Abstract
Objectives
Greater patient activation, defined as having the knowledge, skills, and confidence to manage one’s health, is associated with cancer control behaviors. Cancer risk beliefs may be associated with patient activation, and delineating this relationship could inform cancer control interventions across diverse patient subgroups. This study examines associations between cancer risk beliefs, language preference, and patient activation within a multi-lingual urban primary care setting.
Design
Patients aged 18 and older within a New York City public hospital serving a large proportion of non-native born Americans were surveyed regarding their cancer risk beliefs and patient activation in Haitian Creole, Spanish, or English based on language preference during a health care visit.
Results
The sample (N=460) included 150 Haitian Creole speakers, 159 Spanish speakers, and 151 English speakers, and was primarily non-white (92%). Most participants (84%) had not been born in the United States. Cancer risk beliefs differed across language preference. Beliefs that cancer could be avoided by minimizing thoughts about cancer risk were significantly higher in Haitian Creole speakers compared to others; reported negative emotion when thinking about cancer risk was higher in Spanish and English versus Haitian Creole speakers. These cancer risk beliefs were positively related to patient activation, even when controlling for language preference.
Conclusion
Cancer risk beliefs differ across language preference, and are related to patient activation, making them potential important in cancer control. Consideration of language represents important demographic stratification for understanding the frequency and relevance of different beliefs about cancer and patient activation.
Keywords: Cancer risk beliefs, patient activation, cancer, oncology
Introduction
The term “patient activation” refers to the knowledge, skills, and confidence needed by persons to manage their health and health care [1]. Greater patient activation is related to greater adherence with behaviors that prevent cancer, such as sun protection and tobacco abstinence, as well as cancer screening adherence [2–4], and to greater screening and follow-up care in cancer patients [5]. There are ethnic, racial, acculturation, and other socioeconomic disparities such as language fluency in patient activation [1, 6–8]. This is true in the cancer context, as well; among cancer survivors, poorer patient activation, defined as self-efficacy in making personal medical decisions, has been identified in Asian cancer survivors compared to White survivors [9]. Improving patient activation has been postulated as a means to reduce health disparities, improve quality health care, and reduce costs associated with cancer treatment and prevention [1, 10, 11].
Patient activation combines constructs including information seeking, self-efficacy, behavioral adoption, and stress management [12, 13]. At lower levels of patient activation, patients express lower levels of endorsement concerning whether an active role in their health is important, and low confidence that they can proactively reach out to physicians with their health concerns. At higher levels of patient activation, patients report adopting and maintaining lifestyle changes. The highest level of patient activation is characterized by confidence in one’s ability to maintain lifestyle changes even under stressful conditions [14, 15].
It is well-accepted that beliefs about cancer risk are key motivators of health behavior adoption [16]. This assertion is consistent with most health behavior theories [17] and empirical research [18, 19]. Since health behavior adoption is an important element of patient activation, it is possible that in the cancer context patient activation levels may differ by specific cancer risk beliefs. For example, Haitians may be very heterogeneous in their familiarity with the concept of prevention, having reported that it is “better not to know” about an illness such as cancer. This belief may lead to non-adherence with screening [20–22].
Cancer risk beliefs may be related to patient activation in important ways that differ across ethnicity, race, and acculturation. Examination of cancer risk beliefs across English and non-English speaking populations will contribute to efforts to address cognitive barriers to patient activation in the cancer context, contributing to intervention strategies to activate patients of diverse backgrounds to take action regarding their health.
The current study examines cancer risk beliefs and patient activation across Haitian Creole, Spanish, and English speaking patients seen at an inner-city hospital ambulatory care practice. Queens County in New York City is home to one of the five largest Haitian populations in all United States counties [23]. Of the Haitian population in Queens, 82% claim that they do not speak English at home and, of those not speaking English at home, 14% note not speaking English well or at all [23]. Hispanics comprise 28% of our Queens, New York population, and 30% of Spanish speakers in Queens reported that they do not speak English well [24, 25]. The routine use of English only surveys limits examination of demographic variation solely to that variation represented among English speakers. Thus, one of the primary goals of the current research program is to extend our examination of cancer risk beliefs to non-English speakers in New York City. While language preference is confounded with other important characteristics, such as culture and ethnic background, non-English fluency is an important marker for cancer health disparities [26] that warrants focus in the context of patient activation issues.
The study has two objectives. First, we examine the extent to which cancer risk beliefs differ across three urban primary care subpopulations (Haitian Creole, Spanish, and English speakers). This objective will also allow us to examine whether there are specific cancer risk beliefs that are particularly prominent in one or more subpopulations. Second, we examine associations between cancer risk beliefs, survey language, and patient activation in this population. Based on prior work indicating that cancer risk beliefs can present a barrier to cancer screening [27, 28], we hypothesize that cancer risk beliefs will be lower in those with higher patient activation. Given the importance of patient activation in cancer control, understanding the relation of specific cancer risk beliefs to patient activation levels will enhance opportunities for tailored cancer control intervention development across diverse, multi-lingual populations.
