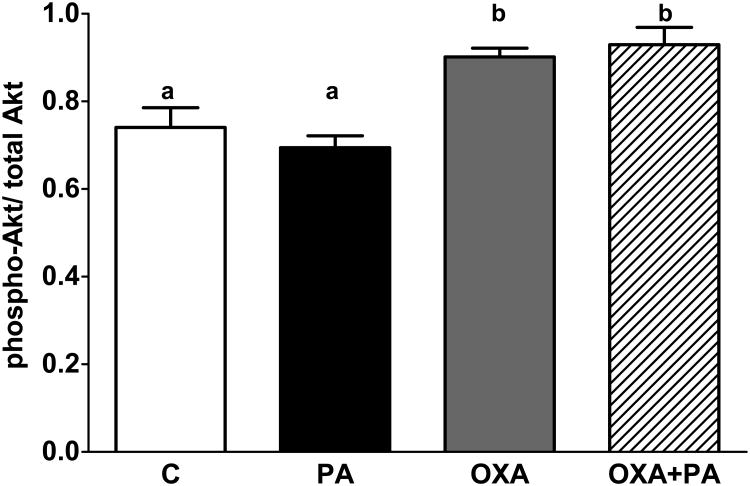
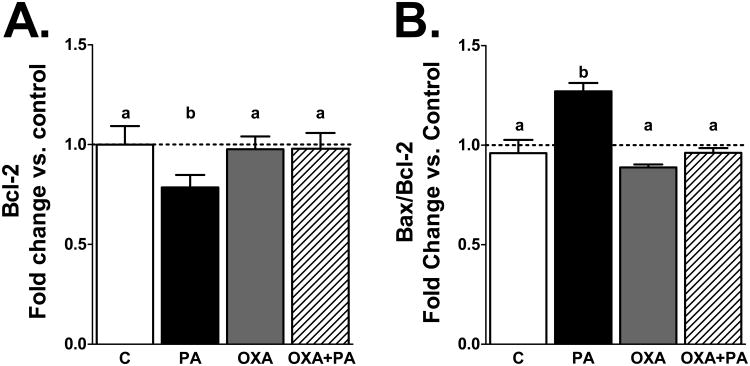
Figure 3-4: OXA increases Akt activation and stabilizes expression of anti-apoptotic gene Bcl-2
Figure 3. Orexin A increases Akt phosphorylation.
mHypo-A1/2 cells were pretreated with OXA for 24 h and then exposed to PA in the presence or absence of OXA for an additional 1 h. Phosphorylated Akt is significantly increased following exposure to OXA in the presence or absence of PA (p<0.05 vs. C, p<0.001 vs. PA).
Figure 4. OXA stabilizes expression of anti-apoptotic gene Bcl-2.
mHypoA-1/2 cells were pretreated with OXA for 24 h and then challenged with PA in the presence or absence of OXA for an additional 2 h. A) PA significantly reduces Bcl-2 expression (p<0.05 vs. C). OXA stabilizes Bcl-2 expression (p<0.05 vs. OXA only and OXA+PA). B) The Bax/Bcl-2 ratio is significantly increased following PA challenge (p<0.05 vs. C). However, OXA stabilizes Bax/Bcl-2 ratio (p<0.001 vs. OXA only, p<0.05 vs. OXA+PA).
Prior reports indicate OXA activates Akt phosphorylation (Sokolowska et al., 2014). Likewise, we demonstrate OXA significantly increases Akt phosphorylation with or without PA exposure (Fig 3; p<0.05 vs. C, p<0.001 vs. PA). To determine if apoptotic gene expression is altered following exposure to PA and OXA, changes in the anti-apoptotic gene Bcl-2 and pro-apoptotic gene Bax were assessed. Following 2 h PA exposure, Bcl-2 expression was significantly reduced (Fig 4A; p<0.05 vs. C). Cells treated with OXA in the presence or absence of PA had stabilized Bcl-2 gene expression compared to those treated with PA only (Fig 4A; p<0.05 vs. OXA only and OXA plus PA). The ratio of Bax/Bcl-2 expression was significantly upregulated in mHypoA-1/2 cells treated with PA only (Fig 4B; p<0.05 vs. C). In contrast, cells treated with OXA or OXA plus PA had stabilized Bax/Bcl-2 ratios (Fig 4B; p<0.001 OXA vs. PA, p<0.05 OXA+PA vs. PA). Additionally, orexin receptors (OX1R and OX2R) gene expression in mHypoA-1/2 cells is unchanged following acute 2 h PA exposure (supplemental Fig 1A-B).
