ABSTRACT
Environmental stress conditions such as high light, extreme temperatures, salinity or drought trigger oxidative stress and eventually protein misfolding in plants. In chloroplasts, chaperone systems refold proteins after stress, while proteases degrade misfolded and aggregated proteins that cannot be refolded. We observed that reduced activity of chloroplast Hsp70 chaperone or Clp protease systems both prevented growth of Arabidopsis thaliana seedlings after treatment with the oxidative agent methyl viologen. Besides showing a role for these particular protein quality control components on the protection against oxidative stress, we provide evidence supporting the existence of a yet undiscovered pathway for Clp-mediated degradation of the damaged proteins.
KEYWORDS: Chaperone, chloroplast, Clp, Hsp70, methyl viologen, oxidative stress, paraquat, protease, protein quality control
Abbreviations
- MV
methyl viologen
- Hsp70
heat shock protein 70
- Clp
caseinolytic protease
- DXS
deoxyxylulose 5-phosphate synthase
Living cells are constantly challenged with potentially harmful situations. To survive stress episodes that damage cell proteins, different families of chaperones and proteases form protein quality control systems that ensure a proper lifespan and activity of proteins. Molecular chaperones such as those of the Heat shock protein 70 (Hsp70) family target hydrophobic stretches present in unfolded or misfolded polypeptides to promote correct folding and assembly, prevent the wasteful formation of inactive and toxic aggregates, or deliver irreversibly damaged proteins to proteolytic degradation.1 In plants, cytosolic Hsp70 chaperones are involved in the ubiquitination of unfolded proteins, targeting them for degradation by the 26S proteasome.2 Hsp70 chaperones are also found in the chloroplast, where they appear to be crucial for resistance to high temperatures and import of proteins into the chloroplast.3-5 The absence of proteasome in the chloroplast suggested that Hsp70 might be collaborating for protein quality control with one of the prokaryotic-type proteases that are found in these organelles. Among them, the ATP-dependent stromal Clp proteolytic complex is one of the major proteases in chloroplasts.6-8 In Arabidopsis, the proteolytic core of the complex is formed by 2 heptameric rings of plastome-encoded ClpP1 and nuclear-encoded ClpP3-P6 and ClpR1-R4 proteins stabilized by plant-specific ClpT1-T2 subunits. A dynamically interacting hexameric ring of Hsp100 chaperones (ClpC1-C2 and ClpD) unfolds protein substrates for translocation into the proteolytic chamber.6,9 While some Clp subtrates can be directly recognized by these Hsp100 chaperones, others are delivered to Clp-mediated degradation by a dedicated binary adaptor system formed by ClpS and ClpF proteins.10,11 A number of substrates of the Clp protease have been identified, including enzymes involved in chlorophyll/tetrapyrrole/isoprenoid metabolism and proteins required for chloroplast homeostasis.6,7
We previously showed that non-functional and aggregated forms of the chloroplast isoprenoid pathway enzyme deoxyxylulose 5-phosphate synthase (DXS) are delivered to Hsp70 by the DNAJ-like co-chaperone J2012. In addition to promoting the correct folding of DXS, the J20/Hsp70 chaperone system was also involved in the degradation of DXS upon stress. Recently, we unveiled that Hsp70 collaborates with specific Hsp100 chaperones to degrade the inactivated enzyme (via ClpC1 and Clp protease) or disaggregate it and refold it back to its active form (via direct interaction with the Hsp100 unfoldase ClpB3) when necessary.13 It is likely that both Hsp70 and Clp protease systems share other protein substrates besides DXS. As a proof of concept, we aimed to investigate whether mutants defective in Hsp70 or Clp protease activity had a similar phenotype of enhanced sensibility to environmental stress. Because most environmental challenges (including excess light, high temperature, water shortage or nutrient starvation) can eventually result in oxidative stress due to the production of reactive oxygen species (ROS), we investigated the phenotypic response of mutant plants to methyl viologen (MV), also called paraquat, an oxidative agent that produces ROS. MV has been shown to trigger protein carbonylation and consequently protein misfolding in many organisms.14-16 In plants, carbonylated proteins are degraded in the cytosol by the ubiquitin-26S proteasome system.17 In chloroplasts, the most important source of MV-generated ROS in plant cells (as MV accepts electrons from photosystem I producing superoxide anion18), proteins such as the Calvin cycle enzyme sedoheptulose-bisphosphatase are also carbonylated and hence inactivated after treatment with MV.19 However, there is no direct evidence that carbonylated (i.e. misfolded) proteins are degraded by the Clp protease. Our data reported here show that both plastidial Hsp70 chaperone and Clp protease systems contribute to the survival of the plant under MV-triggered oxidative stress.