Methods
Sample
The study was reviewed and approved as exempt research by the Institutional Review Boards at The City College of New York (CCNY), Memorial Sloan Kettering Cancer Center (MSKCC), and Queens Hospital Center. This cross-sectional study surveyed people who were aged 18 and older and attended Queens Hospital Center Ambulatory Center between June 2011 and August 2012. Queens Hospital Center is a member of the New York City Health and Hospitals Corporation, the public safety net healthcare system of New York City, and serves communities in central and southeastern Queens with large proportions of non-native born Americans.
Procedure
Research study assistants (RSA) were bilingual and fluent in both their native languages (Haitian Creole or Spanish) as well as English. Patients were approached in the waiting room prior to a health care visit. They were told that participation was voluntary and the interview would be anonymous and confidential. Participants were given the choice of having the study administered in English, Spanish, or Haitian Creole, based on the language with which they had the highest level of fluency. Participants received a transportation card ($15.00) for completing the study.
Measures
Cancer risk beliefs
Cancer risk beliefs were assessed via five scales [29]. The first factor, Cognitive Causation, includes 10 items that tap into the belief that thoughts about cancer risk may encourage the development of disease, and that minimizing such thoughts could actually reduce cancer risk. Higher cognitive causation is related to reduced colorectal cancer screening adherence [27]. The second factor, Negative Affect in Risk, includes 6 items and taps feelings generated during the risk perception process; this factor is also associated with reduced colorectal cancer screening intentions [28]. The third factor, Unpredictability of Cancer, keys into beliefs about irreducible uncertainties regarding whether any one person might get cancer. The fourth factor, Preventability, assesses beliefs around the extent to which cancer development is controllable. The fifth factor, Defensive Pessimism, taps beliefs around the potential negative outcomes associated with being too optimistic about avoiding cancer. Each of these scales used 4-level response options indicating level of agreement, from “strongly disagree” to “strongly agree.” These scales have been translated and adapted into Haitian-Creole and Spanish with high comprehensibility [30]. Scales were scored to range from 0 to 100 in order to achieve comparable relative levels across scales with different numbers of items and for ease of interpretability [31].
Patient activation
Patient activation was measured using the PAM, which has strong psychometric properties and has been shown to be valid and reliable [14, 15]. This measure is a 13-item scale indicating the degree to which individuals take an active role in managing their health and health care [15]. The five possible responses on the PAM range from disagree strongly to agree strongly and include not applicable (NA). The PAM score is based on a scale of 0–100 and falls into one of four levels of activation: (1) not yet taking an active role, (2) gaining confidence and knowledge to take action, (3) taking action, and (4) maintaining behavior. Spanish and Haitian Creole versions of the PAM were available from Insignia Health [12].
Demographics
The final section contained a number of sociodemographic characteristics, including age, sex, education, race and ethnicity, years living in the United States, country of birth, employment, and marital status.
Statistical approach
Differences in patient characteristics across language preference were compared using the Chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables. Internal consistency of the cancer risk belief scales was assessed using Cronbach’s alpha, where an alpha value ≥ 0.7 is considered reliable. Differences in cancer risk beliefs across language preference were assessed using the Fisher’s exact test for individual items and the Kruskal-Wallis test for sub-scale scores. Associations with PAM score were assessed using univariable and multivariable linear regression. Variables associated with PAM with p<0.05 on univariable analysis were incorporated into multivariable analysis.
Statistical significance was defined as p<0.05. All analyses were conducted using SAS software, Version 9.4 of the SAS System for Windows [32].
Results
Sample Characteristics
Of all approached patients, 80% agreed to participate. The overall sample (N=460) was balanced across language preference by design, with 150 Haitian Creole speakers, 159 Spanish speakers, and 151 English speakers. Overall, this primary care sample was non-white (91.5%), had not been born in the United States (non US nativity, 84.3%), and was very diverse in terms of income and educational attainment. English speakers had significantly higher patient activation scores, followed by Spanish and Haitian Creole speakers (Table 1).
Table 1.
Selected characteristics of 460 survey participants.
