Summary
HLA-B*53:01 is strongly associated with DRESS syndrome in patients treated with raltegravir. Virtual modeling suggests that raltegravir may bind within the peptide- binding groove of HLA-B*53:01 and potentially alter the repertoire of self-peptides presented to CD8 T cells.
Keywords: DRESS syndrome, raltegravir, HLA_B*53:01
Abstract
Background.
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a rare, severe adverse event during treatment with raltegravir. The occurrence of DRESS syndrome during treatment with other drugs is strongly associated with particular HLA alleles.
Methods.
We performed HLA testing in 3 of the 5 patients previously reported to have developed raltegravir-induced DRESS syndrome and in 1 previously unreported patient. We then used virtual modeling to visualize interactions between raltegravir and the imputed HLA molecule.
Results.
Five of the 6 patients who developed raltegravir-induced DRESS syndrome were African, and 1 was Hispanic. HLA typing was performed in 4 patients, all of whom carried both the HLA-B*53 allele and the HLA-C*04 allele to which it is commonly haplotypic. No other HLA alleles were shared by all of the tested patients. Given the approximate prevalence of HLA-B*53 carriage in African (20%) and Hispanic (6%) populations, the probability of all 4 patients being HLA-B*53 carriers, and 2 of 3 African patients being homozygous for HLA-B*53:01, is approximately 0.00002.
Conclusions.
These data implicate the prevalent African allele HLA-B*53:01 in the immunopathogenesis of raltegravir-induced DRESS syndrome. Although the immunopathogenic mechanisms are currently unknown, virtual modeling suggests that raltegravir may bind within the antigen binding cleft of the HLA-B*53:01 molecule, but not within the closely related HLA-B*35:01 molecule. Further studies are necessary to confirm the strength of the association between carriage of the HLA-B*53:01 allele and raltegravir-induced DRESS syndrome, and the potential utility of HLA screening before raltegravir treatment.
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a rare, potentially life-threatening, T-cell-mediated, hypersensitivity reaction that may occur during treatment with various drugs, such as dapsone, allopurinol, and nevirapine [1]. DRESS syndrome, and other T-cell–mediated drug hypersensitivity reactions, have been shown to occur in association with specific HLA alleles. For example, a CD8 T-cell–dependent hypersensitivity reaction during treatment with abacavir, a guanosine analogue human immunodeficiency virus (HIV) reverse-transcriptase inhibitor, is strongly associated with the presence of the HLA-B*57:01 allele [1]. An initial, important clue to the HLA association with this syndrome was the strikingly lower incidence of abacavir hypersensitivity in persons of African ethnicity, who have a much lower prevalence of HLA-B*57:01 than white persons. Similar associations with ethnicity and particular HLA alleles have been observed in relation to delayed hypersensitivity reactions during treatment with another antiretroviral drug, nevirapine. In patients treated with nevirapine the development of Stevens-Johnson syndrome/toxic epidermal necrolysis and DRESS syndrome is associated with the presence of the HLA-C*04:01 allele in persons of African, European, or Asian ethnicity [2].
Raltegravir is an HIV integrase inhibitor that has been used to treat persons with HIV infection since 2007 [3–5]. There have been 5 published reports of patients who developed DRESS syndrome while being treated with antiretroviral regimens that included raltegravir [6–10]. Strikingly, 4 of these patients were of African and 1 was of Hispanic ethnicity, with no cases reported in persons of European or Asian ethnicity. This ethnic disparity suggests the presence of a risk allele confined to, or substantially overrepresented, in persons of African or Hispanic ethnicity. We have recently cared for an African HIV-infected man who developed DRESS syndrome while being treated with an antiretroviral regimen that included raltegravir. In view of the strong association between T-cell–mediated drug hypersensitivity syndromes and particular HLA alleles, and the ethnic disparity in reported cases of DRESS syndrome during treatment with raltegravir, we hypothesized that an HLA allele prevalent in African and Hispanic populations, but rare in European populations, would be associated with DRESS syndrome during treatment with raltegravir. We aimed to perform HLA ABC, DRB, and DQB typing of all patients who had been reported to have developed DRESS syndrome during treatment with raltegravir.
