Abstract
Pre-diabetes and diabetes are a global epidemic, and the associated neuropathic complications create a substantial burden on both the afflicted patients and society as a whole. Given the enormity of the problem and the lack of effective therapies, there is a pressing need to understand the mechanisms underlying diabetic neuropathy (DN). In this review, we present the structural components of the peripheral nervous system that underlie its susceptibility to metabolic insults and then discuss the pathways that contribute to peripheral nerve injury in DN. We also discuss systems biology insights gleaned from the recent advances in biotechnology and bioinformatics, emerging ideas centered on the axon-Schwann cell relationship and associated bioenergetic crosstalk, and the rapid expansion in our knowledge of the mechanisms contributing to neuropathic pain in diabetes. These recent advances in our understanding of DN pathogenesis are paving the way for critical mechanism-based therapy development.
Keywords: Diabetic neuropathy, Bioenergetics, Schwann cell, Pain, Axon-glia crosstalk, Peripheral nervous system, Type 2 diabetes, Type 1 diabetes, Pre-diabetes
In Brief
Nearly a quarter of a billion people worldwide have diabetic neuropathy. Yet despite high morbidity, there are no disease-modifying therapies for the disorder. Four international experts review new experimental advances in the field with an eye towards future mechanism-based therapies.
Introduction
Peripheral neuropathy is a highly complex and prevalent disease. Over 8% of the general population has peripheral neuropathy, and this number increases to 15% in individuals 40 years of age or older (Gregg et al., 2004). The most common causes of peripheral neuropathy in the US and Europe are pre-diabetes and type 2 diabetes (T2D). At least half of all diabetic patients, including those patients with Type 1 diabetes (T1D), develop some form of neuropathy during their lifetime. Pre-diabetes and T2D are recognized as a global epidemic, with an incidence that continues to rise, particularly in countries adopting a more Westernized diet (International Diabetes Federation, 2014). It is estimated that over 20 million Americans currently have neuropathy secondary to either pre-diabetes, T1D, or T2D, and this number will double as more Americans develop pre-diabetes and T2D (Menke et al., 2015). Moreover, pre-diabetes and diabetes affect 316 million and 387 million people worldwide, respectively, and while exact figures are lacking, at least 200 million of those affected worldwide have an associated neuropathy.
Diabetes can produce several types of peripheral nervous system (PNS) damage. The most common type of nerve damage is bilateral and symmetric damage to nerves of the feet, with a distal to proximal gradient of severity, known as a stocking-glove neuropathy. Because this pattern of nerve injury is so common, this neuropathy is synonymously deemed diabetic neuropathy (DN) (Callaghan et al., 2012a). A similar pattern of injury occurs with pre-diabetes, supporting the idea that nerve injury secondary to diabetes is a continuum from normal glycemia to varying levels of hyperglycemia (Lee et al., 2015). DN is primarily a disorder of sensory nerves, and early in the course of DN patients commonly experience positive sensory symptoms in the feet such as pain, tingling, and prickling sensations (paresthesias), as well as negative symptoms such as numbness; disordered sensory processing may evoke pain when the feet are touched (allodynia) and increase sensitivity to noxious stimuli (hyperalgesia). Only much later in the course of the disease is there evidence of motor nerve dysfunction, with distal weakness of the toes, or in extreme cases, the ankles and calves. Why sensory axons are particularly vulnerable to diabetes as compared to motor axons is unknown, but potential mechanisms will be discussed during this review. The progressive loss of lower extremity sensation and over time motor weakness results in loss of balance, falls, and the numb, insensate foot (Pop-Busui et al., 2017).
Unfortunately, despite decades of research, there are no modifiable treatments for DN other than improved lifestyle and diabetes control (Pop-Busui et al., 2017). A Cochrane Review of all available clinical studies reveals that rigorous glucose control can decrease the incidence of DN in T1D, but has little to no effect in T2D, despite over 10 years of improved glucose control (Table 1) (Callaghan et al., 2012b). This unexpected difference is highly informative and supports the contention that different mechanisms underlie DN in T1D and T2D, and it may be that DN is actually two diseases with a similar clinical presentation, not one (Callaghan et al., 2012b; Callaghan et al., 2012c). Despite this conundrum, DN research in the last two decades has focused on glucose and the T1D rat as the pre-clinical model to understand disease pathogenesis. In the US, all clinical trials aimed at altering the progressive course of DN have failed (Pop-Busui et al., 2017). Large pharma has now walked away from DN as a result of our lack of basic understanding of the disease, while the enormity of the problem has reached epidemic proportions (Berger et al., 2013). The societal costs are only dwarfed by the individual costs to each patient that include pain and inability to work along with poor quality of life (Stewart et al., 2007). The enormity of the problem, at a personal and societal level, mandates a better understanding of the disease, with the aim of mechanism-based targets (Zochodne, 2014) and demands early diagnosis to prevent poor patient outcomes (Vas and Edmonds, 2016).
Table 1.
Glycemic intervention differentially affects diabetic polyneuropathy in type 1 diabetes mellitus versus type 2 diabetes mellitus.
| Trail size | Length of study (years) | Positive effects of enhanced glycemic control? | |
|---|---|---|---|
| T1D | |||
| Holman et al. (Holman et al., 1983) | 74 | 2 | Yes |
| Dahl-Jorgensen et al. (Dahl-Jorgensen et al., 1986) | 45 | 2 | Yes |
| DCCT (DCCT Research Group, 1993) | 1441 | 5 | Yes |
| Reichard et al. (Reichard et al., 1993) | 102 | 7.5 | Yes |
| Linn et al. (Linn et al., 1996) | 49 | 5 | Yes |
| T2D | |||
| UKPDS (UKPDS Study Group, 1998) | 3867 | 10 | Noa |
| Azad et al. (Azad et al., 1999) | 153 | 2 | No |
| Gaede et al. (Gaede et al., 2003) | 160 | 8 | No |
| Duckworth et al. (Duckworth et al., 2009) | 1791 | 5.6 | No |
| Ismail-Beigi et al. (Ismail-Beigi et al., 2010) | 10251 | 3.7 | No |
DCCT, Diabetes Control and Complications Trial; T1D, type 1 diabetes; T2D, type 2 diabetes; UKPDS, United Kingdom Prospective Diabetes Study.
The UKPDS did not show a statistically significant benefit at 9 or 12 years but did show a benefit at 15 years of follow-up. Of note, only 277 individuals were evaluated for neuropathy at 15 years.
Reprinted with permission from: Callaghan, B.C., Hur, J., and Feldman, E.L. (2012) Diabetic neuropathy: one disease of two? Current Opinion in Neurology. 25, 536-541. (Callaghan et al., 2012c)
This review will begin with an overview of PNS structure and the innate susceptibly of the PNS to diabetes-mediated damage. This section will provide the framework for subsequent sections that briefly outline the well-studied pathways implicated in DN pathogenesis, the use of transcriptomics to enhance our understanding of DN, and a discussion of the newly emerging idea that DN is actually a disorder of energy transfer between the axon and the supporting glia, an idea that advocates a new way forward in our understanding of DN and the development of mechanism-based therapies. The review will end with a discussion of the current mechanisms underlying the pain associated with DN, a problem of great enormity for the individual patient and society as a whole.
Peripheral Nervous System Structure and Function
The peripheral nervous system (PNS) consists of 12 cranial nerves and 31 pairs of spinal nerves, and similar to the central nervous system (CNS), the PNS is composed of both neurons and supporting glia, specifically Schwann cells (SCs) in the PNS. Efferent axons from motor neurons carry information from the CNS to muscles and glands, while afferent axons from sensory neurons carry information from peripheral sensory receptors to the CNS (Figure 1). Support of axons presents a unique challenge to the PNS, where axons can be up to 3 feet or more in length and frequently 20,000 times the length of the motor or sensory neuronal cell body (Wang et al., 2012). The location of the neuron cell body is also important; sensory neurons, specifically dorsal root ganglion (DRG) neurons, lie outside the blood nerve barrier, as do the peripheral sensory receptors, while motor neurons are located within the ventral horn of the spinal cord, under the protection of the blood brain barrier. The anatomical difference in these protective barriers may partly explain the particular vulnerability of sensory neurons in diabetes; while motor neurons remain protected, dorsal root ganglion neurons are exposed to systemic metabolic and hypoxic stressors, making them much more susceptible to injury.
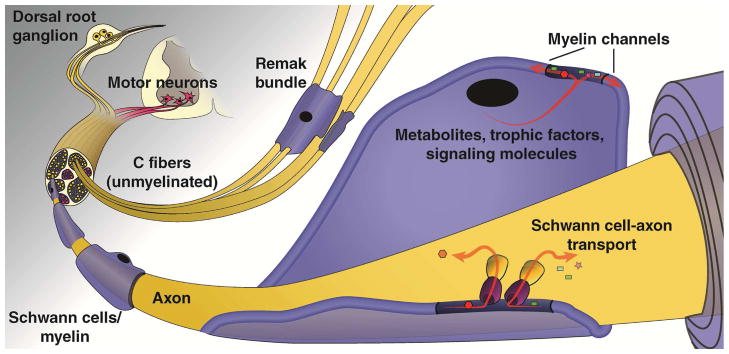
Figure 1. The peripheral nervous system.
The peripheral nervous system (PNS) is comprised of both neurons and Schwann cells (SCs), and the structure, location, and interaction of these components have important implications for PNS function. Efferent axons of motor neurons, whose cell bodies are located in the ventral horn of the spinal cord, carry signals from the central nervous system (CNS) to muscles and glands, whereas afferent axons of sensory neurons, whose cell bodies are located in the dorsal root ganglia, relay information from peripheral sensory receptors to the CNS. Thin and unmyelinated sensory axons, also known as C-fibers or small fibers, are associated with non-myelinating SCs and are grouped as Remak bundles and represent a large portion of the PNS neurons. Myelinated sensory axons, on the other hand, are surrounded by myelin sheaths made by SCs that form distinct nodal domains important for saltatory conduction and a tubular network of myelinic channels that connect the SC cytoplasm with the periaxonal space to provide a source of energy to the axonal compartment
A second aspect of the anatomy of the sensory system beyond the blood nerve barrier may also explain its unique vulnerability. In the PNS, there are thin (<1 μm) unmyelinated axons, known as C-fiber axons or small fibers. These axons are engulfed by and associated with non-myelinating SCs in a pattern reminiscent of axon-glia interactions in invertebrates and are grouped as “Remak bundles” when viewed in cross-section. C-fibers carry information for the autonomic nervous system as well as afferent impulses in response to temperature and noxious stimuli such as potentially injurious chemicals, extreme temperatures, and mechanical forces that could cause tissue damage. There are more unmyelinated than myelinated axons in the PNS, and these C-fibers were deemed the “foot soldiers” of the PNS by the late Jack Griffin, a pioneer in peripheral neuropathy (Feldman et al., 2015). An obvious consequence of the lack of myelin is slow continuous impulse conduction in unmyelinated C fiber axons due to the uniform distribution of ion channels along the axolemma. In addition to the unmyelinated C-fibers, small myelinated Aδ fibers subserving cold detection and high threshold mechanoreceptors are designated small afferent fibers. Essential to our ability to perceive pain, congenital loss of small fibers leads to ulcers, digital mutilation, and amputations (Scherer, 2006).