| Survey language | |||||
|---|---|---|---|---|---|
| Overall (n=460) | Haitian creole (n=150; 33%) | Spanish (n=159; 34%) | English (n=151; 33%) | p-value1 | |
| Age (years), Mean (SD) | 48 (15) | 49 (15) | 50 (13) | 44 (15) | <.001 |
| Sex, N (%) | 0.093 | ||||
| Male | 185 (40.2) | 62 (41.3) | 54 (34.0) | 69 (45.7) | |
| Female | 272 (59.1) | 88 (58.7) | 104 (65.4) | 80 (53.0) | |
| Missing | 3 (0.7) | 0 (0.0) | 1 (0.6) | 2 (1.3) | |
| Family history of cancer, N (%) | 0.022 | ||||
| Yes | 162 (35.2) | 38 (25.3) | 63 (39.6) | 61 (40.4) | |
| No | 271 (58.9) | 98 (65.3) | 90 (56.6) | 83 (55.0) | |
| Missing | 27 (5.9) | 14 (9.3) | 6 (3.8) | 7 (4.6) | |
| Hispanic ethnicity | <.001 | ||||
| No | 281 (61.1) | 146 (97.3) | 0 (0.0) | 135 (89.4) | |
| Yes | 173 (37.6) | 4 (2.7) | 157 (98.7) | 12 (7.9) | |
| Missing | 6 (1.3) | 0 (0.0) | 2 (1.3) | 4 (2.6) | |
| Race, N (%) | <.001 | ||||
| White | 39 (8.5) | 0 (0.0) | 32 (20.1) | 7 (4.6) | |
| Black | 64 (13.9) | 23 (15.3) | 1 (0.6) | 40 (26.5) | |
| American Indian or Alaskan Native | 6 (1.3) | 0 (0.0) | 2 (1.3) | 4 (2.6) | |
| Asian/Pacific Islander | 15 (3.3) | 0 (0.0) | 0 (0.0) | 15 (9.9) | |
| Other | 139 (30.2) | 0 (0.0) | 113 (71.1) | 26 (17.2) | |
| Caribbean Black | 192 (41.7) | 127 (84.7) | 9 (5.7) | 56 (37.1) | |
| Missing | 5 (1.1) | 0 (0.0) | 2 (1.3) | 3 (2.0) | |
| Born in US, N (%) | <.001 | ||||
| Yes | 66 (14.3) | 6 (4.0) | 7 (4.4) | 53 (35.1) | |
| No | 388 (84.3) | 143 (95.3) | 150 (94.3) | 95 (62.9) | |
| Missing | 6 (1.3) | 1 (0.7) | 2 (1.3) | 3 (2.0) | |
| Years living in US, N (%) | <.001 | ||||
| < 1 year | 18 (3.9) | 9 (6.0) | 2 (1.3) | 7 (4.6) | |
| 1–10 | 110 (23.9) | 46 (30.7) | 30 (18.9) | 34 (22.5) | |
| 11–20 | 112 (24.3) | 47 (31.3) | 41 (25.8) | 24 (15.9) | |
| >20 | 149 (32.4) | 42 (28.0) | 77 (48.4) | 30 (19.9) | |
| NA | 66 (14.3) | 6 (4.0) | 7 (4.4) | 53 (35.1) | |
| Missing | 5 (1.1) | 0 (0.0) | 2 (1.3) | 3 (2.0) | |
| Living with anyone, N (%) | 0.012 | ||||
| Yes | 379 (82.4) | 122 (81.3) | 142 (89.3) | 115 (76.2) | |
| No | 72 (15.7) | 28 (18.7) | 14 (8.8) | 30 (19.9) | |
| Missing | 9 (2.0) | 0 (0.0) | 3 (1.9) | 6 (4.0) | |
| Marital status, N (%) | <.001 | ||||
| Married/living with partner | 246 (53.5) | 58 (38.7) | 120 (75.5) | 68 (45.0) | |
| Single | 126 (27.4) | 50 (33.3) | 22 (13.8) | 54 (35.8) | |
| Divorced/Separated | 61 (13.3) | 31 (20.7) | 11 (6.9) | 19 (12.6) | |
| Widowed | 21 (4.6) | 11 (7.3) | 4 (2.5) | 6 (4.0) | |
| Missing | 6 (1.3) | 0 (0.0) | 2 (1.3) | 4 (2.6) | |
| Education, N (%) | <.001 | ||||
| Less than 7th grade | 42 (9.1) | 11 (7.3) | 26 (16.4) | 5 (3.3) | |
| Junior HS or partial HS | 70 (15.2) | 25 (16.7) | 25 (15.7) | 20 (13.2) | |
| High school/GED | 139 (30.2) | 40 (26.7) | 60 (37.7) | 39 (25.8) | |
| Partial college or vocational | 122 (26.5) | 40 (26.7) | 34 (21.4) | 48 (31.8) | |
| Standard college + | 80 (17.4) | 34 (22.7) | 12 (7.5) | 34 (22.5) | |
| Missing | 7 (1.5) | 0 (0.0) | 2 (1.3) | 5 (3.3) | |
| Employment status, N (%) | <.001 | ||||
| Employed | 245 (53.3) | 80 (53.3) | 86 (54.1) | 79 (52.3) | |
| Disabled | 31 (6.7) | 8 (5.3) | 11 (6.9) | 12 (7.9) | |
| Homemaker | 44 (9.6) | 3 (2.0) | 34 (21.4) | 7 (4.6) | |
| Retired | 48 (10.4) | 22 (14.7) | 18 (11.3) | 8 (5.3) | |
| Unemployed | 48 (10.4) | 18 (12.0) | 5 (3.1) | 25 (16.6) | |
| Student | 35 (7.6) | 19 (12.7) | 2 (1.3) | 14 (9.3) | |
| Missing/other | 9 (2.0) | 0 (0.0) | 3 (1.9) | 6 (4.0) | |
| Income, N (%) | 0.003 | ||||
| <$10,000 | 78 (17.0) | 14 (9.3) | 31 (19.5) | 33 (21.9) | |
| $10,000–$29,999 | 134 (29.1) | 44 (29.3) | 53 (33.3) | 37 (24.5) | |
| $29,999–$49,999 | 110 (23.9) | 38 (25.3) | 43 (27.0) | 29 (19.2) | |
| ≥ $50,000 | 31 (6.7) | 8 (5.3) | 5 (3.1) | 18 (11.9) | |
| Missing | 107 (23.3) | 46 (30.7) | 27 (17.0) | 34 (22.5) | |
| CC score, mean (SD) | 39.3 (21.5) | 44.1 (20.4) | 41.9 (20.9) | 31.8 (21.4) | <.001 |
| NA score, mean (SD) | 64.6 (24.1) | 59.9 (22.5) | 66.7 (23.0) | 67.1 (26.1) | 0.006 |
| PAM score, mean (SD) | 64.4 (16.7) | 60.8 (16.8) | 63.5 (15.2) | 69.2 (17.2) | <.001 |
p-value from Chi-square test when categorical and Kruskal-Wallis test when continuous.
