Summary
Hepatitis C virus reinfection rates after cure in HIV coinfection were highest in those injecting cocaine or methamphetamines and in men who have sex with men reporting risky sexual behavior. Interventions addressing these risks are needed to achieve HCV elimination.
Keywords: HIV, hepatitis C virus, reinfection, sustained virologic response, hepatitis C treatment.
Abstract
Background.
Highly effective hepatitis C virus (HCV) therapies have spurred a scale-up of treatment to populations at greater risk of reinfection after sustained virologic response (SVR). Reinfection may be higher in HIV–HCV coinfection, but prior studies have considered small selected populations. We assessed risk factors for reinfection after SVR in a representative cohort of Canadian coinfected patients in clinical care.
Methods.
All patients achieving SVR after HCV treatment were followed with HCV RNA measurements every 6 months in a prospective cohort study. We used Bayesian Cox regression to estimate reinfection rates according to patient reported injection drug use (IDU) and sexual activity among men who have sex with men (MSM).
Results.
Of 497 patients treated for HCV, 257 achieved SVR and had at least 1 subsequent RNA measurement. During 589 person-years of follow-up (PYFU) after SVR, 18 (7%) became HCV RNA positive. The adjusted reinfection rate (per 1000 PYFU) in the first year after SVR was highest in those who reported high-frequency IDU (58; 95% credible interval [CrI], 18–134) followed by MSM reporting high-risk sexual activity (26; 95% CrI, 6–66) and low-frequency IDU (22; 95% CrI, 4–68). The rate in low-risk MSM (16; 95% CrI, 4–38) was similar to that in reference patients (10; 95% CrI, 4–20). Reinfection rates did not diminish with time.
Conclusions.
HCV reinfection rates varied according to risk. Measures are needed to reduce risk behaviors and increase monitoring in high-risk IDU and MSM if HCV elimination targets are to be realized.
Hepatitis C virus (HCV) coinfection is common among human immunodeficiency virus (HIV)-infected individuals and is associated with higher HCV RNA and more rapid liver disease progression compared with HCV infection alone [1]. HCV treatment is now highly effective in coinfected patients and can lead to marked reductions in liver disease and all-cause mortality [2]. Regardless, HCV treatment uptake in coinfected patients currently remains low as many providers are concerned about ongoing substance use and risk behaviors that could impact adherence and lead to reinfection after successful treatment [3].
In a recent metaanalysis, the rate of HCV reinfection after sustained virologic response (SVR) was higher among HIV-coinfected (32 per 1000 person-years of follow-up [PYFU]; 95% confidence interval [CI], 0–123) than among monoinfected patients (22 per 1000 PYFU; 95% CI, 13–33) [4], raising concerns about the long-term impact of treating HCV in coinfected patients. However, only 4 studies with coinfected patients were included in the analysis and reinfection rates were highly variable, ranging from zero in 2 clinical trials where people who inject drugs (PWID) were excluded [5, 6] to 96 per 1000 PYFU in high-risk men who have sex with men (MSM) [7] and 134 reinfections per 1000 PYFU in prisoners [8]. The variability in reinfection rates may be explained by the disparate clinical populations studied and the few reinfections in each study. Similar variability in reinfection rates has been reported in small studies of PWID with [9] and without HIV coinfection [10]. Therefore, there remains considerable uncertainty as to the true risk of reinfection in coinfected patients following SVR and the relative importance of various risk factors for reinfection.
New direct-acting antivirals (DAAs) for HCV are highly effective but expensive. Reinfections come at a cost to individuals who may not be able to access retreatment in many jurisdictions [11, 12]; to public health, through increased transmissions; and to the healthcare system. Identifying patients at greatest risk of reinfection who would benefit most from monitoring and targeted counseling is important when making HCV treatment decisions. Therefore, using statistical methods appropriate for small samples, we assessed rates and risk factors for HCV reinfection after SVR in a broadly representative cohort of Canadian HIV-coinfected patients in clinical care.
METHODS
Study Population
Data from the Canadian Co-infection Cohort, an ongoing prospective study with visits scheduled every 6 months, were analyzed [13]. The cohort includes more than 1600 patients recruited from HIV clinic populations at 18 centers across 6 Canadian provinces. We followed all coinfected patients who achieved SVR with HCV treatment between January 2003 and July 2016 having at least 1 post-SVR study visit with an available HCV RNA measurement. SVR was defined as a negative HCV RNA at least 12 weeks after the end-of-treatment date (because >95% of late relapses occur within this period [14]). The study was approved by the community advisory committee of the Canadian Institutes of Health Research–Canadian HIV Trials Network and by all institutional ethics boards of participating centers.
Outcome
Patients were followed post-SVR with HCV RNA measurements every 6 months until reinfection or their last study visit prior to July 2016. We defined reinfection as a single detectable HCV RNA measurement post-SVR measured in local laboratories using either a qualitative assay (COBAS Ampliprep/TaqMan HCV Test, v2.0, Roche Molecular Systems) or a quantitative assay (Abbott RealTime PCR; Abbott Molecular Inc.).
Risk Factors
We categorized patients according to principal risk factors for incident HCV infection in HIV-coinfected persons (injection drug use [IDU] and high-risk sexual activity in MSM [15, 16]) using information that could be readily obtained by clinicians. Recent high-frequency IDU was defined as any self-reported use of injection cocaine or methamphetamines in the last 6 months because these drugs are associated with multiple injections per day and a high degree of risk-taking behavior [17]. Low-frequency IDU was defined as the self-report of any other injection drug (mainly opiates; Supplementary Table 1). Shared IDU equipment was defined as the reported sharing of needles or other paraphernalia such as containers and spoons. Recent high-risk sexual behavior among MSM was defined as reporting both more than 1 sexual partner and less than 100% condom use in the preceding 6 months. In a sensitivity analysis, we alternatively defined high-risk behavior among men as reporting a sexually transmitted infection (STI) in the previous 6 months.