METHODS
The clinicians who had reported the occurrence of DRESS syndrome in patients being treated with raltegravir were invited to collaborate in a study to determine the patients’ HLA genotypes. Those who agreed to collaborate then explained the study to their patients, and with the patients’ informed consent, requested HLA ABC, DRB and DQB typing, which was performed at the relevant local laboratory. We used the RegiSCAR scoring system to classify each case as a possible, probable, or definite case of DRESS syndrome [11], and we used the Naranjo adverse drug reaction probability scale to estimate for each case the probability that DRESS syndrome was secondary to treatment with raltegravir [12].
The structures of the relevant HLA molecules were compared using the crystal structure of HLA-B*53:01, Protein Data Bank code 1A1M. Next, DOCKER was used to align the sequence of HLA-B*53:01 with HLA-B*35:01 (PileUp software; GCG Wisconsin Package), calculate sequence similarity based on a BLOSUM62 matrix, and output values for each protein position to correspond to atomic coordinates, which were plotted in 3 dimensions using PyMOL software (PyMOL Molecular Graphics System, version 1.8; Schrödinger).
RESULTS
The features of DRESS syndrome in the current patient were very similar to those in the 5 previously reported patients. The RegiSCAR score classified 2 cases as possible, 3 as probable, and 1 as definite DRESS syndrome. The Naranjo scoring system classified the likelihood that DRESS syndrome was secondary to treatment with raltegravir as possible in 4 patients and probable in 2 (Table 1).
Table 1.
Demographic and Clinical Features of Patients With DRESS Syndrome During Raltegravir Treatment
| Features | Patient [Reference] | |||||
|---|---|---|---|---|---|---|
| 1 [Current] | 2 [6] | 3 [7] | 4 [8] | 5 [9] | 6 [10] | |
| Age, y | 44 | 64 | 46 | 55 | 39 | 18 |
| Sex | Male | Female | Female | Female | Male | Female |
| Ethnicity (birthplace) | African (Zambia) | African (USa) | African (Congo) | African (UKa) | African (Ivory Coast) | Hispanic (Mexico) |
| Therapy at onset of DRESS syndrome (treatment duration, wk) |
Raltegravir (4), darunavir (4), ritonavir (4), zidovudine (4), lamivudine (4) | Raltegravir (6), darunavir (6), ritonavir (6) | Raltegravir (8), lopinavir (>20), ritonavir (>20), tenofovir (>20) | Raltegravir (4), tenofovir (>20), emtricitabine (>20), sulfamethoxazole (4), trimethoprim (4), codeine (4) | Raltegravir (4), darunavir (>20), ritonavir (>20), atovaquone (4) |
Raltegravir (5), lopinavir (>20), ritonavir (>20), zidovudine (>20), lamivudine (>20) |
| Subsequent therapy during resolution of DRESS syndrome |
Dolutegravir, atazanavir, zidovudine, lamivudine |
NK | Lopinavir, ritonavir, tenofovir |
“A protease inhibitor,” tenofovir, emtricitabine |
Darunavir, ritonavir, abacavir, lamivudine |
Lopinavir, ritonavir, tenofovir, emtricitabine |
| Main clinical features of DRESS syndrome | Generalized rash, malaise, diarrhea, no fever | Generalized rash, facial edema, lymphadenopathy, no fever | Generalized rash, abdominal pain, lymphadenopathy, fever | Generalized rash, malaise, fever | Rash, oral ulcers, fever | Generalized rash, edema of face hands and feet, lymphadenopathy, fever |
| Peak creatinine, µmol/L | 149 | NK | 2× ULN | NK | NK | NK |
| ALT, U/L | 295 | 520 | Normal | 65 | 617 | 147 |
| Eosinophil count, ×109/L | 1.9 | 2.2 | Normal | 1.5 | 1.3 | 1.3 |
| Outcome | Resolved | Resolved | Resolved | Resolved | Resolved | Resolved |
| RegiSCAR score | 4 (probable) | 3 (possible) | 5 (probable) | 5 (probable) | 2 (possible) | 8 (definite) |
| Naranjo score | 4 (possible) | 4 (possible) | 7 (probable) | 3 (possible) | 4 (possible) | 7 (probable) |
Abbreviations: ALT, alanine aminotransferase; DRESS, drug reaction with eosinophilia and systemic symptoms; NK, not known; UK, United Kingdom; ULN, upper limit of normal; US, United States.
