Summary
We evaluated 3 modified 2-tiered testing protocols for Lyme disease, which substitute noncomplex, objectively interpreted, inexpensive serologic assays for Western blotting. Compared with conventional 2-tiered testing, each modified protocol provided comparable or greater sensitivity in early disease and was comparably specific.
Keywords: Lyme, Borrelia burgdorferi, VlsE, C6, serology.
Abstract
Background.
The conventional 2-tiered serologic testing protocol for Lyme disease (LD), an enzyme immunoassay (EIA) followed by immunoglobulin M and immunoglobulin G Western blots, performs well in late-stage LD but is insensitive in patients with erythema migrans (EM), the most common manifestation of the illness. Western blots are also complex, difficult to interpret, and relatively expensive. In an effort to improve test performance and simplify testing in early LD, we evaluated several modified 2-tiered testing (MTTT) protocols, which use 2 assays designed as first-tier tests sequentially, without the need of Western blots.
Methods.
The MTTT protocols included (1) a whole-cell sonicate (WCS) EIA followed by a C6 EIA; (2) a WCS EIA followed by a VlsE chemiluminescence immunoassay (CLIA); and (3) a variable major protein-like sequence, expressed (VlsE) CLIA followed by a C6 EIA. Sensitivity was determined using serum from 55 patients with erythema migrans; specificity was determined using serum from 50 patients with other illnesses and 1227 healthy subjects.
Results.
Sensitivity of the various MTTT protocols in patients with acute erythema migrans ranged from 36% (95% confidence interval [CI], 25%–50%) to 54% (95% CI, 42%–67%), compared with 25% (95% CI, 16%–38%) using the conventional protocol (P = .003–0.3). Among control subjects, the 3 MTTT protocols were similarly specific (99.3%–99.5%) compared with conventional 2-tiered testing (99.5% specificity; P = .6–1.0).
Conclusions.
Although there were minor differences in sensitivity and specificity among MTTT protocols, each provides comparable or greater sensitivity in acute EM, and similar specificity compared with conventional 2-tiered testing, obviating the need for Western blots.
Conventional 2-tiered testing for Lyme disease (LD) begins with a whole-cell sonicate (WCS) enzyme immunoassay (EIA) or immunofluorescence assay (IFA). If the first-tier test is positive or equivocal, the specimen is further analyzed using standardized immunoglobulin M (IgM) and immunoglobulin G (IgG) Western blots (WBs). Seropositive status is only established when 1 or both of the WBs is positive according to specific interpretive criteria [1, 2].
Next-generation first-tier assays have been approved for diagnostic use in the United States [3–6]. These assays are prepared from recombinant proteins or synthetic peptides that correspond to spirochetal antigens known to induce relatively early and robust humoral immune responses [7]. Because they contain far fewer antigens than WCS assays, these alternative first-tier assays are typically more specific [3, 5, 6, 8], although none is completely specific. At present, these assays must still be used in the first tier of a 2-tiered testing protocol, with WBs used in the second tier.
Two-tiered testing protocols involving WBs are sensitive and specific in the later stages of LD [5, 9, 10], but they are insensitive in early infection [3, 5, 7, 9, 10] due to the slow development of the humoral immune response. Therefore, a negative result does not rule out the diagnosis of early Lyme disease, if signs and symptoms have been present for ≤30 days [2]. Because first-tier assays typically become reactive earlier during seroconversion than WBs, the WB component substantially reduces the sensitivity of 2-tiered testing in patients with the earliest manifestation of LD, erythema migrans (EM) [3, 5, 8, 10, 11]. Although EM is the most common clinical manifestation of LD, only 10%–20% of EM lesions present in the form of a characteristic targetoid rash [12, 13], creating diagnostic uncertainty and strong demand for a diagnostic test to support suspected LD. A recent survey of large commercial reference laboratories revealed that the great majority of LD serologic tests are performed on patients with suspected EM [14], despite the fact that conventional 2-tiered testing is insensitive for this purpose [15].
In addition to their low sensitivity in early LD, WBs are complex assays and must often be performed at reference laboratories. This prolongs turnaround time and adds expense [16]. Furthermore, WBs are sometimes interpreted subjectively by visual examination, and current interpretive guidelines indicate that a positive IgM WB alone should not be used to support a diagnosis of active LD if signs and symptoms have been present for >1 month [1]. In practice, IgM WBs may be overinterpreted, either because faint bands are incorrectly scored as present, or because seropositive status is assigned on the basis of a positive IgM WB despite the presence of symptoms for >1 month [17, 18].