Statistical Analyses
Regression methods are valid when large samples are used; small samples can lead to biased results. The consequence of too few events is a “sparse-data” bias away from the null [18]. Bayesian analyses with informative priors offer a solution to this problem [18] because appropriate informative priors anchor each parameter estimate to a range of values that is clinically sensible and reduce the possibility of extreme values that no knowledgeable clinician would find credible [19, 20].
We used Bayesian methods to fit an adjusted Cox regression model appropriate for interval censored data with an offset that allows for any variation in the time between follow-up visits [21]. To allow some variation in the baseline hazard over time, we estimated the rate of reinfection in each of the following 3 periods: within the first year, between 1 and 3 years, and beyond 3 years. For each parameter we asserted “weakly” informative priors. These are defined as distributions where “the percentiles of the prior distribution would be viewed as at least reasonable if not liberally inclusive by all those working in the research topic” [22]. We categorized potential risk factors as possibly or probably harmful, possibly or probably protective, or of uncertain direction. We then assigned wide log-normal distributions to each category such that these distributions reduced the probability of extreme hazard ratios (HRs; Supplementary Table 2). We used a prior HR of 1.5 (95% credible interval [CrI], 0.38–6.0) for possibly harmful risk factors, 2.0 (95% CrI, 0.5–8.0) for probably harmful risk factors, 1.0 (95% CrI, 0.25–4.0) for factors of uncertain direction, and 0.69 (95% CrI, 0.17–2.7) for possibly protective risk factors [20, 23]. We used R 3.1.0, R2WinBUGS 2.1–20 and WinBUGS 1.4.3 for our analysis.
Covariates that represent potential risk factors for reinfection were time-varying, taking the value measured at the visit prior to each measurement of HCV RNA. We considered that MSM, high-risk sexual behavior among MSM, and low-frequency IDU were all possibly harmful, while high-frequency IDU and shared equipment were both probably harmful. We also included covariates for sex, Aboriginal ethnicity, age (per 10 years), and CD4 cell count (per 100 cells/µL) as a measure of patient health. We considered sex and ethnicity to be risk factors of uncertain direction and increasing age and increasing CD4 cell count to be possibly protective.
We defined reference patients as males of other than Aboriginal ethnicity who reported neither male sexual partners nor IDU in the past 6 months and achieving SVR at age 45 with a CD4 cell count of 400 cells/µL. Our prior for the reinfection rate in reference patients was, however, based on an estimate from an earlier study that was similar to ours, rather than “weakly” informative. In this hospital-based Spanish coinfection cohort, 4 of 84 patients were reinfected in a mean follow-up of 34 months after an SVR [9]. Our prior for the reinfection rate (10 per 1000 PYFU; 95% CrI, 3–32) is as close to the estimate from this study (12; 95% CI, 3–31) as we could get with a log-normal prior.
With Bayesian methods, results should be interpreted with reference to the priors [19]. The shift from prior estimate to posterior estimate (“the results”) reflects the information in the data. We therefore present both prior and posterior estimates and, in sensitivity analyses, we considered posterior estimates under different priors and under alternative assumptions.
Sensitivity Analysis
In a first sensitivity analysis, we replaced our prior for the rein-fection rate with a “weakly” informative prior (7 per 1000 PYFU; 95% CrI, 1–50). As an example, this distribution is far wider than the pooled estimate for high-risk HCV-monoinfected patients in a recent metaanalysis (22 per 1000 PYFU; 95% CI, 13–33) [4]. In a second sensitivity analysis, we made different assumptions about the date of reinfection for 3 patients for whom the date was uncertain because of missed follow-up visits. In a third sensitivity analysis, we used an alternative measure of high-risk MSM, that is, a male reporting an STI in the previous 6 months.