Cancer risk beliefs across languages
As shown in Table 1, levels of cancer risk beliefs differed across language preference. As noted in the Supplementary File, cognitive causation and negative affect in risk scales showed high internal consistency across the entire sample, as well as within each language group (all alpha reliabilities ≥ .80). The correlation between negative affect in risk and cognitive causation was low, r=0.057. Cognitive causation levels were significantly higher in Haitian Creole speakers compared to the other two groups. For example, 42% of Haitian Creole speakers agreed that, “If I think too hard about the possibility of getting cancer, I could get it,” compared to 28% and 23% of Spanish and English speakers, respectively. In contrast, negative affect in risk levels were highest in Spanish and English speakers. For example, 79% and 70% of Spanish and English speakers, respectively, agreed that, “I can’t think about getting cancer without feeling afraid,” compared to 54% of Haitian Creole speakers. Also shown in the Supplementary File, items not in the cognitive causation or negative affect in risk scales did not reliably load onto specific factors (all alpha reliabilities < 0.7), but individual items still showed systematic differences across language preference.
Cancer risk perceptions and patient activation
Both cognitive causation and negative affect in risk were significantly and positively related to patient activation (alpha reliability for patient activation = 0.84). As such, those with greater beliefs in the role of wishful thinking and avoiding too much thought about risk in helping them avoid cancer (characterized by higher cognitive causation scores), and those with greater emotion when thinking about their cancer risk (characterized by higher negative affect in risk scores) both demonstrated higher levels of patient activation (b=0.07, SE(b)=0.04, p=0.046 and b=0.12, SE(b)=0.03, p<.001, respectively, see Figure 1). High levels of patient activation represent the belief in personal ability to sustain health behaviors even in times of stress. These findings remained significant in multivariable analysis adjusting for language preference (Haitian Creole, Spanish, or English) and years living in the US (Table 2). Interaction effects between cognitive causation and language preference on patient activation, and between negative affect in risk and language preference on patient activation were not significant (p=0.590 and p=0.600, respectively).
Figure 1.
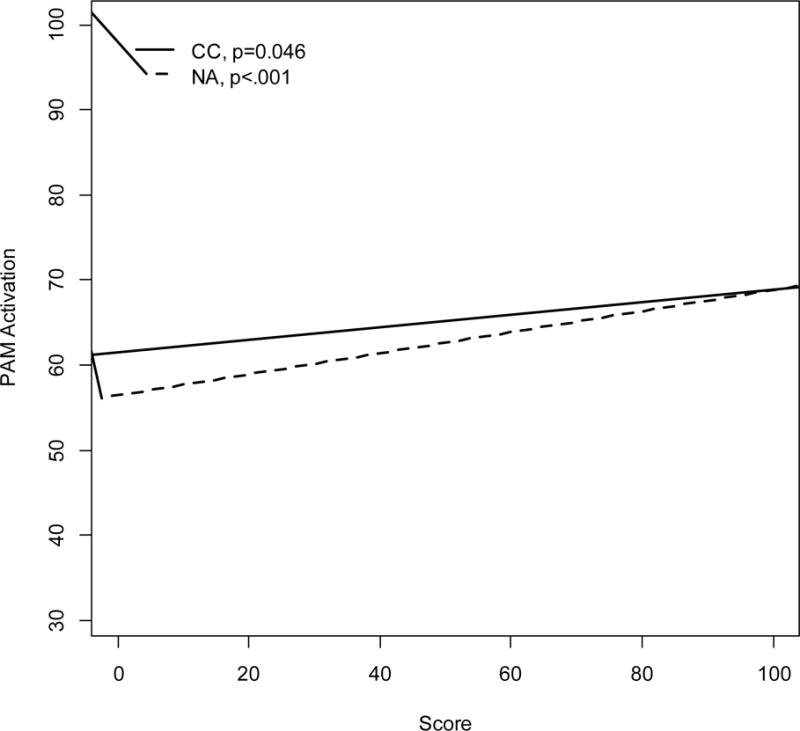
Univariable associations between CC and NA scores with continuous PAM activation
Table 2.