Presumed birthplace.
The previously unreported patient is a 44-year-old Zambian man, who had HIV infection diagnosed in Auckland during 2006, with an initial HIV load of 4.7 log10 copies/mL and a CD4 lymphocyte count of 8 × 106/L. Eight years after starting antiretroviral therapy a period of poor treatment compliance led to the development of multidrug resistance, and the patient’s treatment was changed to raltegravir (400 mg twice daily), darunavir (600 mg twice daily), ritonavir (100 mg twice daily), zidovudine (300 mg twice daily), and lamivudine (150 mg twice daily). Four weeks later he presented with mild fatigue and an intermittently itchy rash on his arms and legs. He continued his antiretroviral regimen, unchanged, but 5 days later he presented again with significant worsening of both his fatigue and his rash, without fever. The rash was present on his face, arms, and trunk, with diffuse, confluent, infiltrating erythema, with scaling and follicular accentuation. At this time he had eosinophilia (eosinophil count, 1.44 × 109/L), and biochemical evidence of hepatic and renal dysfunction (γ-glutamyltransferase, 383 U/L; alanine aminotransferase, 295 U/L; creatinine, 149 µmol/L) and mild myositis (creatine kinase, 523 U/L). The patient had no urinary eosinophilia and no detectable human herpesvirus 6, Epstein-Barr virus, or cytomegalovirus DNA in his peripheral blood.
Antiretroviral therapy was stopped, and the patient’s symptoms and biochemical and hematologic abnormalities improved during the next 3 weeks. He then restarted antiretroviral therapy with darunavir (600 mg twice daily), ritonavir (100 mg twice daily), etravirine (200 mg twice daily), zidovudine (300 mg twice daily), and lamivudine (150 mg twice daily), but 3 days later he experienced nasal congestion, itchy eyes, and a sensation of chills, without fever or rash. Blood tests performed 6 days after restarting antiretroviral therapy showed deterioration in hepatic and renal function (γ-glutamyltransferase, 249 U/L; alanine aminotransferase, 126 U/L; creatinine, 116 µmol/L) and a recurrence of eosinophilia (eosinophil count, 1.14 × 109/L) Antiretroviral therapy was again stopped. Nine weeks later antiretroviral therapy was restarted with atazanavir (400 mg once daily), dolutegravir (50 mg once daily), zidovudine (300 mg twice daily), and lamivudine (150 mg twice daily), with no subsequent recurrence of rash, lethargy or abnormal laboratory test results. The patient has since remained in good health.
The current patient, along with 3 of the 5 who had previously been reported to have DRESS syndrome develop during treatment with raltegravir [7, 9, 10], consented to provide a blood sample for HLA testing. The other 2 patients did not undergo HLA testing [6, 8]. The HLA test results for the 4 patients who were tested are shown in Table 2. The HLA-B*53 allele and the HLA-C*04 allele, with which the HLA-B*53 allele is commonly haplotypic, were present in all 4 patients. High-resolution HLA testing, performed in 3 of the 4 patients, demonstrated that 2 were homozygous and 1 was heterozygous, for the HLA-B*53:01 allele. No other HLA alleles were shared by all tested patients.
Table 2.
HLA Typing Results in 4 Patients With DRESS Syndrome During Raltegravir Treatmenta
| HLA Type | Patient [Reference] | |||
|---|---|---|---|---|
| 1 [Current] | 3 [7] | 5 [9] | 6 [10] | |
| HLA-A | 30:01, 36:01 | 30:02, 74:01 | 02:05, 23:01 | 68, 68 |
| HLA-B | 42:01, 53:01 | 53:01, 53:01 | 53:01, 53:01 | 39, 53 |
| HLA-C | 04:01, 17:01 | 04:01, 04:01 | 04:01, 08:01 | 04, 07 |
| HLA-DRB1 | 03:02, 03:02 | 01:02, 08:04 | 03:02, 13:02 | 04, 13 |
| HLA-DQB1 | 04, 04 | 03:19, 05:01 | 04:01, 05:01 | 04, 06 |
Abbreviations: DRESS, drug reaction with eosinophilia and systemic symptoms.
