ABSTRACT
VASCULAR PLANT ONE-ZINC FINGER (VOZ)1/and VOZ2 have an ability to bind to the specific cis-element in the AVP1 promoter of Arabidopsis, which function on the PhyB-dependent flowering and possibly in various stress responses as potential transcription factors, although nuclear localization of VOZ proteins is still unclear. In this study, we found that VOZ2 is dispersed throughout the cytoplasm under normal growth conditions, whereas VOZ2 is transferred not only to the nucleus but also to the cytoplasmic foci under heat stress conditions. The VOZ2 foci predominantly co-localized with a marker of stress granules (SGs), which were cytoplasmic granular structures for mRNA storage and decay under abiotic stress conditions. We also demonstrated that GFP-VOZ2 with a nuclear localization signal was rapidly degraded via the ubiquitin/proteasome pathway under the heat stress conditions. Also, stress-related expression of DREB2A in the voz1voz2 mutant was significantly upregulated by heat stress as compared with that in the wild-type Arabidopsis. Our results suggest that VOZ2 is localized to SGs and nucleus under heat stress conditions, and functions as a transcriptional repressor of DREB2A in Arabidopsis.
KEYWORDS: Arabidopsis, DREB2A, Heat stress, processing body, stress granule, VOZ
Introduction
Plants are continuously exposed to various abiotic and biotic stressors from the natural environment, because being sessile organisms, plants cannot escape from such a stressful environment. To survive in such stressful environments, plants have developed complicated regulatory mechanisms for adapting to the changes in environmental conditions promptly. Abiotic stressors include temperature, drought, salinity, and osmotic stresses. Among these abiotic stressors, heat stress has a strong effect on plants in the natural environment and causes extensive losses in agricultural fields.1-3
To adapt to heat stress conditions, plants induce a lot of heat shock protein (HSP) genes necessary for heat stress (HS) tolerance under HS conditions.4 The expression of HSP genes is regulated by heat shock transcription factors (HSFs). Among HSFs, HsfA1a, 1b, and 1d function as the main positive regulators of HS-responsive gene expression. Also, heat-inducible HsfA2 and A7 in addition to HsfB1, B2a and B2b are critical for HS response.5-7 In Arabidopsis, DEHYDRATION-RESPONSIVE ELEMENT BINDING PROTEIN 2A (DREB2A) is one of the transcription factors involved in the activation of the expression of not only drought-responsive but also HS-responsive genes. DREB2A recognizes a specific cis-acting element called the dehydration-responsive element (DRE) in drought- and HS-responsive genes. DREB2A expression is induced rapidly by HS but only gradually induced by drought stress.8
In eukaryotic cells, the control of gene expression is determined by transcription and by post-transcriptional control.9 Eukaryotic cells under stressful conditions halt translation initiation and induce formation of cytoplasmic granular structures formed by RNA–protein complexes, which are referred to as stress granules (SGs) or processing bodies (P-bodies).10 SGs contain untranslated mRNAs, translation initiation factors, and proteins involved in the mRNA-associated protein translation, and function to serve as RNA triage centers that sort transcripts to various cellular destinations, whereas P-bodies are another type of cytoplasmic granules containing untranslated mRNA, translation repressors, and mRNA degradation machinery.11 SGs and P-bodies dynamically exchange with each other to change the biochemical composition and messenger ribonucleoproteins (mRNPs) for the control of mRNA function.12 In plants, environmental stressors such as heat, oxidative stress, UV irradiation, and translation inhibition lead to the formation of SGs and P-bodies.13,14
We previously identified transcription factors called vascular plant one-zinc-finger protein (VOZ) 1 and VOZ2, which have been strongly conserved in land plant evolution. The VOZ2 protein has been demonstrated to bind to GCGTNx7ACGC in vitro in the V-PPase (AVP1) promoter. VOZ proteins share a considerable homology with the NAC protein family in the C-terminal basic region and are classified into NAC subgroup VIII-2.15 Recently, VOZs were found to interact with phytochrome B and to upregulate flowering locus T (FT) and downregulate flowering locus C (FLC).16 We previously reported that the voz1voz2 double mutant shows increased cold and drought tolerance but decreased resistance to pathogens and heat stress.17 Although the green fluorescent protein-fused VOZ2 signal was predominantly observed in the cytoplasm under normal growth conditions, nuclear-localization-signalcontaining but not nuclear-export-signal-containing VOZ2 protein can complement the late-flowering phenotype of the voz1voz2 double mutant, indicating that VOZ proteins relocate from the cytoplasm to the nucleus and regulate the flowering signaling pathways.16 Nonetheless, no clear nuclear localization of VOZ proteins under normal growth conditions has been observed in Arabidopsis, these data indicate possible transcriptional regulatory function of VOZ proteins under particular growth conditions.
Here, we demonstrated that VOZ2 protein relocates from the cytosol to the nucleus and SGs under heat stress conditions. Besides, stress-related expression of DREB2A in the voz1voz2 mutant was significantly upregulated by heat stress as compared with that in the wild-type Arabidopsis by heat stress treatment.