Univariable and multivariable associations with continuous PAM activation.
| Est (SE) | p-value1 | Est (SE) | p-value2 | |
|---|---|---|---|---|
| Cognitive causation | 0.07 (0.04) | 0.046 | 0.12 (0.04) | <.001 |
| Negative affect in risk | 0.12 (0.03) | <.001 | 0.11 (0.03) | <.001 |
| Survey language | <.001 | <.001 | ||
| English | Ref | Ref | ||
| Haitian Creole | −8.33 (2.07) | −8.9 (2.04) | ||
| Spanish | −6.68 (2.13) | −8.0 (2.03) | ||
| Years living in US | 0.005 | 0.006 | ||
| Born in US | Ref | Ref | ||
| <1 | −12.84 (4.52) | −10 (4.42) | ||
| 1–10 | −8.22 (2.62) | −4.8 (2.66) | ||
| 11–20 | −5.51 (2.60) | −.55 (2.71) | ||
| >20 | −3.59 (2.49) | 1.46 (2.63) |
p-value from univariable linear regression;
p-value from multivariable linear regression adjusted for all variables included in table
Discussion
In this paper we examine cancer risk beliefs and patient activation among primary care patients with diverse language preferences. Patient activation is gaining acceptance as an important goal in maximizing health and healthcare for the general population as well as those diagnosed with illnesses such as cancer [1, 33]. Patient activation may be particularly important in cancer control across diverse populations [5], yet little is known about the cancer-relevant beliefs that may underlie patient activation levels. We found that patient activation and cancer risk beliefs differed by language preference, but that cancer risk beliefs and patient activation remained positively associated when controlling for language preference. These findings have implications for patient activation interventions in cancer prevention and control.
Our findings regarding cancer risk beliefs and language preference indicated that the Haitian Creole speakers endorsed higher superstitious thinking about cancer risk, as evidenced by their higher cognitive causation scores, and that the Spanish speakers endorsed more negative affect as they think about their cancer risk, as evidenced by their higher negative affect in risk scores. There were also patterns across language preference within the other cancer risk belief items. Most cancer prevention and control research uses a narrow range of risk perception items, generally magnitude judgments [17]. The findings from the current study indicate that different subpopulations may have different beliefs about risk. Future research on cancer risk beliefs must continue to engage diverse populations, including non-English speakers, to fully capture cultural, racial, and socioeconomic variation in how people think about and manage their health [21, 34–37]. The use of multiple cancer risk belief assessments – that assess both judgments about magnitude as well as intuitive beliefs about risk – are necessary to examine potentially culturally different aspects of risk beliefs.
Cancer risk beliefs, including both cognitive causation and negative affect in risk, were significantly related to patient activation scores even when controlling for language. Counter to our hypotheses, these relations were in the positive direction, with those at highest patient activation, characterized by high levels of confidence in their ability to manage health and maintain health behaviors even under stressful conditions, endorsing high levels of cognitive causation and negative affect in risk. Superstitious thinking has been examined in the social psychological literature [38–41], but only recently in health [29, 42]. Orom and colleagues [42] found that inquiries regarding cancer risk perceptions generated reports of wishful thinking and a desire to avoid thinking negatively among African American community members. Superstitious thinking about risk – characterized by beliefs such as hopefulness in protecting against the development of cancer – as assessed via cognitive causation, is significantly associated with internal and external health locus of control [43]. It is possible that high perceived control is associated with endorsement of patient activation at the highest levels, such that high levels of perceived control are also operative among those who believe that cancer risk can be influenced by thoughts. Indeed, prior work confirms that a common superstitious behavior, knocking on wood, is higher among those with a greater desire for control [44]. As such, the highest levels of patient activation may be characterized by strong – perhaps unrealistically strong – confidence that health behaviors can be well maintained even under stress. In other words, there may be an element of wishful thinking or overestimation of personal control that is operative at the very highest levels of patient activation. Interestingly, AuYoung and colleagues [45] recently found that obese individuals with higher patient activation were able to adopt fruit and vegetable consumption but not necessarily regular physical activity. Further research could examine whether there are subsets of highly activated patients who are more or less realistic concerning their ability to maintain health behaviors under challenging, stressful conditions. For our Haitian Creole speakers, in particular, who had the highest levels of superstitious thinking about risk, examining these beliefs may help us to better understand the cognitive underpinnings of patient activation, as well as Haitian Creole speakers’ differential receptivity to health interventions that seek to raise cancer risk awareness and cancer control behaviors.
In terms of negative affect in risk, those with the highest levels of patient activation may also have more salient emotions and affect when they think about cancer risk, which may motivate them to remain actively focused on their health behaviors even under stress. Affect can highlight salient concerns [46] and tends to promote cancer screening behaviors rather than impede them [47, 48]. Other cancer risk belief items were also systematically related to patient activation, and require further study given their prevalent endorsement; they may have important implications for behaviors to prevent and control cancer.
Superstitious beliefs that certain thoughts or behaviors ward off negative events [35, 39, 41, 49–53] are likely embedded in cultural beliefs systems. They may be more common in Latino or Asian populations [39, 54]. Such cultural belief systems may also encompass supernatural causes of illness, influencing cancer decision-making among individuals already diagnosed with a malignancy [55]. African-Americans, Latinos, and Asians are more likely to endorse supernatural causes of illness than whites [56]. Haitians, in particular, may believe in the role of spirits or the supernatural in the development of disease [57]. Nonetheless, superstitious beliefs are quite ubiquitous in the general population [53]. For instance, the widespread acceptance of superstitions concerning risk is evidenced by the lack of 13th floors in many hospitals and hotels, the continued presence of horoscopes in magazines and newspapers, the ubiquity of “knock on wood” and “crossing fingers” to avoid tempting fate [41, 49], as well as rituals meant to gain the best outcome among sports figures and fans, and gamblers [52, 58, 59]. Patient activation interventions that engage diverse patient groups – across language and other cultural factors – may need to directly address and minimize superstitious beliefs that could encourage unrealistic perceptions of control about health behavior maintenance under challenging conditions. Relevant interventions may have physician communication as a primary intervention channel [60] or may target patients directly through broader public health messaging such as mass media channels. For example, such interventions may focus on finding alternative and potentially less superstitious ways to maintain actual control over health, by engagement with risk reduction behaviors and screening.