To address these limitations, we recently proposed a modified 2-tiered testing (MTTT) protocol, in which a WCS EIA is followed by a C6 EIA, the latter being a next-generation assay designed as a first-tier test [8]. This “2-EIA” algorithm, which does not involve WBs, improves sensitivity in early LD compared with conventional 2-tiered testing, is equally sensitive in later stages of LD, is equally specific, and is less expensive [3, 8, 16]. The 2-EIA algorithm is more specific than either EIA alone, because the assays involved are orthogonal to some degree. Cross-reactive antibodies that affect the specificity of WCS EIAs often do not react with the C6 EIA, and vice versa [3, 5, 8].
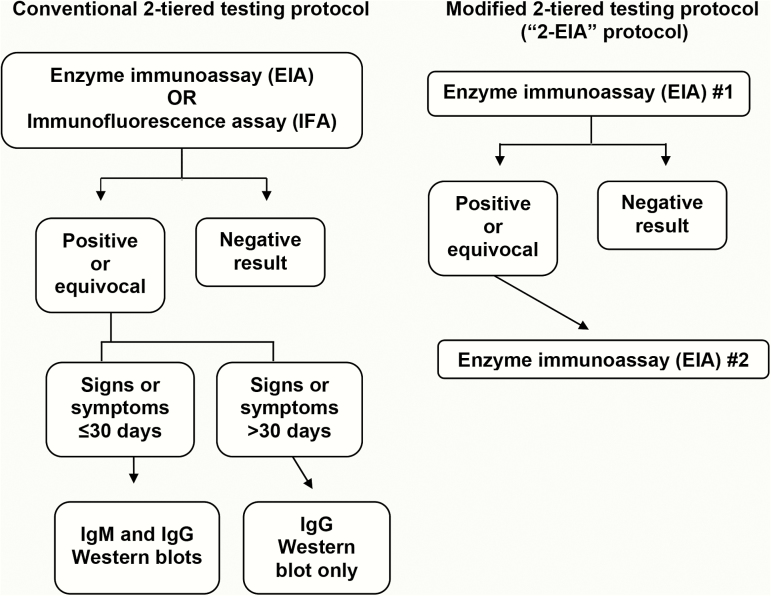
In the present study, we reevaluated the original 2-EIA protocol using serum samples from a distinct cohort of patients with EM, and also evaluated 2 additional MTTT protocols, involving different pairings of assays designed as first-tier tests, which might improve the performance of the original 2-EIA algorithm. The various MTTT protocols were compared with conventional 2-tiered testing as the reference standard (Figure 1).
Figure 1.
Serodiagnostic testing protocols evaluated in this study. Adapted from Moore et al [2]. Abbreviations: EIA, enzyme immunoassay; IFA, immunofluorescence assay; IgG, immunoglobulin G; IgM, immunoglobulin M.
METHODS
Patient Samples
The study was approved by the Partners Healthcare Human Research Committee. Sensitivity of the diagnostic algorithms under evaluation was determined using serum samples collected from patients who had physician-diagnosed EM, confirmed by culture in 62% of cases. The patients were evaluated by experienced physicians in 2 highly endemic areas for LD: Nantucket, Massachusetts (T. J. L., Nantucket Cottage Hospital) or Wakefield, Rhode Island (N. S. D., South County Internal Medicine). Samples were collected during the summer tick-transmission seasons in 2012 or 2015. Acute-phase serum samples were obtained at initial presentation; convalescent-phase samples were obtained after the completion of standard oral antimicrobial regimens, 3–6 weeks after study entry. In addition, a 2.0-mm punch skin biopsy was performed for Borrelia burgdorferi culture [19] during the initial visit.
Specificity of the algorithms was determined using serum samples from 2 groups of control patients: (1) patients referred to A. C. S. for possible LD, but diagnosed with other illnesses; (2) healthy, asymptomatic patients who donated blood in Boston (an endemic area) or New Zealand (a nonendemic area), or who presented for routine well-office visits in Rhode Island or Connecticut clinics. All control sera were collected as part of previous studies [8, 9, 20].