Results
The VOZ2 protein is localized to the nucleus and granular structures in the cytoplasm under heat stress
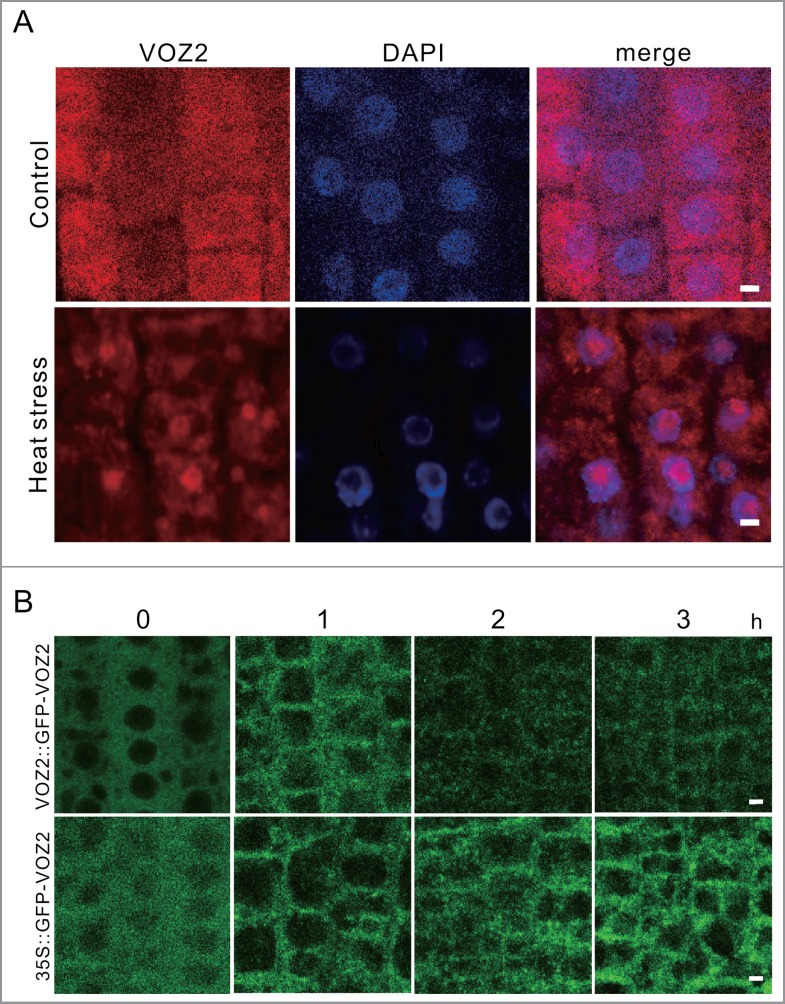
We previously reported that the voz1voz2 double mutant shows higher sensitivity to high-temperature conditions, suggesting that VOZ1 and VOZ2 are involved in heat stress responses. Since we previously reported that around 42°C is a critical temperature of thermotolerance of Arabidopsis (ecotype Columbia-0),17 we decided to set 42°C as the heat stress temperature in this study. To explore the function of VOZs under heat stress conditions, we first examined the subcellular localization of VOZ2 by immunostaining with an anti-VOZ2 antibody. As shown in Fig. 1A, VOZ2 was found to be dispersed throughout the cytosol under normal growth conditions, but VOZ2 localization was dramatically changed to both the nucleus and cytoplasmic granular structures after the seedlings were incubated at 42 °C for 2 h.
Figure 1.
High-temperature treatment induced relocalization of VOZ2 to the nucleus and cytoplasmic foci. (A) Immunostaining of the root epidermal cells of a 5-day-old WT plant with the anti-VOZ2 antibody. The seedlings were grown under normal growth conditions (22 °C) or incubated for 2 h at 42 °C. Scale bars = 5 µm. (B) Localization of GFP-VOZ2 in the 7-day-old voz1voz2 mutant expressing VOZ2::GFP-VOZ2 and CaMV35S::GFP-VOZ2. The images of epidermal cells were taken in the root division zone at 42°C for up to 3 hours. Scale bars = 5 µm.
Next, we observed the dynamics of the changes in the localization of VOZ2 under heat stress by means of transgenic Arabidopsis plants expressing a translational fusion of VOZ2 with green fluorescent protein (GFP) under the control of the cauliflower mosaic virus (CaMV) 35S promoter or its promoter, and we found that the distribution of GFP-VOZ2 was changed to the cytosolic foci after 20 min of 42°C heat stress treatment. Then, the fluorescence of GFP-VOZ2 foci gradually decreased after 2- h of heat stress treatment at 42°C (Fig. 1B, C). The formation of GFP-VOZ2 foci was apparently delayed for 40 min at 40 °C or 120 min at 39°C (Fig. S1).
Heat stress induces degradation of VOZ2 in the nucleus and cytosol via different kinetics
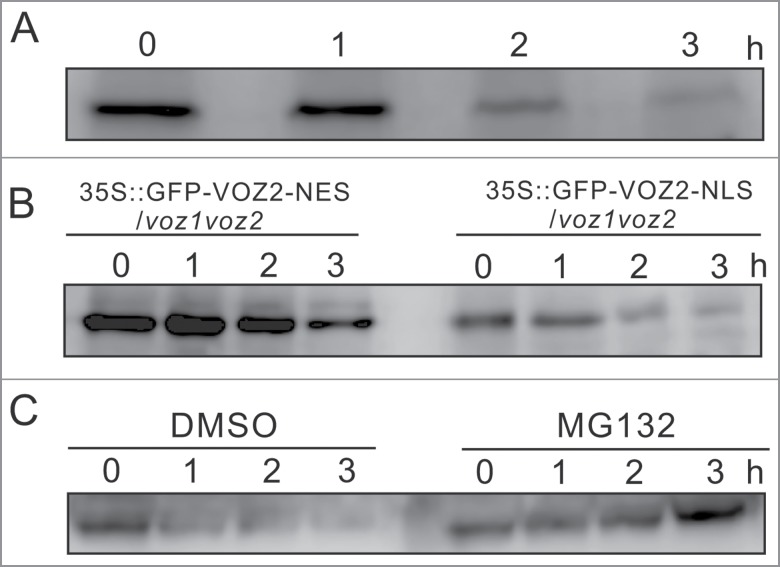
We analyzed the degradation kinetics of VOZ2 by western blotting with the anti-VOZ2 antibody. Two-week-old WT seedlings were incubated at 42°C and then were collected at 4-time points (0, 1, 2, and 3 h), and subjected to SDS-PAGE followed by western blotting with the anti-VOZ2 antibody. In line with the changes of GFP-VOZ2 fluorescence under the high-temperature conditions, VOZ2 was not degraded until 1 h of high-temperature incubation, but this protein was gradually degraded after 2 h of incubation (Fig. 2A).