Figure 5-7. OXA alters metabolic respiration
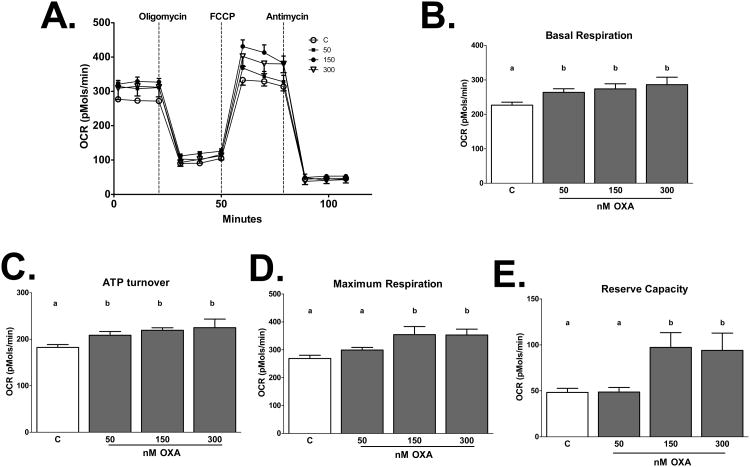
Figure 5. OXA alters metabolic respiration in a dose dependent manner.
Oxygen consumption rates (OCRs) were determined in mHypoA-1/2 cells treated with increasing concentrations of OXA (50, 150, 300 nM) for 2 h. OXA increases B) basal respiration (p<0.05 vs. C), C) ATP turnover (p<0.05 50, 300 nM OXA vs. C, p<0.001 150 nM OXA vs. C), D) maximum respiration (p<0.05 150, 300 nM OXA vs. C), and E) reserve capacity (p<0.05 150, 300 nM OXA vs. C).
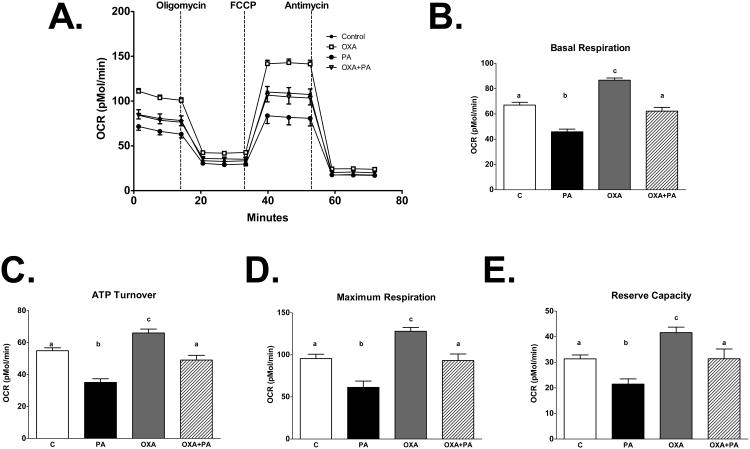
Figure 7. Orexin stabilizes metabolic respiration.
Oxygen consumption rates (OCRs) were determined in mHypoA-1/2 cells pretreated with OXA (300 nM) for 24 h and challenged with PA in the presence or absence of OXA for 6 h. PA significantly reduces B) basal respiration (p<0.0001 vs. C, OXA, OXA+PA), C)ATP turnover (p<0.0001 vs. C, OXA, p<0.001 vs. OXA+PA), D) maximum respiration (p<0.0001 vs. OXA, p<0.001 vs. C, OXA+PA), and E) reserve capacity (p<0.05 vs. C, OXA+PA, p<0.0001 vs. OXA). Treatment with OXA stabilizes PA-reduced B) basal respiration, C) ATP turnover, D) maximum respiration, and E) reserve capacity.
