Summary
The Malawi Adult Meningitis Score prediction tool accurately estimates risk of clinical outcome from bacterial meningitis in sub-Saharan Africa. Clinical trial analysis by risk stratification reveals more severe outcomes in low-risk groups receiving adjunctive glycerol compared to placebo.
Keywords: bacterial meningitis, HIV, Africa, outcome, severity score,
Abstract
Background.
Acute bacterial meningitis (ABM) in adults residing in resource-poor countries is associated with mortality rates >50%. To improve outcome, interventional trials and standardized clinical algorithms are urgently required. To optimize these processes, we developed and validated an outcome prediction tool to identify ABM patients at greatest risk of death.
Methods.
We derived a nomogram using mortality predictors derived from a logistic regression model of a discovery database of adult Malawian patients with ABM (n = 523 [65%] cerebrospinal fluid [CSF] culture positive). We validated the nomogram internally using a bootstrap procedure and subsequently used the nomogram scores to further interpret the effects of adjunctive dexamethasone and glycerol using clinical trial data from Malawi.
Results.
ABM mortality at 6-week follow-up was 54%. Five of 15 variables tested were strongly associated with poor outcome (CSF culture positivity, CSF white blood cell count, hemoglobin, Glasgow Coma Scale, and pulse rate), and were used in the derivation of the Malawi Adult Meningitis Score (MAMS) nomogram. The C-index (area under the curve) was 0.76 (95% confidence interval, .71–.80) and calibration was good (Hosmer-Lemeshow C-statistic = 5.48, df = 8, P = .705). Harmful effects of adjunctive glycerol were observed in groups with relatively low predicted risk of poor outcome (25%–50% risk): Case Fatality Rate of 21% in the placebo group and 52% in the glycerol group (P < .001). This effect was not seen with adjunctive dexamethasone.
Conclusions.
MAMS provides a novel tool for predicting prognosis and improving interpretation of ABM clinical trials by risk stratification in resource-poor settings. Whether MAMS can be applied to non-HIV-endemic countries requires further evaluation.
(See the Editorial Commentary by Quagliarello on pages 420–1.)
Acute bacterial meningitis (ABM) in sub-Saharan Africa is common and associated with high rates of death and comorbidity in both adults and children [1, 2]. Throughout the African continent, bacterial meningitis is predominately caused by Streptococcus pneumoniae [3–5], with the exception of the “meningitis belt” Sahel region, where seasonal epidemic meningococcal meningitis also occurs [6]. In Malawi, the incidence of ABM in adults is 12 per 100000, and the associated mortality is 54% compared with 20%–30% in Europe and the United States [1, 2, 7, 8]. Despite intensive efforts, high mortality remains unchanged region-wide; adjunctive treatments such as corticosteroids and glycerol are ineffective and possibly harmful [9–12].
To improve ABM outcome, interventional trials testing new treatment approaches and resource-appropriate standardized clinical algorithms are urgently required. A robust outcome prediction tool would help optimize these processes and provide a basis to examine why previous adjunctive interventions have been unsuccessful [13].
Well-validated scores used for risk stratification in life-threatening infection include Confusion Urea Respiratory Rate age 65 (CURB-65) for pneumonia, and Acute Physiology And Chronic Health Evaluation (APACHE II) and Sepsis-related Organ Failure Assessment (SOFA) for sepsis [14–17]. Two good-quality scores have been reported for ABM in developed settings [18, 19]. A meningitis score has been developed in the African subcontinent [20] but was developed specifically for outcome prediction during meningococcal outbreaks.
We have previously identified clinical predictors of death from adult ABM in Malawi [1]. In the present study, we grouped these outcome predictors to generate and validate a severity score nomogram for use in resource-poor settings. We used the validated score to analyze data from large placebo-controlled trials of adjunctive dexamethasone and glycerol in Malawi, to better understand why these treatments were ineffective.