High-resolution (4-digit) HLA testing was performed in patients 1, 3, and 5, but only low-resolution testing could be performed in patient 6.
These data suggested that HLA-B*53:01 molecules may be directly or indirectly involved in the mechanisms that induce DRESS syndrome in patients treated with raltegravir. Of significance, the allele for the HLA-B*35:01 molecule, which is very similar to the HLA-B*53:01 molecule, is carried in approximately 10% of persons of European ancestry [13], but there have not been any reports of DRESS syndrome or other cutaneous reactions to raltegravir in persons of European ethnicity. This suggested that structural features unique to the HLA-B*53:01 molecule, and not shared by the HLA-B*35:01 molecule, may be essential for raltegravir binding. We therefore plotted sequence variability between the HLA-B*53:01 and HLA-B*35:01 molecules to identify unique structural elements.
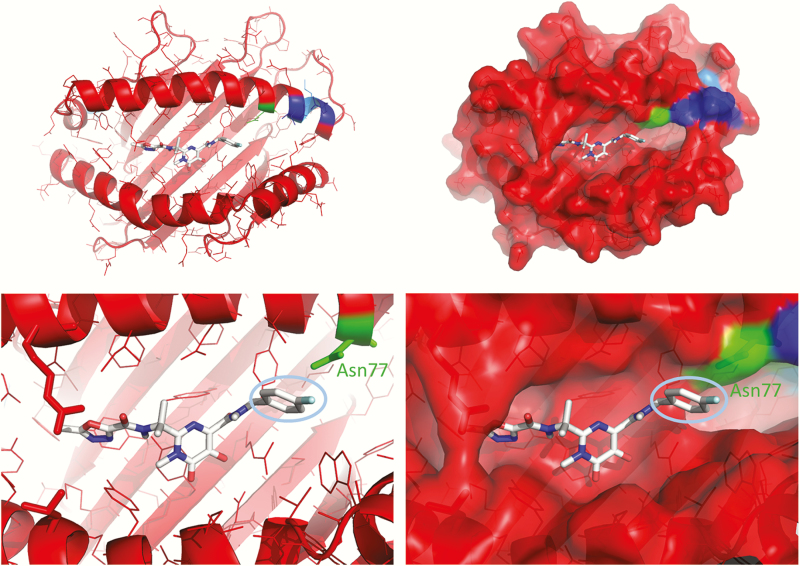
As shown in Figure 1, there are 4 polymorphic differences between HLA-B*53:01 and HLA-B*35:01 in the C-terminal end of the α1 helix flanking the antigen binding cleft. To determine whether side chains of the amino acids at these polymorphic sites may be involved in direct interactions with raltegravir, we used molecular docking to predict intermolecular contacts between the drug and the antigen binding cleft of HLA-B*53:01. Figure 1 shows that raltegravir was predicted to bind at a site corresponding to positions 1–7 of the HLA-B*53:01 peptide-binding cleft. The 4-fluorobenzyl group of raltegravir was predicted to contact Asparagine77, a position that differs between HLA-B*53:01 and the closely related HLA-B*35:01. The most significant difference between HLA-B*53:01 and HLA-B*35:01 is located at position 83, Arginine in HLA-B*53:01. This side chain was solvent accessible in the HLA-B*35:01 crystal structure and oriented toward the T-cell antigen receptor (20566715).
Figure 1.