Study limitations include the fact that patient activation is a self-report measure. Prior empirical work has established that patient activation is related to higher knowledge, skills, and confidence to manage health and healthcare. Ongoing research is examining the relations of risk beliefs to behavior adoption and maintenance. Second, patient activation is not specific to cancer-related prevention or control behavior per se, even though it has been found to be related to cancer screening activities [4]. Third, our study is limited by the cross-sectional dataset that precludes us making causal inferences. Finally, despite the fact that culture, ethnic background, and language preference are related but not identical constructs, we were unable to fully examine how culture may influence cancer risk beliefs and patient activation due to our limited assessment battery; future work is needed to accomplish this important goal.
In conclusion, we identify different patterns of cancer risk beliefs across language preferences in inner-city non-Native born Americans seeking primary care services. These cancer risk beliefs are significantly related to patient activation in diverse populations, regardless of language group, warranting continued research of this important outcome in cancer prevention and control, making cancer risk beliefs potentially informative in understanding activation and behavior in diverse populations.
Supplementary Material
Acknowledgments
We acknowledge the vital contributions of our interviewer Kathy Isaac, and our Queens Hospital Center collaborator Linda Bulone. This research was supported by a grant from the National Cancer Institute #U54CA137788 City College of New York/Memorial Sloan-Kettering Cancer Center Partnership.
References
- 1.Hibbard JH, Cunningham PJ. How Engaged Are Consumers In Their Health and Health Care, And Why Does It Matter? Research Briefs. 2008;(8):1–9. [PubMed] [Google Scholar]
- 2.Harvey L, et al. When Activation Changes, What Else Changes? The Relationship Between Change In Patient Activation Measure (PAM) and Employees’ Health Status and Health Behaviors. Patient Education and Counseling. 2012;88(2):338–43. doi: 10.1016/j.pec.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Greene J, Hibbard JH. Why Does Patient Activation Matter? An Examination Of The Relationships Between Patient Activation and Health-Related Outcomes. Journal of General Internal Medicine. 2012;27(5):520–6. doi: 10.1007/s11606-011-1931-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katz ML, et al. Patient Activation Increases Colorectal Cancer Screening Rates: A Randomized Trial Among Low-Income Minority Patients. Cancer Epidemiology, Biomarkers and Prevention. 2012;21(1):45–52. doi: 10.1158/1055-9965.EPI-11-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nonzee NJ, et al. Delays in Cancer Care Among Low-Income Minorities Despite Access. J Womens Health (Larchmt) 2015;24(6):506–14. doi: 10.1089/jwh.2014.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hibbard JH, et al. Racial/Ethnic Disparities and Consumer Activation In Health. Health Affairs. 2008;27(5):1442–53. doi: 10.1377/hlthaff.27.5.1442. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham PJ, Hibbard J, Gibbons CB. Raising Low ‘Patient Activation’ Rates Among Hispanic Immigrants May Equal Expanded Coverage In Reducing Access Disparities. Journal Affairs (Millwood) 2011;30(10):1888–94. doi: 10.1377/hlthaff.2009.0805. [DOI] [PubMed] [Google Scholar]
- 8.Alegria M, et al. The Role Of Patient Activation On Patient-Provider Communication and Quality Of Care For US and Foreign Born Latino Patients. Journal of General Internal Medicine. 2009;24(Suppl 3):534–41. doi: 10.1007/s11606-009-1074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer NR, et al. Racial and Ethnic Disparities In Patient-Provider Communication, Quality-Of-Care Ratings, and Patient Activation Among Long-Term Cancer Survivors. Journal of Clinical Oncology. 2014;32(36):4087–94. doi: 10.1200/JCO.2014.55.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hibbard JH, Greene J, Overton V. Patients With Lower Activation Associated With Higher Costs; Delivery Systems Should Know Their Patients’ ‘Scores’. Health Affairs (Millwood) 2013;32(2):216–22. doi: 10.1377/hlthaff.2012.1064. [DOI] [PubMed] [Google Scholar]
- 11.Hibbard JH, Greene J. What The Evidence Shows About Patient Activation: Better Health Outcomes and Care Experiences; Fewer Data On Costs. Health Affairs (Millwood) 2013;32(2):207–14. doi: 10.1377/hlthaff.2012.1061. [DOI] [PubMed] [Google Scholar]
- 12.Health, I. Insignia Health Patient Activation Measure License Package. Portland, OR: [Google Scholar]