PATIENTS AND METHODS
Patient Selection
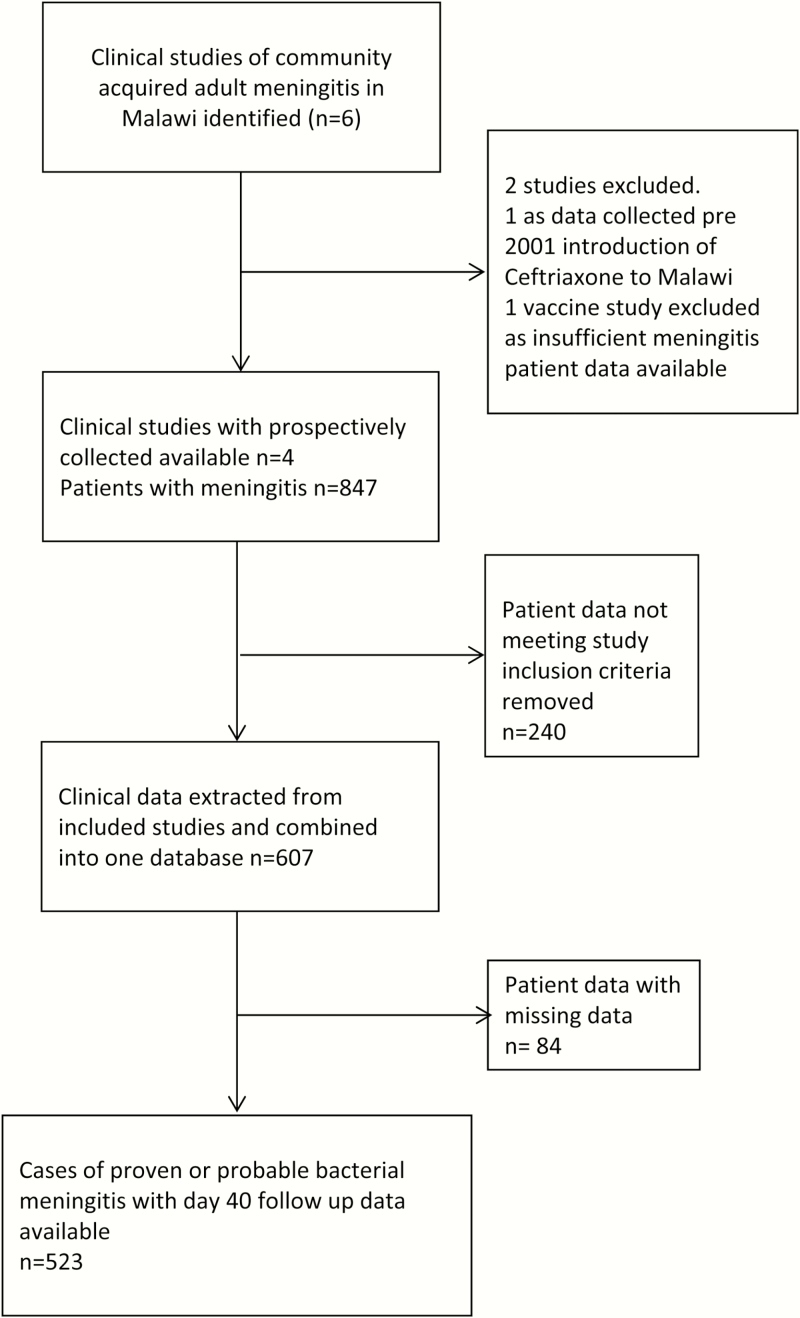
Clinical data from the Malawi Meningitis Database (MMD) [1], and patient data from a recent clinical trial of goal-directed therapy (ISRCTN96218197) were used (Figure 1). All patients had been enrolled in clinical studies of adult ABM in Queen Elizabeth Central Hospital in Blantyre, Malawi, between 2000 and 2013 (Figure 1). The inclusion criteria for the MMD were age >14 years with proven cerebrospinal fluid (CSF) infection on culture, polymerase chain reaction, or Gram stain of bacteria known to cause meningitis (proven meningitis), or appropriate clinical history <5 days with a CSF white blood cell (WBC) count >50 cells/µL and >50% neutrophils (probable meningitis). In human immunodeficiency virus (HIV)–coinfected individuals, the CSF WBC count threshold for probable meningitis was lowered to >5 cells/µL, (100% neutrophils), to include HIV-coinfected individuals with low CD4 counts who mount a weak CSF inflammatory response in ABM [1, 4].
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram demonstrating selection of data for the Malawi Meningitis Database.
Cases were excluded if there was CSF evidence of Cryptococcus neoformans or Mycobacterium tuberculosis, CSF WBC count >50% lymphocytes with no documented prehospital antibiotics, or trial-specific exclusion criteria such as diabetes for the glycerol trial [9, 10]. Diagnostic testing for viral meningitis was not available and is an uncommon cause of neurological infection in our center [21]. MMD patients randomized to receive glycerol were excluded from the nomogram development, as glycerol receipt was an independent predictor of poor outcome [10].
Ascertainment of Clinical Characteristics and Outcomes
All prospective clinical trial data were utilized with permission from the principal investigator. Individual study protocols were examined; only identical variables across the studies were collected (data collected in the same units, at similar time points, using the same normal ranges). All studies were undertaken in the same hospital, using the same laboratories and similar case record forms. Physiological data are from the first recording to minimize both heterogeneity and biases associated with delays to antibiotic therapy, and increase applicability for clinical use in the region. Outcome was measured as death or survival at day 40 postadmission to account for additional acute postdischarge mortality, which has been reported to occur due to late complications in 5%–10% of initial survivors of ABM [9, 22]. Data on in-hospital delays and morbidity (principally deafness) were collected variably across the studies and were not included to minimize heterogeneity.
Ethical Approvals
All contributing clinical studies were granted ethical approval by the College of Medicine Research and Ethics Committee, University of Malawi, and the Liverpool School of Tropical Medicine Research Ethics Committee and conformed to institutional guidelines. All trial participants or named patient guardians provided written informed consent.
Selection of Predictor Variables
A literature search was performed to determine predictor variables for adults with bacterial meningitis [1]. Individual predictors of mortality from the published analysis of the MMD (coma, seizures, anemia, and tachycardia) [1] and variables associated in other studies with poor outcome (age, sex, HIV status, CSF WBC count, and CSF culture) were finally used [7, 19, 23, 24].