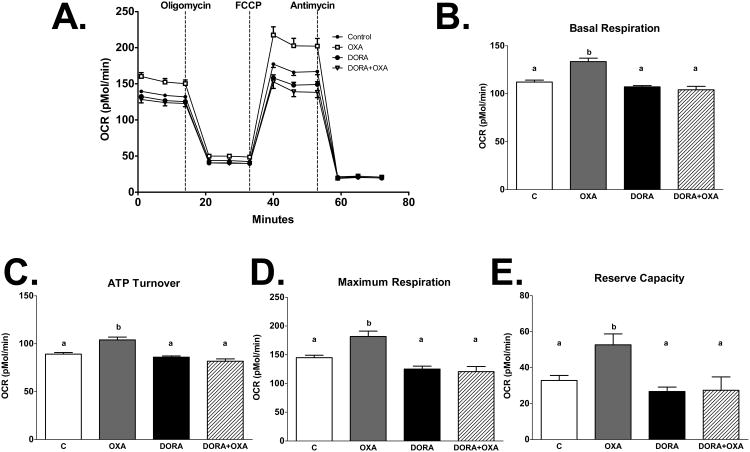
To determine the effects of OXA on metabolic respiration, mHypoA-1/2 cells were treated with increasing concentrations of OXA (50, 150, and 300 nM). We show that OXA increased basal respiration (p<0.05 vs. C), ATP turnover (p<0.05 50, 300 nM OXA vs. C, p<0.001 150 nM OXA vs. C) maximum respiration (p<0.05 150, 300 nM OXA vs. C), and reserve capacity (p<0.05 150, 300 nM OXA vs. C) in a dose-dependent manner (Fig. 5A-E). This effect is directly mediated by orexin receptors, as treatment with DORA attenuated OXA-increased basal respiration (p<0.0001 DORA, DORA+OXA vs. OXA), ATP turnover (p<0.0001 DORA, DORA+OXA vs. OXA), maximum respiration (p<0.0001 DORA, DORA+OXA vs. OXA), and reserve capacity (p<0.0001 DORA vs. OXA, p<0.001 DORA+OXA vs. OXA; Fig. 6A-E). We next sought to determine if PA alters metabolic respiration and if OXA treatment would negate this effect. We demonstrate PA significantly reduced basal respiration (p<0.0001 vs. C, OXA, OXA+PA), ATP turnover (p<0.0001 vs. C, OXA, p<0.001 vs. OXA+PA), maximum respiration (p<0.0001 vs. OXA, p<0.001 vs. C, OXA+PA), and reserve capacity (p<0.05 vs. C, OXA+PA, p<0.0001 vs. OXA) (Fig 7A-E). Importantly, OXA attenuated PA-induced reduction in metabolic respiration (Fig 7A-E).
Figure 6. Orexin induced metabolic respiration is directly mediated by orexin receptors.
Oxygen consumption rates (OCRs) were determined in mHypoA-1/2 cells treated with OXA (300 nM) and/or DORA (1.16 nM) for 2 h. OXA increases B) basal respiration (p<0.0001 vs. C), C) ATP turnover (p>0.001 vs. C), D) maximum respiration (p<0.0001 vs. C), and E) reserve capacity (p<0.05 vs. C). Treatment with DORA attenuates OXA-increased B) basal respiration (p<0.0001 vs. OXA), C) ATP turnover (p<0.0001 vs. OXA), D) maximum respiration (p<0.0001 vs. OXA), and E) reserve capacity (p<0.0001 DORA vs. OXA, p<0.001 OXA+DORA vs. OXA).
Discussion
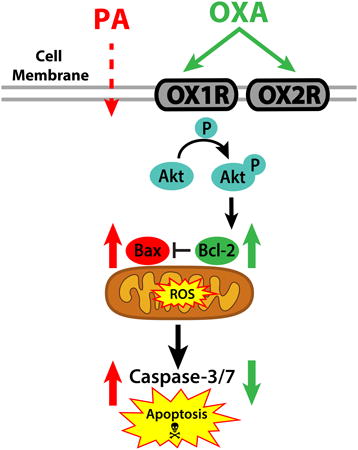
The hypothalamus is vital in sensing changes in fuel availability and maintaining energy balance. High fat diets contribute to hypothalamic dysregulation and obesity in part via increased inflammation, oxidative stress, and apoptosis (Duffy et al., 2015; Moraes et al., 2009; Valdearcos et al., 2014; Zhang et al., 2008). Therefore, identifying mechanisms to prevent HFD-induced hypothalamic dysregulation is of major importance to treating obesity. Data support that OXA is a mediator of obesity resistance through increased spontaneous physical activity (Butterick et al., 2013; Kotz et al., 2012), yet the intracellular components of how this occurs are unclear. Prior reports demonstrate that OXA is neuroprotective against oxidative stress in hypothalamic and cortical neurons (Butterick et al., 2012; Sokolowska et al., 2014). Likewise, we show that OXA prevents PA-induced apoptosis through inhibition of caspase-3/7 activity (Fig 1A-B). Our data show that PA increases ROS in hypothalamic cells (Fig 2), and notably, we show that OXA attenuates PA-induced ROS production (Fig 2). We also demonstrate that OXA increases Akt phosphorylation and stabilizes both Bcl-2 expression and the Bax/Bcl-2 ratio following PA challenge (Fig 4-5). These data indicate that neuroprotective mechanisms of OXA rely in part on attenuation of ROS signaling and caspase-dependent apoptosis. To the best of our knowledge, this is the first report demonstrating that OXA attenuates PA-induced hypothalamic cell death. Diets rich in PA exacerbate the production of ROS throughout the brain (Park et al., 2011; Pipatpiboon et al., 2013; Pipatpiboon et al., 2012). Further, PA induces neuronal cell death in part via increased ROS production (Almaguel et al., 2009). Although modest amounts of ROS act as cell signaling molecules and are important in nutrient sensing, chronic and sudden increases in ROS, such as those observed after HFD exposure, alter cell physiology and induce pathways of cell death (Gyengesi et al., 2012). Hypothalamic neuropeptide Y (NPY) and pro-melanocortin (POMC) neurons are in part regulated by ROS signaling to control food intake (Gyengesi et al., 2012). Further, orexin neurons directly interact with POMC and NPY neurons (Muroya et al., 2004), suggesting one mechanism through which OXA suppression of ROS could contribute to obesity resistance.