Figure 2.
The VOZ2 protein was degraded under heat stress in the ubiquitin/proteasome-dependent manner. (A) Degradation time course of VOZ2. Fourteen-day-old seedlings of WT Arabidopsis were incubated at 42 °C for various lengths of time (0, 1, 2, or 3 h). (B) The degradation time course of the transgenic Arabidopsis lines expressing GFP-VOZ2-NES or GFP-VOZ2-NLS under the control of the CaMV35S promoter in the voz1voz2 mutant background. Fourteen-day-old seedlings of the transgenic Arabidopsis lines were incubated at 42 °C for various periods of time (0, 1, 2, or 3 h). (C) High-temperature dependent degradation of GFP-VOZ2-NLS is completely inhibited by a 26S proteasome inhibitor, MG132. Root tissues of the WT and GFP-VOZ2-NES- or GFP-VOZ2-NLS-expressing lines were subjected to SDS-PAGE, and then the VOZ2 protein amounts were analyzed by western blotting using an anti-VOZ2 polyclonal antibody.
Next, we investigated where the VOZ2 protein is mainly degraded in the cell under the high-temperature conditions using the plants expressing a fusion protein 35S::GFP-VOZ2 with either a nuclear export signal (NES) or a nuclear localization signal (NLS). Cytosol-resident VOZ2 (GFP-VOZ2-NES) was slightly degraded after 3 h of incubation at 42°C, whereas nucleus-localized VOZ2 (GFP-VOZ2-NLS) was rapidly degraded under the high-temperature conditions, and the protein almost disappeared after 3 h of incubation (Fig. 2B). The degradation of GFP-VOZ2-NLS was completely inhibited by the addition of a 26S proteasome inhibitor MG-132 (Fig. 2C), suggesting that the nucleus-localized VOZ protein is rapidly degraded by the ubiquitin/26S proteasome system.
VOZ2 is associated with SGs under the high-temperature conditions
Heat stress treatment induced granule structures of VOZ2 and GFP-VOZ2 in the cytosol (Fig. 1). In eukaryotic cells, granular structures that form in the cytosol are referred to as SGs or P-bodies. In plants, SGs and P-bodies are known to be formed under various stressful conditions including heat, oxidative stress, UV irradiation, and translation inhibition.18
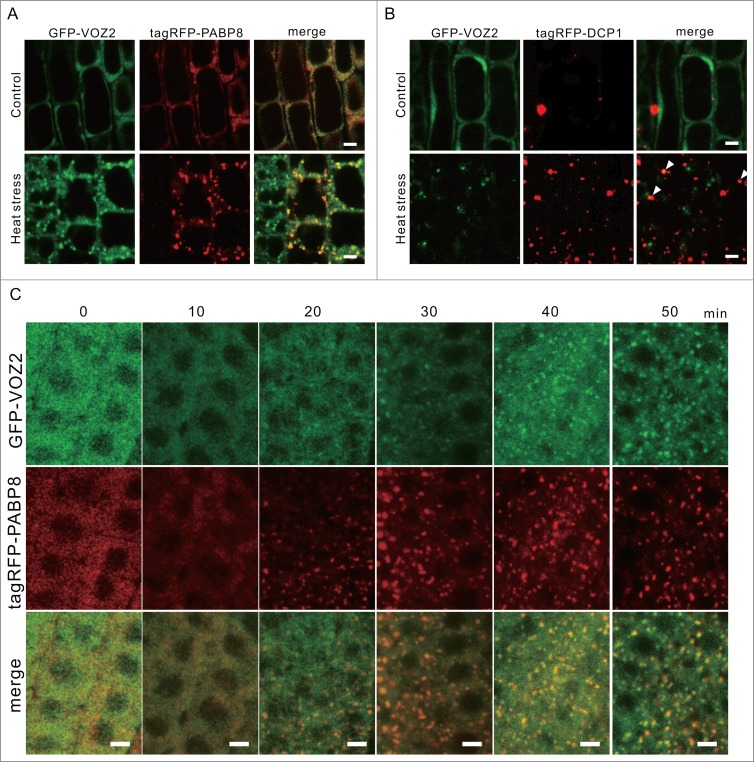
We speculated that the VOZ2 foci formed under the heat stress conditions were associated with SGs or P-bodies. To confirm that VOZ2 colocalizes with PABP8 and DCP1,14 marker proteins of SGs and P-bodies, respectively, we generated transgenic Arabidopsis plants expressing both tagRFP-PABP8 or tagRFP-DCP1 and GFP-VOZ2 under the control of the CaMV35S promoter. Under normal temperature conditions, both tagRFP-PABP8 and GFP-VOZ2 were dispersed throughout the cytosol, whereas after the incubation at 42°C, the cytoplasmic granular structures of tagRFP-PABP8 and GFP-VOZ2 were formed after 20 min under the high-temperature condition, and the 2 types of granular structures merged completely (Fig. 3A, C). In contrast, GFP-VOZ2 foci were partially merged with the P-body marker tagRFP-DCP1 after the heat stress treatment (Fig. 3B). From these results, we concluded that the VOZ2 protein is associated mainly with SGs and partially with P-bodies under heat stress conditions.