RESULTS
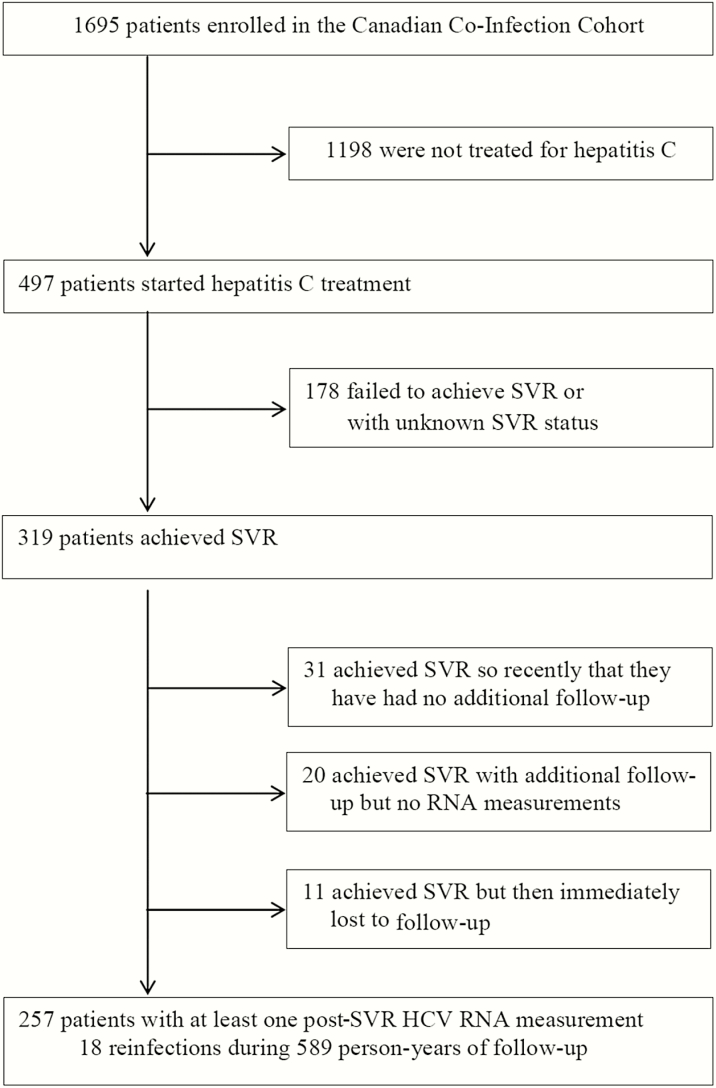
As of July 2016, 497 coinfected patients initiated HCV treatment, of whom 319 achieved SVR. Of these, 257 had at least 1 post-SVR HCV RNA measurement available and were included in the analysis (31 had recently completed follow-up and 31 had no available measures; Figure 1). Most of the patients were male (82%) with a history of IDU (74%); 14% were actively using injection drugs and 33% reported being MSM (Table 1). Fifty-one patients were treated with interferon-free regimens. The majority were receiving antiretrovirals (92%) and had undetectable HIV RNA (87%) with median CD4 cell count of 450 cells/µL. The median total post-SVR follow-up time was 1.5 years (interquartile range [IQR], 0.6, 3.2). All patients had more than 1 post-SVR HCV measurement (median, 3; IQR, 2, 6), with a median time between measurements of 6 months (IQR, 6, 8). Of the 239 who were censored without reinfection, 183 were administratively censored, 11 died, and 45 were lost to follow-up post-SVR (Supplementary Table 3).
Figure 1.
Patient flow diagram. As of July 2016, 497 coinfected patients initiated hepatitis C virus (HCV) treatment, of whom 319 achieved sustained virologic response (SVR). Of these, 257 patients had at least 1 post-SVR HCV RNA measurement available and were included in the analysis. Patients excluded from the analysis (n = 62) were older (median age 52 years), more likely to be Aboriginal (15%), and less likely to be former (65%) or current (8%) injection drug users than those included. Abbreviations: HCV, hepatitis C virus; SVR, sustained virologic response.
Table 1.
Patient Characteristics and Recent (in the Previous 6 Months) Risk Behavior Reported at the Time of Sustained Virologic Response (Baseline) and at the End of Follow-up
| Characteristic | At Sustained Virologic Response | At the End of Follow-up | |
|---|---|---|---|
| At Visit Prior to Reinfection | At Visit Prior to Censoring (Without Reinfection) | ||
| (n = 257) | (n = 18) | (n = 239) | |
| Follow-up in years, median (IQR) |
– | 2.5 (1.6, 3.2) | 1.4 (0.6, 3.2) |
| Demographics and risk behaviors | |||
| Age in years, median (IQR) | 49 (43, 53) | 48 (42, 54) | 51 (45, 56) |
| Male sex, % | 82 | 89 | 82 |
| Aboriginal ethnicity, % | 8 | 11 | 8 |
| Ever IDU, % | 74 | 72 | 74 |
| Type of recent IDU, % a,b | |||
| No IDU | 86 | 50 | 86 |
| Low frequency | 3 | 6 | 2 |
| High frequency | 11 | 44 | 12 |
| Recent shared IDU equipment, % a | 0 | 0 | 1 |
| Recent men who have sex with men activity, % a,c,d | 33 | 40 | 29 |
| Recent condom use, % a,d | |||
| Not sexually active | 43 | 50 | 47 |
| Always | 32 | 25 | 28 |
| Sometimes or never | 25 | 25 | 25 |
| Recent sexually transmitted infection diagnosis, % a,d | 3 | 11 | 4 |
| Clinic type, % | |||
| Tertiary care | 79 | 72 | 80 |
| Community based | 17 | 28 | 16 |
| Rural | 4 | 0 | 4 |
| HIV characteristics | |||
| Time since HIV diagnosis in years, median (IQR) | 15 (9, 22) | 19 (12, 24) | 17 (12, 23) |
| CD4 cell count in cells/µL, median (IQR) d | 450 (310, 640) | 465 (350, 590) | 540 (390, 750) |
| HIV viral load >50 copies/mL, % d | 13 | 28 | 8 |
| On antiretroviral therapy, % | 92 | 72 | 95 |
| HCV characteristics | |||
| Duration of HCV infection in years, median (IQR) | 21 (12, 29) | 21 (13, 28) | 24 (15, 31) |
| HCV genotype at initial infection, % e | |||
| 1 | 62 | 44 | 63 |
| 2 | 8 | 17 | 7 |
| 3 | 20 | 28 | 20 |
| 4 | 2 | 0 | 2 |
| Unknown | 8 | 11 | 8 |
| IL-28B haplotype, % | |||
| CC | 41 | 56 | 40 |
| CT | 27 | 22 | 27 |
| TT | 9 | 5 | 9 |
| Unknown | 23 | 17 | 24 |
| Median AST to platelet ratio index (IQR) | 0.42 (0.31, 0.71) | 0.38 (0.25, 0.51) | 0.40 (0.30, 0.55) |
| Cirrhosis, % | 21 | 28 | 23 |
| HCV treatment, % | |||
| Interferon-free | 20 | 0 | 21 |
Canadian Co-infection Cohort, n = 257.