Activation of protein kinase phosphatidylinositol 3-kinase (PI3K/Akt) is required for stabilization of Bcl-2 expression and suppression of apoptosis in part through accumulation of Akt in the mitochondria (Bijur and Jope, 2003; Jiang et al., 2011; Zhou et al., 2000). Others have demonstrated OXA is neuroprotective against oxidative stress via involvement of the PI3K/Akt signaling pathway (Sokolowska et al., 2014; Zhang et al., 2010). Moreover, OXA may rely on increase mitochondrial accumulation of Akt to inhibit the onset of apoptosis (Bijur and Jope, 2003). Under oxidative stress conditions, Akt accumulation in the mitochondria is inhibited (Bijur and Jope, 2003) When PI3K/Akt activity is downregulated, neurons are more susceptible to oxidative stress-induced cell death (Taylor et al., 2005). Likewise, increased ROS leads to dephosphorylation of PI3K/Akt (Cao et al., 2009), indicating ROS levels play a crucial role in apoptosis. Phosphorylated PI3K/Akt upregulates Bcl-2 expression; conversely, oxidative stress downregulates Bcl-2 expression (Hildeman et al., 2003; Pugazhenthi et al., 2003; Pugazhenthi et al., 2000), therefore it is logical that OXA not only influences Bcl-2 expression but also alters mitochondrial function to inhibit apoptosis (Bijur and Jope, 2003). Although others demonstrate OXA is neuroprotective in cortical neurons without any pretreatment (Sokolowska et al., 2014; Sokolowska et al., 2012), our prior work (Butterick et al., 2012) and data in the current study demonstrate that pretreating hypothalamic neurons with OXA for 24 h prior to challenge produces the most robust neuroprotective response (supplemental Fig 2). Orexin pretreatment prior to insults such as PA could lead to heightened activation of neuroprotective pathways, including increased Akt, mitogen-activated protein kinase, and hypoxia-inducible factor 1 alpha (HIF-1α) (Butterick et al., 2013; Sikder and Kodadek, 2007; Yuan et al., 2011).