- 13.Hibbard JH, et al. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39(4 Pt 1):1005–26. doi: 10.1111/j.1475-6773.2004.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hibbard JH, et al. Development of the Patient Activation Measure (PAM): Conceptualizing and Measuring Activation In Patients and Consumers. Health Services Research. 2004;39(4 Pt 1):1005–26. doi: 10.1111/j.1475-6773.2004.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hibbard JH, et al. Development and Testing Of A Short Form Of The Patient Activation Measure. Health Services Research. 2005;40(6 Pt 1):1918–30. doi: 10.1111/j.1475-6773.2005.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slovic P, et al. Affect, Risk, and Decision Making. Health Psychology. 2005;24(4 Suppl):S35–40. doi: 10.1037/0278-6133.24.4.S35. [DOI] [PubMed] [Google Scholar]
- 17.Conner M, Norman P. Predicting Health Behaviour: Research and Practice with Social Cognition Models. Berkshire, England: Open University Press; 2005. [Google Scholar]
- 18.Woloshin S, et al. A New Scale For Assessing Perceptions Of Chance: A Validation Study. Medical Decision Making. 2000;20(3):298–307. doi: 10.1177/0272989X0002000306. [DOI] [PubMed] [Google Scholar]
- 19.Watts BG, et al. Intention To Be Screened Over Time For Colorectal Cancer In Male Automotive Workers. Cancer Epidemiology, Biomarkers & Prevention. 2003;12(4):339–49. [PubMed] [Google Scholar]
- 20.Gany FM, et al. Attitudes, Knowledge, and Health-Seeking Behaviors Of Five Immigrant Minority Communities In The Prevention and Screening Of Cancer: A Focus Group Approach. Ethnicity and Health. 2006;11(1):19–39. doi: 10.1080/13557850500391394. [DOI] [PubMed] [Google Scholar]
- 21.Francois F, et al. Colon Cancer Knowledge and Attitudes In An Immigrant Haitian Community. Journal of Immigrant and Minority Health. 2009;11(4):319–25. doi: 10.1007/s10903-008-9126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coughlin SSLE, Hay JL, Raphael R, Smith SA. Promoting colorectal cancer screening among Haitian Americans. J GA Public Health Association. 2015 In press. [PMC free article] [PubMed] [Google Scholar]
- 23.Ruggles S, et al. American Community Survey 2006–2008 in Integrated Public Use Microdata Series: Version 5.0 [Machine-Readable Database] University of Minnesota; Minneapolis, MN: 2010. [Google Scholar]
- 24.U.S. Census Bureau. U.S. Census Quick Facts 2011 [Google Scholar]
- 25.U.S. Census Bureau. American Community Survey 2011. 2011 [Google Scholar]
- 26.Jacobs EA, et al. Limited English proficiency and breast and cervical cancer screening in a multiethnic population. Am J Public Health. 2005;95(8):1410–6. doi: 10.2105/ajph.2004.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hay JL, et al. Deliberative and intuitive risk perceptions as predictors of colorectal cancer screening over time. J Behav Med. 2016;39(1):65–74. doi: 10.1007/s10865-015-9667-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boonyasiriwat W, et al. Intention to Undergo Colonoscopy Screening Among Relatives of Colorectal Cancer Cases: A Theory-Based Model. Ann Behav Med. 2013 doi: 10.1007/s12160-013-9562-y. [DOI] [PubMed] [Google Scholar]
- 29.Hay JL, et al. Examining intuitive risk perceptions for cancer in diverse populations. Health Risk Soc. 2014;16(3):227–242. doi: 10.1080/13698575.2014.911822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hay JL, et al. Examining Intuitive Cancer Risk Perceptions in Haitian-Creole and Spanish-Speaking Populations. Journal of Transcultural Nursing. 2014 doi: 10.1177/1043659614561679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baser R, et al. Measurement Invariance of Intuitive Cancer Risk Perceptions in Diverse Populations. In Preparation. 2015 doi: 10.1177/1359105317693910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.SAS Institute Inc. SAS System for Windows (Version 9.4) SAS Institute Inc.; Cary, NC: 2009. [Google Scholar]
- 33.Fowles JB, et al. Measuring Self-Management Of Patients’ and Employees’ Health: Further Validation of The Patient Activation Measure (PAM) Based On Its Relation To Employee Characteristics. Patient Education and Counseling. 2009;77(1):116–22. doi: 10.1016/j.pec.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 34.Hofstede G. Culture’s Consequences: Comparing Values, Behaviors, Institutions, and Organizations Across Nations. Thousand Oaks, CA: Sage Publications; 2001. [Google Scholar]
- 35.Huerta EE, Macario E. Communicating Health Rsk To Ethnic Groups: Reaching Hispanics As a Case Study. Journal of the National Cancer Institute Monographs. 199925:23–26. doi: 10.1093/oxfordjournals.jncimonographs.a024202. [DOI] [PubMed] [Google Scholar]
- 36.Joseph G, et al. Perceived Susceptibility To Illness and Perceived Benefits Of Preventive Care: An Exploration Of BehavioralTheory Constructs In A Transcultural Context. Health Education and Behavior. 2009;36(5 Suppl):71S–90S. doi: 10.1177/1090198109338915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pasick RJ, et al. Behavioral Theory In A Diverse Society: Like A Compass On Mars. Health Education and Behavior. 2009;36(5 Suppl):11S–35S. doi: 10.1177/1090198109338917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wegner DM, Wheatley T. Apparent Mental Causation. Sources Of The Experience Of Will. The American Psychologist. 1999;54(7):480–92. doi: 10.1037//0003-066x.54.7.480. [DOI] [PubMed] [Google Scholar]