Serologic Testing
All testing was performed according to the manufacturers’ instructions. Serum samples were analyzed using the C6 B. burgdorferi EIA (Immunetics), which uses a variable major protein-like sequence, expressed (VlsE) C6 peptide, and the Liaison B. burgdorferi CLIA (DiaSorin), which uses full-length, recombinant VlsE, as well as 1 of 2 polyvalent WCS EIAs. Serum samples from patients with EM and from Boston blood donors were analyzed using the Wampole B. burgdorferi IgG/M enzyme-linked immunosorbent assay II (Alere). The remaining serum samples, which represent the smaller control groups, were analyzed using the VIDAS Lyme IgG/IgM assay (bioMérieux). WBs were performed using Borrelia B31 IgM and IgG ViraBlot or ViraStripe test strips (Viramed Biotech AG). WBs were interpreted using standard Centers for Disease Control and Prevention criteria [1]. As done previously, bands that were fainter than the cutoff control band were scored as absent [8, 20]. Serologic testing of the control sera, except using the Liaison CLIA, was performed as part of previous studies [8, 20], and the results are reported again here.
Statistical Analysis
Differences between proportions were considered statistically significant if the 2-tailed P value was ≤.05, as determined using Fisher exact test. The 95% confidence intervals (CIs) were calculated by the modified Wald method.
RESULTS
First-Tier Assays
When all patients with acute EM were considered together, there were nonsignificant differences in sensitivity between the first-tier tests (C6 EIA, 65%; VlsE CLIA, 55%; WCS EIA, 49%; P ≥ .12 for all comparisons; Table 1). However, in the subcategory of patients with acute EM whose skin biopsy culture was positive for B. burgdorferi, the C6 EIA was significantly more sensitive than the WCS EIA (79% vs 50%, P = .02), while the VlsE CLIA was not (71% vs 50%, P = .14). The sensitivity of each first-tier assay was greater during convalescence than in the acute phase of EM; the difference was significant for the WCS EIA (77% vs 49% sensitivity, P = .008), but not for the C6 EIA (81% vs 65%, P = .12) or the VlsE CLIA (72% vs 55%, P = .07).
Table 1.
Serologic Responses in Patients with Erythema Migrans
| No. Positive or Equivocal (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| First-Tier Tests Alone | 2-Tiered Protocols Using WBs | Modified 2-Tiered Protocols | |||||||
| Serology | WCS EIA | C6 EIA | VlsE CLIA | WCS EIA f/b WBs | C6 EIA f/b WBs | VlsE CLIA f/b WBs | WCS EIA f/b C6 EIA | WCS EIA f/b VlsE CLIA | VlsE CLIA f/b C6 EIA |
| Patients with erythema migrans | |||||||||
| Acute phase (n = 55) | 27 (49) [36–62] | 36 (65) [52–77] | 30 (55) [42–67] | 14 (25) [16–38] | 20 (36) [25–50] | 19 (35) [23–48] | 21 (38) [26–51] | 20 (36) [25–50] | 30 (54) [42–67] |
| Culture-confirmed (n = 34) | 17 (50) [34–66] | 27 (79) [63–90] | 24 (71) [54–83] | 10 (29) [17–46] | 16 (47) [31–63] | 15 (44) [29–61] | 14 (41) [26–58] | 14 (41) [26–58] | 24 (71) [54–83] |
| Culture-negative (n = 21) | 10 (48) [28–68] | 9 (43) [24–63] | 6 (29) [14–50] | 4 (19) [7–41] | 4 (19) [7–41] | 4 (19) [7–41] | 7 (33) [17–55] | 6 (29) [14–50] | 6 (29) [14–50] |
| Convalescent phase (n = 47) | 36 (77) [63–87] | 38 (81) [67–90] | 34 (72) [58–83] | 26 (55) [41–69] | 28 (60) [45–72] | 27 (57) [43–71] | 34 (72) [58–83] | 31 (66) [52–78] | 34 (72) [58–83] |
| Culture-confirmed (n = 29) | 26 (90) [73–97] | 29 (100) [86–100] | 26 (90) [73–97] | 20 (69) [51–83] | 22 (76) [58–88] | 21 (72) [54–86] | 26 (90) [73–97] | 23 (79) [61–91] | 26 (90) [73–97] |
| Culture-negative (n = 18) | 10 (56) [34–75] | 9 (50) [29–71] | 8 (44) [25–66] | 6 (33) [16–56] | 6 (33) [16–56] | 6 (33) [16–56] | 8 (44) [25–66] | 8 (44) [25–66] | 8 (44) [25–66] |
Bracketed numbers represent the 95% confidence intervals for the listed percentages.
Abbreviations: CLIA, chemiluminescence immunoassay; EIA, enzyme immunoassay; f/b, followed by; VlsE, variable major protein-like sequence, expressed; WB, Western blot; WCS, whole-cell sonicate.