Abbreviations: AST, aspartate aminotransferase; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IDU, injection drug use; IQR, interquartile range.
aPatient reported behavior for the previous 6 months.
bHigh frequency: patient reported injecting cocaine or methamphetamines. Low frequency: patient reported injecting some other drug.
cAmong males only (n = 212).
dNotes on missing data: Recent men who have sex with men: at baseline, 3; at end of follow-up, 1 of those reinfected, 2 of those censored. Recent condom use: at baseline, 9; at end of follow-up, 2 of those reinfected, 5 of those censored. Recent sexually transmitted infection: at baseline, 6; at end of follow-up, 5 of those censored. CD4 cell count: at baseline, 4; at end of follow-up, 6 of those censored. HIV viral load: at baseline, 8; at end of follow-up, 7 of those censored.
ePrior to HCV treatment.
During 589 PYFU after achieving SVR, 18 patients (7%) became HCV RNA positive (Table 2), with a median time to reinfection of 2.5 years (IQR, 1.6, 3.2) and an unadjusted reinfection rate of 31 per 1000 PYFU. Of 18 reinfections, 5 (28%) spontaneously cleared and 13 became chronically infected, 9 of whom had a genotype switch. The adjusted reinfection rate per 1000 PYFU was 10 (95% CrI, 4–20) for reference patients in the first year after SVR, 20 (95% CrI, 8–38) in the first 1 to 3 years, and 18 (95% CrI, 8–36) after 3 years (Supplementary Table 4). In our second sensitivity analysis these rates (per 1000 PYFU) were 12 (95% CrI, 6–24) in the first year after SVR, 18 (95% CrI, 8–32) in the first 1 to 3 years, and 16 (95% CrI, 6–32) after 3 years. Thus, the estimated rate of reinfection was lower in the first year than in later years regardless of when reinfection was assumed to have occurred for the 3 patients with an uncertain reinfection date (Supplementary Figure 1).
Table 2.
Characteristics of the 18 Hepatitis C Virus Reinfections seen in the Canadian Co-infection Cohort
| Sex | Risk Factor Reported 6 Months Prior to Reinfection | First Positive HCV RNA Value After SVR | Genotype at Baseline | Genotype at Reinfection | Time from SVR to Reinfection (days) | Number of RNA Measures Before/ After Reinfection | Average Time Between Measures (days) | HCV RNA (copies/ mL) Values Post- Reinfection | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | Low-risk MSM, high-frequency IDU |
90000 | 1 | Not tested | 1281 | 7/1 | 183 | Subsequent visit undetectable |
| 2 | Male | None reported | Detectable (qualitative) | 2b | 3a | 259 | 2/4 | 259 | All subsequent visits detectable >3 000 000 |
| 3 | Male | High-frequency IDU | 126000 | 2 | 1a | 925 | 4/5 | 308 | All subsequent visits detectable > 1 000 000 |
| 4 | Male | High-risk MSM | Detectable (qualitative) | 1a | 3a | 573 | 4/12 | 191 | All subsequent visits detectable (qualitative) |
| 5 | Male | High-risk MSM | Detectable (qualitative) | 1a | 3a | 370 | 3/1 | 185 | Subsequent visit detectable (qualitative) |
| 6 | Male | Low-risk MSM | Detectable (qualitative) | 3a | 1 | 698 | 2/2 | 698 | All subsequent visits detectable > 900 000 |
| 7 | Female | High-frequency IDU | Detectable (qualitative) | 1a | 3a | 1297 | 8/2 | 185 | All subsequent visits detectable (qualitative) |
| 8 | Male | High-frequency IDU | Detectable (qualitative) | 2b | 1a | 1309 | 6/4 | 262 | All subsequent visits detectable (qualitative) |
| 9 | Male | High-frequency IDU | Detectable (qualitative) | 3a | Not tested | 1115 | 4/1 | 372 | Subsequent visit undetectable |
| 10 | Male | High-risk MSM | Detectable (qualitative) | 1a | Not tested | 1088 | 7/4 | 181 | All subsequent visits undetectable |
| 11 | Male | High-frequency IDU | 26000 | 3a | 1a | 548 | 2/2 | 274 | Subsequent visits detectable > 26100 |
| 12 | Female | None reported | 1400000 | 3a | 1 | 719 | 3/1 | 360 | Subsequent visit detectable at 5 000 000 |
| 13 | Male | None reported | Detectable (qualitative) | 3 | 3a | 350 | 3/1 | 175 | Subsequent visit detectable at 9000 |
| 14 | Male | Low-risk MSM | 27000 | 1a | 1a | 1167 | 6/2 | 233 | All subsequent visits detectable > 242000 |
| 15 | Male | None reported | Detectable (qualitative) | 3a | Not tested | 169 | 2/1 | 169 | Subsequent visit undetectable |
| 16 | Male | Low-frequency IDU | 178 | Unknown | 3a | 1063 | 6/1 | 213 | Subsequent visit undetectable |
| 17 | Male | High-frequency IDU | 412000 | 1a | 1a | 1396 | 5/1 | 349 | Subsequent visit detectable at 1500 |
| 18 | Male | High-frequency IDU | 531000 | 1a | 1a | 549 | 2/1 | 549 | Subsequent visit detectable at 322000 |
Abbreviations: HCV, hepatitis C virus; IDU, injection drug use; MSM, men who have sex with men; SVR, sustained virologic response.
Patients who became reinfected were 3 times more likely to report having engaged in high-frequency IDU at the visit before reinfection compared to those who were censored (Table 1). Patients who became reinfected were also somewhat more likely to report being MSM in the last 6 months. Among the 6 MSM who became reinfected, none reported low-frequency IDU and only 1 reported high-frequency IDU; 3 reported recent high-risk sexual behavior and 2 reported a recent STI. At the time of reinfection, median CD4 cell count was lower and more patients were off antiretroviral therapy with a detectable HIV RNA compared to those not reinfected at the end of follow-up.