The PNS also contains afferent myelinated fibers that carry information from peripheral receptors on position sense and touch. The myelin sheaths made by SCs that surround these fibers differ from central myelin, with much longer internodal segments, the presence of Schmidt-Lantermann incisures, and a different composition of myelin structural proteins (Nave and Werner, 2014). Myelination of these afferent fibers by SCs is highly controlled and includes the formation of distinct nodal domains: the nodes of Ranvier, the paranodes, and the juxtaparanodes. Enrichment of voltage gated ion channels in the nodes of Ranvier and paranodes permits rapid saltatory impulse conduction, while tight junction adhesion complexes in the paranodes protect axons against toxic metabolites entering the periaxonal space (Wang et al., 2012).
Pre-diabetes and diabetes alters the anatomy, i.e. the structure of both the aforementioned unmyelinated and myelinated fibers. While Auche first described changes in PNS structure with DN in 1890 (Auche, 1890), there were no pathological studies beyond small or single case reports for the next 60 years (reviewed in (Colby, 1965)). With the advent of the electron microscope, P.K. Thomas, P.J. Dyck, and their colleagues began careful anatomical studies in the 1960’s of sural nerve biopsies from cohorts of patients with varying degrees of DN. These studies along with more current reports (Malik et al., 2005) reveal that the earliest changes of DN occur at the level of the unmyelinated C-fibers, with initial degeneration and regeneration of C fibers resulting in pain, allodynia, and hyperesthesias (Green et al., 2010). Over time degeneration overrides regeneration and there is loss of C fibers, a common early finding also seen in patients with pre-diabetes (Ziegler et al., 2009). It is likely that C fibers are more susceptible to metabolic injury in large part because they lack the degree of protection and nutrient supplementation afforded to myelinated axons by SCs. As small fiber pathology proceeds, mild segmental axonal demyelination and remyelination begin to occur in the course of DN (Malik et al., 2005). As the disease course progresses, there is continued evidence of demyelination with frank axonal degeneration of myelinated fibers. This temporal course of nerve pathology in DN argues for a loss of protective and nutrient flow from the SCs to the myelinated axons, leading then to eventual axonal loss (Mizisin, 2014). The pathological changes of DN are present in a distal to proximal course along the nerve, paralleling the patient’s symptomatology.
To summarize, in addition to the distinct anatomical differences of the blood nerve barrier between motor and sensory axons, there are also distinct anatomical and molecular relationships between sensory axons and SCs when comparing unmyelinated and myelinated fibers. Accumulating data suggest that SCs are much more than “passive” insulators for axons, as once previously thought, but active partners for providing metabolic support to axons that may be disturbed in disease states such as diabetes. We will now review the metabolic pathways that are known to be dysregulated in DN, and then address the concept of how glia may provide metabolic support to axons and how such support may be disturbed by diabetes.
Pathways Implicated in Diabetic Neuropathy
Over the last 3 decades, research in the field of DN, including our own, has focused on DN-linked pathways related to the metabolic and/or redox state of the DRG and SC. We and others have focused our work on understanding the pathological significance of polyol pathway activation, of the hexosamine pathway and protein kinase C (PKC) isoforms, of accumulation of advanced glycation end products (AGEs) in the diabetic nerve, and of excess glucose and/or fatty acid flux. While each pathway may be injurious to the nerve alone, collectively they cause an imbalance in the mitochondrial redox state and lead to excess formation of mitochondrial and cytosolic reactive oxygen species (ROS) (Figures 2 and 3).
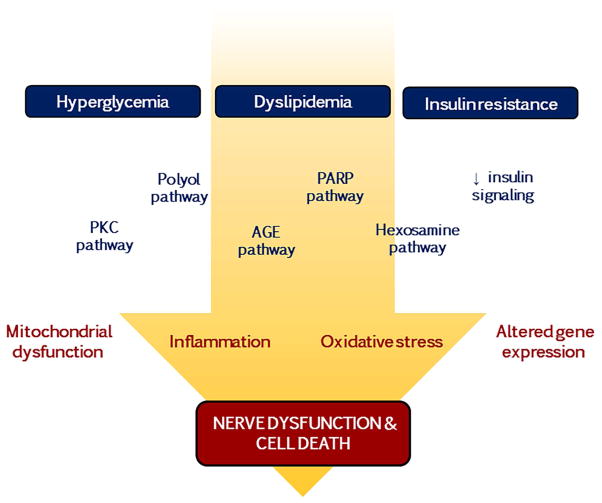
Figure 2. Pathways implicated in diabetic neuropathy pathogenesis.
Nerve dysfunction and cell death in DN results from a complex myriad of events that are triggered by the metabolic imbalances associated with diabetes. Hyperglycemia, dyslipidemia, and/or insulin resistance promotes activation of the protein kinase C (PKC), polyol, advanced glycation end-product (AGE), poly (ADP-ribose) polymerase (PARP), and hexosamine pathways, as well as loss of insulin signalling, which culminate in deleterious effects on mitochondrial function and gene expression along with inflammation and oxidative stress.
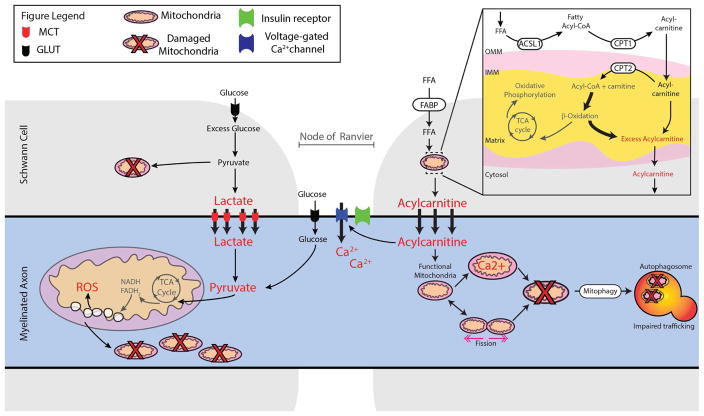
Figure 3. Bioenergetic mechanisms of nerve injury in diabetes.
Glucose enters SCs via Glut3 and LCFA enter via FABP for production of ATP via the TCA cycle and OxPhos. In T2D, excess glucose undergoes glycolysis but excess pyruvate overwhelms the TCA cycle with loss of OxPhos, and the SC enters anaerobic metabolism with increased lactate production. Lactate is shuttled to the axon via the MCT. Glucose can also enter the axon directly via Glut3 on the Node of Ranvier. How the insulin receptor alters glucose entry or nerve viability is unknown. In T2D, excess LCFA can also enter the SC and CPT1 transports the LCFA acyl-CoA esters, along with the carnitine shuttle and CPT2, to the mitochondrial matrix to undergo β-oxidation, with repeat cleavage of 2 carbons to form acetyl-CoA with each cycle. Excess acetyl-CoA overwhelms the TCA cycle with loss of OxPhos, and a secondary increase in large quantities of acylcarnitines, which are shuttled to the axon. Finally, excess lactate and acylcarnitines mediate mitochondrial axonal damage in T2D. Lactate overload results in TCA/OxPhos dysfunction, producing mitochondrial injury, mitochondrial fission, and ROS. Acylcarnitines may trigger flux of extracellular Ca2+ into the axon and intracellular Ca2+ into the mitochondria, which alters mitochondrial trafficking and induces mitochondrial apoptosis, respectively. Impaired trafficking mechanisms will likely impede the crucial mitophagy clearance pathways that shuttle damaged mitochondria back to the soma via retrograde transport. Cumulative continued substrate excess results in loss of axonal mitochondrial function with subsequent loss of axon structure and function, leading to DN. ACSL1: long chain acyl-CoA synthetase 1; CPT1/2: Carnitine palmitoyltransferase 1/2; FABP: fatty acid binding protein; FFA: Free fatty acids; Glut3: Glucose transporter 3; IMM/OMM: Inner/outer mitochondrial membrane; LCFA: Long chain fatty acids; MCT: Monocarboylase transporter 1 or 4; ROS: Reactive oxygen species.
Polyol Pathway
The most highly studied pathway thought to underlie DN is the polyol pathway. Excess glucose is converted to sorbitol by aldose reductase; this results in osmotic imbalance in the cell secondary to increased sorbitol, with resultant osmotic stress and compensatory efflux of myoinositol and taurine. In turn, loss of myoinositol, an essential component of sodium/potassium (Na/K) ATPase, impairs normal nerve physiology. A second set of neuronal insults occur as aldose reductase activity depletes cellular stores of NADPH, needed for nitric oxide (NO) generation and regeneration of the essential antioxidant glutathione. The resulting generation of cytoplasmic ROS leads to ROS-mediated intracellular injury and cellular dysfunction. This idea, known as the “metabolic flux” hypothesis, is now generally accepted as the more significant biological consequence of polyol pathway activation (reviewed in (Oates, 2008)).