Among control subjects, differences in specificity between the first-tier assays were primarily confined to symptomatic patients with other illnesses, rather than asymptomatic, healthy control subjects (Table 2). In patients with other illnesses, the specificity of the C6 EIA (98%) or VlsE CLIA (96%) was significantly greater than the specificity of the WCS EIA (78%; P = .004 and P = .01, respectively). Among asymptomatic control subjects, each of the first-tier assays was comparably specific.
Table 2.
Serologic Responses in Control Subjects
| No. Positive (% Negative) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| First-Tier Tests Alone | 2-Tiered Protocols Using WBs | Modified 2-Tiered Protocols | |||||||
| Subjects | WCS EIA | C6 EIA | VlsE CLIA | WCS EIA f/b WBs | C6 EIA f/b WBs | VlsE CLIA f/b WBs | WCS EIA f/b C6 EIA | WCS EIA f/b VlsE CLIA | VlsE CLIA f/b C6 EIA |
| All control subjects (n = 1277) | 33 (97.4) [96.4–98.2] | 20 (98.4) [97.6–99.0] | 24 (98.1) [97.2–98.8] | 7 (99.5) [98.9–99.8] | 4 (99.7) [99.2–99.9] | 4 (99.7) [99.2–99.9] | 7 (99.5) [98.9–99.8] | 7 (99.5) [98.9–99.8] | 9 (99.3) [98.6–99.7] |
| Other illnesses (n = 50) | 11 (78) [65–87] | 1 (98) [89–100] | 2 (96) [86–100] | 0 (100) [91–100] | 0 (100) [91–100] | 0 (100) [91–100] | 0 (100) [91–100] | 0 (100) [91–100] | 1 (98) [89–100] |
| Asymptomatic (n = 1227) | 22 (98.2) [97.3–98.8] | 19 (98.5) [97.6–99.0] | 22 (98.2) [97.3–98.8] | 7 (99.4) [98.8–99.8] | 4 (99.7) [99.1–99.9] | 4 (99.7) [99.1–99.9] | 7 (99.4) [98.8–99.8] | 7 (99.4) [98.8–99.8] | 8 (99.4) [98.7–99.7] |
| CT or RI field sites (n = 47) | 3 (94) [82–98] | 3 (94) [82–98] | 1 (98) [88–100] | 1 (98) [88–100] | 1 (98) [88–100] | 1 (98) [88–100] | 1 (98) [88–100] | 1 (98) [88–100] | 1 (98) [88–100] |
| Boston blood donors (n = 1080) | 16 (98.5) [97.6–99.1] | 16 (98.5) [97.6–99.1] | 19 (98.2) [97.3–98.9] | 6 (99.4) [98.8–99.8] | 3 (99.7) [99.2–100] | 3 (99.7) [99.2–100] | 6 (99.4) [98.8–99.8] | 6 (99.4) [98.8–99.8] | 7 (99.4) [98.6–99.7] |
| NZ blood donors (n = 100) | 3 (97) [91–99] | 0 (100) [96–100] | 2 (98) [93–100] | 0 (100) [96–100] | 0 (100) [96–100] | 0 (100) [96–100] | 0 (100) [96–100] | 0 (100) [96–100] | 0 (100) [96–100] |
Bracketed numbers represent the 95% confidence intervals for the listed percentages.
Abbreviations: CLIA, chemiluminescence immunoassay; CT, Connecticut; EIA, enzyme immunoassay; f/b, followed by; NZ, New Zealand; RI, Rhode Island; VlsE, variable major protein-like sequence, expressed; WB, Western blot; WCS, whole-cell sonicate.
Two-Tiered Testing Protocols Using Western Blots in the Second Tier
Regardless of which first-tier test was used, the 2-tiered algorithms involving WBs in the second tier were comparably sensitive. Whereas each individual protocol was more sensitive in patients with convalescent-phase EM compared with acute-phase EM (P ≤ .03), within these categories there were no significant differences in sensitivity between the protocols. Each first-tier assay, when considered as a stand-alone test, was approximately 2-fold more sensitive in acute EM compared with the corresponding 2-tiered protocol using WBs in the second tier (49% vs 25% with the WCS EIA, P = .02; 65% vs 36% with the C6 EIA, P = .004; and 55% vs 35% with the VlsE CLIA, P = .05).
All of the 2-tiered algorithms involving WBs in the second tier were comparably specific in the control group as a whole (99.5%–99.7% specificity, P ≥ .55), or in any of the subcategories of control subjects. Each of the protocols was significantly more specific compared with any of the first-tier assays used alone (P ≤ .02 for all comparisons).