Two genes encode plastid-targeted Hsp70 chaperones in Arabidopsis, cpHsp70.1 (At4g24280) and cpHsp70.2 (At5g49910). Since mutants defective in both cpHsp70.1 and cpHsp70.2 are lethal,3 we analyzed the response to MV of single mutant lines, named hsp70.1 and hsp70.2. As shown in Fig. 1, both single mutants showed enhanced sensitivity to MV. Compared to the wild-type, hsp70.1 and hsp70.2 plants showed much reduced size and weight (Fig. 1A) in response to MV treatment. The higher sensitivity of Hsp70 mutants to oxidative stress could somehow be expected considering previous reports that showed less resistance of the mutants to heat shock.3 If plastidial Hsp70 chaperones and the Clp protease are part of the same mechanism regulating the responses of chloroplasts to oxidative stress, then mutants defective in Clp activity should also display an increased sensitivity to MV treatment. Indeed, mutants defective in the proteolytic core subunit ClpR1 showed a strong sensitivity to MV (Fig. 1B). Destabilization of the complex derived from loss of either ClpT1 or ClpT2 subunits in individual mutants20 had a weaker impact on the response to oxidative stress, whereas the clpt1 clpt2 double mutant displayed a MV sensitivity phenotype very similar to that observed in the clpr1 mutant (Fig. 1B). In agreement with these data, comparative proteomics of clpt1 clpt2 plants showed a molecular phenotype that shared several key features of mutants defective in ClpPR subunits such as ClpR2, ClpR4, and ClpP320. For instance, chaperones of the Hsp100/ClpB3, Hsp90, Hsp70 and Hsp60/Cpn60 families were all significantly overaccumulated in these mutants, suggesting that they are the common consequence of reduced Clp protease capacity.
Figure 1.
Methyl viologen sensitivity of mutants defective in plastidial Hsp70 and Clp protease subunits. Mutants for the indicated proteins of the chloroplast Hsp70 (A) and Clp protease (B) systems were treated with the oxidative agent methyl viologen (MV). Representative picture of individual 25-day-old plants of the indicated lines are shown in control or after treatment with 20 μM MV. Data of fresh weight are represented relative to those in each individual line grown in the absence of MV. Mean and SE values of n ≥ 6 independent experiments are shown (t test: *P < 0.05 and **P < 0.01).
Strikingly, mutants defective in ClpC-like Hsp100 chaperones (ClpC1, ClpC2 and ClpD) as well as those defective in the ClpS adaptor displayed a wild-type phenotype in terms of growth response to MV (Fig. 1B). ClpC-like chaperones possess a tripeptide P-loop that allows the interaction with the proteolytic subunits of the Clp complex.21, 22 ClpD significantly differs from ClpC isoforms at the sequence level, suggesting that ClpD activity might be required for specific substrates under certain stress situations.23-25 By contrast, ClpC1 and ClpC2 are very similar in sequence and they have been demonstrated to be functionally redundant.26,27 Overlapping functions of these chaperones in plastids might explain the absence of a clear MV sensitivity phenotype in single clpc1 and clpc2 mutants. However, this is in sharp contrast with the results obtained when testing the sensitivity of Arabidopsis plants to the bleaching herbicide clomazone (CLM), a specific inhibitor of DXS that has been used to estimate the activity of the enzyme in vivo.12 While mutants defective in ClpC2 showed no statistical difference with the wild-type in terms of DXS levels and CLM resistance, those impaired in ClpC1 were found to be less sensitive to CLM than the wild-type because they accumulated higher levels of active DXS enzyme.13 This and other results led to conclude that DXS was targeted to Clp-mediated degradation mainly by ClpC1 (via J20 and Hsp70). The observation that the single clpc1 mutant shows a virtually wild-type phenotype after MV treatment therefore suggests that ClpC1 might participate in the degradation of particular enzymes (such as DXS) but it is not specifically required for the response to oxidative stress.