Statistical Methods, Derivation of the Nomogram, and Validation Methods
Data were summarized as follows: continuous normally distributed data using means and standard deviations, skewed data using medians and interquartile range, and categorical data using percentages. Analyses using a univariate logistic regression model were performed on all relevant covariates and factors to assess any association with day 40 outcome. The unadjusted estimates were reported using odds ratios (ORs) and 95% confidence intervals (CIs). Multivariable analyses followed on the discovery cohort using a logistic regression model to assess factors and covariates independently associated with the day 40 outcome; this model included all variables with statistical significance on univariate analysis. Variables with high proportions (>50%) of missing data were excluded to reduce risks of overfitting. All analyses were performed using IBM SPSS software version 20.0 (IBM Statistics, Armonk, New York) and Stata software version 13.0 (StataCorp, College Station, Texas). Statistical significance was set at P < .05.
All variables with significance in the multivariable model were used to model the predictive tool, using R software version R-i386 3.0.3 (www.r-project.org) using rms package and the lrm and nomogram commands.
The developed model was validated to estimate the potential model performance. Because external data for validation have not yet been identified, an internal validation procedure was performed using bootstrapping (5000 samples), based on calibration, and discrimination [25, 26]. This procedure was selected to optimize the number of cases available while minimizing risks of overfitting.
A logistic regression model was used to obtain the predicted deaths. The Hosmer-Lemeshow C-statistic was used to assess calibration; low insignificant Hosmer-Lemeshow C-statistic indicated good fit [27]. The calibration assessment was supplemented by the use of a Brier score (range 0 and 1); an α value <.05 (5% significance level) was used to determine model fit [28, 29]. A receiver operating characteristic (ROC) curve was plotted and used to calculate the validated concordance index (C-index). The ROC curve was obtained with the C-index (area under the curve [AUC]) and associated 95% CIs. The sensitivity and specificity of the model is based on a cutoff of 50% risk score. The individual Malawi Adult Meningitis Score (MAMS) was calculated for patients using IBM SPSS software version 20.0.
Analysis of Clinical Trial Data Using MAMS
The MAMS was calculated for all patients who had been recruited to clinical trials testing adjunctive dexamethasone and glycerol. Cases were risk stratified by quartile risk of poor outcome, and separated by placebo or intervention. Case fatality rate (CFRs) were calculated within each quartile group and compared to the predicted risk of poor outcome; statistical significance was determined using χ2 test. Data were analyzed using both original complete case and bootstrapped data.
RESULTS
Clinical Characteristics of the Patients
Data on patients meeting the inclusion criteria were available for 607 ABM cases and were used for univariate analyses. Five hundred ninety-three patients had CSF results available; of these, 384 (65%) were CSF culture positive (Supplementary Table 1). Complete case data were available for 523 cases of bacterial meningitis, which were used for the multivariable analyses and development of the nomogram. Mean age was 32.5 years (SD, 10.8 years), 50.7% (308/607) were female, 87% (506/584) were HIV coinfected, and 31% (171/551) had an acute seizure within the first 6 hours of admission. Presenting characteristics by outcome group are detailed in Table 1.
Table 1.
Results of the Univariate Analysis and Predictors of Outcome From the Complete Case Data
| Parameter | Day 40 | Univariate Analysis (Unadjusted) | Multivariable Analysis (Adjusted)a | |||
|---|---|---|---|---|---|---|
| Alive | Dead | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| Sample size n = 607 | 273 | 320 | ||||
| Age, y, mean (SD) | 31.5 (10.9) | 32 (10.6) | 1.01 (1.00–1.03) | .01 | 1.00 (.95–1.06) | .84 |
| >40 y | 42 (14.6) | 53 (16.6) | 1.30 (.67–2.5) | .42 | ||
| Sex, male | 136 (47) | 163 (50.9) | 1.17 (.85–1.62) | .32 | ||
| HIV status | ||||||
| Infected | 226 (78) | 280 (87) | 0.79 (.32–1.95) | .61 | ||
| Unknown | 10 (3.5) | 13 (4.1) | ||||
| Admission out of hours | 122 (42.5) | 139 (43.9) | 1.20 (.68–1.28) | .40 | ||
| Unknown | 30 (10.5) | 29 (9.1) | ||||
| GCS, mean (SD) | 12 (2.8) | 9.9 (3.5) | 0.82 (.78–.86) | <.001 | 0.77 (.72–.83) | <.001 |
| Acute seizure episodes | ||||||
| 1 seizure | 45 (16) | 70 (22) | 0.61 (.30–1.23) | .16 | ||
| 2 seizures | 18 (6.3) | 37 (11.6) | 0.75 (.33–1.70) | .49 | ||
| Temperature, °C, mean (SD) | 38.2 (1.1) | 38.4 (1.3) | 1.17 (1.02–1.34)b | .02 | c | |
| SpO2, %, mean (SD) | 95.5 (2.5) | 96 (6.5) | 0.90 (.82–1.00)b | .07 | c | |
| Pulse rate, beats/min, mean (SD) | 98.6 (19) | 104.8 (19.8) | 1.01 (1.00–1.02)b | <.001 | 1.01 (1.00–1.03) | .01 |
| MAP, mm Hg, mean (SD) | 89.9 (15.1) | 90.5 (16.7) | 1.00 (.99–1.01) | .81 | ||
| Respiratory rate, breaths/min, mean (SD) | 24.7 (6.6) | 26.9 (7.9) | 1.04 (.99–1.09)b | .11 | c | |
| CSF WBC count, cells/µLd, median (IQR) | 356 (64–1280) | 215 (35–630) | 0.49 (.37–.64)b | <.001 | 0.66 (.53–.80) | <.001 |
| CSF culture positive for Streptococcus pneumoniae |
160 (55) | 165 (51.6) | 1.06 (.66–17.1)b | .96 | 0.36 (.23–.57) | <.001 |
| Plasma glucose, mmol/L, mean (SD) | 7.5 (2.7) | 7.6 (3.8) | 1.00 (.95–1.05) | .87 | c | |
| Hemoglobin, g/dL, mean (SD) | 11.2 (2.6) | 10.1 (2.6) | 0.86 (.80–.91)b | <.001 | 0.84 (.78–.90) | <.001 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CI, confidence interval; CSF, cerebrospinal fluid; GCS, Glasgow Coma Scale; HIV, human immunodeficiency virus; IQR, interquartile range; MAP, mean arterial pressure; SD, standard deviation; SpO2, Oxygen Saturations (%); WBC, white blood cell.