Figure 3.
GFP-VOZ2 associates with SGs and P-bodies under heat stress. Colocalization of GFP-VOZ2 with an SG marker, tagRFP-PABP8 (A), and a P-body marker, tagRFP-DCP1 (B), respectively, in the root epidermal cells of 7-day-old seedlings under the standard growth conditions (22 °C) or after incubating for 3 h at 42 °C (heat stress condition). Arrowheads in B is merged structures of GFP-VOZ2 and tagRFP-DCP1. (C) Time course of the formation of granular structures of GFP-VOZ2 and tagRFP-PABP8 in root tip cells at 42°C. Scale bars = 5 µm.
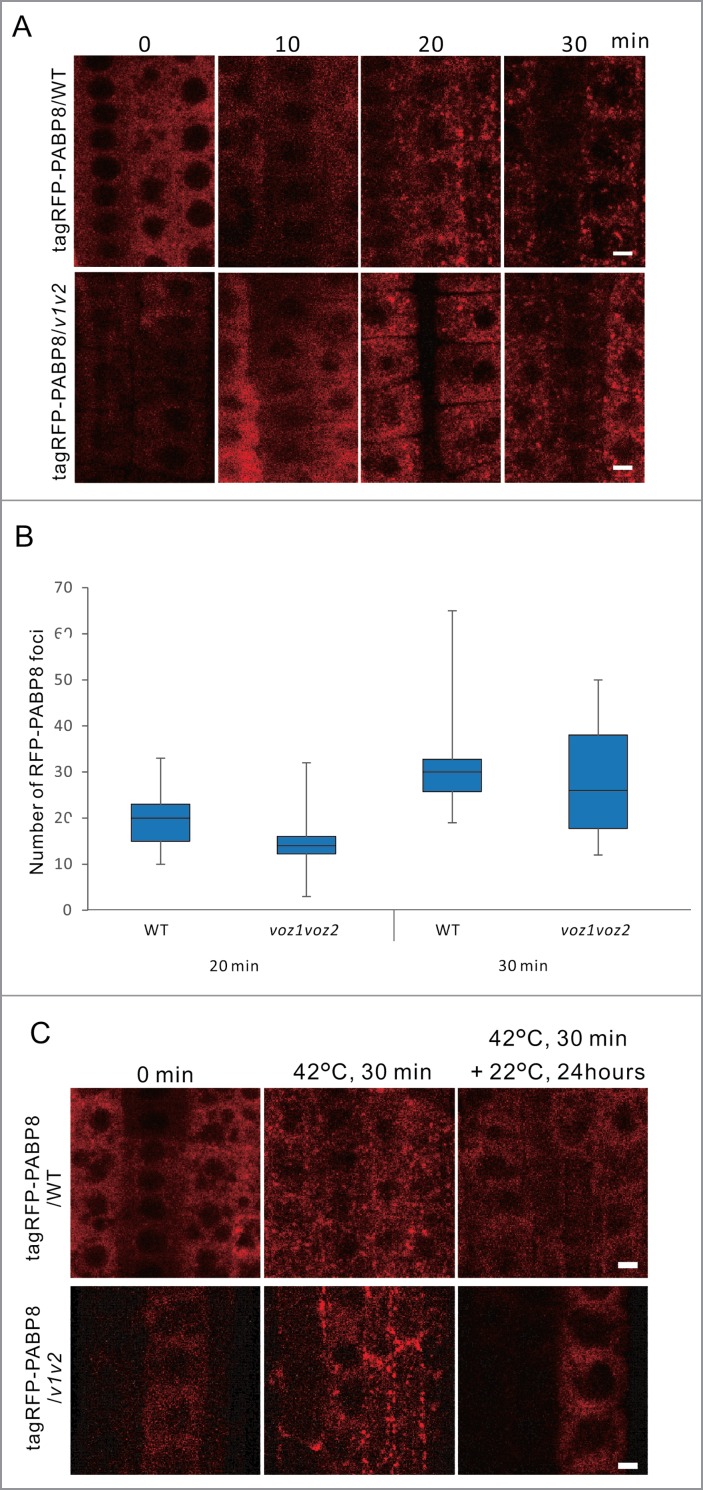
Next, to determine whether VOZ2 is one of the essential components of SG assembly or disassembly under heat stress, we analyzed changes in the number of tagRFP-PABP8 signals of Arabidopsis seedlings in the WT or voz1voz2 background. PABP8 foci were induced not only in WT but also in the voz1voz2 background under heat stress (Fig. 4A). No statistically significant differences in the formation rate and the number of the granule structures were observed between plants with the WT and voz1voz2 background (Fig. 4B). After 24 h recovery at 22°C from 30 min of the heat stress treatment, tagRFP-PABP8 foci were completely disappeared, and the tagRFP-PABP8 fluorescence were dispersed throughout the cytosol not only in WT but also in the voz1voz2 mutant background (Fig. 4C). From these results, we concluded that VOZ2 is not essential for processes of assembly or disassembly of SGs.
Figure 4.
The granule structures of PABP8 in WT or the voz1voz2 mutant under heat stress conditions. (A) Subcellular localization of tagRFP-PABP8 was observed in 7-day-old WT and voz1voz2 seedlings. (B) The box-and-whisker plot shows the number of tagRFP-PABP8 foci (SG markers) in root tip cells of WT and voz1voz2 lines were treated at 42 °C for 20 min or 30 min. P values were calculated by the Mann–Whitney U test (*P < 0.05). (C) Seven-day-old WT and voz1voz2 seedlings expressing tagRFP-PABP8 were incubated at 42 °C for 30 min, followed by incubation at 22 °C for 24 h, and images of root tip cells were captured using the confocal microscope. Scale bars = 5 µm.