Pre-clinical rodent studies, especially in the streptozotocin (STZ) rat model of T1D, confirmed polyol pathway activation and the subsequent deleterious downstream effects outlined above that impair PNS structure and function (reviewed in (Cameron and Cotter, 1993)). These studies led to the development and pre-clinical testing of a family of aldose reductase inhibitors in the STZ rat, studies that produced robust and encouraging results. Specifically, in the STZ rat, aldose reductase inhibitors ameliorated diabetes-mediated deficits in behavior, nerve conduction velocity (NVC), and nerve structure (reviewed in (Schemmel et al., 2010)). In parallel, aldose reductase deficient mice had normalized nerve function and physiology (Ho et al., 2006) while transgenic overexpression of aldose reductase worsened neuropathy in STZ treated mice (Yagihashi et al., 2001). Consequently, aldose reductase inhibitors became the focus of the diabetes complications community in the 1990’s, and 32 clinical trials were undertaken in the US, Canada, Europe, and Japan (Chalk et al., 2007). Unfortunately, despite the early promise of these drugs in pre-clinical animal models of DN, all clinical trials in man failed in the US, Canada, and Europe. Indeed, since 1981, ten different aldose reductase inhibitors have failed in the treatment of patients with DN. Failure has been attributed to trial design, the inability of the compounds to reach the PNS, or the inability to administer sufficiently high doses of the drugs secondary to hepatoxicity (Chalk et al., 2007; Grewal et al., 2015). More recently, however, there has been an emergence in the use of aldose reductase inhibitors for their proposed anti-inflammatory properties, not in DN, but in sepsis and cancer (Grewal et al., 2015).
Hexosamine and Protein Kinase C Pathways
Increased glycolysis in response to excess glucose also disrupts several metabolic pathways that can promote neuronal injury. The glycolysis intermediate, fructose-6-phosphate, enters the hexosamine pathway and undergoes a series of reactions to form uridine 5-diphosphate-N-acetylglucosamine (GlcNac). GlcNac is one of the sugar moieties that binds serine/threonine residues on common transcription factors, such as Sp-1, promoting lipid dyshomeostasis, inflammation, and injury of complication prone tissues, including peripheral nerves (Du et al., 2000). Increased glycolysis also leads to the accumulation of another intermediate, dihydroxy-acetone phosphate, which is converted to diacylglycerol (DAG). Increases in DAG are also well documented in complication prone tissues, especially nerve, where DAG activates neuronal PKC (Eichberg, 2002). Activated PKC leads to multiple metabolic impairments that range from insulin resistance to disrupting the function of Na/K ATPase to altering gene expression of vascular endothelial growth factor (VEGF) and transforming growth factor beta (TGFβ), leading to vasoconstriction, hypoxia, and neuronal damage in rodent models of diabetes, especially the STZ rat (Geraldes and King, 2010).
As with aldose reductase, these pre-clinical data led to multiple studies using PKC inhibitors as a therapeutic agent for DN in the STZ rat, where improvement in rodent nerve physiology provided the enthusiasm to more towards the development of specific PKC inhibitors for the treatment of DN (reviewed in (Geraldes and King, 2010)). The PKC-β inhibitor ruboxistaurin (LY333531, RBX or Arxxant™; Eli Lilly and Company, Indianapolis, IN) was the most highly studied drug of its class for diabetic microvascular complications in man. Unfortunately, ruboxistaurin was ineffective in the treatment of diabetic retinopathy, nephropathy (reviewed in (Geraldes and King, 2010)) and in a single well conducted study of DN (Vinik et al., 2005). The primary reason cited for the underlying failure of ruboxistaurin in DN was inability of the drug to reach the PNS (Bansal et al., 2013). Like aldose reductase inhibitors, the use of this class of drugs (along with PKC activators) has expanded in the last decade into cancer and metabolic disorders (Mochly-Rosen et al., 2012), and is a source of current investigation.
Advanced Glycation Endproducts
In the Maillard reaction, glucose reacts with amino groups on proteins to form Amadori products that, over time, produce irreversible glycation products, known as AGEs. AGEs cross-link essential proteins, altering their function and causing cellular damage. AGEs also bind cell surface receptors, primarily the Receptor for AGE (RAGE), that activates an injurious downstream signaling cascade mediated in part by activation of nuclear factor-kappa B (NF-κB). Vasoconstriction, inflammation, and loss of neurotrophic support are all reported in the rodent PNS secondary to AGE activation of RAGE (Lukic et al., 2008) while AGE accumulation is present in the peripheral nerves of patients with DN and T2D (Misur et al., 2004). In a rodent model of diabetes and DN, aminoguanidine prevented the formation of AGE and had robust pre-clinical promise (Miyauchi et al., 1996), but in humans, the side effects of the drug prohibited its therapeutic development (Thornalley, 2003). A soluble extracellular domain of RAGE, sRAGE, blocks RAGE, and serum levels of sRAGE are strongly associated in man with both cardiovascular disease and diabetic nephropathy, but not as of yet in human DN (Humpert et al., 2007; Thomas et al., 2015). More detailed studies, similar to what has been done for diabetic nephropathy, are proposed to determine if RAGE activation is a meaningful therapeutic target in DN (Zochodne, 2014).
Bioenergetics and Inflammation
In primary DRG neurons and axons, high glucose (via glycolysis and the TCA cycle) and excess fatty acids (via β-oxidation) produce the electron donors NADH and FADH2 which are shuttled via oxidative phosphorylation (OxPhos) through complexes I thru IV until they are donated to molecular oxygen to form water. The electron transfer produces a proton gradient at the outer mitochondrial membrane, generating a potential between the inner and outer mitochondrial membrane that drives ATP synthesis and is crucial for mitochondrial viability, function, and normal neuronal metabolism. As electrons are passed from complex II to complex III, small quantities of ROS are produced as by-products and are detoxified in the nerve by cellular antioxidants, such as glutathione, catalase, and superoxide dismutase (Vincent et al., 2011). In particular, SCs have a high anti-oxidant capacity and easily detoxify the accumulating ROS under normal metabolic conditions (Vincent et al., 2011).
Under conditions of excess glucose and high fatty acid flux, however, excess substrate is metabolized through the glycolytic/TCA cycles and β-oxidation/TCA cycles, respectively, providing the DRG neurons with an over-abundance of NADH and FADH2 electron donors. This produces a high proton gradient across the inner mitochondrial membrane, uncoupling the normal gradient, which disrupts OxPhos and in turn decreases ATP production while markedly increasing ROS. In both animal models of DN and in vitro cultures of DRG from rodents with DN, the anti-oxidant response is overwhelmed and ROS initiate an extensive injury cascade that is intensified by loss of ATP production and neuronal dysfunction (Fernyhough, 2015).
Neuronal oxidative/nitrosative stress can also activate multiple downstream kinases such as PKC, mitogen activated protein kinase (MAPK), jun N-terminal kinase (JNK), and redox-sensitive transcriptional factors including NF-κB, producing a feed-forward loop of injury (Stavniichuk et al., 2014). These factors play a critical role in triggering a cascade of cytokine and chemokine production, including proinflammatory interleukin-1β, -2, -6, and -8 (IL1β, IL2, IL6, and IL8), tumor necrosis factor- α (TNFα), chemokine (C-C motif) ligand 2 (CCL2), and chemokine (C-X-C motif) ligand 1 (CXCL1). Cytokines and chemokines not only enhance existing inflammatory and immune responses, but also increase cellular oxidative/nitrosative stress, promoting even more neuronal damage in experimental models of DN (Vincent et al., 2013). In the axons of murine models of DN, there are increased numbers of dense, depolarized mitochondria undergoing metabolic fission that correlate with the degree and severity of cytosolic ROS and mitochondrial ROS and DN (Edwards et al., 2010; Vincent et al., 2010). In contrast, SCs appear generally healthy, have a much higher innate anti-oxidant response as discussed above, and are at least partially protected against ROS damage. SCs are further protected by their remarkable and just discovered ability to survive with anaerobic glycolysis only, which will be discussed more in detail below.
Antioxidant treatment of rodents with DN aimed at neutralizing ROS injury of the PNS restores normal nerve electrophysiology and nerve structure (reviewed in (Low et al., 1997)). One antioxidant, α-lipoic acid, has robust therapeutic effects in experimental rodent models of DN (reviewed in (Cameron and Cotter, 1999)) and is approved for clinical use in Europe (Papanas and Ziegler, 2014) but failed regulatory approval for the treatment of DN in the United States. Currently, combination therapies that include antioxidants like α-lipoic acid are being tested in experimental DN with the goal of future combination therapy in man (Yorek et al., 2016). These combination treatments are also targeted at ameliorating the inflammatory response, which may provide further therapeutic benefit in experimental DN (Chen et al., 2016) with the hopes that blocking both ROS and inflammation will yield a more robust therapeutic effect in DN (Yorek et al., 2016).
Insulin, Insulin Receptors, and Insulin Resistance
As DN research has evolved from a glucocentric viewpoint, a broader understanding has emerged that views DN as a complex disorder secondary to multiple linked metabolic and inflammatory insults, as described above. The clinical studies comparing DN in patients with T1D and T2D point to different mechanisms underlying DN in each disorder, at least early in the course of the disease (Callaghan et al., 2012b). Experimental research to understand these clinical findings is dependent on rodent models of prediabetes, T1D and T2D that develop neuropathy, yet there is no one rodent model that accurately reflects all aspects of human disease. Careful selection of an experimental animal model is required with the goal to select a model that most closely parallels the specific aspects of DN under investigation. This requires attention to diabetes type, animal background strain and selected use of transgenic animals, gender, age and diet. With the recent advent of over 20 murine models of DN, there is a rich variety of experimental models from which to choose from for DN research beyond the STZ rat. These experimental models provide the tools for future research to more fully understand the differences between DN in T1D and T2D (Islam, 2013; O’Brien et al., 2014b; Yorek, 2016).