Performed on complete case data only (n = 523).
Odds ratio for a unit change in predictor variable.
Proportion of missing data >50%, so variable was excluded from multivariable analysis.
Odds ratios calculated on log10 data. Fifteen patients were CSF culture negative with CSF WBC count >50 cells/µL; an additional 6 were HIV coinfected white cell counts >5 cells/µL.
The case fatality rate (CFR) was 44% (267/607) at day 10 and 54% (320/593) at day 40. The following associations with death at day 40 were observed univariately in the discovery cohort (Table 1): age, temperature, low Glasgow Coma Scale (GCS), low oxygen saturation, high pulse rate, low CSF WBC count, and low hemoglobin level (Table 1). Other included variables showed no association with outcome on univariate analysis: sex, HIV serostatus, mean arterial blood pressure, respiratory rate, CSF culture, seizures, and blood glucose (Table 1).
All variables tested were included in the multivariable analysis model, with the exception of oxygen saturation, temperature, and respiratory rate (>50% missing data) (Table 1). All variables with univariate significance, with the exception of age, also had an association with outcome on multivariable analysis (ORs per unit of measure change): GCS: OR, 0.77 [95% CI, .72–.83], P < .001; pulse rate: OR, 1.01 [95% CI, 1.00–1.03], P = .01; log10 CSF WBC count: OR, 0.66 [95% CI, .53–.80], P < .001; and hemoglobin: OR, 0.84 [95% CI, .78–.90], P < .001.
Although CSF pneumococcal culture had no significant association with outcome on univariate analysis, the multivariable model demonstrated a significant association with survival (OR, 0.36 [95% CI, .23–.57], P < .001). Outcome data by organism (Supplementary Table 1) showed that increased mortality was observed in patients with nonpneumococcal meningitis. Although numbers were small, increased mortality in the nonpneumococcal groups (CFR, 56.5% vs 51.5% for S. pneumoniae) was correlated with lower GCS and thus associated with poor outcome in the multivariable model. The lack of significant association with mortality on univariate analysis for the remainder of the variables, including HIV status, was retained on multivariable analysis.
Results of the Nomogram
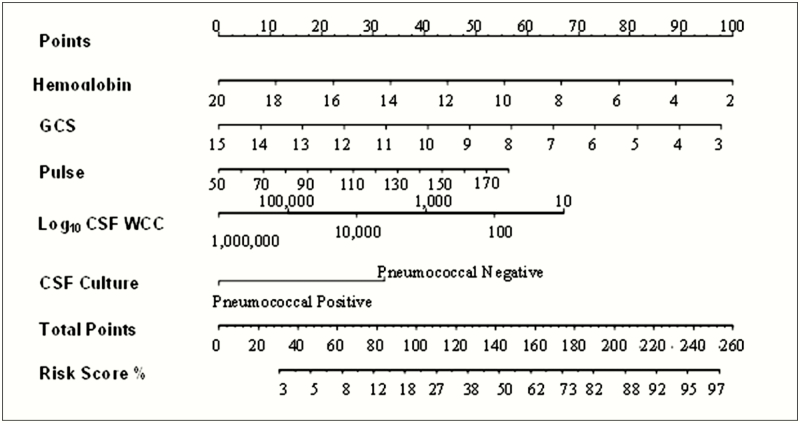
Five variables strongly associated with day 40 mortality were used to develop the nomogram: GCS, pulse rate, CSF WBC count, pneumococcal culture, and hemoglobin level (Figure 2). To calibrate the nomogram, the continuous variables were categorized either by quartile around the median, or by standard normal clinical ranges. To optimize the nomogram, calibration data derived from the categorical data were compared with continuous data [30]. Categorical data led to overfitting and poorer performance; therefore, the nomogram using continuous data is reported (Figure 2).
Figure 2.