VOZ functions as a suppressor of DREB2A under heat stress conditions
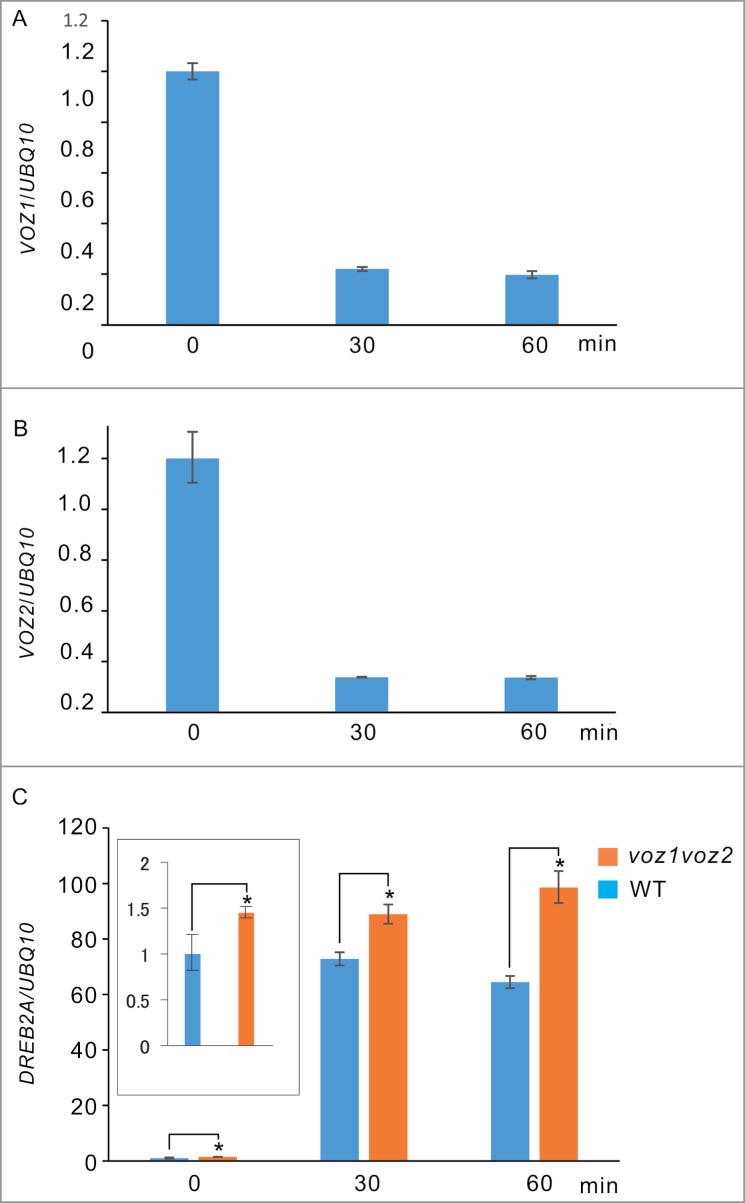
To determine how high temperature affects the expression of VOZ proteins, we measured the levels of VOZ1 and VOZ2 expression in WT and voz1voz2 plants by real-time qRT-PCR. Both VOZ1 and VOZ2 expression levels were reduced dramatically under the high temperature conditions (Fig. 5A, B). In contrast, expression of a drought and heat stress responsive transcription factor, DREB2A, was significantly enhanced in voz1voz2 plants compared with the WT under the high-temperature conditions (Fig. 5C). Note that DREB2A expression was already suppressed even in the non-heat stress conditions (Fig. 5C inset). These results suggest that VOZ2 protein function as transcriptional repressors of DREB2A not only under heat stress but also non-heat stress conditions.
Figure 5.
Relative expression levels of the VOZ1, VOZ2, and DREB2A genes in the root under heat stress. The gene expression levels were analyzed by quantitative reverse transcription polymerase chain reaction (qRT-PCR). Seven-day-old seedlings were incubated at 42 °C at 3 time points (0, 30, and 60 min). The expression levels of VOZ1 (A) and VOZ2 (B) in the WT are presented as the values relative to the data at 0 min. (C) DREB2A gene expression levels in WT are presented as a value relative to the data at 0 min. UBQ10 served as the internal expression control. Data are represented as a mean ± standard error (SE) from 3 replications. Significant differences are indicated by asterisks above the bars (t-test; P < 0.05). The experiments were repeated 3 times and yielded similar results. Inset; the expression profile of DREB2A at 0 min in the high temperature condition.
Discussion
Although VOZ proteins were speculated to function as transcriptional activators,15 no clear localization of VOZ has ever been observed in the nucleus under normal growth conditions. In this study, we showed that VOZ2 is transferred from the cytosol to the nucleus under heat stress conditions (Fig. 1). We also found that heat stress leads to the rapid degradation of nucleus-localized GFP-VOZ2 proteins (Fig. 2B). The degradation was completely inhibited by the proteasome inhibitor MG132. These results suggested that VOZ2 proteins relocated into the nucleus, subsequently were rapidly degraded via the ubiquitin/26S proteasome pathway under heat stress.