Mitochondria play an essential role in modulating ATP production, ROS, and apoptosis (Sun et al., 2016). Because the brain is a highly metabolically active organ, maintaining mitochondrial function and integrity is critically important. Mutations in mitochondrial proteins, impaired mitochondrial dynamics and respiration, and increased ROS production are associated with neurodegenerative diseases such as obesity, Alzheimer's disease, and Parkinson's disease (Cai and Tammineni, 2016; Petrov et al., 2015; Raza et al., 2015; Van Laar and Berman, 2009; Zorzano and Claret, 2015). Therefore, understanding mechanisms to prevent mitochondrial disturbances are important for developing therapeutics for neurodegenerative diseases. Prior data indicate that OXA increases ATP production, indicating that OXA alters mitochondrial function (Sikder and Kodadek, 2007). We demonstrate for the first time that OXA increases basal respiration, maximum respiration, ATP turnover, and reserve capacity in a dose-dependent manner in real time (Fig 5A-E). Additionally, we show OXA mediated changes of metabolic function are attenuated in the presence of DORA (Fig 6A-E). Increased reserve capacity is linked to promoting neuronal survival in models of oxidative stress (Choi et al., 2009; Yadava and Nicholls, 2007). Thus, the increased reserve capacity observed after OXA exposure could represent a bioenergetic advantage for neuronal survival during PA challenge. To the best of our knowledge, we demonstrate for the first time that OXA stabilizes basal respiration, maximum respiration, ATP turnover, and reserve capacity in the presence of PA (Fig 7A-E). These data indicate increasing mitochondrial bioenergetics as a potential mechanism through which OXA protects against insults such as HFDs. Maintaining hypothalamic circuitry would be beneficial in preventing the pathophysiology of neurodegenerative diseases such as obesity. Mitofusin 2, a mitochondrial membrane protein, participates in mitochondrial fusion and has recently been implicated in obesity pathogenesis. Diaz et al demonstrated rodents fed a high saturated fat diet had impaired hypothalamic mitochondrial function and reduction in mitofusin 2 (Diaz et al., 2015). In addition, mitochondrial dynamics governed by mitofusin 2 are integral in maintaining hypothalamic neurons that dictate metabolic status (Diaz et al., 2015; Dietrich et al., 2013; Schneeberger et al., 2013). As such, OXA-induced neuroprotective mechanisms may rely in part on altering proteins involved in mitochondrial dynamics. Future studies aimed at targeting the effects of OXA on mitochondrial dynamics and a potential role of mitofusin 2 should be considered in the context of obesity and neurodegenerative diseases. Additionally, data suggest the neuroprotective and obesity resistant mechanisms triggered by OXA rely on a HIF-1α-dependent pathway (Yuan et al., 2011). OXA upregulates HIF-1α, increasing ATP production and oxidative phosphorylation by shunting pyruvate into the tricarboxylic acid (TCA) cycle (Butterick et al., 2013; Butterick et al., 2012; Kodadek and Cai, 2010; Sikder and Kodadek, 2007). This HIF-1α-triggered increase in ATP could represent a biochemical mechanism through which OXA-producing neurons could prompt increases in cellular activity in target cell populations, subsequently stimulating wakefulness and other behavioral and physiological processes (Kodadek and Cai, 2010; Sikder and Kodadek, 2007).
In summary, these data support that OXA is neuroprotective against PA-induced neuronal insult in part through a ROS/caspase-dependent mechanism. Although in vitro studies have limitations, this work demonstrates an important mechanism in which OXA alters mitochondrial function and promotes neuronal survival following PA challenge. Future work will explore the effects of OXA signaling and the relationship of long and short-term changes in mitochondrial function and proteins in response to HFD. Understanding the role of OXA in maintaining neuronal survival will provide therapeutic targets for prevention or treatment of neurodegenerative diseases such as obesity.
Supplementary Material
Highlights.
Palmitic acid (PA) induces hypothalamic apoptosis
Orexin A attenuates PA-induced apoptosis and suppresses reactive oxygen species
Orexin A increases intracellular respiration and ATP in hypothalamic cells
Orexin A stabilizes PA-induced reduction of mitochondrial respiration
Acknowledgments
This work was funded by the US Department of Veterans Affairs BLR&D IK2 BX001686 and IS1 BX003083A (to TAB), the University of Minnesota Healthy Foods, Healthy Lives Institute (to JPN and TAB), and the Minnesota Veterans Medical Research & Education Foundation (to TAB). We also thank the Minnesota Obesity Center, Dr. David A Bernlohr, and Dr. Rocio Foncea for their assistance with the Seahorse assays.