Comparison of posterior with prior HRs (Table 3) showed that high-frequency IDU was even more detrimental than we anticipated. There was no information in the data about the risk of sharing IDU equipment; none of the reinfected patients reported sharing IDU equipment at the visit prior to reinfection. Estimates for other risk factors were broadly in line with our expectations in that posterior intervals were contained within prior intervals. Estimates for high-risk sexual activity in MSM were similar regardless of how this was defined (posterior HR, 1.8; 95% CrI, 0.56–4.4, and posterior HR, 2.0; 95% CrI, 0.57–5.1 in the main and third sensitivity analysis, respectively).
Table 3.
Prior and Posterior Estimates of Risk Factors for Reinfection With Hepatitis C in Patients With a Sustained Virologic Response After Treatment
| Risk Factor | Prior HR (95% CrI) | Posterior HR (95% CrI) |
|---|---|---|
| MSM (vs heterosexual male) a | 1.5 (0.38–6.0) | 1.7 (0.62–3.4) |
| High-risk sexual behavior in MSM a,b | 1.5 (0.38–6.0) | 1.8 (0.56–4.4) |
| Low frequency IDU a,c | 1.5 (0.38–6.0) | 2.3 (0.53–6.3) |
| High frequency IDU a,d | 2.0 (0.50–8.0) | 6.1 (2.5–12) |
| Shared IDU equipment a,e | 2.0 (0.50–8.0) | 2.0 (0.44–5.6) |
| Female (vs heterosexual male) | 1.0 (0.25–4.0) | 1.0 (0.32–2.5) |
| Aboriginal ethnicity | 1.0 (0.25–4.0) | 1.6 (0.42–4.1) |
| Age at sustained virologic response (per 10-year increase) | 0.67 (0.17–2.7) | 0.90 (0.48–1.5) |
| Latest CD4+ cell count (per 100 cells/μL increase) | 0.67 (0.17–2.7) | 0.82 (0.62–1.0) |
Canadian Co-infection Cohort, n = 257.
Abbreviations: CrI, credible interval; HR, hazard ratio; IDU, injection drug use; MSM, men who have sex with men.
aPatient report of behavior in the previous 6 months.
bPatient reports more than 1 male sexual partner and less-than-perfect condom use.
cPatient reports injecting drugs other than cocaine or methamphetamines (mainly opiates).
dPatient reports injecting cocaine or methamphetamines.
ePatient reports shared use of needles or of other paraphernalia, such as containers and spoons.
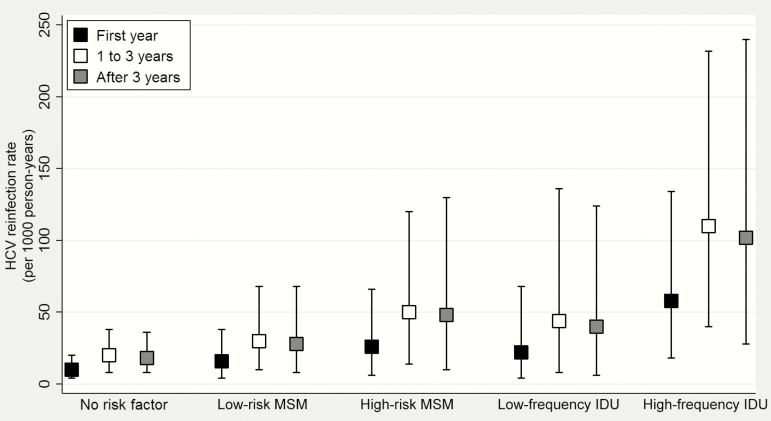
The reinfection rate in those reporting high-frequency IDU was therefore potentially much higher than in reference patients; 58 per 1000 PYFU (95% CrI, 18–134) during the first year after SVR (Figure 2). By comparison, rates for high-risk MSM and for low-frequency IDU were intermediate (26; 95% CrI, 6–66 and 22; 95% CrI, 4–68 per 1000 PYFU, respectively). The rate for MSM reporting low-risk sexual activity (16; 95% CrI, 4–38 per 1000 PYFU) was lower and similar to that of reference patients.
Figure 2.
Hepatitis C reinfection rates and 95% credible intervals per 1000 person-years for up to 1 year, 1 to 3 years, and more than 3 years after a sustained virologic response (Canadian Co-infection Cohort, n = 257). Reference patients are those with no risk factors. Abbreviations: HCV, hepatitis C virus; IDU, injection drug use; MSM, men who have sex with men.
DISCUSSION
Safe and simple short-course DAAs have spurred a rapid scale-up of HCV treatment to populations at greater risk of reinfection. Given the costly consequences of reinfection, it is essential to have reasonable estimates of the risk of reinfection and to understand which subgroups need risk reduction measures. In a large and diverse cohort representative of the Canadian coinfection population in care [13], we found that those engaging in high-frequency IDU (cocaine and methamphetamines) were at greatest risk of becoming reinfected at a rate roughly 6 times that of low-risk reference patients. This rate was higher than we had anticipated. We estimated intermediate, and comparable, risk for high-risk MSM and low-frequency IDU. Reinfections among MSM appear to have been sexually transmitted with one exception. Reinfection rates were low in low-risk MSM and in reference patients, the majority of whom were former PWID, emphasizing the safety of treating HCV in this subgroup.