Modified 2-Tiered Testing Protocols, Involving Sequential Use of Assays Designed as First-Tier Tests, without the Use of Western Blots
Three MTTT protocols were evaluated. Although sensitivity values were higher in patients with acute EM for all 3 MTTT protocols compared with the conventional 2-tiered testing algorithm (WCS EIA followed by WBs), the difference was only significant for the MTTT protocol involving a VlsE CLIA in the first tier, followed by a C6 EIA in the second tier (54% vs 25% sensitivity, P = .003; Table 3). In the subcategory of patients with acute EM who were culture-positive, this MTTT was also significantly more sensitive compared with the conventional algorithm (71% vs 29% sensitivity, P = .001). During the convalescent phase of EM, the 3 MTTT protocols were similarly sensitive compared with any of the 2-tiered protocols involving WBs in the second tier. Compared with each other, the 3 MTTT algorithms were comparably sensitive in the convalescent phase of EM, and in patients with culture-negative acute EM. However, in patients with culture-confirmed acute EM, the VlsE CLIA followed by C6 EIA protocol was significantly more sensitive than the other 2 MTTT protocols (71% vs 41% and 41%, P = .03).
Table 3.
Sensitivity and Specificity of Serodiagnostic Algorithms for Acute Erythema Migrans
| Sensitivity and Specificity | First-Tier Tests Alone | Modified 2-Tiered Testing Protocols |
Conventional 2-Tiered Testing |
||||
|---|---|---|---|---|---|---|---|
| WCS EIA | C6 | VlsE CLIA | WCS EIA f/b C6 EIA | WCS EIA f/b VlsE CLIA |
VlsE CLIA f/b C6 EIA | WCS EIA f/b IgM/IgG WBs | |
| Sensitivity, % | |||||||
| Acute-phase EM (n = 55) | 49 [.02] | 65 [<.0001] | 55 [.003] | 38 [.22] | 36 [.30] | 54 [.003] | 25 |
| Convalescent-phase EM (n = 47) | 77 [.05] | 81 [.01] | 72 [.13] | 72 [.13] | 66 [.40] | 72 [.13] | 55 |
| Specificity, % | |||||||
| Overall (n = 1277) | 97.4 [<.0001] | 98.4 [.02] | 98.1 [.003] | 99.5 [1.00] | 99.5 [1.00] | 99.3 [.63] | 99.5 |
Bracketed numbers represent the P values for the comparison with conventional 2-tiered testing.
Abbreviations: CLIA, chemiluminescence immunoassay; EIA, enzyme immunoassay; EM, erythema migrans; f/b, followed by; IgG, immunoglobulin G; IgM, immunoglobulin M; VlsE, variable major protein-like sequence, expressed; WB, Western blot; WCS, whole-cell sonicate.
Among control subjects, the 3 MTTT protocols were similarly specific when compared with each other (99.3%–99.5% specificity), or compared with 2-tiered protocols using WBs in the second tier (99.5%–99.7% specificity; P ≥ .3 for all comparisons between any of these protocols). Each of the 3 MTTT protocols was significantly more specific compared with any of the first-tier assays (WCS, C6, or VlsE) used alone, with the exception of the comparison between the C6 EIA alone and the MTTT protocol using a VlsE CLIA followed by a C6 EIA (P = .06 for that comparison; P ≤ .02 for all other comparisons).
DISCUSSION
In this study, we evaluated the performance of 3 MTTT protocols, in which assays designed as first-tier tests are performed sequentially in 2-tiered algorithms. One of the MTTT protocols, a WCS EIA followed by a C6 EIA, has been evaluated by several independent groups with similar findings to those reported here [3, 5, 6, 8, 21]. Our study furnishes additional evidence that this algorithm provides similar or greater sensitivity in acute- or convalescent-phase EM, compared with any 2-tiered protocol involving WBs, while maintaining the specificity of algorithms involving WBs. Moreover, we demonstrated that the C6 EIA can be replaced in this algorithm with a VlsE CLIA, without any significant difference in sensitivity or specificity.
The highest sensitivity in patients with acute EM was obtained using a third MTTT protocol, involving a VlsE CLIA as the first-tier test, and a C6 EIA as the second-tier test. This was expected; the other 2 MTTT protocols used a WCS EIA in the first tier, and this assay was less sensitive as an individual test compared with the VlsE CLIA or C6 EIA in patients with acute EM. Moreover, the MTTT protocol using a VlsE CLIA followed by a C6 EIA was similarly specific compared with the other MTTT protocols or with 2-tiered protocols involving WBs. Assays using full-length VlsE may be orthogonal to assays using the C6 peptide, at least to some degree. The resolved 3-dimensional structure of VlsE demonstrates that its sixth invariable region (IR6) is buried within the parent molecule, and anti-IR6 antibodies are predicted to have minimal interaction with full-length VlsE [22]. There is also direct evidence that anti-IR6 antibodies often do not react with VlsE and vice versa [23]. Thus, cross-reactive antibodies capable of causing falsely positive results with one assay may not react in the other assay. However, even when used sequentially in a 2-tiered protocol, these tests are not completely specific for B. burgdorferi infection.