In T1D, the responsiveness of DN in patients to improved glucose control achieved with different insulin regimens suggests one or more insulin-responsive pathways participate in PNS function. While insulin does not directly control glucose transport into the PNS, it is a potent neurotrophic factor capable of supporting axonal growth (Mielke and Wang, 2011). Insulin receptors (IRs) are highly expressed on sensory neurons, motor neurons and intraneural mitochondria, and they are highly enriched in SC membranes and at nodes of Ranvier (Waldbillig and LeRoith, 1987). The role of insulin signaling in each of these unique anatomical locations is not fully understood. Activation of IRs via insulin leads to a signaling cascade of essential intracellular pathways for normal cellular maintenance (Kim and Feldman, 2012); yet, there is significant redundancy in these cascades which are also activated by many tyrosine kinase growth factors, in particular insulin-like growth factor I (IGF-I). It is speculated that in T1D and DN, lack of insulin signaling promotes cellular injury by decreasing gene expression of essential proteins, blocking protein synthesis, and promoting cell injury and death pathways (Zochodne, 2014). Supplementation with insulin restores glucose homeostasis and activates IR-insulin mediated pathways, with return of near normal PNS function in rodent models of T1D and DN (Brussee et al., 2004). Whether the beneficial therapeutic effects of insulin are due to normoglycemia or return of insulin-mediated neuronal signaling, or a combination of both, is unknown and a source of controversy in the field of experimental DN (Grote and Wright, 2016). In parallel, the exact mechanism(s) underlying the treatment effects of insulin therapy in patients with T1D and DN is unknown, with the same controversy concerning potential mechanisms (Martin et al., 2014). Insulin deficiency is accompanied by decreased levels of C-peptide in T1D, and targeted intervention with C-peptide in T1D and DN in patients shows modest clinical promise (Wahren et al., 2016).
In contrast to DN in T1D, however, a close interrogation of the clinical data in T2D and DN reveals that improved glucose controls does little to affect the course of DN (Table 1) (Callaghan et al., 2012b). Indeed, in T2D, it is likely that components of the metabolic syndrome promote the onset and progression of DN (Callaghan and Feldman, 2014; Lupachyk et al., 2012). Defined as hypertension, hyperlipidemia, visceral adiposity, and impaired glucose regulation, the metabolic syndrome predisposes individuals with pre-diabetes and diabetes to neuropathy; the greater the number of metabolic syndrome components, the more likely an individual with abnormal glucose regulation will have DN. These data suggest several interesting possibilities concerning the pathogenesis of DN. For example, because individuals with T2D are known to have peripheral insulin resistance of fat and muscle, could a similar resistance develop in the nervous system? If so, then a state of relative insulin deficiency, similar to what is observed in T1D and DN, could in part be underlying the pathobiology of DN in T2D. While both DRG and CNS neurons develop insulin resistance in vitro (Singh et al., 2012) and in murine models of T2D and DN (Grote et al., 2013; Kim and Feldman, 2012), direct confirmation of this idea is lacking in humans at this time.
Transcriptomics and Diabetic Neuropathy
Biomedical research over the past decade has undergone a paradigm shift from focusing on a single pathway, as reviewed above, to analyzing biological systems as a whole. In DN, microarray-based genome-wide profiling was used to examine transcriptomic changes in human sural nerve samples obtained from subjects with DN (Hur et al., 2013; Hur et al., 2011). Transcriptomic profiling of sural nerve biopsies from subjects with progressive or non-progressive DN identified over 500 differentially expressed genes, which were highly enriched in immune response and lipid metabolism pathways (Hur et al., 2011). A literature-derived co-citation network analysis of these genes revealed that apolipoprotein E, leptin, and peroxisome proliferator-activated receptor (PPAR) gamma may play a central role in DN progression (Hur et al., 2010; Hur et al., 2011). A subset of subjects with T1D and T2D and non-progressive DN actually experienced sural nerve regeneration during the course of their disease, and in these subjects lower glycated hemoglobin (HbA1c) was a significant clinical factor that positively correlated with anatomical improvement (Hur et al., 2013). While the mechanisms underlying regeneration are unknown, these data suggest that there is an interplay between dyslipidemia and glucotoxicity in the onset and progression of DN, an area requiring further experimental investigation.
Microarrays have also been widely used to examine the transcriptome changes in peripheral nerves induced by diabetes and anti-diabetic drug-treatment in various experimental animal models of DN (Abdul-Rahman et al., 2012; Hur et al., 2015; O’Brien et al., 2014a; O’Brien et al., 2016; Wiggin et al., 2008). Gene expression changes in SCs, fibroblasts, endothelial cells and sciatic nerve-associated adipocytes were consistent with structural changes of axonal degeneration in sciatic nerves of T2D mice with DN, and pathways such as lipid metabolism and PPAR signaling were also highly dysregulated in diabetic animals (Pande et al., 2011). When treated with the PPAR inhibitor pioglitazone, T2D mice had no improvement in nerve conduction studies and gene expression profiling of sciatic nerves showed that pathways related to adipogenesis, adipokine signaling, and lipoprotein signaling were highly up-regulated, which likely contributed to the blunted therapeutic response (Hur et al., 2015). In parallel, a recent study using RNA-Seq examined transcriptome differences in sensory neurons between non-diabetic and Hsp70 KO mice and found that inflammatory pathways were strongly increased in diabetes (Ma et al., 2015).
Finally, the data emanating from the past 3 decades of DN pathway studies and the more recent transcriptomic approaches strongly align with the genetic risk variants implicated in DN. ACE, AKR1B1, APOE, NF-kB, NOS3, TLR2 and TLR4, and SREBP-1 are among the candidate genes with specific polymorphisms associated with DN and T2D (for a full review see (Witzel et al., 2015)). The functions of these genes primarily correspond to lipid metabolism and inflammatory pathways, the same pathways identified in the transcriptomic analyses cited above and implicated in T2D (Figures 2 and 3).
In summary, transcriptomic analyses of nerve biopsies from DN patients and analyses of human genetic risk variants for DN support a role for lipid metabolism and inflammation in the pathogenesis of DN. In the future DN may be a disease target for mechanism-based pharmacogenetics approaches, and a more personalized treatment of each patient, depending on their genotype.
Novel Functions of Oligodendrocytes in Axonal Energy Metabolism: Relevant Mechanisms for Schwann Cells and Diabetic Neuropathy?
Recently there has been a move towards understanding the bioenergetics profile of the PNS in context of diabetes with an increasing focus on the interactions between the cellular components of the nerve, namely the axonal compartment and SCs. Newly emerging ideas in the field center on metabolic functions of glia, originally discovered in the CNS, which could be relevant also as disease mechanisms in DN (Zenker et al., 2013). In vertebrates, myelination of axons by glia is essential for rapid saltatory conduction; however, it also poses a paradoxal risk to neuronal integrity. Axons, in contrast to the cell body and dendrites, become >99% covered with a myelin sheath that forms a physical barrier between the extracellular milieu and the underlying axonal compartment, thereby restricting free access to glucose and other metabolites (Nave, 2010). The nodal region, flanked by paranodal and juxtaparanodal domains, comprises <1% of the axon surface. In the nodal region, the expression of voltage-gated Na+ and K+ channels and their respective anchoring proteins is well documented (Rasband and Peles, 2016), but there is surprisingly little information about metabolite transporters in these regions, such as glucose transporters (GLUTs) and monocarboxylate transporters (MCTs), that should play a key role in cellular energy metabolism. New concepts on the role of SCs in axonal integrity are based on observations made in the CNS (Funfschilling et al., 2012; Lee et al., 2012). Here, in addition to their well-known role in saltatory conduction, oligodendrocytes serve as a source of energy-rich metabolites (lactate) for the axonal compartment, thereby supporting axonal ATP production and energy homeostasis (Beirowski, 2013; Saab et al., 2013).
For myelinated axons, glial support is a particularly important function, which depends on a connecting network of nanometer-wide “myelinic channels” filled with glial cytoplasm leading to and surrounding the periaxonal space. The extent of this channel system can be better visualized by dye filling experiments at the light microscopic level or high pressure freezing electron microscopy than with histology following conventional fixation techniques. In PNS fibers, these myelinic channels include the inner and outer lip of non-compacted myelin, the lumen of the paranodal loops, and numerous Schmidt-Lantermann incisures (Figure 1). They provide a direct radial pathway for metabolite diffusion via gap junctions. These junctions are formed by connexin 32 and, interestingly, mutations of this gene underlie an X-linked form of peripheral neuropathy (Charcot-Marie-Tooth disease) (Scherer, 2006), where the clinical phenotype further worsens in the presence of diabetes (Ursino et al., 2013).
Do SCs (like oligodendrocytes) provide glycolysis products to the axons they myelinate or are associated with in Remak bundles? This supportive role of all SCs in axonal energy homeostasis is suggested by the outcome of experiments in which mitochondrial respiration was genetically disrupted, yet mutant SCs remained fully viable as glycolytic cells (Funfschilling et al., 2012; Viader et al., 2013). Presumably the SCs survive by aerobic glycolysis and, in turn, glycolysis products are exported to the axonal compartment that metabolize lactate. Direct loss-of-function evidence for the role of SC metabolism in axon health came with the selective genetic targeting of LKB1 in SCs. LKB1 is a serine-threonine kinase regulating SC energy homeostasis.and loss of this protein causes the degeneration of both myelinated and unmyelinated axons (Beirowski et al., 2014; Tzvetanova and Nave, 2014). Interestingly, compared to myelinated motor axons unmyelinated sensory axons were particularly vulnerable to loss of LKB1 in SCs; this situation is reminiscent of DN and suggests that unmyelinated sensory axons may have increased dependence on metabolic support from SCs. Compromised lactate export in MCT1 heterozygous null mice severely delays the regeneration of crushed peripheral axons (Morrison et al., 2015), independently supporting the role of naturally glycolytic SCs in axonal energy metabolism.
To metabolically support axons, SCs must maintain GLUT1, the glucose transporter of all myelinating glia, on the outer cell surface, as well as MCT1 at the adaxonal membrane (Magnani et al., 1996; Morrison et al., 2015). We speculate that if the level of surface expressed GLUT1 falls below a certain “threshold” (similar to the effects of GLUT4 in metabolically active tissues in T2D), ATP generation by glycolysis alone no longer suffices for SCs to survive and to support axons with excess lactate. In this case, SCs are more likely to metabolize pyruvate in their own mitochondria to generate ATP. Consequently, normal lactate supply by SCs to axons would be reduced, causing a reduction of axonal energy metabolism (Domenech-Estevez et al., 2015). Any prolonged failure of axonal ATP generation and membrane repolarization would ultimately lead to axonal degeneration (Kiryu-Seo et al., 2010). As discussed earlier in this review, the small caliber axons of sensory DRG neurons may be more vulnerable to injury, because conduction of unmyelinated axons is more energy consuming than saltatory impulse propagation along myelinated fibers.
Schwann Cell Glucose Transporter GLUT1: A Role in Insulin Resistance?