By having a more broadly representative population than in past studies and evaluating reinfection in specific subgroups, we were able to put previous estimates of reinfection rates into context. For example, we estimated higher rates of reinfection than seen in several other studies of coinfected patients. Studies from clinical trials have reported no reinfections [5, 6]. This is not unexpected given stringent inclusion criteria that led to exclusion of active substance users and short post-trial follow-up [24]. In the study by Pineda et al (on which we based our prior distribution for the rate of reinfection) [9], the overall reinfection rate was 12 per 1000 PYFU (95% CI, 3–31), similar to the rate we estimated for our reference patients in the first year after SVR (Supplementary Table 4). However, while 86% acquired HCV through IDU in their study, the vast majority were no longer using injection drugs. Most of our reference patients were also former PWID. However, these rates are substantially higher than those estimated for HCV monoinfected patients with no reported risk factors (1.9 per 1000 PYFU; 95% CI, 0.7–3.4 [4]) although less than rates in high-risk HCV-monoinfected patients (22 per 1000 PYFU; 95% CI, 13–33 [4]) and patients actively injecting drugs (24 per 1000 PYFU; 95% CI, 9–61 [25]). Previously it has been difficult to estimate the risk of reinfection after SVR according to recency, frequency, or type of IDU as we have done here because of lack of data [10].
Martin et al reported high reinfection rates after SVR among HIV-positive MSM attending a London clinic (96 per 1000 PYFU; 95% CI, 58–105) [7], nearly twice our estimate in high-risk MSM. Their population was clearly at elevated risk, with many patients having second and even third reinfections. High rates of reinfections have also been observed among MSM in Amsterdam after treatment of acute HCV (152 per 1000 PYFU; 95% CI, 80–265) [26]. The recent epidemic of sexually transmitted HCV infection among MSM has underscored the importance of sexual networks [27, 28], and thus reinfection rates may be regionally specific. Lower rates of HCV infection among MSM have been seen in Canada than in Europe [29, 30]. Higher rates of HCV seroconversion have been reported among MSM with a recent STI, especially syphilis [30–32]. A recent STI in our study was suggestive of a higher risk of reinfection and likely serves as a marker of unprotected high-risk sexual activity that should prompt more frequent HCV testing.
In our study, the rate of reinfection did not diminish over time. This ongoing risk underlines that regular monitoring for reinfection is important following SVR, particularly in persons who continue to engage in IDU and for high-risk MSM. In a recent study from Scotland where concerted efforts to treat PWID for HCV have been made, the frequency of HCV RNA testing after SVR was low [33]. Only 61% were tested at least once in 4.5 years of follow-up post-SVR and only 31% received at least 2 tests. The reinfection rate was estimated to be 17 per 1000 PYFU (95% CI, 7–35), but this is likely an underestimate given the low rate of testing. In order to gauge the success of HCV treatment scale-up, it will be essential to routinely monitor for reinfection.
Strengths of our study include a relatively large number of reinfections from a widely generalizable population, although within the cohort fewer women, Aboriginals, and PWID received HCV treatment [3]. While we used methods to minimize small sample bias, we were still limited by a small number of reinfections, which led to imprecision in estimates. Risk behaviors were self-reported; however, we were interested in using reported risk behavior to identify patient subgroups at risk of reinfection. Not all risky behaviors appear to be useful for this purpose. For example, none of those who were reinfected reported sharing injection equipment at the visit 6 months prior to reinfection. While the majority of patients acquired a different HCV genotype at the time of reinfection, we were not able to distinguish reinfection from late relapse in 4 patients with similar genotypes at baseline and in 5 patients who spontaneously cleared following reinfection. The median time to reinfection for those who did not switch genotypes was, however, more than 2.5 years after SVR. There is no evidence thus far that HCV can reemerge from reservoirs after such a long period. While our results are generalizable to coinfected patients who receive care in a Canadian context, there may be very different geographical realities (concentrated epidemics) where infection rates could be considerably higher or lower. It is therefore important to understand local epidemiology when assessing the risk in a given community and in marginalized groups [34, 35]. Finally, the observed rates may be an underestimate of the potential reinfection risk in the era of DAAs, as most patients received interferon-based regimens that arguably could act as a disincentive to becoming reinfected given their poor tolerability.
While we report high rates of reinfection in some subgroups, the majority of patients did not become reinfected after achieving SVR. Our results should not serve to discriminate against offering HCV therapy to coinfected persons but rather should guide clinicians and policy makers on how best to intervene in order to reduce risk and identify subgroups that need more frequent monitoring. Indeed, information obtained from patients about their drug use and sexual activity was useful in determining the risk of reinfection. Patient education, harm reduction measures including substitution therapy [36, 37], enhanced social supports for PWID [38], and behavioral interventions for high-risk MSM [39, 40], while important, have met with mixed success. In particular, there are few therapeutic options to treat the high-frequency cocaine and methamphetamine users at highest risk of reinfection [41, 42]. Given the negative health consequences of HCV and the paucity of options for addressing high-risk behaviors, treatment needs to be rapidly expanded to all high-risk HCV transmitters from whom reinfections occur. Only in this way will the ambitious World Health Organization targets of reducing HCV infections by 90% by 2030 be realized [43].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank all study coordinators and nurses for their assistance with study coordination, participant recruitment, and care.
Financial support. The Canadian Co-infection Cohort is funded by the Canadian Institutes of Health Research (CIHR; FDN-143270), the CIHR-Canadian HIV Trials Network (CTN-222) and the Fonds de recherche Québec- Santé, Réseau SIDA/maladies infectieuses (FRQ-S). M. B. K. is supported by a Chercheur National career award from the FRQ-S.