Although this study demonstrated that the C6 peptide and full-length VlsE assays were more specific compared with WCS EIAs, we do not recommend their use as stand-alone tests. Rather, we think that they should be used in 2-tiered testing protocols, whether standard (involving WBs) or modified (using tests designed as first-tier tests sequentially, without the use of WBs). When used as stand-alone tests, both the C6 and VlsE assays were significantly less specific compared with any 2-tiered algorithm using WBs in the second tier, or compared with any MTTT protocol. The one exception was the comparison between the most specific first-tier assay (the C6 EIA) and the least specific MTTT protocol (VlsE CLIA followed by C6 EIA); however, even this comparison closely approached statistical significance (P = .06). Considering that the prevalence of LD among the tested population is usually low [14], small differences in specificity translate to substantial differences in positive predictive value [8].
Any of the MTTT protocols evaluated in this study could be reversed (putting the second test first, and the first test second), with equivalent sensitivity and specificity (data not shown). In addition, while we have proposed the sequential use of assays designed as first-tier tests, the 2 assays involved in any particular MTTT protocol could instead be performed in parallel. In deciding which assay to perform first in a MTTT protocol, or in choosing between sequential or concurrent testing, there are performance and practical matters to consider. When assays are performed sequentially, there will be more discordant outcomes (one test positive or equivocal, the other test negative) if the more sensitive assay is used first, and fewer if the less sensitive assay is used first. Concurrent performance of both assays will likely produce even more discordant outcomes than sequential testing, regardless of which test is used first during sequential testing. Whether the goal should be to maximize or minimize discordant outcomes depends somewhat on the disease prevalence in the tested population. When prevalence is high, a substantial proportion of patients with discordant results will have true early infection, with an antibody response that is too undeveloped to yield positive results in orthogonal serologic assays. In such patients, discordant results may appropriately support empiric antimicrobial therapy and/or repeated serologic testing after a short time. However, in low-prevalence populations, most patients with discrepant results will not have LD, and in such patients the results might lead to unnecessary monitoring or therapy. Beyond this consideration, the question of whether to perform the assays simultaneously or sequentially, and in which order, and the question of which particular assays to use will depend on practical considerations such as cost, the availability of compatible instrument platforms, and turnaround time.
Although MTTT protocols address several drawbacks of conventional 2-tiered serologic testing, they do not distinguish between active or past infection, as antibody responses may persist for months or years after antibiotic treatment [24–27]. In addition, for the evaluation of complex cases, there is still a need for WBs or similar systems that separately measure multiple antibody specificities. By showing the degree of expansion of the antibody response, and by differentiating antibody classes, they can help to determine the duration of illness. This can aid in determining whether a given clinical manifestation is due to LD. Therefore, the best use of MTTT protocols is in suspected first-stage infection (erythema migrans), or in straightforward cases of suspected second stage infection (Lyme carditis or early neuroborreliosis), where there are clear advantages in sensitivity [3, 5, 8, 21]. There is less advantage in performing MTTT protocols when late-stage LD is suspected (eg, Lyme arthritis or late neuroborreliosis) [8], at least from a performance standpoint. Patients with late manifestations of LD have been infected for months or years, and thus have a well-developed anti–B. burgdorferi antibody response that is reliably detected by any serologic assay, including WBs [5, 9]. However, the practical advantages of MTTT protocols, such as lower cost and complexity compared with Western blotting, are maintained [16].