Malawi Adult Meningitis Score (MAMS) nomogram. A variable is measured and its value is matched to the corresponding point on the first row of the nomogram. The points are then added for all 5 variables for each patient. The total points for the patient are matched to the corresponding risk score percentage at the bottom (ie, match up the 2 last rows of the nomogram). Abbreviations: CSF, cerebrospinal fluid; GCS, Glasgow Coma Scale; WBC, white blood cell count.
The database was subject to 5000 bootstraps, and the entire derivation process was then repeated using the bootstrapped data. The log (OR) and associated bootstrapped CIs are reported (Supplementary Table 2). The data show shrinkage factor (bias) used to adjust the estimated regression coefficients in the final model for overfitting. Supplementary Table 2 details the bootstrapped CIs for estimated coefficients.
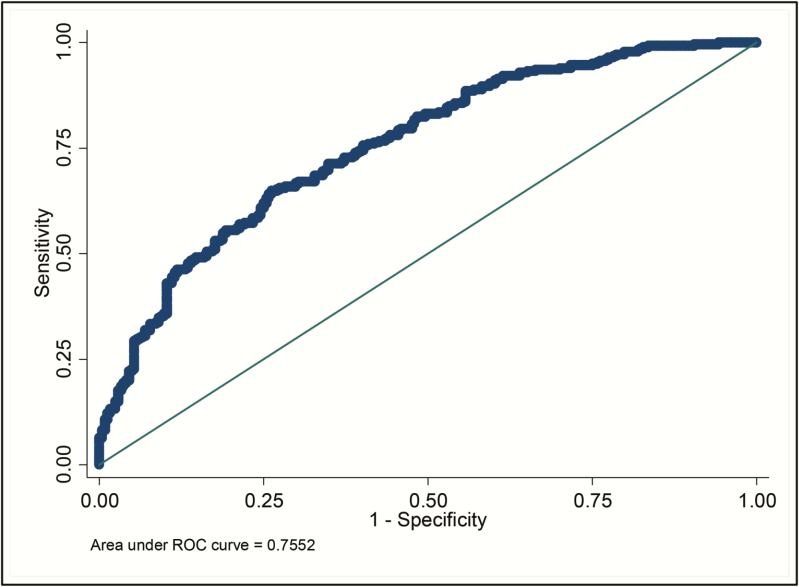
The corresponding ROC curve for the final nomogram is shown in Figure 3. The AUC was 0.76 (95% CI, .71–.80). The sensitivity of this model using a cutoff of 50% risk score is 71.7% (95% CI, 66.0%–76.9%) with a specificity of 63.1% (95% CI, 56.7%–69.2%).
Figure 3.
Receiver operating characteristic (ROC) curve for the Malawi Adult Meningitis Score (MAMS).
Calibration
Goodness-of-fit analysis was assessed to calibrate the nomogram. Hosmer-Lemeshow C-statistic was χ2 = 5.48, df = 8, P = .705. Calibration was good across individual centiles of estimated mortality probability (Table 2). The Brier score was 0.2 (95% bootstrap CI, .185–.215). Both the C-statistic and Brier score indicate good calibration. Discrimination was primarily determined by the C-index (AUC). The observed C-index was 0.755 from the original sample; the optimism corrected or adjusted C-index (mean over 5000 bootstraps) was 0.760 (95% bootstrapped CI, .71–.79).
Table 2.
Summary of the Agreement of the Nomogram per Risk Centile
| Group | Probability Centile of Death | Observed Deaths, No. (%) | Predicted Deaths, No. (%) | Total |
|---|---|---|---|---|
| 1 | 0.234 | 6 (11.3) | 9 (17.0) | 53 |
| 2 | 0.326 | 14 (26.9) | 15 (28.8) | 52 |
| 3 | 0.389 | 23 (44.2) | 19 (36.5) | 52 |
| 4 | 0.460 | 24 (45.3) | 22 (41.5) | 53 |
| 5 | 0.540 | 25 (48.1) | 26 (50.0) | 52 |
| 6 | 0.604 | 30 (57.7) | 30 (57.7) | 52 |
| 7 | 0.677 | 30 (56.6) | 34 (64.2) | 53 |
| 8 | 0.763 | 40 (76.9) | 37 (71.2) | 52 |
| 9 | 0.836 | 42 (80.8) | 42 (80.8) | 52 |
| 10 | 0.962 | 45 (86.5) | 46 (88.5) | 52 |
| Total | 279 | 279 | 523 |
Hosmer-Lemeshow C-statistic, χ2 = 5.48, df = 8, P = .705.
MAMS, the European Meningitis Score (EMS), and an older outcome score developed in the United States were all derived using similar methods. Therefore, when the C-index of MAMS was compared directly to the published C-index of these scores [18, 19, 31], MAMS was equivalent. When tested using Malawi data, the EMS C-index was 0.68 (95% CI, .63–.73), compared with the MAMS C-index of 0.76 (95% CI, .71–.80), suggesting that MAMS may be a better score than EMS in this setting.
Mortality Effects of Glycerol and Dexamethasone
Case fatality rate were compared by quartile of predicted risk in 2 clinical trials of dexamethasone and glycerol [9, 10]. In the dexamethasone study, an increased CFR was seen in the lowest risk quartile, but no differences were observed in other risk quartiles (Table 3). In contrast, glycerol was associated with increased risk of death across all but the highest risk quartile in that study (Table 3). The same trends were observed when nonbootstrapped data were used, but were not statistically significant (data not shown).