Abbreviations
- OXA
Orexin A
- PA
palmitic acid
- ROS
reactive oxygen species
- HFD
high fat diet
- Bcl-2
B cell lymphoma 2
- Bax
B cell lymphoma 2 associated X protein
- OX1R
orexin receptor 1
- OX2R
orexin receptor 2
- DORA
dual orexin receptor antagonist
References
- Almaguel FG, Liu JW, Pacheco FJ, Casiano CA, De Leon M. Activation and reversal of lipotoxicity in PC12 and rat cortical cells following exposure to palmitic acid. J Neurosci Res. 2009;87:1207–1218. doi: 10.1002/jnr.21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsham DD, Cai F, Cui H, Smukler SR, Salapatek AM, Shkreta L. Generation of a phenotypic array of hypothalamic neuronal cell models to study complex neuroendocrine disorders. Endocrinology. 2004;145:393–400. doi: 10.1210/en.2003-0946. [DOI] [PubMed] [Google Scholar]
- Belsham DD, Fick LJ, Dalvi PS, Centeno ML, Chalmers JA, Lee PK, Wang Y, Drucker DJ, Koletar MM. Ciliary neurotrophic factor recruitment of glucagon-like peptide-1 mediates neurogenesis, allowing immortalization of adult murine hypothalamic neurons. Faseb J. 2009;23:4256–4265. doi: 10.1096/fj.09-133454. [DOI] [PubMed] [Google Scholar]
- Benoit SC, Kemp CJ, Elias CF, Abplanalp W, Herman JP, Migrenne S, Lefevre AL, Cruciani-Guglielmacci C, Magnan C, Yu F, Niswender K, Irani BG, Holland WL, Clegg DJ. Palmitic acid mediates hypothalamic insulin resistance by altering PKC-theta subcellular localization in rodents. The Journal of clinical investigation. 2009;119:2577–2589. doi: 10.1172/JCI36714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijur GN, Jope RS. Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J Neurochem. 2003;87:1427–1435. doi: 10.1046/j.1471-4159.2003.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulbul M, Tan R, Gemici B, Ongut G, Izgut-Uysal VN. Effect of orexin-a on ischemia-reperfusion-induced gastric damage in rats. J Gastroenterol. 2008;43:202–207. doi: 10.1007/s00535-007-2148-3. [DOI] [PubMed] [Google Scholar]
- Butterick TA, Billington CJ, Kotz CM, Nixon JP. Orexin: pathways to obesity resistance? Reviews in endocrine & metabolic disorders. 2013;14:357–364. doi: 10.1007/s11154-013-9259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterick TA, Duffy CM, Lee RE, Billington CJ, Kotz CM, Nixon JP. Use of a caspase multiplexing assay to determine apoptosis in a hypothalamic cell model. J Vis Exp. 2014 doi: 10.3791/51305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterick TA, Nixon JP, Billington CJ, Kotz CM. Orexin A decreases lipid peroxidation and apoptosis in a novel hypothalamic cell model. Neurosci Lett. 2012;524:30–34. doi: 10.1016/j.neulet.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Tammineni P. Alterations in Mitochondrial Quality Control in Alzheimer's Disease. Front Cell Neurosci. 2016;10:24. doi: 10.3389/fncel.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callander GE, Olorunda M, Monna D, Schuepbach E, Langenegger D, Betschart C, Hintermann S, Behnke D, Cotesta S, Fendt M, Laue G, Ofner S, Briard E, Gee CE, Jacobson LH, Hoyer D. Kinetic properties of “dual” orexin receptor antagonists at OX1R and OX2R orexin receptors. Frontiers in neuroscience. 2013;7:230. doi: 10.3389/fnins.2013.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Xu D, Wang D, Wu R, Zhang L, Zhu H, He Q, Yang B. ROS-driven Akt dephosphorylation at Ser-473 is involved in 4-HPR-mediated apoptosis in NB4 cells. Free Radic Biol Med. 2009;47:536–547. doi: 10.1016/j.freeradbiomed.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Choi SW, Gerencser AA, Nicholls DG. Bioenergetic analysis of isolated cerebrocortical nerve terminals on a microgram scale: spare respiratory capacity and stochastic mitochondrial failure. J Neurochem. 2009;109:1179–1191. doi: 10.1111/j.1471-4159.2009.06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz B, Fuentes-Mera L, Tovar A, Montiel T, Massieu L, Martinez-Rodriguez HG, Camacho A. Saturated lipids decrease mitofusin 2 leading to endoplasmic reticulum stress activation and insulin resistance in hypothalamic cells. Brain Res. 2015;1627:80–89. doi: 10.1016/j.brainres.2015.09.014. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Liu ZW, Horvath TL. Mitochondrial dynamics controlled by mitofusins regulate Agrp neuronal activity and diet-induced obesity. Cell. 2013;155:188–199. doi: 10.1016/j.cell.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy CM, Yuan C, Wisdorf LE, Billington CJ, Kotz CM, Nixon JP, Butterick TA. Role of orexin A signaling in dietary palmitic acid-activated microglial cells. Neurosci Lett. 2015;606:140–144. doi: 10.1016/j.neulet.