Our study has several limitations. First, some patients who were diagnosed with EM had a negative skin biopsy culture. Because B. burgdorferi infection could not be directly confirmed in these patients, it is possible that some were not truly infected. This could lead to an underestimate of the sensitivity of serologic testing among patients with EM. On the other hand, it is clear that B. burgdorferi cannot be cultured from EM skin lesions in all patients [28]. Among other potential variables, skin biopsy culture may be negative when the spirochetal burden is relatively low [29]. Because the exclusion of patients with low spirochetal burden may overestimate the frequency of seropositivity, the inclusion of culture-negative EM patients might actually provide sensitivity values that are closer to reality. A second limitation is the inclusion of only a relatively small number of control patients with other illnesses (n = 50), compared with healthy subjects (n = 1227). Because symptomatic control subjects may have a higher frequency of falsely positive results [5], this distribution of control subjects may overestimate the specificity of the tests studied here. However, in this study, there were no significant differences in the specificity of any 2-tiered testing protocol between symptomatic and asymptomatic control subjects, suggesting that this theoretical consideration was not a real problem. Finally, while some serum samples were analyzed using typical Western blots prepared by protein electrophoresis, others were analyzed using commercially available immunoblots prepared by direct application of purified proteins to the blot. These “ladder” blots generally give a cleaner result that is easier to score, and their use might have yielded better specificity than would be expected in routine clinical practice, where ordinary Western blots are commonly used.
In summary, MTTT algorithms provide a reliable “positive or negative” result regarding seroreactivity in early LD. The tests involved are cleared by the US Food and Drug Administration for diagnostic use, are not highly complex, can be performed using automated instruments, are objectively interpreted, and are relatively inexpensive. Although there were minor differences in sensitivity or specificity among the 3 MTTT approaches, any would be a valid replacement for conventional 2-tiered testing during the first several weeks of infection.
Notes
Acknowledgments. We are grateful to Brittany Lemos, Vedrana Eleta, Katherine B. Sulka, Stephanie Boynton, Tiffany MacKenzie, and Ashli Rode for their excellent technical assistance. The authors thank Claudia R. Molins and Martin E. Schriefer for their input and support.
Financial support. This work was supported by research grants from DiaSorin, Inc, and the Bay Area Lyme Foundation to J. A. B.
Potential conflicts of interest. J. A. B. has received research support from Immunetics, Alere, bioMérieux, and the National Institute of Allergy and Infectious Diseases (NIAID; award number 1R21AI119457-01) for other research projects, and has served as consultant to T2 Biosystems. A. C. S. has received research support from the NIAID (award number R01A1101175), the English-Bonter-Mitchell Foundation, and the Eshe Fund. K. S. has received research support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases at the National Institutes of Health (NIH; award number K01AR062098). P. M. L. has received research support from the National Center for Advancing Translational Sciences of the NIH (award number KL2 TR001115). L. E. N. has received research support from the Harvard Catalyst, the Boston Children’s Hospital Faculty Council, the Bay Area Lyme Foundation, and the Centers for Disease Control and Prevention. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 1995; 44:590–1. [PubMed] [Google Scholar]
- 2. Moore A, Nelson C, Molins C, Mead P, Schriefer M. Current guidelines, common clinical pitfalls, and future directions for laboratory diagnosis of Lyme disease, United States. Emerg Infect Dis 2016; 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wormser GP, Schriefer M, Aguero-Rosenfeld ME, et al. Single-tier testing with the C6 peptide ELISA kit compared with two-tier testing for Lyme disease. Diagn Microbiol Infect Dis 2013; 75:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ledue TB, Collins MF, Young J, Schriefer ME. Evaluation of the recombinant VlsE-based liaison chemiluminescence immunoassay for detection of Borrelia burgdorferi and diagnosis of Lyme disease. Clin Vaccine Immunol 2008; 15:1796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Molins CR, Delorey MJ, Sexton C, Schriefer ME. Lyme borreliosis serology: performance of several commonly used laboratory diagnostic tests and a large resource panel of well-characterized patient samples. J Clin Microbiol 2016; 54:2726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lipsett SC, Branda JA, McAdam AJ, et al. Evaluation of the C6 lyme enzyme immunoassay for the diagnosis of Lyme disease in children and adolescents. Clin Infect Dis 2016; 63:922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bacon RM, Biggerstaff BJ, Schriefer ME, et al. Serodiagnosis of Lyme disease by kinetic enzyme-linked immunosorbent assay using recombinant VlsE1 or peptide antigens of Borrelia burgdorferi compared with 2-tiered testing using whole-cell lysates. J Infect Dis 2003; 187:1187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis 2011; 53:541–7. [DOI] [PubMed] [Google Scholar]