Table 3.
Day 40 Mortality Outcomes From Dexamethasone and Glycerol Compared to Placebo by Malawi Adult Meningitis Score Risk Centile (Bootstrapped Data)
| MAMS Risk Probability of Death at Day 40 | Placebo (n = 1293) |
Dexamethasone (n = 1268) |
CFR% P Value |
||
|---|---|---|---|---|---|
| Alive, No. | Dead, No. (CFR%) |
Alive, No. | Dead, No. (CFR%) |
||
| <25% | 61 | 0 (0) | 48 | 6 (11) | .010 |
| 25%–50% | 232 | 106 (31) | 236 | 126 (35) | .330 |
| 50%–75% | 252 | 311 (55) | 173 | 264 (60) | .100 |
| >75% | 66 | 265 (80) | 99 | 316 (78) | .210 |
| Total | 611 | 682 (53) | 556 | 712 (56) | .120 |
| Placebo (n = 549) |
Glycerol (n = 592) |
CFR% Significance of CFR Intervention vs Placebo P Value | |||
|---|---|---|---|---|---|
| Alive, No. | Dead, No. (CFR%) |
Alive, No. |
Dead, No. (CFR%) |
||
| <25% | 51 | 6 (10) | 49 | 14 (22) | .080 |
| 25%–50% | 112 | 31 (21) | 85 | 92 (52) | <.001 |
| 50%–75% | 119 | 124 (51) | 69 | 167 (70) | <.001 |
| >75% | 18 | 88 (83) | 29 | 87 (75) | .140 |
| Total | 300 | 249 (45) | 232 | 360 (61) | .001 |
Abbreviations: CFR, case fatality rate; MAMS, Malawi Adult Meningitis Score.
DISCUSSION
This analysis shows that estimation of mortality risk from bacterial meningitis in adults in sub-Saharan Africa is possible using simple clinical parameters. MAMS is derived from community-acquired meningitis data, from an African region with a high HIV prevalence and burden of adult bacterial meningitis. This score is therefore likely to be relevant to similar settings but not to regions where epidemics of both Neisseria meningitidis and S. pneumoniae occur seasonally [2, 6, 32]. We demonstrate the utility of MAMS in the assessment of clinical trials, showing that compared to placebo, adjunctive glycerol is associated with the greatest increased risk of death in those patients predicted to have a lower risk of poor outcome. The only other score published from Africa was derived in the Sahel region and, therefore, while applicable to settings with epidemic of meningococcal meningitis [20], is of limited utility elsewhere.
We used similar data processes to develop MAMS as used in the EMS and a score developed in the United States [18, 19]. The EMS was developed on a discovery cohort of 691 patients [7] and underwent validation on 301 separate patients from the European Dexamethasone Study [33]. Patients receiving either dexamethasone or placebo were included and, interestingly, the authors comment that EMS performed better in those in receipt of dexamethasone who had better outcomes, compared with placebo [18, 33]. In the development of our score, we included patients who received dexamethasone, but excluded those receiving glycerol to minimize biases; glycerol was independently associated with mortality in Malawi [1, 9, 10]. The C-index of MAMS falls within the 95% CI for the C-index of both the EMS and the US-based score, and therefore can be considered of equal strength as the other scores in their validation settings.
The EMS performed less well in an external validation exercise, utilizing data from dexamethasone clinical trials from Vietnam and Malawi [9, 31, 34]. This may simply be due to differing methodology, including the use of categorized data in the EMS nomogram, and the selection of categorical cutoffs may have been less appropriate for these younger African patients [18]. However, we believe that the poorer performance of the EMS in those settings highlights critical differences between developed and developing country ABM populations, including longer time to presentation, a higher prevalence of HIV and anemia, greater frequency of complications, and fewer resources available for clinical management [35, 36].
The original trial of adjunctive glycerol in Malawi was stopped early due to unexpected increase in mortality in the glycerol arm [10]. Why this effect occurred predominately in those with lower predicted risk of death as determined by MAMS is uncertain. However, we hypothesize that in less severely ill patients, glycerol-induced vasodilation of pial microarterioles and osmotic effects may decompensate fragile but balanced fluid compartments in the brain and the systemic circulation, with adverse effects on brain swelling and perfusion [37, 38]. By comparison, in severely ill patients, breakdown of the blood–brain barrier is advanced; hence, glycerol has no additive adverse effect.
A major limitation in the development both of EMS and MAMS was missing data, managed using bootstrapping for both scores to reduce the risk of overfitting [18]. The developed model was validated to estimate the potential for overfitting and optimism in model performance using an internal validation procedure. This methodology is advised in the absence of external validation data, instead of use of split samples or cross-validation without replication [25, 26]. The nomogram was optimized using standard validation exercises to reduce overlap, and high levels of agreement were observed. Cohort assembly was done using data from multiple studies in the same hospital; biases from interobserver disagreement were minimized by only selecting variables that were collected uniformly across all studies. All data were collected prospectively in the same units, by research staff trained in the same institution, using the same equipment to minimize potential biases associated with post hoc cohort assembly. We used data from the first recording after admission to hospital in all studies to minimize the effect of in-hospital delays on alterations on physiological variables that may otherwise affect the prognostic power of the score. Other limitations include use of data from only 1 country, relative infrequency of HIV-uninfected patients, lack of inclusion data on morbidity endpoints and processes of care including antibiotic delays, and relatively few patients with meningococcal vs pneumococcal meningitis [6]. The MAMS nomogram requires external prospective validation in other populations, including specific validation in countries at risk of epidemic meningococcal disease.