In summary, the results reported here demonstrate an involvement of both the Clp protease and plastidial Hsp70 chaperones on the response to MV treatment (i.e., to ROS production and oxidative stress). Both ClpC,27,28 and Hsp704,5 chaperones are also involved in protein import into chloroplasts. Mutants defective in these activities show inefficient protein import and, as shown here, an increased sensibility to MV. By contrast, reducing protein import capacity by directly interfering with the translocation apparatus was previously demonstrated to result in improved tolerance to MV and other stress agents, likely because decreased import of photosynthetic apparatus components attenuates photosynthetic activity and eventually reduces the potential for ROS production.29 We therefore conclude that the lower tolerance to MV observed in mutants defective in Hsp70 or ClpC activities results from mechanisms other than reduced chloroplast protein import. Our results suggest that ClpC-like chaperones might unfold protein substrates that became carbonylated or/and misfolded upon MV treatment to be degraded by the Clp proteolytic complex. The high degree of redundancy between ClpC1 and ClpC2 (Fig. 2) might ensure that the potentially large variety of proteins damaged during oxidative stress episodes could be readily removed to prevent the formation of toxic aggregates. Based on the lack of a clear phenotype in the case of the clps mutant, we propose that the ClpS/F adaptor system might not be a major player in delivering client protein to the Clp protease. The second pathway targeting proteins to Clp-mediated degradation, the DNAJ-Hsp70-ClpC pathway, might be functional during the response to oxidative stress but with decreased specificity (e.g. with ClpC1 and ClpC2 playing redundant functions). Alternatively, other pathways yet to be discovered might exist for Clp-mediated degradation of the proteins damaged by MV treatment (Fig. 2).
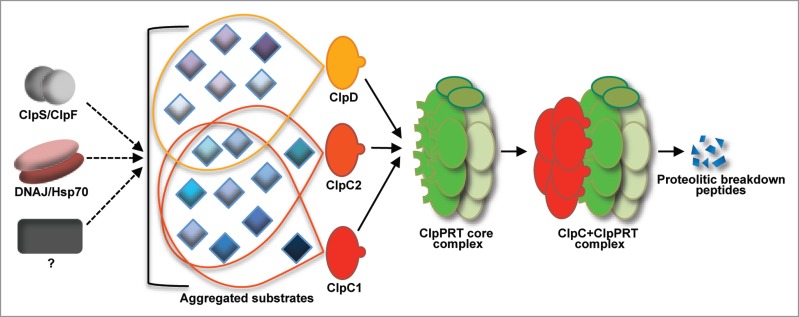
Figure 2.
Schematic model of substrate recognition and degradation by the Clp protease system. Substrate recognition can be mediated by the ClpF/ClpS adaptors, the DNAJ/Hsp70 pathway, or an alternative system whose components are unknown. These 3 pathways likely deliver substrates to the Clp-associated Hsp100 chaperones (ClpC1, ClpC2 and ClpD) for protein unfolding before degradation by the proteolytic core of the complex (ClpPRT, composed by ClpP, ClpR and ClpT subunits).
Plastidial proteases have been proposed to detoxify protein aggregates that form under stress conditions. For example, FtsH and Deg proteases coordinately control the turnover of D1 protein of photosystem II under high light stress.30-34 While disruption of Clp protease activity is known trigger the accumulation of chaperones, likely to deal with the accumulation of misfolded or aggregated proteins that cannot be degraded (the so called unfolded protein response), a role for this complex on stress protection had not been experimentally demonstrated. Besides providing evidence of such a role, our results further suggest the existence of delivery pathways for Clp protein clients that are distinct from those already reported in the literature. Further experiments should determine the molecular nature of such unknown pathways and investigate the collaboration of Hsp70 chaperones and Clp protease in the response of the chloroplast proteome to stress.