HLA-B*53:01 has unique structural features in the antigen binding cleft. The crystal structure (Protein Data Bank code 1A1M) is shown as a ribbon diagram colored by sequence similarity to HLA-B*35:01. BLOSUM62 matrix similarity values are as follows: blue, 40–50; cyan, 50–60; green, 60–70; yellow, 70–80; orange, 80–90; and red, 90–100. Raltegravir was positioned in the antigen binding cleft by molecular docking using AutoDock Vina software, version 1.1.2. The cyan oval in the bottom panels shows the 4-fluorobenzyl group of raltegravir, which is predicted to interact with Asparagine77, a position in the C-terminal of the α1 helix that distinguishes the risk allele HLA-B*53:01 from the closely related and apparently nonrisk allele HLA-B*35:01.
DISCUSSION
Our study strongly suggests an association between HLA-B*53:01 and raltegravir-induced DRESS syndrome. The carriage rate of HLA-B*53 is approximately 20% in African populations [13–15], approximately 6% in American Hispanics [13, 15], and approximately 0.5% in whites [13, 15]. Therefore, the probability that 3 African and 1 Hispanic patient would be carriers of HLA-B*53, with 2 of 3 African patients homozygous for HLA-B*53, is approximately 0.0000192 (0.25 × 0.06). Previous studies have shown that the HLA-B*53:01 allele comprises >95% of all HLA-B*53 alleles [13–15]. Therefore, it is extremely likely that the Hispanic patient in whom high-resolution testing could not be performed also had the HLA-B*53:01 allele. The finding that 2 of the 4 patients overall were homozygous for HLA-B*53:01, and 2 of the 3 of African origin, is also interesting and raises the possibility that the presence of 2 HLA-B*53:01 alleles may lead to a heightened risk of DRESS syndrome during raltegravir treatment . This is of interest in light of the previous demonstration of a potential gene dose effect for HLA-B*13:01 in relation to dapsone hypersensitivity [16], and for HLA-B*58:01 in relation to DRESS syndrome or Stevens-Johnson syndrome/toxic epidermal necrolysis during treatment with allopurinol [17].
Previous reports have highlighted the difficulties that may be encountered in confidently diagnosing DRESS syndrome and determining the drug responsible for initiating the adverse reaction [11, 18, 19]. We used the RegiSCAR scoring system to classify each case as a possible, probable, or definite case of DRESS syndrome [11], and the Naranjo adverse drug reaction probability scale to estimate the probability that DRESS syndrome was secondary to treatment with raltegravir [12]. The proportion of cases considered probable or definite DRESS syndrome in our series (4 of 6:66%) is consistent with that found in the cases enrolled in the RegiSCAR registry (114 of 174:66%), and the proportion in whom causation by raltegravir was considered possible or probable (6 of 6:100%) is similar to the proportion of cases in the RegiSCAR registry in whom a drug was identified as the possible or probable cause of DRESS syndrome (103 of 117:88%) [18]. The recurrence of some symptoms of DRESS syndrome, together with a deterioration in liver function test results, after antiretroviral therapy had been restarted with darunavir, ritonavir, etravirine, zidovudine, and lamivudine raised the possibility that raltegravir was not the causative agent in the current patient. However, the presence of limited symptoms and relatively minor laboratory abnormalities in the absence of rash, on rechallenge with darunavir, was consistent with relapse of DRESS syndrome, and unlike a rechallenge reaction, in which the severity of all symptoms and laboratory findings is intensified compared with the original reaction. Relapse of DRESS syndrome commonly occurs 2–3 weeks after the initial presentation, often when steroids are being weaned, and typically includes a narrower constellation of symptoms and/or less marked laboratory abnormalities compared with the initial presentation [19].
Awareness that the HLA-B*53 allele is very common in African, and to a lesser degree Hispanic, persons, and that it is strongly associated with the development of DRESS syndrome during treatment with raltegravir, potentially could assist clinicians caring for patients with HIV infection. Detection of the HLA-B*53 allele in a patient with HIV infection before starting treatment with raltegravir, particularly if 2 copies of the allele are carried, could alert physicians to the potential risk of a serious, immunologically mediated, adverse drug reaction. The safety and clinical utility of detection of an HLA allele known to be associated with a hypersensitivity drug reaction is influenced by the negative predictive value of the test and the number of patients needed to be tested (NNT) to prevent 1 case. The NNT is influenced by the frequency of the risk HLA allele in the relevant population, the positive predictive value of the risk HLA allele for the development of disease, and the prevalence of the disease itself. The NNT may vary from as few as 13 needed to test to prevent 1 case of abacavir hypersensitivity, to almost 14 000 needed to test to prevent 1 case of flucloxacillin drug-induced liver disease [1]. Our results do not allow us to estimate the negative predictive value, the positive predictive value, or the NNT to prevent 1 case of HLA-B*53–associated, raltegravir-induced, DRESS syndrome.