GLUT1 is generally thought to function independent of insulin (Bryant et al., 2002; Foran et al., 1999); however, it has recently been shown that GLUT1 translocation to the cell surface of oligodendrocytes and into myelin membranes is regulated by NMDA receptor stimulation, presumably in response to the spiking activity of underlying glutamatergic axons (Saab et al., 2016). Although slower, the translocation itself is reminiscent of GLUT4 trafficking in muscle cells or adipocytes in response to insulin signaling and demonstrates that surface expression of GLUT1 (like GLUT4) can be regulated. It is unlikely that SCs, which lack NMDA receptors, respond to glutamate like oligodendrocytes. It is also unclear whether they translocate GLUT1 in response to insulin signaling, although SCs express the insulin receptor (Rachana et al., 2016; Shettar and Muttagi, 2012). Moreover, SC-specific conditional mouse mutants of insulin receptor and IGF-I receptor are transiently hypomyelinated, with elevated G-ratios in the first postnatal weeks (Kungl, Quintes, Brüning and Nave, unpublished observation), a finding consistent with insulin-dependent glucose import that limits myelin synthesis at the peak of development. However, regardless of the receptor system, it is tempting to speculate that SCs can also become “insulin resistant”, similar to hepatocytes, myotubes and adipocytes, in response to the same (systemic) risk factors that lead to T2D. In this scenario, reduced GLUT1 export in DN would cause the underlying axon to “starve”, despite hyperglycemia, because the distal axonal compartments require lactate (not glucose) to maintain its normal energy balance.
Yet, there are data that suggest that the opposite could be true. If hyperglycemia causes SCs to increase glucose uptake and the rate of glycolysis, a corresponding reduction of OxPhos and elevated release of excess lactate to the axonal compartment might perturb axon function by activating extracellular acidosis activating acid sensing ion channels (Wang and Xu, 2011) and the pain-inducing TRPV1 channel (Julius, 2013). Intracellular acidosis suppresses G-protein coupled receptor signaling (Isom et al., 2013), which plays a key role in SC development (Monk et al., 2015). This idea may also explain why in the STZ rat, satellite glial cells become “activated”, possibly by hyperglycemia and lactate overload alone (Hanani et al., 2014). Clearly, the finding that myelinating glial cells actively contribute to glucose utilization and energy metabolism of axons forces us to reconsider their potential role in the pathogenesis of DN and warrants further study.
Schwann Cell Lipid Metabolism: Potential Relevance to Diabetic Neuropathy?
SCs, which have a highly active lipid metabolism, share many molecular signatures with adipocytes (Verheijen et al., 2003). Similar to adipocytes, SCs transport long chain fatty acids (LCFAs) from the extracellular space into the SC cytoplasm in T2D, and cultured SCs increase the expression of carnitine palmitoyltransferase I (CPT1) in response to LCFA treatment (Hinder et al., 2014). CPT1 transports the LCFA acyl-CoA esters, along with the carnitine shuttle and CPT2, to the mitochondrial matrix to undergo β-oxidation, with repeat cleavage of 2 carbons to form acetyl-CoA with each cycle. Acetyl-CoA can enter the TCA cycle and generate NADH and FADH2 for OxPhos and ATP production, while remaining acyl-CoAs re-enter the β-oxidation cycle and undergo another round of 2-carbon cleavage. However, if there is substrate overload, acyl-CoAs are unable to re-enter the cycle, are converted to acylcarnitines (McCoin et al., 2015), accumulate in the SC, and are shuttled out of the SC into the axon (Viader et al., 2013). Hence, as SCs experience LCFA overload, they could undergo a metabolic reprogramming and increase CPT1 and CPT2 in an attempt to shuttle the increased load of LCFA for β-oxidation and energy. With substrate excess, diabetic SCs may undergo loss of OxPhos and ATP production, similar to what is observed in neurons, leading to an accumulation of acylcarnitines (Figure 3). SC transport of these acylcarnitines is toxic to DRG neurons, and similar pathology could be occurring in DN, explaining nerve injury (Viader et al., 2013). Moreover, increased levels of ceramides, a component of sphingomyelin (which constitutes 20% of SC myelin), are recently reported to be highly hepatotoxic in T2D, while their targeted reduction preserves hepatic function (Chaurasia and Summers, 2015). Older literature also shows that ceramides inhibit axonal growth in vitro (de Chaves et al., 2001). If SCs shift the stoichiometry of their lipid production in DN as we hypothesize, this shift would in turn affect the abundance of selected lipid species and could increase toxic ceramide production.
In summary, accumulating data suggest that SCs are much more than “passive” insulators for axons. We believe SCs are critical sensors of axonal activity and provide the needed energy for axonal function. Thus an emerging idea is that disruption of the normal “bioenergetic crosstalk” between SCs and axons during T2D underlies DN (Figure 3). It is possible this reprogramming leads to SC “insulin resistance” and axonal starving, or alternatively substrate overload from the SCs to the myelinated axon resulting in pH changes and/or axonal mitochondrial injury characterized by mitophagy (with different substrate overloads causing specific injury for myelinated versus unmyelinated axons), and/or the transfer of toxic lipid species from affected SCs to the axonal compartment leading to mitochondrial injury.
Why Are Axons More Vulnerable to Injury in Diabetes?
We have speculated that axons are dependent on SCs as a source of energy and that during diabetes, SCs not only lose their ability to provide energy to myelinated and unmyelinated axons but also transfer toxic lipid species to the axons they contact. What remains an important piece of the puzzle in deciphering axonal vulnerability in diabetes is an understanding of the role of the mitochondria and a recognition that mitochondrial integrity, motility, and localization along the axon are all adversely affected by diabetes (reviewed in (Fernyhough, 2015; Fernyhough and McGavock, 2014)). For example, acylcarnitines produced by SCs trigger influx of extracellular Ca2+ into the axon (Viader et al., 2013) that impairs axonal mitochondrial trafficking, resulting in insufficient axonal energy production, and mitochondrial apoptosis in experimental diabetes (Fernyhough, 2015). In parallel, rodent models of DN display increased mitochondrial ROS in axons, facilitating a feedforward mechanism of axonal mitochondrial dysfunction and axonal injury (Fernyhough, 2015). In cultured sensory DRG neurons, blocking mitochondrial complex 1 reduces ATP and increases ROS production within axons; these changes ultimately lead to axonal degeneration, despite preservation of sensory cell bodies (Persson et al., 2013), further emphasizing the vulnerability of the axon to ROS and energy depletion. Impaired trafficking mechanisms in DN also impede the crucial mitophagy clearance pathways that shuttle damaged mitochondria back to the soma via retrograde transport (Cashman and Hoke, 2015). Collectively, these insults to mitochondria in experimental diabetes result in loss of axonal energy stores and axonal injury, promoting DN (Sajic, 2014).
Another reason that axons are highly vulnerable to diabetes-mediated injury lies in their abundant expression of ion channels. Axons are known to express a number of distinct voltage gated sodium channels (VGSCs) as well as the Na+Ca2+ exchanger isoform 2 (NCX2) in their terminals (Persson et al., 2010). Na-K ATPase is required to export intra-axonal Na+ that accumulates following action potential propagation, however this function fails when ATP levels are below normal. This in turn leads to increased intra-axonal Na+, reversal of the NCX2, increased intracellular Ca2+ and axonal degeneration. Blockade of VGSCs using tetrodotoxin (TTX) or prevention of Ca2+ accumulation by restoring NCX2 function prevents axonal degeneration by restoring normal bioenergetics (Persson et al., 2013). These findings emphasize the vulnerability of axons to energetic stress as seen in diabetes and highlight potential therapeutic interventions such as regulating VGSC activity.
Why are sensory axons more vulnerable to diabetes-mediated injury? In part, because sensory axons express distinct VGSCs (Nav1.6, 1.7, 1.8 and 1.9) compared to motor axons (Nav1.6) and these VGSCs have different biophysical characteristics (Dib-Hajj et al., 2010). For example, Nav1.7 and 1.9 in sensory neurons demonstrate a persistent Na+ current near the resting membrane potential. These same neurons have substantially smaller diameter axons when compared to motor axons, resulting in a higher surface area to volume ratio, that can in turn accentuate changes in intracellular Na+ and Ca2+ rendering sensory axons more susceptible to injury. Differences in conduction velocity properties and energy requirements, as discussed earlier, also predispose unmyelinated or thinly myelinated sensory neurons to be more vulnerable to higher energy demands, that cannot be met in the presence of diabetes.
In summary, axonal vulnerability in diabetes leading to DN is secondary to a combination of factors centered on energy production and ion channel expression. With an eye to the future, more research is needed to expand our understanding of the mechanisms underlying axonal vulnerability to bioenergetics stressors, as seen in diabetes, and the early selective injury of sensory over motor axons in DN, discussed earlier in this review. VGSCs may also provide new therapeutic targets, and this idea is discussed below. It is clear that an increased understanding of this emerging field could yield effective mechanism based treatments of DN.
Painful Diabetic Neuropathy
In parallel with the emerging insights into how specific genes, pathways, and SC:axonal bioenergetics contribute to nerve injury in DN, efforts focused on determining the pathophysiological processes underlying pain have also enhanced our understanding of DN pathogenesis. Neuropathic pain is a common and disabling consequence of DN, affecting between 25 and 50% of patients (Abbott et al., 2011). Neuropathic pain is defined as “pain caused by a lesion or disease affecting the somatosensory system” (Finnerup et al., 2016). An important distinguishing feature in neuropathic pain versus other types of pain is the paradoxical combination of sensory loss and pain with or without sensory hypersensitivity phenomena to one or several sensory modalities in the painful area. This pattern is in contrast to the two other major forms of chronic pain – inflammatory and idiopathic types of pain – where the somatosensory nervous system is intact and where the sensory abnormalities are limited to symptoms and signs of hypersensitivity (Jensen and Finnerup, 2014).