Materials and methods
Arabidopsis thaliana mutant lines used here are indicated in Table S1 (all in the Columbia background). Plants were grown in chambers under long day conditions (16h light / 8h darkness) at 22°C. For methyl viologen (paraquat) treatments, plants were sprayed with a solution containing 20 µM methyl viologen and 0,25% Tween20 twice at day 18 and at day 21. Both treated and control plants were analyzed at day 25.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was funded by grants from the Spanish MINECO Ministerio de Economia y Competitividad (BIO2014–59092-P and BIO2015–71703-REDT) and Generalitat de Catalunya (2014SGR-1434) to MRC. We also acknowledge the financial support of the Ministerio de Economia y Competitividad Severo Ochoa (SEV-2015-0533) and Generalitat de Catalunya CERCA Programmes to CRAG. EL was supported by PhD fellowships from the Mexican Consejo Nacional de Ciencia y Tecnologia (421688) and Secretaria de Educacion Publica (Beca Complemento). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mayer MP, Bukau B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell Mol Life Sci 2005; 62:670-84; PMID:15770419; http://dx.doi.org/ 10.1007/s00018-004-4464-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee S, Lee DW, Lee Y, Mayer U, Stierhof YD, Lee S, Jürgens G, Hwang I. Heat shock protein cognate 70-4 and an E3 ubiquitin ligase, CHIP, mediate plastid-destined precursor degradation through the ubiquitin-26S proteasome system in Arabidopsis. Plant Cell 2009; 21:3984-4001; PMID:20028838; http://dx.doi.org/ 10.1105/tpc.109.071548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su PH, Li HM. Arabidopsis stromal 70-kD heat shock proteins are essential for plant development and important for thermotolerance of germinating seeds. Plant Physiol 2008; 146:1231-41; PMID:18192441; http://dx.doi.org/ 10.1104/pp.107.114496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su PH, Li HM. Stromal Hsp70 is important for protein translocation into pea and Arabidopsis chloroplasts. Plant Cell 2010; 22:1516-31; PMID:20484004; http://dx.doi.org/ 10.1105/tpc.109.071415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi LX, Theg SM. A stromal heat shock protein 70 system functions in protein import into chloroplasts in the moss Physcomitrella patens. Plant Cell 2010; 22:205-20; PMID:20061551; http://dx.doi.org/ 10.1105/tpc.109.071464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishimura K, van Wijk KJ. Organization, function and substrates of the essential Clp protease system in plastids. Biochim Biophys Acta 2015; 1847:915-30; PMID:25482260; http://dx.doi.org/ 10.1016/j.bbabio.2014.11.012 [DOI] [PubMed] [Google Scholar]
- 7.Nishimura K, Kato Y, Sakamoto W. Chloroplast proteases: Updates on proteolysis within and across suborganellar compartments. Plant Physiol 2016; 171:2280-93; PMID:27288365; http://dx.doi.org/ 10.1104/pp.16.00330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimura K, Kato Y, Sakamoto W. Essentials of proteolytic machineries in chloroplasts. Mol Plant 2017; 10:4-19; PMID:27585878; http://dx.doi.org/ 10.1016/j.molp.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 9.Peltier JB, Ripoll DR, Friso G, Rudella A, Cai Y, Ytterberg J, Giacomelli L, Pillardy J, van Wijk KJ. Clp protease complexes from photosynthetic and non-photosynthetic plastids and mitochondria of plants, their predicted three-dimensional structures, and functional implications. J Biol Chem 2004; 279:4768-81; PMID:14593120; http://dx.doi.org/ 10.1074/jbc.M309212200 [DOI] [PubMed] [Google Scholar]