The absence of any reported episodes of DRESS syndrome in 359 African and 161 Hispanic patients treated with raltegravir in the STARTMRK, BENCHMRK, SECOND-LINE, and SELECT studies and the small number of cases of DRESS syndrome reported in the postmarketing phase of raltegravir development suggest that although raltegravir-induced DRESS syndrome may be underreported, the overall prevalence is very low [20–22]. This suggests that only a small proportion of patients carrying the HLA-B*53 allele will develop raltegravir-induced DRESS syndrome; however, given the lesser exposure of indigenous African populations to raltegravir, more information is needed regarding the risk associated with this allele. Of additional interest, homozygosity for HLA-B*53 seemed to be overrepresented in our patients. Using allele frequency estimates and assuming adherence to Hardy-Weinberg equilibrium in patients of African origin living in Africa, as opposed to African Americans or African Europeans, homozygosity for HLA-B*53 is predicted to be overrepresented (0.0025–0.04) in continental African populations [13]. This may have additional relevance for the current dose finding trials of raltegravir in African neonates. DRESS syndrome associated with nevirapine has been reported in an HLA-C*04:01–positive 5-month-old infant, demonstrating that T-cell maturation sufficient to allow the development of DRESS syndrome may occur early in life [23].
We found that the occurrence of DRESS syndrome during treatment with raltegravir was strongly associated not only with the HLA-B*53 allele but also with the HLA-C*04 allele with which it commonly is haplotypic. The close proximity of the genes for HLA-B and HLA-C on chromosome 6 results in combinations of specific HLA-B and HLA-C alleles occurring much more commonly than would be expected by chance. The HLA-B*53/HLA-C*04 haplotype is the most common HLA-B/C haplotype in African Americans (17%) and the 14th most common in Hispanics (4%), but it is very infrequent in white, Asian, and indigenous North Americans and in Europeans (all <1%) [13, 15]. It is important to note that HLA-C*04 is also in linkage disequilibrium with a number of other HLA-B alleles both in African and in other ethnic groups, and therefore it is likely that HLA-B*53 rather than HLA-C*04 is driving the association of the haplotype with raltegravir hypersensitivity. The presence of only 1 HLA-C*04 allele in 1 patient who was homozygous for the HLA-B*53 allele is also supportive of this hypothesis. Interestingly, presence of the HLA-B*53 allele is associated with protection from severe malaria, and it is proposed that this allele arose as a result of a gene conversion event and has been selected for by malaria [24]. Presence of the HLA-B*53:01 allele is associated with an increased risk of progression to AIDS in HIV-1–infected persons [25], but a reduced risk of progression to AIDS in HIV-2–infected persons [26].
Further functional, molecular, biochemical, and structural studies are needed to determine the specific mechanism by which treatment with raltegravir leads to the development of DRESS syndrome in persons with the HLA-B*53 allele. However, our findings suggest that raltegravir-induced DRESS syndrome in HLA-B*53:01–positive patients may be caused by an mechanism analogous to that causing the abacavir hypersensitivity reaction in HLA-B*57:01–positive patients. Noncovalent binding of abacavir within the antigen presenting cleft of the HLA-B*57:01 molecule changes the shape and chemistry of the cleft, alters the repertoire of self-peptides that are bound and presented to CD8 T cells, and culminates in the clinical syndrome known as abacavir hypersensitivity [27, 28]. Although our findings suggest that raltegravir may bind within the antigen binding cleft of the HLA-B*53:01 molecule, it is currently unknown whether it acts in a manner similar to abacavir and alters the repertoire of self-peptides presented to CD8 T cells. Just as abacavir hypersensitivity exclusively occurs as a result of binding to the HLA-B*57:01 molecule and does not occur in persons with other very similar HLA molecules, such as HLA-B*57:03 or HLA-B*58:01 [27, 28], so it seems that raltegravir-induced DRESS syndrome may occur exclusively as a result of binding to the HLA-B*53:01 molecule and not occur in persons with other very similar HLA molecules, such as HLA-B*35:01. These data suggest that raltegravir has the potential to interact with HLA-B*53:01 because the antigen binding cleft has unique structural features complementary to the drug. Alternative possible explanations for the induction of DRESS syndrome by raltegravir include the formation of raltegravir metabolites and/or the induction of specific peptides that are presented in the HLA-B*53:01 binding cleft.