Potential conflicts of interest. J. G. received personal fees for being a member of the national advisory boards of AbbVie, Gilead, Merck, Janssen, and ViiV Healthcare. S. W. received grants, consulting fees, lecture fees, nonfinancial support, and fees for the development of educational presentations from Merck, ViiV Healthcare, GlaxoSmithKline, Pfizer, Gilead, AbbVie, Bristol-Myers Squibb, and Janssen. C. C. reports receipt of consulting fees from AbbVie, Gilead, and Merck and grants from AbbVie and Gilead. J. C. received consulting fees from Bristol-Meyers Squibb, grants from ViiV Healthcare and Gilead, and payment for lectures from Merck. V. M.-L. reports receipt of consulting fees from Merck and Gilead, a grant from Gilead, and lecture fees from AbbVie, Merck, and Gilead. B. C. reports receipt of grants, travel support, personal fees for speakers bureau and advisory board participation from AbbVie, Gilead, and Merck. N. P. received personal fees for being a member of advisory boards from Gilead, ViiV Healthcare, and Merck. M.-L. V. has received consulting fees from Boehringer Ingelheim and Merck; consulting fees and lecture honoraria from Janssen Pharmaceuticals, Gilead, Hoffman–La Roche, and Vertex Pharmaceuticals; and speaker fees from Gilead. M. B. K. has received research grants for investigator-initiated trials from Merck and ViiV Healthcare; consulting fees from ViiV Healthcare, Bristol-Meyers Squibb, Merck, Gilead, and AbbVie. All other authors: No potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Peters L, Klein MB. Epidemiology of hepatitis C virus in HIV-infected patients. Curr Opin HIV AIDS 2015; 10:297–302. [DOI] [PubMed] [Google Scholar]
- 2. Limketkai BN, Mehta SH, Sutcliffe CG, et al. Relationship of liver disease stage and antiviral therapy with liver-related events and death in adults coinfected with HIV/HCV. JAMA 2012; 308:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Young J, Potter M, Cox J, et al. Variation between Canadian centres in the uptake of treatment for hepatitis C by patients coinfected with HIV: a prospective cohort study. CMAJ Open 2013; 1:E106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simmons B, Saleem J, Hill A, Riley RD, Cooke GS. Risk of late relapse or reinfection with hepatitis C virus after achieving a sustained virological response: a systematic review and meta-analysis. Clin Infect Dis 2016; 62:683–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Swain MG, Lai MY, Shiffman ML, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology 2010; 139:1593–601. [DOI] [PubMed] [Google Scholar]
- 6. Soriano V, Núñez M, Camino N, et al. Hepatitis C virus-RNA clearance in HIV-coinfected patients with chronic hepatitis C treated with pegylated interferon plus ribavirin. Antivir Ther 2004; 9:505–9. [PubMed] [Google Scholar]
- 7. Martin TC, Martin NK, Hickman M, et al. Hepatitis C virus reinfection incidence and treatment outcome among HIV-positive MSM. AIDS 2013; 27:2551–7. [DOI] [PubMed] [Google Scholar]
- 8. Marco A, Esteban JI, Solé C, et al. Hepatitis C virus reinfection among prisoners with sustained virological response after treatment for chronic hepatitis C. J Hepatol 2013; 59:45–51. [DOI] [PubMed] [Google Scholar]
- 9. Pineda JA, Núñez-Torres R, Téllez F, et al. ; HEPAVIR Group of the Andalusian Society of Infectious Diseases Hepatitis C virus reinfection after sustained virological response in HIV-infected patients with chronic hepatitis C. J Infect 2015; 71:571–7. [DOI] [PubMed] [Google Scholar]
- 10. Grady BP, Schinkel J, Thomas XV, Dalgard O. Hepatitis C virus reinfection following treatment among people who use drugs. Clin Infect Dis 2013; 57Suppl 2:S105–10. [DOI] [PubMed] [Google Scholar]
- 11. Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med 2015; 163:215–23. [DOI] [PubMed] [Google Scholar]
- 12. Marshall AD, Saeed S, Barrett L, et al. ; Canadian Network on Hepatitis C Restrictions for reimbursement of direct-acting antiviral treatment for hepatitis C virus infection in Canada: a descriptive study. CMAJ Open 2016; 4:E605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klein MB, Saeed S, Yang H, et al. Cohort profile: the Canadian HIV-hepatitis C co-infection cohort study. Int J Epidemiol 2010; 39:1162–9. [DOI] [PubMed] [Google Scholar]
- 14. Medrano J, Barreiro P, Resino S, et al. Rate and timing of hepatitis C virus relapse after a successful course of pegylated interferon plus ribavirin in HIV-infected and HIV-uninfected patients. Clin Infect Dis 2009; 49:1397–401. [DOI] [PubMed] [Google Scholar]
- 15. Kim AY, Onofrey S, Church DR. An epidemiologic update on hepatitis C infection in persons living with or at risk of HIV infection. J Infect Dis 2013; 207 Suppl 1:S1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marcellin F, Lorente N, Demoulin B, et al. ; ANRS VESPA2 Study Group Comparison of risk factors in HIV-infected men who have sex with men, coinfected or not with hepatitis C virus (ANRS VESPA2 French cross-sectional national survey). Sex Transm Infect 2015; 91:21–3. [DOI] [PubMed] [Google Scholar]
- 17. Leri F, Stewart J, Tremblay A, Bruneau J. Heroin and cocaine co-use in a group of injection drug users in Montréal. J Psychiatry Neurosci 2004; 29:40–7. [PMC free article] [PubMed] [Google Scholar]
- 18. Greenland S, Schwartzbaum JA, Finkle WD. Problems due to small samples and sparse data in conditional logistic regression analysis. Am J Epidemiol 2000; 151:531–9. [DOI] [PubMed] [Google Scholar]