- 10.Nishimura K, Asakura Y, Friso G, Kim J, Oh SH, Rutschow H, Ponnala L, van Wijk KJ. ClpS1 is a conserved substrate selector for the chloroplast Clp protease system in Arabidopsis. Plant Cell 2013; 25:2276-301; PMID:23898032; http://dx.doi.org/ 10.1105/tpc.113.112557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishimura K, Apitz J, Friso G, Kim J, Ponnala L, Grimm B, van Wijk KJ. Discovery of a unique Clp component, ClpF, in chloroplasts: A proposed binary ClpF-ClpS1 adaptor complex functions in substrate recognition and delivery. Plant Cell 2015; 27:2677-91; PMID:26419670; http://dx.doi.org/ 10.1105/tpc.15.00574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pulido P, Toledo-Ortiz G, Phillips MA, Wright LP, Rodriguez-Concepcion M. Arabidopsis J-protein J20 delivers the first enzyme of the plastidial isoprenoid pathway to protein quality control. Plant Cell 2013; 25:4183-94; PMID:24104567; http://dx.doi.org/ 10.1105/tpc.113.113001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pulido P, Llamas E, Llorente B, Ventura S, Wright LP, Rodriguez-Concepcion M. Specific Hsp100 chaperones determine the fate of the first enzyme of the plastidial isoprenoid pathway for either refolding or degradation by the stromal Clp protease in Arabidopsis. PLoS Genet 2016; 12:e1005824; PMID:26815787; http://dx.doi.org/ 10.1371/journal.pgen.1005824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nystrom T. Role of oxidative carbonylation in protein quality control and senescence. EMBO J 2005; 24:1311-7; PMID:15775985; http://dx.doi.org/ 10.1038/sj.emboj.7600599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aguilaniu H, Gustafsson L, Rigoulet M, Nystrom T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science 2003; 299:1751-3; PMID:12610228; http://dx.doi.org/ 10.1126/science.1080418 [DOI] [PubMed] [Google Scholar]
- 16.Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: Paraquat and alpha-synuclein. J Biol Chem 2002; 277:1641-4; PMID:11707429; http://dx.doi.org/ 10.1074/jbc.C100560200 [DOI] [PubMed] [Google Scholar]
- 17.Kurepa J, Toh EA, Smalle JA. 26S proteasome regulatory particle mutants have increased oxidative stress tolerance. Plant J 2008; 53:102-14; PMID:17971041; http://dx.doi.org/ 10.1111/j.1365-313X.2007.03322.x [DOI] [PubMed] [Google Scholar]
- 18.Babbs CF, Pham JA, Coolbaugh RC. Lethal hydroxyl radical production in paraquat-treated plants. Plant Physiol 1989; 90:1267-70; PMID:16666920; http://dx.doi.org/ 10.1104/pp.90.4.1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu XL, Yu HD, Guan Y, Li JK, Guo FQ. Carbonylation and loss-of-function analyses of SBPase reveal its metabolic interface role in oxidative stress, carbon assimilation, and multiple aspects of growth and development in Arabidopsis. Mol Plant 2012; 5:1082-99; PMID:22402261; http://dx.doi.org/ 10.1093/mp/sss012 [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Kimber MS, Nishimura K, Friso G, Schultz L, Ponnala L, van Wijk KJ. Structures, functions, and interactions of ClpT1 and ClpT2 in the Clp protease system of Arabidopsis chloroplasts. Plant Cell 2015; 27:1477-96; PMID:25921872; http://dx.doi.org/ 10.1105/tpc.15.00106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YI, Levchenko I, Fraczkowska K, Woodruff RV, Sauer RT, Baker TA. Molecular determinants of complex formation between Clp/Hsp100 ATPases and the ClpP peptidase. Nat Struct Biol 2001; 8:230-3; PMID:11224567; http://dx.doi.org/ 10.1038/84967 [DOI] [PubMed] [Google Scholar]
- 22.Weibezahn J, Tessarz P, Schlieker C, Zahn R, Maglica Z, Lee S, Zentgraf H, Weber-Ban EU, Dougan DA, Tsai FT, et al.. Thermotolerance requires refolding of aggregated proteins by substrate translocation through the central pore of ClpB. Cell 2004; 119:653-65; PMID:15550247; http://dx.doi.org/ 10.1016/j.cell.2004.11.027 [DOI] [PubMed] [Google Scholar]