- 9. Steere AC, McHugh G, Damle N, Sikand VK. Prospective study of serologic tests for Lyme disease. Clin Infect Dis 2008; 47:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Molins CR, Sexton C, Young JW, et al. Collection and characterization of samples for establishment of a serum repository for Lyme disease diagnostic test development and evaluation. J Clin Microbiol 2014; 52:3755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wormser GP, Nowakowski J, Nadelman RB, Visintainer P, Levin A, Aguero-Rosenfeld ME. Impact of clinical variables on Borrelia burgdorferi–specific antibody seropositivity in acute-phase sera from patients in North America with culture-confirmed early Lyme disease. Clin Vaccine Immunol 2008; 15:1519–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wormser GP, Masters E, Nowakowski J, et al. Prospective clinical evaluation of patients from Missouri and New York with erythema migrans-like skin lesions. Clin Infect Dis 2005; 41:958–65. [DOI] [PubMed] [Google Scholar]
- 13. Steere AC, Sikand VK. The presenting manifestations of Lyme disease and the outcomes of treatment. N Engl J Med 2003; 348:2472–4. [DOI] [PubMed] [Google Scholar]
- 14. Hinckley AF, Connally NP, Meek JI, et al. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis 2014; 59:676–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lantos PM, Brinkerhoff RJ, Wormser GP, Clemen R. Empiric antibiotic treatment of erythema migrans-like skin lesions as a function of geography: a clinical and cost effectiveness modeling study. Vector Borne Zoonotic Dis 2013; 13:877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wormser GP, Levin A, Soman S, Adenikinju O, Longo MV, Branda JA. Comparative cost-effectiveness of two-tiered testing strategies for serodiagnosis of Lyme disease with noncutaneous manifestations. J Clin Microbiol 2013; 51:4045–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect 2012; 18:1236–40. [DOI] [PubMed] [Google Scholar]
- 18. Lantos PM, Lipsett SC, Nigrovic LE. False positive Lyme disease IgM immunoblots in children. J Pediatr 2016; 174: 267–9 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vaz A, Glickstein L, Field JA, et al. Cellular and humoral immune responses to Borrelia burgdorferi antigens in patients with culture-positive early Lyme disease. Infect Immun 2001; 69:7437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Branda JA, Aguero-Rosenfeld ME, Ferraro MJ, Johnson BJ, Wormser GP, Steere AC. 2-tiered antibody testing for early and late Lyme disease using only an immunoglobulin G blot with the addition of a VlsE band as the second-tier test. Clin Infect Dis 2010; 50:20–6. [DOI] [PubMed] [Google Scholar]
- 21. Branda JA, Strle F, Strle K, Sikand N, Ferraro MJ, Steere AC. Performance of United States serologic assays in the diagnosis of Lyme borreliosis acquired in Europe. Clin Infect Dis 2013; 57:333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eicken C, Sharma V, Klabunde T, et al. Crystal structure of Lyme disease variable surface antigen VlsE of Borrelia burgdorferi. J Biol Chem 2002; 277:21691–6. [DOI] [PubMed] [Google Scholar]
- 23. Embers ME, Jacobs MB, Johnson BJ, Philipp MT. Dominant epitopes of the C6 diagnostic peptide of Borrelia burgdorferi are largely inaccessible to antibody on the parent VlsE molecule. Clin Vaccine Immunol 2007; 14:931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aguero-Rosenfeld ME, Nowakowski J, McKenna DF, Carbonaro CA, Wormser GP. Serodiagnosis in early Lyme disease. J Clin Microbiol 1993; 31:3090–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Glatz M, Golestani M, Kerl H, Müllegger RR. Clinical relevance of different IgG and IgM serum antibody responses to Borrelia burgdorferi after antibiotic therapy for erythema migrans: long-term follow-up study of 113 patients. Arch Dermatol 2006; 142:862–8. [DOI] [PubMed] [Google Scholar]
- 26. Kalish RA, McHugh G, Granquist J, Shea B, Ruthazer R, Steere AC. Persistence of immunoglobulin M or immunoglobulin G antibody responses to Borrelia burgdorferi 10–20 years after active Lyme disease. Clin Infect Dis 2001; 33:780–5. [DOI] [PubMed] [Google Scholar]
- 27. Feder HM, Jr, Gerber MA, Luger SW, Ryan RW. Persistence of serum antibodies to Borrelia burgdorferi in patients treated for Lyme disease. Clin Infect Dis 1992; 15:788–93. [DOI] [PubMed] [Google Scholar]
- 28. Liveris D, Schwartz I, McKenna D, et al. Comparison of five diagnostic modalities for direct detection of Borrelia burgdorferi in patients with early Lyme disease. Diagn Microbiol Infect Dis 2012; 73:243–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li X, McHugh GA, Damle N, Sikand VK, Glickstein L, Steere AC. Burden and viability of Borrelia burgdorferi in skin and joints of patients with erythema migrans or Lyme arthritis. Arthritis Rheum 2011; 63:2238–47. [DOI] [PMC free article] [PubMed] [Google Scholar]