Yasui et al. reported that VOZ proteins function downstream of phyB to promote flowering to regulate FT and FLC expression; besides, VOZ proteins in the nucleus are subjected to proteasome-dependent degradation under far-red and dark conditions in a phytochrome-dependent manner. Yasui et al. also reported that nuclear localization signal-containing but not nuclear export signal-containing VOZ2 protein could complement the late-flowering phenotype of the voz1voz2 double mutant, indicating that VOZ proteins function in the nucleus for regulation of flowering.16 We previously reported that VOZ2 is degraded by the ubiquitin-proteasome system under cold-stress conditions.17 These results suggest that VOZ proteins are transferred to the nucleus and function as transcriptional regulators when the plant cells perceive certain environmental signals (e.g., light, cold or heat), and then are rapidly subjected to the ubiquitin/proteasome-dependent degradation for inactivation. Nevertheless, we cannot rule out the possibility that VOZ2 continuously moves to the nucleus and subjects a subsequent degradation in non-stressful conditions, and disappears from the nucleus under heat stress conditions because of the decrease of VOZ2 transcription and the sequestration of VOZ2 in the SGs or P-bodies. In this case, the nuclear-resided VOZ2 protein might function in the normal growth conditions as a transcriptional regulator.
In addition to the transcriptional regulation of gene expression, post-transcriptional regulation is also important for various physiologic processes especially in the stress response for rapid and dominant expression of specific stress-induced transcripts.9 Under various stressful conditions, eukaryotic cells form cytosolic granular structures composed of RNA-protein complexes; these structures are called SGs and P-bodies and are intended for mRNA turnover and translational repression.12
SGs and P-bodies contain several RNA-binding proteins, for instance, 2 homologous nucleoplasmic RNA-binding proteins, TIA-1 (T-cell internal antigen 1) and TIAR (TIA-1-related protein). These proteins work as translational regulators by binding to specific mRNA to form untranslated messenger ribonucleoproteins. Under stressful conditions, TIA or TIAR proteins dramatically change their subcellular distribution to associate with SGs or P-bodies.19
In higher plants, proteins of the oligouridylate-binding protein 1 family (UBP1s) are known as TIA-1/TIAR-related proteins, which were found to bind to a polyuridylate tract to enhance intron recognition, splicing, and mRNA accumulation. UBP1s are assembled into SGs under various stressful conditions.20 Heat treatment changes the localization of UBP1b from the nucleus to SGs in Arabidopsis. UBP1 acts as SGs' central component, and protects heat stress tolerance-related transcripts from exonucleolytic degradation via interactions with the 3′-UTR of mRNAs to play a fundamental role in thermotolerance.13,21,22 In plants, another DNA- and RNA-biding family of proteins, the CCCH-type tandem zinc finger proteins (TZFs), colocalized with both SGs and P-bodies under various stressful conditions including heat and hypoxia. TZFs are highly conserved among eukaryotes and perform critical functions in mRNA metabolism in animals and yeast. In plants, a much higher number of TZFs has been found, and among them, a class of TZF family proteins containing an arginine-rich (RR) motif, which are referred to as RR-TZF.23 It has been reported that RR-TZF proteins are associated with P-bodies and SGs to perform functions crucial for plant growth, development, and stress response. One of RR-TZF proteins, AtTZF1, can bind to both DNA and RNA in vitro and colocalizes with P-bodies and SGs under certain stress conditions.14
In this study, we demonstrated that VOZ2 colocalized with both SGs and P-bodies under heat stress conditions (Fig. 3). We previously reported that VOZ proteins in higher plants have a well-conserved unconventional CCCH zinc-finger motif, which functions as the DNA-binding domain of VOZ-family proteins.15 Taking VOZ proteins as P-body- and SG-associated CCCH-type zinc proteins into consideration, VOZs may have an ability to bind to RNA and to participate in sequestration of particular mRNAs under heat stress for turnover and translational repression of specific mRNAs.
We previously reported that VOZ proteins are required for resistance to higher temperatures in Arabidopsis.17 Nevertheless, little is known about the molecular mechanism by which VOZ proteins function under heat stress conditions. In this study, we found that localization of the VOZ2 protein is dramatically changed (to the nucleus and cytoplasmic foci) under high-temperature conditions. We also found that heat stress-induced DREB2A is significantly upregulated in the voz1voz2 mutant. We previously reported that the expression levels of several cold-responsive genes including CBF4, ZAT12, COR15A, and RD29B are increased under low-temperature conditions in the voz1voz2 mutant. Thus, VOZ proteins function as negative regulators of abiotic stress response pathways.17 Arabidopsis HsfA1 transcription factors serve as the main-positive regulators in heat stress-responsive gene expression. The expression of DREB2A under heat stress is also regulated by HsfA1s. Nuclear localization of HsfA1 under heat stress is negatively regulated by the interaction with HSP90.7
In this study, we demonstrated that some of the VOZ2 molecules are transferred from the cytosol to the nucleus and then are degraded by the ubiquitin/proteasome system, and the rest of VOZ2 molecules in the cytosol are associated with SG or P-body structures under heat stress conditions. These observations suggest that VOZ2 possibly functions as a negative regulator of DREB2A to directly associated with DREB2A or indirectly regulate its expression to suppress the up-stream regulator HsfA1 function in the cytosol under normal growth conditions. Further studies are needed to clarify how the dual localization of VOZ2 in the nucleus and cytoplasm contributes to a heat stress response.
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana Col-0 was used as the wild-type plants in this study. The voz1voz2–2 mutant was generated by crossing voz1 (WISCDSLOX489–492O10) and voz2 (SALK_115813). Plants expressing 35S::GFP-VOZ2 were kindly provided by T. Kohchi. GFP-VOZ2 with NES or NLS was expressed by introducing the plasmid donated by Y. Yasui into the voz1voz2–2. All plants used in this study were grown on 1/2 Murashige-Skoog (MS) agar plates at 22°C under a 16-h light, 8-h dark photoperiod.