The symptoms and signs in DN reflect sensory loss, with reduced sensitivity to touch, pinprick, and hot and cold temperatures, and pain hypersensitivity with hyperalgesia or allodynia as well as on-going (i.e. stimulus independent) pain. The distribution of symptoms and signs depends on the nerves affected in DN; in the most common form of DN, distal symmetric polyneuropathy, pain and sensory findings are located in toes, feet, and calves and occasionally also in fingers and hands, resulting in the characteristic stocking-glove distribution. Pain has different characteristics, but with burning, stinging, and shooting and deep aching types being the most typical ones within the territory of damaged nerve fibers. It remains unexplained why some patients develop neuropathic pain and others remain pain free, although this is likely to relate to complex interactions between the nature of the inciting lesion interacting with genotype and the environment (von Hehn et al., 2012). Given the significant heritability of pain traits demonstrated by twin studies, it is likely that genetics has an important role (Norbury et al., 2007). Although there are several large genome wide association studies investigating the relationship between genotype and risk of developing diabetes (Horikoshi et al., 2016) the study of neuropathic pain is in it’s infancy; some genotype associations are reported but these are yet to be replicated (Meng et al., 2015). While neuronal hyperexcitability is a key feature in several neuropathic pain conditions (Jensen and Finnerup, 2014), the clinical manifestations of such hyperexitability are not always present in painful DN. Detailed studies combining interviews of patients with clinical examinations in T2D demonstrate a phenotypic diversity in patients with and without pain. Results show that increased sensitivity to mechanical and thermal stimuli are the best discriminating features between DN patients with and without neuropathic pain (Scholz et al., 2016). It is possible that the dominance of sensory loss versus the presence of hyperalgesia and allodynia (Maier et al., 2010; Themistocleous et al., 2016) may explain the failure to find clear distinguishing features in painful versus painless DN.
Psychological factors are also likely to be critically important. Although the initial insult of DN may be peripheral, brain networks sub-serving pain and its modulation differ between individuals, can be shaped by experience, and are likely to have a key role in the expression of pain. There may be some relatively simple explanations for the development of neuropathic pain that relate to the degree of the inciting lesion to the somatosensory nervous system. For example, there is a positive correlation between both neuropathy severity and poor glycemic control with both the risk and intensity of neuropathic pain (Themistocleous et al., 2016); however, this is by no means a linear relationship, and there are patients with severe neuropathy who never develop pain. In relation to genetic predisposition, data are now emerging (discussed below) that certain ion channel variants may predispose to the development of painful DN.
In summary, in the following sections we will discuss potential pathophysiological mechanisms underlying neuropathic pain, both within the PNS and CNS. A complete understanding of the relative importance of such processes in patients would need prospective natural history studies combined with distinct outcome measures to assess pathophysiological drivers in individual patients; unfortunately, these studies are yet to be done. Finally, previous studies in painful DN have generally not distinguished between T1D and T2D, and given the fact that the pathophysiological mechanisms may differ between these conditions, future studies should distinguish between these two patient classes.
Peripheral Mechanisms of Painful Diabetic Neuropathy
Sensory neuron hyper-excitability in the forms of spontaneous activity and altered stimulus-response function is reported both in patients (Orstavik et al., 2006) and in experimental models of DN (Suzuki et al., 2002). Sensory neuron excitability is governed by the pattern of expression, trafficking, and function of ligand and voltage gated ion channels (Bennett and Woods, 2014). These ion channels are critical for the initial transduction of sensory stimuli, action potential generation and propagation, and finally neurotransmitter release within the dorsal horn of the spinal cord. Given the selective expression of many of these ion channels by sensory neurons, they are important targets for analgesic development. Figure 4 illustrates potential mechanisms whereby ion channel dysfunction could lead to neuropathic pain in DN. Painful DN shares common pathophysiological mechanisms with other types of neuropathic pain, for instance dysregulated ion channel expression (Craner et al., 2002). An example are the VGSCs which are important drivers of excitability of DRG neurons that mediate noxious stimuli, termed nociceptors. Nav1.7, 1.8, and 1.9 are the most abundant α subunits expressed in nociceptors, all of which have been implicated in human pain states (Waxman et al., 2014). Nav1.8 and 1.9 are relatively resistant to TTX and TTX Na+ currents are a known pharmacological feature of nociceptors. Patch clamp recordings of small diameter DRG neurons show remarkable heterogeneity in the characteristics of Na+ currents between neurons indicating that not only different Nav sub-unit expression but also dynamic post-translational modifications of these subunits are likely to be important in tuning these neurons (Rizzo et al., 1994). Nav1.8 makes a major contribution to Na+ currents during the generation of action potentials in nociceptors, and furthermore, pain related behavior is reduced in animals lacking this ion channel (Blair and Bean, 2002). The expression of Nav1.8 increases in painful DN, and recent findings in experimental DN suggest that the increase in the TTX current mediated by Nav1.8 results in reduced conduction failure in C-fiber nociceptors, enhancing impulse conduction to the CNS and contributing to neuropathic pain (Sun et al., 2012).
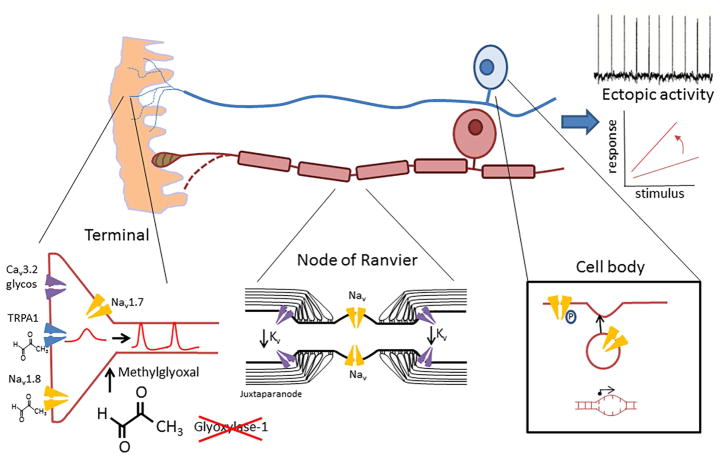
Figure 4. Peripheral mechanisms contributing to neuropathic pain in diabetic neuropathy.
The axons of both unmyelinated (blue) and myelinated axons (brown) undergo length-dependent degeneration (dotted lines). At the terminals of nociceptors, ion channels undergo post-translational modifications, for instance due to increased levels of the reactive metabolite methylglyoxal and enhanced glycosylation, resulting in gain of function. In myelinated axons, the distribution of voltage gated ion channels at the node of Ranvier changes with at the juxtaparanode leading to hyperexcitability. At the reduced expression of shaker type Kv cell body there is increased expression and trafficking of pro-excitatory voltage gated sodium channels such as Nav1.8. Inheriting rare variants of Nav1.7 may also confer risk of developing neuropathic pain. The end result of these changes is reflected in hyperexcitability with the development of enhanced stimulus-response and ectopic activity.
There is emerging evidence that inheriting genetic variants in VGSCs can also modulate an individual’s risk of developing neuropathic pain. For instance, the Val991Leu/Met932Leu variant of Nav1.7, which can cause DRG neuron hyperexcitability (Faber et al., 2012), is associated with increased risk and severity of painful DN (Li et al., 2015). Nav1.7 can amplify external stimuli and depolarize membrane potentials closer to the threshold for Nav1.8 activation so that, for example, Nav1.7 gain-of-function mutations may give rise to a diabetes-induced increased sensitivity of DRG neurons (Hoeijmakers et al., 2014). Such gain of function mutations may not only be related to pain severity but could also act as drivers of axonal degeneration through energetic stress. The G856D mutation of Nav1.7, associated with small fiber neuropathy, results in reduced ATP and increased ROS within sensory axons. This mutation is also associated with increased levels of intracellular Na+ and Ca2+ and increased axonal degeneration after axonal depolarization that is prevented by blocking the reverse mode of NCX (Estacion et al., 2015; Rolyan et al., 2016). Whether these phenomena can be amplified by the energetic stress imposed by diabetes has not yet been studied, but it is plausible that individuals carrying such mutations may be particularly susceptible to painful DN.
There are, in addition, specific post-translational modifications of ion channels as a consequence of diabetes. Methylglyoxal is an example of a highly reactive metabolite that increases in diabetes and produces rapid non-enzymatic glycation of proteins. Glyoxylase-1, the enzyme that metabolizes methylglyoxal, has low expression in the PNS and this enzyme activity is further reduced by diabetes. The concentration of methylglyoxal has been shown in one study (Bierhaus et al., 2012) but not in another (Hansen et al., 2015) to be higher in patients with painful versus painless DN. Administration of methylglyoxal to rodents can produce pain-related hypersensitivity (Bierhaus et al., 2012). This effect is partly mediated by post-translational modification of the VGSC Nav1.8. Methylglyoxal modifies the amino acid arginine within the inactivation domain of Nav1.8, resulting in a depolarizing shift in the voltage dependence of fast inactivation. Reduced inactivation of Nav1.8 results in hyperexcitability of nociceptors. A further interesting feature of the effects of methylglyoxal is that large diameter low threshold mechanoreceptors, that do not express the TTX channels Nav1.8 and 1.9, demonstrate slowed conduction following treatment with this agent. This appears to be due to methylglyoxal modifying TTXs channels (such as Nav 1.6 and 1.7), resulting in enhanced inactivation leading to loss of function. This emphasizes the molecular and functional diversity of sensory neurons, such that the same metabolite has opposite functional effects on sensory neurons mediating distinct VGSC channels and sensory modalities.
Another ion channel of interest is transient receptor potential cation channel, subfamily A, member 1 (TRPA1), a ligand-gated ion channel which is implicated in the pathogenesis of painful DN due to its ability to respond to environmental irritants. This is a non-selective cation channel expressed in a sub-set of nociceptors that is activated by a variety of exogenous reactive compounds (such as mustard oil and cinnamaldehyde), noxious cold, as well as a number of endogenous ligands (Andersson et al., 2008). Methylglyoxal, which like many agonists of TRPA1 is an electrophile, can modify critical intracellular cysteine residues in TRPA1, resulting in strong activation of this ion channel and in turn hyperexcitability of nociceptive afferents (Andersson et al., 2013). Diabetes is associated with a high level of ROS, resulting in the production of a number of compounds that can activate TRPA1, including H2O2 and 4- hydroxynonenaL (HNE) (Andersson et al., 2008), and there is some evidence that the effect of these agents can potentiate activation by methylglyoxal. A TRPA1 antagonist reduced pain-related hypersensitivity following in STZ rats with DN (Wei et al., 2009), although an important caveat is that STZ itself can directly activate TRPA1 and evoke sensory axonal injury (Andersson et al., 2015). The experimental validation of TRPA1 antagonists in painful DN, therefore, awaits replication in one or more of the newer animal models, discussed earlier in this review (O’Brien et al., 2014b).