- 39.Subbotsky E, Quinteros G. Do Cultural Factors Affect Causal Beliefs? Rational and Magical Thinking In Britain and Mexico. British Journal Of Psychology. 2002;93:519–543. doi: 10.1348/000712602761381385. [DOI] [PubMed] [Google Scholar]
- 40.Pronin E, et al. Everyday Magical Powers: The Role Of Apparent Mental Causation In The Overestimation Of Personal Influence. Journal of Personality and Social Psychology. 2006;91(2):218–31. doi: 10.1037/0022-3514.91.2.218. [DOI] [PubMed] [Google Scholar]
- 41.Risen JL, Gilovich T. Why People Are Reluctant To Tempt Fate. Journal of Personality and Social Psychology. 2008;95(2):293–307. doi: 10.1037/0022-3514.95.2.293. [DOI] [PubMed] [Google Scholar]
- 42.Orom H, et al. Perceived Cancer Risk and Risk Attributions Among African-American Residents Of A Low-Income, Predominantly African-American Neighborhood. Ethnicity and Health. 2014:1–14. doi: 10.1080/13557858.2014.950197. [DOI] [PubMed] [Google Scholar]
- 43.Hay LJ, et al. Correlates Of Intuitive Beliefs About Cancer In Diverse Primary Care. Under Review. 2016 [Google Scholar]
- 44.Keinan G. The Effects of Stress and Desire for Control on Superstitious Behavior. Personality and Social Psychology Bulletin. 2002;28(1):102–108. doi: 10.1177/0146167202281009. [DOI] [Google Scholar]
- 45.AuYoung M, et al. Patient Activation is Inconsistently Associated with Positive Health Behaviors Among Obese Safety Net Patients. J Immigr Minor Health. 2015 doi: 10.1007/s10903-015-0285-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCaul KD, Mullens AB. Affect, Thought, and Self-Protective Health Behavior: The Case of Worry and Cancer Screening. In: Suls J, Wallston K, editors. Social Psychological Foundations of Helath and Illness. Malden, MA: Blackwell Publishing; 2003. pp. 137–168. [Google Scholar]
- 47.Consedine NS, et al. Fear, Anxiety, Worry, and Breast Cancer Screening Behavior: A Critical Review. Cancer Epidemiology, Biomarkers and Prevention. 2004;13(4):501–10. [PubMed] [Google Scholar]
- 48.Hay JL, McCaul KD, Magnan RE. Does Worry About Breast Cancer Predict Screening Behaviors? A Meta-Analysis of The Prospective Evidence. Preventive Medicine. 2006;42(6):401–8. doi: 10.1016/j.ypmed.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 49.Sherman SJ, et al. Imagining can heighten or lower the perceived likelihood of contracting a disease – the mediating effect of ease of imagery. Personality and Social Psychology Bulletin. 1985;11(1):118–127. [Google Scholar]
- 50.Cartwright-Hatton S, Wells A. Beliefs about worry and intrusions: the Meta-Cognitions Questionnaire and its correlates. J Anxiety Disord. 1997;11(3):279–96. doi: 10.1016/s0887-6185(97)00011-x. [DOI] [PubMed] [Google Scholar]
- 51.James A, Wells A. Death beliefs, superstitious beliefs and health anxiety. Br J Clin Psychol. 2002;41(Pt 1):43–53. doi: 10.1348/014466502163787. [DOI] [PubMed] [Google Scholar]
- 52.Torgler B. Determinants of superstition. The Journal of Socio-Economics. 2007;36:713–733. [Google Scholar]
- 53.Wiseman R, Watt C. Measuring superstitious belief: Why lucky charms matter. Journal of behavioral decision making. 2004;37(8):1533–1541. [Google Scholar]
- 54.Darke PR, Freedman JL. The belief in good luck scale. Journal of Research In Personality. 1997;31(4):486–511. [Google Scholar]
- 55.Ashing-Giwa KT, et al. Understanding the breast cancer experience of women: a qualitative study of African American, Asian American, Latina and Caucasian cancer survivors. Psychooncology. 2004;13(6):408–28. doi: 10.1002/pon.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Landrine H, Klonoff EA. Cultural diversity in causal attributions for illness: The role of the supernatural. Journal of Behavioral Medicine. 1994;17(2):181–193. doi: 10.1007/BF01858104. [DOI] [PubMed] [Google Scholar]
- 57.Meade CD, et al. Addressing cancer disparities through community engagement: improving breast health among Haitian women. Oncol Nurs Forum. 2009;36(6):716–22. doi: 10.1188/09.onf.716-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cartwright-Hatton S, Wells A. Beliefs about worry and intrusions: The Meta-Cognitions Questionnaire and its correlates. Journal of anxiety disorders. 1997;11(3):279–296. doi: 10.1016/s0887-6185(97)00011-x. [DOI] [PubMed] [Google Scholar]
- 59.James A, Wells A. Death beliefs, superstitious beliefs and health anxiety. The British Journal of Clinical Psychology. 2002;41(Pt 1):43–53. doi: 10.1348/014466502163787. [DOI] [PubMed] [Google Scholar]
- 60.Ledford CJ, Ledford CC, Childress MA. Extending Physician ReACH: influencing patient activation and behavior through multichannel physician communication. Patient Educ Couns. 2013;91(1):72–8. doi: 10.1016/j.pec.2012.11.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