Notes
Author contributions. Study concept and design: M. T., C. H., E. D., D. A. O., and E. P. Data collection: all authors. Data analysis: M. T. and E. P. Drafting of the manuscript: M. T., D. A. O., and E. P. Review and approval of the final manuscript: all authors.
Acknowledgments We are grateful to the patients who agreed to have HLA testing performed, and to the staff of the tissue typing laboratories who performed the HLA testing. Simon Mallal kindly provided advice on the manuscript.
Financial support. This work was supported by the National Health and Medical Research Council of Australia (E. P.), the National Institutes of Health (grant R01AI103348 to D. A. O. and grants P50GM115305-1 and R01AI103348-01 to E. P.), and the Australian Centre for Hepatitis and HIV Research (E. P.).
Potential conflicts of interest. E. P. is codirector of IIID Pty Ltd, which holds a patent for HLA-B*57:01 testing, but reports no conflicts directly related to the work at hand. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Pavlos R, Mallal S, Ostrov D, et al. T cell-mediated hypersensitivity reactions to drugs. Annu Rev Med 2015; 66:20.1–20.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carr DF, Chaponda M, Jorgensen AL, et al. Association of human leukocyte antigen alleles and nevirapine hypersensitivity in a Malawian HIV-infected population. Clin Infect Dis 2013; 56:1330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Markowitz M, Nguyen BY, Gotuzzo E, et al. ; Protocol 004 Part II Study Team. Rapid and durable antiretroviral effect of the HIV-1 Integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study. J Acquir Immune Defic Syndr 2007; 46:125–33. [DOI] [PubMed] [Google Scholar]
- 4. Lennox JL, Dejesus E, Berger DS, et al. ; STARTMRK Investigators. Raltegravir versus efavirenz regimens in treatment-naive HIV-1-infected patients: 96-week efficacy, durability, subgroup, safety, and metabolic analyses. J Acquir Immune Defic Syndr 2010; 55:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steigbigel RT, Cooper DA, Kumar PN, et al. ; BENCHMRK Study Teams. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med 2008; 359:339–54. [DOI] [PubMed] [Google Scholar]
- 6. Zhang KS, Modi GM, Hsu S. DRESS syndrome associated with raltegravir. Dermatol Online J 2011; 17:14. [PubMed] [Google Scholar]
- 7. Loulergue P, Mir O. Raltegravir-induced DRESS syndrome. Scand J Infect Dis 2012; 44:802–3. [DOI] [PubMed] [Google Scholar]
- 8. Perry MEO, Almaani N, Desai N, Larbalestier N, Fox J, Chilton D. Raltegravir-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome – implications for clinical practice and patient safety. Int J STD AIDS 2013; 24:675–7. [DOI] [PubMed] [Google Scholar]
- 9. Ripamonti D, Benatti SV, Di Filippo E, Ravasio V, Rizzi M. Drug reaction with eosinophilia and systemic symptoms associated with raltegravir use: case report and review of the literature. AIDS 2014; 28:1077–9. [DOI] [PubMed] [Google Scholar]
- 10. Yee BE, Nguyen NH, Lee D. Extensive pulmonary involvement with raltegravir-induced DRESS syndrome in a postpartum woman with HIV. BMJ Case Rep 2014. doi:10.1136/bcr-2013–201545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kardaun SH, Sidoroff A, Valeyrie-Allanore L, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol 2007; 156:609–11. [DOI] [PubMed] [Google Scholar]
- 12. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30:239–45. [DOI] [PubMed] [Google Scholar]