It is clear that in the development of a severity score, other factors beyond those measured in the score may be important in determining outcome of adults with bacterial meningitis in sub-Saharan Africa. Although HIV coinfection was not associated with mortality and not included in the nomogram, it is possible that low CSF WBC count and low hemoglobin level may both correlate with advanced immunosuppression. CD4 cell count data are currently lacking in this setting.
In conclusion, the MAMS risk stratification tool has a good predictive power for use in ABM in adults in sub-Saharan Africa outside the epidemic meningitis belt. MAMS can be used in the analysis of prospective clinical trial data, testing efficacy of interventions in different risk groups. The differences between MAMS and EMS scores highlight important differences between ABM patients in these contrasting environments. Further work is required to explore these differences, to test MAMS prospectively, and to design optimal interventions to reduce the unacceptable mortality from meningitis in Africa.
Supplementary Material
Notes
Acknowledgments. The authors thank Philip Gichiru for his help with the generation of the original Malawi database; Malango Msukwa for his assistance with data from the Bundles of care for Adult Meningitis trial; and Professor Diedrik van de Beek for his sharing his experience with the generation of meningitis severity scores. In addition, we thank Professor E. Ziljstra for his support of the data collection for both the steroid and glycerol trials.
Disclaimer. The funding body had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Financial support. This work was supported by a clinical PhD fellowship in Global Health (089671/B/09/Z) to E. C. W. from the Wellcome Trust; and the MLW core grant from the Wellcome Trust. The original constituent studies in the discovery cohort were funded by the Meningitis Research Foundation (Steroids for Adult Meningitis and Glycerol for Adult Meningitis) and by the MLW core grant from the Wellcome Trust.
Potential conflicts of interest. Author certifies no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Wall EC, Cartwright K, Scarborough M, et al. High mortality amongst adolescents and adults with bacterial meningitis in sub-Saharan Africa: an analysis of 715 cases from Malawi. PLoS One 2013; 8:e69783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wall EC, Everett DB, Mukaka M, et al. Bacterial meningitis in Malawian adults, adolescents, and children during the era of antiretroviral scale-up and Haemophilus influenzae type b vaccination, 2000–2012. Clin Infect Dis 2014; 58:e137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pelkonen T, Roine I, Monteiro L, et al. Risk factors for death and severe neurological sequelae in childhood bacterial meningitis in sub-Saharan Africa. Clin Infect Dis 2009; 48:1107–10. [DOI] [PubMed] [Google Scholar]
- 4. Jarvis JN, Meintjes G, Williams A, Brown Y, Crede T, Harrison TS. Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infect Dis 2010; 10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mudhune S, Wamae M; Network Surveillance for Pneumococcal Disease in the East African Region Report on invasive disease and meningitis due to Haemophilus influenzae and Streptococcus pneumoniae from the network for surveillance of pneumococcal disease in the East African Region. Clin Infect Dis 2009; 48:S147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Daugla DM, Gami JP, Gamougam K, et al. Effect of a serogroup A meningococcal conjugate vaccine (PsA-TT) on serogroup A meningococcal meningitis and carriage in Chad: a community study [corrected]. Lancet 2014; 383:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med 2004; 351:1849–59. [DOI] [PubMed] [Google Scholar]
- 8. Thigpen MC, Whitney CG, Messonnier NE, et al. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 2011; 364:2016–25. [DOI] [PubMed] [Google Scholar]
- 9. Scarborough M, Gordon SB, Whitty CJ, et al. Corticosteroids for bacterial meningitis in adults in sub-Saharan Africa. N Engl J Med 2007; 357:2441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ajdukiewicz KM, Cartwright KE, Scarborough M, et al. Glycerol adjuvant therapy in adults with bacterial meningitis in a high HIV seroprevalence setting in Malawi: a double-blind, randomised controlled trial. Lancet Infect Dis 2011; 11:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Molyneux EM, Walsh AL, Forsyth H, et al. Dexamethasone treatment in childhood bacterial meningitis in Malawi: a randomised controlled trial. Lancet 2002; 360:211–8. [DOI] [PubMed] [Google Scholar]
- 12. Molyneux EM, Kawaza K, Phiri A, et al. Glycerol and acetaminophen as adjuvant therapy did not affect the outcome of bacterial meningitis in Malawian children. Pediatr Infect Dis J 2014; 33:214–6. [DOI] [PubMed] [Google Scholar]