- 19. Greenland S. Bayesian perspectives for epidemiological research: I. Foundations and basic methods. Int J Epidemiol 2006; 35:765–75. [DOI] [PubMed] [Google Scholar]
- 20. Greenland S. Bayesian perspectives for epidemiological research. II. Regression analysis. Int J Epidemiol 2007; 36:195–202. [DOI] [PubMed] [Google Scholar]
- 21. Carlin JB, Wolfe R, Coffey C, Patton GC. Analysis of binary outcomes in longitudinal studies using weighted estimating equations and discrete-time survival methods: prevalence and incidence of smoking in an adolescent cohort. Stat Med 1999; 18:2655–79. [DOI] [PubMed] [Google Scholar]
- 22. Greenland S. Prior data for non-normal priors. Stat Med 2007; 26:3578–90. [DOI] [PubMed] [Google Scholar]
- 23. Young J, Scherrer AU, Günthard HF, et al. ; Swiss HIV Cohort Study Efficacy, tolerability and risk factors for virological failure of darunavir-based therapy for treatment-experienced HIV-infected patients: the Swiss HIV Cohort Study. HIV Med 2011; 12:299–307. [DOI] [PubMed] [Google Scholar]
- 24. Saeed S, Strumpf EC, Walmsley SL, et al. ; Canadian Co-Infection Cohort Study How generalizable are the results from trials of direct antiviral agents to people coinfected with HIV/HCV in the real world? Clin Infect Dis 2016; 62:919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aspinall EJ, Corson S, Doyle JS, et al. Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clin Infect Dis 2013; 57Suppl 2:S80–9. [DOI] [PubMed] [Google Scholar]
- 26. Lambers FA, Prins M, Thomas X, et al. ; MSM Observational Study of Acute Infection With Hepatitis C Study Group Alarming incidence of hepatitis C virus re-infection after treatment of sexually acquired acute hepatitis C virus infection in HIV-infected MSM. AIDS 2011; 25:F21–7. [DOI] [PubMed] [Google Scholar]
- 27. van de Laar T, Pybus O, Bruisten S, et al. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology 2009; 136:1609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matthews GV, Hellard M, Haber P, et al. ; Australian Trial in Acute Hepatitis C Study Group Characteristics and treatment outcomes among HIV-infected individuals in the Australian Trial in Acute Hepatitis C. Clin Infect Dis 2009; 48:650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boesecke C, Grint D, Soriano V, et al. ; EuroSIDA in EuroCoord Hepatitis C seroconversions in HIV infection across Europe: which regions and patient groups are affected? Liver Int 2015; 35:2384–91. [DOI] [PubMed] [Google Scholar]
- 30. Burchell AN, Gardner SL, Mazzulli T, et al. Hepatitis C virus seroconversion among HIV-positive men who have sex with men with no history of injection drug use: results from a clinical HIV cohort. Can J Infect Dis Med Microbiol 2015; 26:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bottieau E, Apers L, Van Esbroeck M, Vandenbruaene M, Florence E. Hepatitis C virus infection in HIV-infected men who have sex with men: sustained rising incidence in Antwerp, Belgium, 2001–2009. Euro Surveill 2010; 15:19673. [PubMed] [Google Scholar]
- 32. Wandeler G, Gsponer T, Bregenzer A, et al. ; Swiss HIV Cohort Study Hepatitis C virus infections in the Swiss HIV Cohort Study: a rapidly evolving epidemic. Clin Infect Dis 2012; 55:1408–16. [DOI] [PubMed] [Google Scholar]
- 33. Weir A, McLeod A, Innes H, et al. Hepatitis C reinfection following treatment induced viral clearance among people who have injected drugs. Drug Alcohol Depend 2016; 165:53–60. [DOI] [PubMed] [Google Scholar]
- 34. Centers for Disease Control and Prevention. Hepatitis C virus infection among adolescents and young adults: Massachusetts, 2002–2009. Morb Mortal Wkly Rep 2011; 60:537–41. [PubMed] [Google Scholar]
- 35. Public Health Agency of Canada. Hepatitis C in Canada: 2005–2010 surveillance report 2011. Available at: http://publications.gc.ca/collections/collection_2012/aspc-phac/HP40-70-2012-eng.pdf. Accessed June 30, 2016.
- 36. Vickerman P, Page K, Maher L, Hickman M. Commentary on Nolan et al. (2014): opiate substitution treatment and hepatitis C virus prevention: building an evidence base? Addiction 2014; 109:2060–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ 2012; 345:e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Raycraft T, Hakobyan S, Vafadary S, et al. Retrospective analysis of recurrent HCV viremia in high-risk HIV co-infected people who inject drugs (PWID). J Viral Hepat 2016; 2:2. Available at: http://hepatitis.imedpub.com/. Accessed March 7, 2017. [Google Scholar]
- 39. Melendez-Torres GJ, Bonell C. Systematic review of cognitive behavioural interventions for HIV risk reduction in substance-using men who have sex with men. Int J STD AIDS 2014; 25:627–35. [DOI] [PubMed] [Google Scholar]
- 40. Berg R. The effectiveness of behavioural and psychosocial HIV/STI prevention interventions for MSM in Europe: a systematic review. Euro Surveill 2009; 14. [DOI] [PubMed] [Google Scholar]
- 41. Andraka-Christou B. A pressing need for pharmacotherapy development to treat drug addiction: an editorial from a legal perspective. Int Rev Neurobiol 2016; 126:15–38. [DOI] [PubMed] [Google Scholar]
- 42. Ling W, Hillhouse MP, Saxon AJ, et al. Buprenorphine + naloxone plus naltrexone for the treatment of cocaine dependence: the Cocaine Use Reduction with Buprenorphine (CURB) study. Addiction 2016; In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. World Health Organization. Combating hepatitis B and C to reach elimination by 2030. Advocacy brief. 2016. Available at: http://www.who.int/hepatitis/publications/hep-elimination-by-2030-brief/en/ Accessed June 29, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.