- 23.Weaver LM, Froehlich JE, Amasino RM. Chloroplast-targeted ERD1 protein declines but its mRNA increases during senescence in Arabidopsis. Plant Physiol 1999; 119:1209-16; PMID:10198079; http://dx.doi.org/ 10.1104/pp.119.4.1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh A, Singh U, Mittal D, Grover A. Genome-wide analysis of rice ClpB/HSP100, ClpC and ClpD genes. BMC Genomics 2010; 11:95; PMID:20141629; http://dx.doi.org/ 10.1186/1471-2164-11-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muthusamy SK, Dalal M, Chinnusamy V, Bansal KC. Differential regulation of genes coding for organelle and cytosolic ClpATPases under biotic and abiotic stresses in wheat. Front Plant Sci 2016; 7:929; PMID:27446158; http://dx.doi.org/ 10.3389/fpls.2016.00929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovacheva S, Bedard J, Wardle A, Patel R, Jarvis P. Further in vivo studies on the role of the molecular chaperone, Hsp93, in plastid protein import. Plant J 2007; 50:364-79; PMID:17376159; http://dx.doi.org/ 10.1111/j.1365-313X.2007.03060.x [DOI] [PubMed] [Google Scholar]
- 27.Flores-Perez U, Bedard J, Tanabe N, Lymperopoulos P, Clarke AK, Jarvis P. Functional analysis of the Hsp93/ClpC chaperone at the chloroplast envelope. Plant Physiol 2016; 170:147-62; PMID:26586836; http://dx.doi.org/ 10.1104/pp.15.01538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sjogren LL, Tanabe N, Lymperopoulos P, Khan NZ, Rodermel SR, Aronsson H, Clarke AK. Quantitative analysis of the chloroplast molecular chaperone ClpC/Hsp93 in Arabidopsis reveals new insights into its localization, interaction with the Clp proteolytic core, and functional importance. J Biol Chem 2014; 289:11318-30; PMID:24599948; http://dx.doi.org/ 10.1074/jbc.M113.534552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ling Q, Jarvis P. Regulation of chloroplast protein import by the ubiquitin E3 ligase SP1 is important for stress tolerance in plants. Curr Biol 2015; 25:2527-34; PMID:26387714; http://dx.doi.org/ 10.1016/j.cub.2015.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindahl M, Spetea C, Hundal T, Oppenheim AB, Adam Z, Andersson B. The thylakoid FtsH protease plays a role in the light-induced turnover of the photosystem II D1 protein. Plant Cell 2000; 12:419-31; PMID:10715327; http://dx.doi.org/ 10.1105/tpc.12.3.419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haussuhl K, Andersson B, Adamska I. A chloroplast DegP2 protease performs the primary cleavage of the photodamaged D1 protein in plant photosystem II. Embo J 2001; 20:713-22; PMID:11179216; http://dx.doi.org/ 10.1093/emboj/20.4.713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun X, Peng L, Guo J, Chi W, Ma J, Lu C, Zhang L. Formation of DEG5 and DEG8 complexes and their involvement in the degradation of photodamaged photosystem II reaction center D1 protein in Arabidopsis. Plant Cell 2007; 19:1347-61; PMID:17449806; http://dx.doi.org/ 10.1105/tpc.106.049510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner R, Aigner H, Pruzinska A, Jankanpaa HJ, Jansson S, Funk C. Fitness analyses of Arabidopsis thaliana mutants depleted of FtsH metalloproteases and characterization of three FtsH6 deletion mutants exposed to high light stress, senescence and chilling. New Phytol 2011; 191:449-58; PMID:21438879; http://dx.doi.org/ 10.1111/j.1469-8137.2011.03684.x [DOI] [PubMed] [Google Scholar]
- 34.Kato Y, Sakamoto W. Possible compensatory role among chloroplast proteases under excess-light stress condition. Plant Signal Behav 2013; 8:e23198; PMID:23299325; http://dx.doi.org/ 10.4161/psb.23198 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.