- 13. González-Galarza FF, Takeshita LY, Santos EJ, et al. Allele frequency net 2015 update: new features for HLA epitopes, KIR and disease and HLA adverse drug reaction associations. Nucleic Acids Res 2015; 43(Database issue):D784–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cao K, Moormann AM, Lyke KE, et al. Differentiation between African populations is evidenced by the diversity of alleles and haplotypes of HLA class I loci. Tissue Antigens 2004; 63:293–325. [DOI] [PubMed] [Google Scholar]
- 15. Cao K, Hollenbach J, Shi X, Shi W, Chopek M, Fernández-Viña MA. Analysis of the frequencies of HLA-A, B, and C alleles and haplotypes in the five major ethnic groups of the United States reveals high levels of diversity in these loci and contrasting distribution patterns in these populations. Hum Immunol 2001; 62:1009–30. [DOI] [PubMed] [Google Scholar]
- 16. Zhang FR, Liu H, Irwanto A, et al. HLA-B*13:01 and the dapsone hypersensitivity syndrome. N Engl J Med 2013; 369:1620–8. [DOI] [PubMed] [Google Scholar]
- 17. Chung WH, Pan RY, Chu MT, et al. Oxypurinol-specific T cells possess preferential TCR clonotypes and express granulysin in allopurinol-induced severe cutaneous adverse reactions. J Invest Dermatol 2015; 135:2237–48. [DOI] [PubMed] [Google Scholar]
- 18. Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. ; RegiSCAR study group. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol 2013; 169:1071–80. [DOI] [PubMed] [Google Scholar]
- 19. Picard D, Vellar M, Janela B, Roussel A, Joly P, Musette P. Recurrence of drug-induced reactions in DRESS patients. J Eur Acad Dermatol Venereol 2015; 29:801–4. [DOI] [PubMed] [Google Scholar]
- 20. Teppler H, Brown DD, Leavitt RY, et al. Long-term safety from the raltegravir clinical development program. Curr HIV Res 2011; 9:40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. SECOND-LINE Study Group. Ritonavir-boosted lopinavir plus nucleoside or nucleotide reverse transcriptase inhibitors versus ritonavir-boosted lopinavir plus raltegravir for treatment of HIV-1 infection in adults with virological failure of a standard first-line ART regimen (SECOND-LINE): a randomised, open-label, non-inferiority study. Lancet 2013; 381:2091–9. [DOI] [PubMed] [Google Scholar]
- 22. La Rosa AM, Harrison LJ, Taiwo B, et al. ; ACTG A5273 Study Group. Raltegravir in second-line antiretroviral therapy in resource-limited settings (SELECT): a randomised, phase 3, non-inferiority study. Lancet HIV 2016; 3:e247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Keane NM, Pavlos RK, McKinnon E, et al. HLA class I restricted CD8+ and class II restricted CD4+ T cells are implicated in the pathogenesis of nevirapine hypersensitivity. AIDS 2014; 28:1891–901. [DOI] [PubMed] [Google Scholar]
- 24. Hill AV, Allsopp CE, Kwiatkowski D, et al. Common west African HLA antigens are associated with protection from severe malaria. Nature 1991; 352:595–600. [DOI] [PubMed] [Google Scholar]
- 25. Gao X, Nelson GW, Karacki P, et al. Effect of a single amino acid change in MHC class I molecules on the rate of progression to AIDS. N Engl J Med 2001; 344:1668–75. [DOI] [PubMed] [Google Scholar]
- 26. Diouf K, Sarr AD, Eisen G, Popper S, Mboup S, Kanki P. Associations between MHC class I and susceptibility to HIV-2 disease progression. J Hum Virol 2002; 5:1–7. [PubMed] [Google Scholar]
- 27. Illing PT, Vivian JP, Dudek NL, et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature 2012; 486:554–8. [DOI] [PubMed] [Google Scholar]
- 28. Ostrov DA, Grant BJ, Pompeu YA, et al. Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire. Proc Natl Acad Sci U S A 2012; 109:9959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]