T-type Ca2+ channels are also important; they regulate the sub-threshold excitability of nociceptors, and this current increases in nociceptors during experimental diabetes. Down-regulation of the Cav3.2 T type channel can ameliorate pain-related hypersensitivity in the STZ rat (Messinger et al., 2009). The enhanced activity of this channel is related to glycosylation of extracellular arginine residues, leading to altered kinetics, increased current density, and surface expression, ultimately producing DRG neuron hyperexcitability (Orestes et al., 2013).
Of note, the nodal region of myelinated peripheral nerves is a region of high metabolic demand, and so may be particular susceptible to the molecular changes associated with diabetes and potentially the generation of pain. These nodal regions, including the nodes of Ranvier, the paranode, and juxtaparanodal region, have a high number of ion channels and glucose transporters that can contribute to neuronal hyperexcitability and act as ectopic impulse generators, as discussed earlier in this review (Salzer et al., 2008; Zenker et al., 2012). Indeed, certain K+ channels (that act as excitability breaks) are reduced in animals with T1D and T2D including the expression of shaker type K+ channels within the juxtaparanodes (Zenker et al., 2012).
In summary, ion channel dysfunction is central to the pathogenesis of painful DN. Using newer animal models for experimental work along with visualization of these channels in carefully phenotyped and genotyped patients will help determine to what extent the altered expression/trafficking of these ion channels contributes to pain in clinical DN. These changes in ion channel function occur in the context of bombardment of the dorsal horn of the spinal cord with increased neuronal activity from sensitized nociceptors and from ectopic foci in degenerating and regenerating nerve fibers. These diverse stimuli generate and increase synaptic transmission within the dorsal horn of the spinal cord, known as central sensitization, the topic of the next portion of this review.
Central Mechanisms in Painful Diabetic Neuropathy
The cellular consequence of the central amplification of peripheral neuronal activity is generally manifested by an exaggerated response to synaptic inputs, a reduction in threshold to activate neurons, an increased response to suprathreshold stimuli, and an expansion of receptive fields (Jensen and Finnerup, 2014). Accumulating data suggest that these central mechanisms can contribute to painful DN. In STZ rats, wide dynamic range dorsal horn neurons show altered Rac-1 mediated dendritic spine morphology that is associated with spontaneous activity and hyperexcitability to peripheral stimuli. Reversal of these changes using a Rac1 inhibitor prevents hypersensitivity, both at the electrophysiological and behavioral level (Tan et al., 2012). The dorsal horn of the spinal cord is under control by a descending pain modulatory system that can either inhibit or facilitate transmission of nociceptive information (West et al., 2015). At a clinical level, conditioned pain modulation (in which a painful conditioning stimulus applied to one body site reduces pain in response to a stimulus applied to a different body site) is a technique that is used to test these descending controls; importantly, conditioned pain modulation is impaired in some patients with DN (Yarnitsky et al., 2012).
Aberrant signalling between neurons and glia also has a role in pain in the context of maladaptive plasticity. Following traumatic nerve injury, microglia within the dorsal horn of the spinal cord release factors such as brain-derived neurotrophic factor (BDNF) that result in the amplification of nociceptive synaptic processing, and thus ‘gating’ of neuropathic pain (Beggs et al., 2012). While DN is associated with a slower rate of de-afferentation compared to traumatic neuropathy, there is also evidence for a role of microglia in the development of neuropathic pain in DN (Tsuda et al., 2008). An important consideration is that recent studies report that microglia are required for the development of mechanical pain hypersensitivity following traumatic nerve injury in male but not in female rodents (Sorge et al., 2015). Such gender-specific effects have not been studied in experimental models of painful DN, and emphasize the importance of gender when investigating pathophysiological pain mechanisms. More recently oligodendrocytes are also implicated to play a role in central pain mechanisms (Gritsch et al., 2014). Loss of oligodendrocytes in the spinal cord leads to both an exaggerated pain response and axon loss in the spinal dorsal horn with damage to the spinothalamic tract. At present, it is unknown whether damage to oligodendrocytes in the CNS also contributes to pain in DN. Nevertheless, the studies by Gritsch and colleagues suggest that separate CNS mechanisms independent of a peripheral driven central sensitization may be involved in pain following glia damage
In addition to axonal damage in the dorsal horn and spinothalamic tract, plasticity is also seen at higher brain centers. In STZ rats with confirmed behavioral hypersensitivity, electrophysiological recordings within the ventro-postero-lateral nucleus of the thalamus demonstrate neuronal hyperexcitability in the form of spontaneous activity as well as increased evoked activity to brush, pinch, and pressure stimuli (Fischer et al., 2009). MR spectroscopy reveals a reduction of N-acetyl-aspartate (NAA), which is a marker for neuronal and axonal activity, in the dorsolateral prefrontal cortex in patients with DN with and without pain, and a reduction of NAA in the thalamus only in those diabetic patients that also had neuropathic pain (Sorensen et al., 2008). Using functional MRI, a reduced functional state connectivity between the thalamus and cortex is observed in patients with painful DN compared to healthy individuals (Cauda et al., 2009). In a morphometric MRI study of a group of T1D DN patients with and without pain compared to a group of healthy volunteers and non-neuropathic diabetics, Selvarajah and colleagues (Selvarajah et al., 2014) found reduced gray matter in primary somatosensory cortex and supramarginal gyrus only in the neuropathy groups. In addition, a greater thalamic gray matter loss occurred in those patients with DN pain compared to DN patients without pain. This reduction in thalamic gray matter in painful DN may correspond to the observed reduced flow and metabolism seen with positron emission tomography in other peripheral and central neuropathic pain states (Iadarola et al., 1995). While the exact mechanism for these central changes in painful DN are unclear, the findings do suggest that the co-existence of pain may give rise to a set of maladaptive neuroplastic changes that involves the thalamus and other parts of the central projections of the somatosensory system.
New Approaches to Treatment of Neuropathic Pain
While there has been a rapid and major expansion in our knowledge of potential pathophysiological mechanisms underlying neuropathic pain in DN which span the whole somatosensory nervous system, from primary afferent terminals to the sensory cortex, this has yet to translate into a clear mechanism based approach in treating painful DN. In a recent study this was attempted, by stratifying patients with neuropathic pain into those with and without irritable nociceptors, and treating both groups with a nonspecific Na+ channel blocker. In patients with irritable nociceptors, treatment provided more pain control than in those patients with non-irritable nociceptors (Demant et al., 2014). Another approach is to target ion channels more selectively expressed in nociceptors such as VGSCs (Nav 1.7, 1.8 and 1.9) and TRPA1 in order to avoid CNS side effects, a common problem with current agents used to treat neuropathic pain (Finnerup et al., 2015). The increased knowledge about nociceptor function has facilitated drug discovery efforts, particularly in developing more selective blockers of VGSCs such as Nav1.7 (which has a crucial role in nociceptor function) (Emery et al., 2016). Both small molecule (McCormack et al., 2013) and biologic therapeutic approaches (Lee et al., 2014) are being used to gain sub-type selectivity, and clinical trials of selective Nav1.7 blockers have begun in neuropathic pain conditions such as trigeminal neuralgia (Zakrzewska et al., 2013). Trials in DN are now starting and new Nav1.8 blockers are under development for possible use in painful DN (Han et al., 2016; Payne et al., 2015). A particularly exciting prospect of VGSC blockers in painful DN would be if such therapy could be disease modifying given the potential role for these ion channels in axonal degeneration in circumstances of diabetes-mediated energetic stress.
In summary, given the multiplicity of mechanisms, which may overlap, a major future goal is to better stratify patients in order to individualize treatment to block key pathophysiological driver(s) in each patient. An increased understanding of ion channels, their biology and distinct expression along with mechanism based research in new experimental murine models, will yield new treatments for painful DN and potentially also provide disease modifying improvements. There is now tentative evidence that such stratification will yield clinical benefits (Yarnitsky et al., 2012). Ongoing advances in sensory phenotyping, genotyping, electrophysiology, and imaging outcomes, which can be applied to patients, should aid in this translation of current and future preclinical advances to improved patient outcomes in DN.
Conclusions
In summary, DN research has gone through an evolution from studies aimed at specific dysregulated pathways, such as aldose reductase, protein kinase C, and AGEs, to a new era of DN research focused on understanding global whole nerve metabolism, insulin sensitivity and resistance, nutrient overload, and the axoglial sharing of energy in diabetes. The vulnerability of the axon to diabetes-mediated injury is a recent area of interest among DN researchers, with an increased understanding of the pivotal role of axonal mitochondria in normal axonal function and disease states. How specific ion channels determine not only pain states but may also be modifying disease in DN has fostered an emerging series of therapeutic interventions targeting specific ion channels in the treatment of painful DN. In parallel, advances in understanding the roles of both the CNS and PNS in the pathogenesis of pain in DN are occurring at a rapid pace, implying new cross-talk among the different components of the nervous system. While there is still much to discover, the recent advances in DN research are bringing new ideas to the field, which will likely result in much needed mechanism based treatments.