- 13. Aronin SI, Quagliarello VJ. Utility of prognostic stratification in adults with community-acquired bacterial meningitis. Compr Ther 2001; 27:72–7. [DOI] [PubMed] [Google Scholar]
- 14. Bauer TT, Ewig S, Marre R, Suttorp N, Welte T; CAPNETZ Study Group CRB-65 predicts death from community-acquired pneumonia. J Intern Med 2006; 260:93–101. [DOI] [PubMed] [Google Scholar]
- 15. Yandiola PP, Capelastegui A, Quintana J, et al. Prospective comparison of severity scores for predicting clinically relevant outcomes for patients hospitalized with community-acquired pneumonia. Chest 2009; 135:1572–9. [DOI] [PubMed] [Google Scholar]
- 16. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13:818–29. [PubMed] [Google Scholar]
- 17. Vincent JL, de Mendonça A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 1998; 26:1793–800. [DOI] [PubMed] [Google Scholar]
- 18. Weisfelt M, van de Beek D, Spanjaard L, Reitsma JB, de Gans J. A risk score for unfavorable outcome in adults with bacterial meningitis. Ann Neurol 2008; 63:90–7. [DOI] [PubMed] [Google Scholar]
- 19. Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med 1998; 129:862–9. [DOI] [PubMed] [Google Scholar]
- 20. Ajayi-Obe EK, Lodi E, Alkali AS, et al. Prognostic scores for use in African meningococcal epidemics. Bull World Health Organ 1998; 76:149–52. [PMC free article] [PubMed] [Google Scholar]
- 21. Benjamin LA, Kelly M, Cohen D, et al. Detection of herpes viruses in the cerebrospinal fluid of adults with suspected viral meningitis in Malawi. Infection 2013; 41:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lucas MJ, Brouwer MC, van de Beek D. Delayed cerebral thrombosis in bacterial meningitis: a prospective cohort study. Intensive Care Med 2013; 39:866–71. [DOI] [PubMed] [Google Scholar]
- 23. Almirante B, Cortés E, Pigrau C, et al. Treatment and outcome of pneumococcal meningitis in adults. Study of a recent series of 70 episodes. Med Clin (Barc) 1995; 105:681–6. [PubMed] [Google Scholar]
- 24. Dzupova O, Rozsypal H, Prochazka B, Benes J. Acute bacterial meningitis in adults: predictors of outcome. Scand J Infect Dis 2009; 41:348–54. [DOI] [PubMed] [Google Scholar]
- 25. Moons KG, Kengne AP, Woodward M, et al. Risk prediction models: I. development, internal validation, and assessing the incremental value of a new (bio)marker. Heart 2012; 98:683–90. [DOI] [PubMed] [Google Scholar]
- 26. Smith GC, Seaman SR, Wood AM, Royston P, White IR. Correcting for optimistic prediction in small data sets. Am J Epidemiol 2014; 180:318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arabi Y, Abbasi A, Goraj R, et al. External validation of a modified model of acute physiology and chronic health evaluation (APACHE) II for orthotopic liver transplant patients. Crit Care 2002; 6:245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Minne L, Abu-Hanna A, de Jonge E. Evaluation of SOFA-based models for predicting mortality in the ICU: a systematic review. Crit Care 2008; 12:R161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toma T, Abu-Hanna A, Bosman RJ. Discovery and integration of univariate patterns from daily individual organ-failure scores for intensive care mortality prediction. Artif Intell Med 2008; 43:47–60. [DOI] [PubMed] [Google Scholar]
- 30. Cook CE. Potential pitfalls of clinical prediction rules. J Man Manip Ther 2008; 16:69–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schut ES, Brouwer MC, Scarborough M, et al. Validation of a Dutch risk score predicting poor outcome in adults with bacterial meningitis in Vietnam and Malawi. PLoS One 2012; 7:e34311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gessner BD, Mueller JE, Yaro S. African meningitis belt pneumococcal disease epidemiology indicates a need for an effective serotype 1 containing vaccine, including for older children and adults. BMC Infect Dis 2010; 10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Gans J, van de Beek D; European Dexamethasone in Adulthood Bacterial Meningitis Study Investigators Dexamethasone in adults with bacterial meningitis. N Engl J Med 2002; 347:1549–56. [DOI] [PubMed] [Google Scholar]
- 34. Nguyen TH, Tran TH, Thwaites G, et al. Dexamethasone in Vietnamese adolescents and adults with bacterial meningitis. N Engl J Med 2007; 357:2431–40. [DOI] [PubMed] [Google Scholar]
- 35. Weerkamp N, van de Beek D, de Gans J, Koehler PJ. Herpes reactivation in patients with bacterial meningitis. J Infect 2008; 57:493–4. [DOI] [PubMed] [Google Scholar]
- 36. Adriani KS, Brouwer MC, Geldhoff M, et al. Common polymorphisms in the complement system and susceptiblity to bacterial meningitis. J Infect 2013; 66:255–62. [DOI] [PubMed] [Google Scholar]
- 37. Gerber J, Nau R. Mechanisms of injury in bacterial meningitis. Curr Opin Neurol 2010; 23:312–8. [DOI] [PubMed] [Google Scholar]
- 38. Lapi D, Marchiafava PL, Colantuoni A. Pial microvascular responses to transient bilateral common carotid artery occlusion: effects of hypertonic glycerol. J Vasc Res 2008; 45:89–102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.