Plasmid construction
Primer sequences are listed in Table S1. For expression of the N-terminal tagRFP fusion under the control of the CaMV 35S promoter, PABP8 (At1g49760) or DCP1 (At1g08370) were amplified by PCR from a cDNA derived from seedlings of Arabidopsis. tagRFP fragment and either PABP8 or DCP1 were inserted into pENTR using In-Fusion Cloning. The tagRFP-PABP8 and tagRFP-DCP1 fragments were transferred into the pFAST-G01 vector by Gateway LR recombination reactions.
To generate transgenic Arabidopsis expressing tagRFP-DCP1 or tagRFP-PABP8 and GFP-VOZ2, transgenic Arabidopsis expressing 35S::GFP-VOZ2 was transformed by Agrobacterium-mediated floral dip transformation.24
Western blot analysis
Total protein was extracted from roots of 14-day-old Arabidopsis seedlings. The sample ground with a mortar and pestle and dissolved in a buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM CaCl2, 1% Triton X-100) containing a proteinase inhibitor cocktail (Nacalai Tesque) was centrifuged at 10,000 × g for 10 min at 4°C. Then, 12.5 μg of the supernatant of samples was subjected to SDS-PAGE. Western blotting was performed using a 5,000-fold diluted anti-VOZ2 antibody17 as the primary antibody and a 10,000-fold diluted goat anti-rabbit IgG antibody.
Quantitative RT-PCR (qRT-PCR) analysis
For qRT-PCR, total RNA was isolated from WT and voz1voz2 double-mutant plants with the Nucleo Spin RNA kit (TaKaRa). cDNAs were synthesized from 1 μg of total RNA of each plant using the ReverTra Ace qPCR RT Master Mix (TOYOBO). Quantitative real-time PCR was conducted using the THUNDERBIRD SYBR qPCR Mix (TOYOBO) and Eco Real-Time PCR System (Illumina) according to the manual. The expression level was normalized to an internal control gene (UBQ10, At4g05320). The primers are listed in Table S1.
Drug treatments
Fourteen-day-old seedlings were transferred to a liquid half-strength MS medium supplemented (or not supplemented) with 50 μM MG132 (Nacalai Tesque, Kyoto, Japan) and incubated for 5-h, with 10 μM wortmannin (Wako, Japan), and for 24-h. Total protein was extracted and subjected to SDS-PAGE followed by protein gel blotting using the anti-VOZ2 antibody.
Confocal microscopy and statistical analyses
GFP and tagRFP fluorescent images were obtained using a confocal laser microscope (ECLIPSE E600; Nikon). The captured images were processed in the Nikon EZ-C1 software. The image analysis was done using the ImageJ software. SGs and P-bodies whose size was larger than 0.5 μm were scored as positive. The analyzed image data are presented as the box-and-whisker plots. Boxes and solid lines in the boxes show the upper (75th) and lower (25th) quartiles and median values, respectively. Whiskers indicate the 95% confidence intervals.
Supplementary Material
Disclosure of potential conflicts of interest
The authors declare that they have no competing financial interests. Correspondence and requests for materials should be addressed to M.H.S. ( mhsato@kpu.ac.jp).
Acknowledgments
We thank T. Shiina (Kyoto Prefectural University, Japan), T. Hamada, Y. Watanabe (University of Tokyo, Japan) and K. Yamaguchi-Shinozaki (University of Tokyo, Japan) for the fruitful discussion.
Funding
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan; a grant-in-aid for Scientific Research (B) (No. 16H05068), and the Strategic Research Funds of Kyoto Prefectural University to M.H.S.
References
- 1.Yamaguchi-Shinozaki K, Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 2006; 57:781-803; PMID:16669782; http://dx.doi.org/21828105 10.1146/annurev.arplant.57.032905.105444 [DOI] [PubMed] [Google Scholar]
- 2.Mittler R. Abiotic stress, the field environment and stress combination. Trends Plant Sci 2006; 11:15-9; PMID:16359910; http://dx.doi.org/21828105 10.1016/j.tplants.2005.11.002 [DOI] [PubMed] [Google Scholar]
- 3.Qin F, Shinozaki K, Yamaguchi-Shinozaki K. Achievements and challenges in understanding plant abiotic stress responses and tolerance. Plant Cell Physiol 2011; 52:1569-82; PMID:21828105; http://dx.doi.org/ 10.1093/pcp/pcr106 [DOI] [PubMed] [Google Scholar]
- 4.Kotak S, Larkindale J, Lee U, von Koskull-Döring P, Vierling E, Scharf KD. Complexity of the heat stress response in plants. Curr Opin Plant Biol 2007; 10:310-6; PMID:17482504; http://dx.doi.org/15625403 10.1016/j.pbi.2007.04.011 [DOI] [PubMed] [Google Scholar]
- 5.Mittler R, Finka A, Goloubinoff P. How do plants feel the heat? Trends Biochem Sci 2012; 37:118-25; PMID:22236506; http://dx.doi.org/15625403 10.1016/j.tibs.2011.11.007 [DOI] [PubMed] [Google Scholar]
- 6.Baniwal SK, Bharti K, Chan KY, Fauth M, Ganguli A, Kotak S, Mishra SK, Nover L, Port M, et al.. Heat stress response in plants: a complex game with chaperones and more than twenty heat stress transcription factors. J Biosci 2004; 29:471-87; PMID:15625403; http://dx.doi.org/ 10.1007/BF02712120 [DOI] [PubMed] [Google Scholar]