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliyazova NS, Huh EY, Ibeanu GC. A novel phenoxy thiophene sulphonamide molecule protects against glutamate evoked oxidative injury in a neuronal cell model. BMC Neurosci. 2013;14:93. doi: 10.1186/1471-2202-14-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Feng P. OX2R activation induces PKC-mediated ERK and CREB phosphorylation. Experimental cell research. 2012;318:2004–2013. doi: 10.1016/j.yexcr.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyengesi E, Paxinos G, Andrews ZB. Oxidative Stress in the Hypothalamus: the Importance of Calcium Signaling and Mitochondrial ROS in Body Weight Regulation. Curr Neuropharmacol. 2012;10:344–353. doi: 10.2174/157015912804143496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada S, Fujita-Hamabe W, Tokuyama S. Effect of orexin-A on post-ischemic glucose intolerance and neuronal damage. J Pharmacol Sci. 2011;115:155–163. doi: 10.1254/jphs.10264fp. [DOI] [PubMed] [Google Scholar]
- Hildeman DA, Mitchell T, Aronow B, Wojciechowski S, Kappler J, Marrack P. Control of Bcl-2 expression by reactive oxygen species. Proc Natl Acad Sci U S A. 2003;100:15035–15040. doi: 10.1073/pnas.1936213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Liang P, Deng G, Tu Z, Liu M, Xiao X. Increased stability of Bcl-2 in HSP70-mediated protection against apoptosis induced by oxidative stress. Cell Stress Chaperones. 2011;16:143–152. doi: 10.1007/s12192-010-0226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinridders A, Schenten D, Konner AC, Belgardt BF, Mauer J, Okamura T, Wunderlich FT, Medzhitov R, Bruning JC. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 2009;10:249–259. doi: 10.1016/j.cmet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodadek T, Cai D. Chemistry and biology of orexin signaling. Molecular bioSystems. 2010;6:1366–1375. doi: 10.1039/c003468a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz C, Nixon J, Butterick T, Perez-Leighton C, Teske J, Billington C. Brain orexin promotes obesity resistance. Annals of the New York Academy of Sciences. 2012;1264:72–86. doi: 10.1111/j.1749-6632.2012.06585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Moraes JC, Coope A, Morari J, Cintra DE, Roman EA, Pauli JR, Romanatto T, Carvalheira JB, Oliveira AL, Saad MJ, Velloso LA. High-fat diet induces apoptosis of hypothalamic neurons. PLoS One. 2009;4:e5045. doi: 10.1371/journal.pone.0005045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroya S, Funahashi H, Yamanaka A, Kohno D, Uramura K, Nambu T, Shibahara M, Kuramochi M, Takigawa M, Yanagisawa M, Sakurai T, Shioda S, Yada T. Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca 2+ signaling in a reciprocal manner to leptin: orexigenic neuronal pathways in the mediobasal hypothalamus. Eur J Neurosci. 2004;19:1524–1534. doi: 10.1111/j.1460-9568.2004.03255.x. [DOI] [PubMed] [Google Scholar]
- Park HR, Kim JY, Park KY, Lee J. Lipotoxicity of palmitic Acid on neural progenitor cells and hippocampal neurogenesis. Toxicol Res. 2011;27:103–110. doi: 10.5487/TR.2011.27.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov D, Pedros I, Artiach G, Sureda FX, Barroso E, Pallas M, Casadesus G, Beas-Zarate C, Carro E, Ferrer I, Vazquez-Carrera M, Folch J, Camins A. High-fat diet-induced deregulation of hippocampal insulin signaling and mitochondrial homeostasis deficiences contribute to Alzheimer disease pathology in rodents. Biochim Biophys Acta. 2015;1852:1687–1699. doi: 10.1016/j.bbadis.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Pipatpiboon N, Pintana H, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. DPP4-inhibitor improves neuronal insulin receptor function, brain mitochondrial function and cognitive function in rats with insulin resistance induced by high-fat diet consumption. Eur J Neurosci. 2013;37:839–849. doi: 10.1111/ejn.12088. [DOI] [PubMed] [Google Scholar]
- Pipatpiboon N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. PPARgamma agonist improves neuronal insulin receptor function in hippocampus and brain mitochondria function in rats with insulin resistance induced by long term high-fat diets. Endocrinology. 2012;153:329–338. doi: 10.1210/en.2011-1502. [DOI] [PubMed] [Google Scholar]
- Pugazhenthi S, Nesterova A, Jambal P, Audesirk G, Kern M, Cabell L, Eves E, Rosner MR, Boxer LM, Reusch JE. Oxidative stress-mediated down-regulation of bcl-2 promoter in hippocampal neurons. J Neurochem. 2003;84:982–996. doi: 10.1046/j.1471-4159.2003.01606.x. [DOI] [PubMed] [Google Scholar]
- Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000;275:10761–10766. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- Raza H, John A, Howarth FC. Increased oxidative stress and mitochondrial dysfunction in zucker diabetic rat liver and brain. Cell Physiol Biochem. 2015;35:1241–1251. doi: 10.1159/000373947. [DOI] [PubMed] [Google Scholar]
- Schneeberger M, Dietrich MO, Sebastian D, Imbernon M, Castano C, Garcia A, Esteban Y, Gonzalez-Franquesa A, Rodriguez IC, Bortolozzi A, Garcia-Roves PM, Gomis R, Nogueiras R, Horvath TL, Zorzano A, Claret M. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell. 2013;155:172–187. doi: 10.1016/j.cell.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellayah D, Bharaj P, Sikder D. Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab. 2011;14:478–490. doi: 10.1016/j.cmet.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Sikder D, Kodadek T. The neurohormone orexin stimulates hypoxia-inducible factor-1 activity. Genes Dev. 2007;21:2995–3005. doi: 10.1101/gad.1584307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowska P, Urbanska A, Bieganska K, Wagner W, Ciszewski W, Namiecinska M, Zawilska JB. Orexins protect neuronal cell cultures against hypoxic stress: an involvement of Akt signaling. J Mol Neurosci. 2014;52:48–55. doi: 10.1007/s12031-013-0165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowska P, Urbanska A, Namiecinska M, Bieganska K, Zawilska JB. Orexins promote survival of rat cortical neurons. Neurosci Lett. 2012;506:303–306. doi: 10.1016/j.neulet.2011.11.028. [DOI] [PubMed] [Google Scholar]
- Sun N, Youle RJ, Finkel T. The Mitochondrial Basis of Aging. Molecular cell. 2016;61:654–666. doi: 10.1016/j.molcel.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JM, Ali U, Iannello RC, Hertzog P, Crack PJ. Diminished Akt phosphorylation in neurons lacking glutathione peroxidase-1 (Gpx1) leads to increased susceptibility to oxidative stress-induced cell death. J Neurochem. 2005;92:283–293. doi: 10.1111/j.1471-4159.2004.02863.x. [DOI] [PubMed] [Google Scholar]
- Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschop MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. The Journal of clinical investigation. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell reports. 2014;9:2124–2138. doi: 10.1016/j.celrep.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laar VS, Berman SB. Mitochondrial dynamics in Parkinson's disease. Exp Neurol. 2009;218:247–256. doi: 10.1016/j.expneurol.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur J Neurosci. 2004;19:1699–1707. doi: 10.1111/j.1460-9568.2004.03246.x. [DOI] [PubMed] [Google Scholar]
- Xu H, Hertzel AV, Steen KA, Bernlohr DA. Loss of Fatty Acid Binding Protein 4/aP2 Reduces Macrophage Inflammation Through Activation of SIRT3. Molecular endocrinology. 2016;30:325–334. doi: 10.1210/me.2015-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Hertzel AV, Steen KA, Wang Q, Suttles J, Bernlohr DA. Uncoupling lipid metabolism from inflammation through fatty acid binding protein-dependent expression of UCP2. Molecular and cellular biology. 2015;35:1055–1065. doi: 10.1128/MCB.01122-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadava N, Nicholls DG. Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex I with rotenone. J Neurosci. 2007;27:7310–7317. doi: 10.1523/JNEUROSCI.0212-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106:14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan LB, Dong HL, Zhang HP, Zhao RN, Gong G, Chen XM, Zhang LN, Xiong L. Neuroprotective effect of orexin-A is mediated by an increase of hypoxia-inducible factor-1 activity in rat. Anesthesiology. 2011;114:340–354. doi: 10.1097/ALN.0b013e318206ff6f. [DOI] [PubMed] [Google Scholar]
- Zhang L, Qu Y, Tang J, Chen D, Fu X, Mao M, Mu D. PI3K/Akt signaling pathway is required for neuroprotection of thalidomide on hypoxic-ischemic cortical neurons in vitro. Brain Res. 2010;1357:157–165. doi: 10.1016/j.brainres.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Zhang X, Dong F, Ren J, Driscoll MJ, Culver B. High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex. Exp Neurol. 2005;191:318–325. doi: 10.1016/j.expneurol.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Li XM, Meinkoth J, Pittman RN. Akt regulates cell survival and apoptosis at a postmitochondrial level. J Cell Biol. 2000;151:483–494. doi: 10.1083/jcb.151.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzano A, Claret M. Implications of mitochondrial dynamics on neurodegeneration and on hypothalamic dysfunction. Front Aging Neurosci. 2015;7:101. doi: 10.3389/fnagi.2015.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.