- 7.Yoshida T, Ohama N, Nakajima J, Kidokoro S, Mizoi J, Nakashima K, Maruyama K, Kim JM, Seki M, Todaka D, et al.. Arabidopsis HsfA1 transcription factors function as the main positive regulators in heat shock-responsive gene expression. Mol Genet Genomics 2011; 286:321-32; PMID:21931939; http://dx.doi.org/ 10.1007/s00438-011-0647-7 [DOI] [PubMed] [Google Scholar]
- 8.Sakuma Y, Maruyama K, Qin F, Osakabe Y, Shinozaki K, Yamaguchi-Shinozaki K. Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heat-stress-responsive gene expression. Proc Natl Acad Sci U S A 2006; 103:18822-7; PMID:17030801; http://dx.doi.org/ 10.1073/pnas.0605639103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA Translation and Stability by microRNAs. Annu Rev Biochem 2010; 79:351-79; PMID:20533884; http://dx.doi.org/ 10.1146/annurev-biochem-060308-103103 [DOI] [PubMed] [Google Scholar]
- 10.Kedersha N, Anderson P. Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem Soc Trans 2002; 30:963-9; PMID:1244095518643965 [DOI] [PubMed] [Google Scholar]
- 11.Belostotsky DA, Sieburth LE. Kill the messenger: mRNA decay and plant development. Curr Opin Plant Biol 2009; 12:96-102; PMID:18990607; http://dx.doi.org/18643965 10.1016/j.pbi.2008.09.003 [DOI] [PubMed] [Google Scholar]
- 12.Buchan JR, Parker R. Eukaryotic stress granules: The ins and out of translation. Mol Cell 2009; 36:932-941; PMID:20064460; http://dx.doi.org/18643965 10.1016/j.molcel.2009.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber C, Nover L, Fauth M. Plant stress granules and mRNA processing bodies are distinct from heat stress granules. Plant J 2008; 56:517-30; PMID:18643965; http://dx.doi.org/ 10.1111/j.1365-313X.2008.03623.x [DOI] [PubMed] [Google Scholar]
- 14.Pomeranz MC, Hah C, Lin PC, Kang SG, Finer JJ, Blackshear PJ, Jang JC. The Arabidopsis tandem zinc finger protein AtTZF1 traffics between the nucleus and cytoplasmic foci and binds both DNA and RNA. Plant Physiol 2010; 152:151-65; PMID:19897605; http://dx.doi.org/15295067 10.1104/pp.109.145656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitsuda N, Hisabori T, Takeyasu K, Sato MH. VOZ; Isolation and characterization of novel vascular plant transcription factors with a one-zinc finger from Arabidopsis thaliana. Plant Cell Physiol 2004; 45:845-54; PMID:15295067; http://dx.doi.org/ 10.1093/pcp/pch101 [DOI] [PubMed] [Google Scholar]
- 16.Yasui Y, Mukougawa K, Uemoto M, Yokofuji A, Suzuri R, Nishitani A, Kohchi T. The phytochrome-interacting VASCULAR PLANT ONE-ZINC FINGER1 and VOZ2 redundantly regulate flowering in Arabidopsis. PlantCell 2012; 24:3248-63; PMID:22904146; http://dx.doi.org/23167462 10.1105/tpc.112.101915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakai Y, Nakahira Y, Sumida H, Takebayashi K, Nagasawa Y, Yamasaki K, Akiyama M, Ohme-Takagi M, Fujiwara S, Shiina T, et al.. Vascular plant one-zinc-finger protein 1/2 transcription factors regulate abiotic and biotic stress responses in Arabidopsis. Plant J 2013; 73:761-75; PMID:23167462; http://dx.doi.org/ 10.1111/tpj.12069 [DOI] [PubMed] [Google Scholar]
- 18.Zawoznik MS, Groppa ML, Tomaro MP, Benavides MP. Endogenous salicylic acid potentiates cadmium-induced oxidative stress in Arabidopsis thaliana. Plant Science 2007; 173:190–7; http://dx.doi.org/ 10.1016/j.plantsci.2007.05.004 [DOI] [Google Scholar]
- 19.Anderson P, Kedersha N. Visibly stressed: the role of eIF2, TIA-1, and stress granules in protein translation. Cell Stress Chaperones 2002; 7:213-21; PMID:12380690; http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorenson R, Bailey-Serres J. Selective mRNA sequestration by OLIGOURIDYLATE-BINDING PROTEIN 1 contributes to translational control during hypoxia in Arabidopsis. Proc Natl Acad Sci U S A 2014; 111:2373-8; PMID:24469793; http://dx.doi.org/ 10.1073/pnas.1314851111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen CC, Nakaminami K, Matsui A, Kobayashi S. Oligouridylate binding protein 1b plays an integral role in plant heat stress tolerance. Front Plant Sci 2016; 7:853; PMID:27379136; http://dx.doi.org/ 10.3389/fpls.2016.00853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambermon MHL, Simpson GG, Kirk DAW, Hemmings-mieszczak M, Klahre U, Filipowicz W. UBP1 , a novel hnRNP-like protein that functions at multiple steps of higher plant nuclear pre-mRNA maturation. EMBO J 2000; 19:1638-49; PMID:10747031; http://dx.doi.org/ 10.1093/emboj/19.7.1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bogamuwa SP, Jang JC. Tandem CCCH zinc finger proteins in plant growth, development and stress response. Plant Cell Physiol 2014; 55:1367-75; PMID:24850834; http://dx.doi.org/ 10.1093/pcp/pcu074 [DOI] [PubMed] [Google Scholar]
- 24.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 1998; 16:735-43; PMID:10069079; http://dx.doi.org/ 10.1046/j.1365-313x.1998.00343.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.