Acknowledgments
The authors would like to thank Dr. Stacey Sakowski Jacoby for expert editorial assistance with the preparation of this manuscript. ELF is supported by the National Institutes of Health (1DP3DK094292, 1R24082841), the Novo Nordisk Foundation (NNF14OC0011633), and the Program for Neurology Research & Discovery. DLHB is a Welcome senior clinical scientist (ref. no. 095698z/11/z) and is supported by the European Union’s Horizon 2020 research and innovation program under grant agreement No 633491 to the ‘DOLORisk’ consortium. KAN is supported by the DFG (Research Center Molecular Physiology of the Brain), DFG SPP-1757 and an ERC Advanced Investigators Grant. TSJ is supported by Novo Nordisk Foundation (NNF14OC0011633).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care. 2011;34:2220–2224. doi: 10.2337/dc11-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul-Rahman O, Sasvari-Szekely M, Ver A, Rosta K, Szasz BK, Kereszturi E, Keszler G. Altered gene expression profiles in the hippocampus and prefrontal cortex of type 2 diabetic rats. BMC Genomics. 2012;13:81. doi: 10.1186/1471-2164-13-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DA, Filipovic MR, Gentry C, Eberhardt M, Vastani N, Leffler A, Reeh P, Bevan S. Streptozotocin stimulates the ion channel TRPA1 directly: Involvement of peroxynitrite. The Journal of Biological Chemistry. 2015;290:15185–15196. doi: 10.1074/jbc.M115.644476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DA, Gentry C, Light E, Vastani N, Vallortigara J, Bierhaus A, Fleming T, Bevan S. Methylglyoxal evokes pain by stimulating TRPA1. PloS One. 2013;8:e77986. doi: 10.1371/journal.pone.0077986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. The Journal of Neuroscience. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auche B. Des alteration des nerfs peripheriques chez les diabetiques. Arch de Med Exper et d’anet Path. 1890;2:635. [Google Scholar]
- Azad N, Emanuele NV, Abraira C, Henderson WG, Colwell J, Levin SR, Nuttall FQ, Comstock JP, Sawin CT, Silbert C, Rubino FA. The effects of intensive glycemic control on neuropathy in the VA cooperative study on type II diabetes mellitus (VA CSDM) Journal of Diabetes and Its Complications. 1999;13:307–313. doi: 10.1016/s1056-8727(99)00062-8. [DOI] [PubMed] [Google Scholar]
- Bansal D, Badhan Y, Gudala K, Schifano F. Ruboxistaurin for the treatment of diabetic peripheral neuropathy: a systematic review of randomized clinical trials. Diabetes & Metabolism Journal. 2013;37:375–384. doi: 10.4093/dmj.2013.37.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs S, Trang T, Salter MW. P2X4R+ microglia drive neuropathic pain. Nature Neuroscience. 2012;15:1068–1073. doi: 10.1038/nn.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirowski B. Concepts for regulation of axon integrity by enwrapping glia. Frontiers in Cellular Neuroscience. 2013;7:256. doi: 10.3389/fncel.2013.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirowski B, Babetto E, Golden JP, Chen YJ, Yang K, Gross RW, Patti GJ, Milbrandt J. Metabolic regulator LKB1 is crucial for Schwann cell-mediated axon maintenance. Nature Neuroscience. 2014;17:1351–1361. doi: 10.1038/nn.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DL, Woods CG. Painful and painless channelopathies. The Lancet Neurology. 2014;13:587–599. doi: 10.1016/S1474-4422(14)70024-9. [DOI] [PubMed] [Google Scholar]
- Berger JR, Choi D, Kaminski HJ, Gordon MF, Hurko O, D’Cruz O, Pleasure SJ, Feldman EL. Importance and hurdles to drug discovery for neurological disease. Annals of Neurology. 2013;74:441–446. doi: 10.1002/ana.23997. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Fleming T, Stoyanov S, Leffler A, Babes A, Neacsu C, Sauer SK, Eberhardt M, Schnolzer M, Lasitschka F, et al. Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nature Medicine. 2012;18:926–933. doi: 10.1038/nm.2750. [DOI] [PubMed] [Google Scholar]
- Blair NT, Bean BP. Roles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons. The Journal of Neuroscience. 2002;22:10277–10290. doi: 10.1523/JNEUROSCI.22-23-10277.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussee V, Cunningham FA, Zochodne DW. Direct insulin signaling of neurons reverses diabetic neuropathy. Diabetes. 2004;53:1824–1830. doi: 10.2337/diabetes.53.7.1824. [DOI] [PubMed] [Google Scholar]
- Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nature Reviews Molecular Cell Biology. 2002;3:267–277. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]
- Callaghan B, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurology. 2012a;11:521–534. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B, Little A, Feldman EL, Hughes RAC. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database of Systematic Reviews. 2012b;6:CD007543. doi: 10.1002/14651858.CD007543.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BC, Feldman EL. Painful diabetic neuropathy: many similarly effective therapies with widely dissimilar costs. Annals of Internal Medicine. 2014;161:674–675. doi: 10.7326/M14-2157. [DOI] [PubMed] [Google Scholar]
- Callaghan BC, Hur J, Feldman EL. Diabetic neuropathy: one disease or two? Current Opinion in Neurology. 2012c;25:536–541. doi: 10.1097/WCO.0b013e328357a797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron NE, Cotter MA. Potential therapeutic approaches to the treatment or prevention of diabetic neuropathy: evidence from experimental studies. Diabetic Medicine. 1993;10:593–605. doi: 10.1111/j.1464-5491.1993.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Cameron NE, Cotter MA. Effects of antioxidants on nerve and vascular dysfunction in experimental diabetes. Diabetes Research and Clinical Practice. 1999;45:137–146. doi: 10.1016/s0168-8227(99)00043-1. [DOI] [PubMed] [Google Scholar]
- Cauda F, Sacco K, Duca S, Cocito D, D’Agata F, Geminiani GC, Canavero S. Altered resting state in diabetic neuropathic pain. PloS One. 2009;4:e4542. doi: 10.1371/journal.pone.0004542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalk C, Benstead TJ, Moore F. Aldose reductase inhibitors for the treatment of diabetic polyneuropathy. Cochrane Database of Systematic Reviews. 2007:CD004572. doi: 10.1002/14651858.CD004572.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia B, Summers SA. Ceramides - Lipotoxic inducers of metabolic disorders. Trends in Endocrinology and Metabolism. 2015;26:538–550. doi: 10.1016/j.tem.2017.09.005. [DOI] [PubMed] [Google Scholar]
- Chen L, Li B, Chen B, Shao Y, Luo Q, Shi X, Chen Y. Thymoquinone alleviates the experimental diabetic peripheral neuropathy by modulation of inflammation. Science Reports. 2016;6:31656. doi: 10.1038/srep31656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby AO. Neurologic disorders of diabetes mellitus. I. Diabetes. 1965;14:424–429. doi: 10.2337/diab.14.7.424. [DOI] [PubMed] [Google Scholar]
- Craner MJ, Klein JP, Renganathan M, Black JA, Waxman SG. Changes of sodium channel expression in experimental painful diabetic neuropathy. Annals of Neurology. 2002;52:786–792. doi: 10.1002/ana.10364. [DOI] [PubMed] [Google Scholar]
- Dahl-Jorgensen K, Brinchmann-Hansen O, Hanssen KF, Ganes T, Kierulf P, Smeland E, Sandvik L, Aagenaes O. Effect of near normoglycaemia for two years on progression of early diabetic retinopathy, nephropathy, and neuropathy: the Oslo study. Brittish Medical Journal (Clinical Research Ed) 1986;293:1195–1199. doi: 10.1136/bmj.293.6556.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial research group. The New England Journal of Medicine. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- de Chaves EP, Bussiere M, MacInnis B, Vance DE, Campenot RB, Vance JE. Ceramide inhibits axonal growth and nerve growth factor uptake without compromising the viability of sympathetic neurons. The Journal of Biological Chemistry. 2001;276:36207–36214. doi: 10.1074/jbc.M104282200. [DOI] [PubMed] [Google Scholar]
- Demant DT, Lund K, Vollert J, Maier C, Segerdahl M, Finnerup NB, Jensen TS, Sindrup SH. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. Pain. 2014;155:2263–2273. doi: 10.1016/j.pain.2014.08.014. [DOI] [PubMed] [Google Scholar]
- Domenech-Estevez E, Baloui H, Repond C, Rosafio K, Medard JJ, Tricaud N, Pellerin L, Chrast R. Distribution of monocarboxylate transporters in the peripheral nervous system suggests putative roles in lactate shuttling and myelination. Journal of Neuroscience. 2015;35:4151–4156. doi: 10.1523/JNEUROSCI.3534-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proceedings of the National Academy of Sciences. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, et al. Glucose control and vascular complications in veterans with type 2 diabetes. The New England Journal of Medicine. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- Edwards JL, Quattrini A, Lentz SI, Figueroa-Romero C, Cerri F, Backus C, Hong Y, Feldman EL. Diabetes regulates mitochondrial biogenesis and fission in mouse neurons. Diabetologia. 2010;53:160–169. doi: 10.1007/s00125-009-1553-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichberg J. Protein kinase C changes in diabetes: is the concept relevant to neuropathy? International Review of Neurobiology. 2002;50:61–82. doi: 10.1016/s0074-7742(02)50073-8. [DOI] [PubMed] [Google Scholar]
- Emery EC, Luiz AP, Wood JN. Nav1.7 and other voltage-gated sodium channels as drug targets for pain relief. Expert Opinion on Therapeutic Targets. 2016;20:975–983. doi: 10.1517/14728222.2016.1162295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estacion M, Vohra BP, Liu S, Hoeijmakers J, Faber CG, Merkies IS, Lauria G, Black JA, Waxman SG. Ca2+ toxicity due to reverse Na+/Ca2+ exchange contributes to degeneration of neurites of DRG neurons induced by a neuropathy-associated Nav1.7 mutation. Journal of Neurophysiology. 2015;114:1554–1564. doi: 10.1152/jn.00195.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber CG, Hoeijmakers JG, Ahn HS, Cheng X, Han C, Choi JS, Estacion M, Lauria G, Vanhoutte EK, Gerrits MM, et al. Gain of function Nanu1.7 mutations in idiopathic small fiber neuropathy. Annals of Neurology. 2012;71:26–39. doi: 10.1002/ana.22485. [DOI] [PubMed] [Google Scholar]
- Feldman EL, Hughes RA, Willison HJ. Progress in inflammatory neuropathy -the legacy of Dr Jack Griffin. Nature Reviews Neurology. 2015;11:646–650. doi: 10.1038/nrneurol.2015.192. [DOI] [PubMed] [Google Scholar]
- Fernyhough P. Mitochondrial dysfunction in diabetic neuropathy: a series of unfortunate metabolic events. Current Diabetes Reports. 2015;15:89. doi: 10.1007/s11892-015-0671-9. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpaa M, Hansson P, Jensen TS, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurology. 2015;14:162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DL, Bouhassira D, Cruccu G, Freeman R, Hansson P, Nurmikko T, et al. Neuropathic pain: An updated grading system for research and clinical practice. Pain. 2016;157:1599–1606. doi: 10.1097/j.pain.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer TZ, Tan AM, Waxman SG. Thalamic neuron hyperexcitability and enlarged receptive fields in the STZ model of diabetic pain. Brain Research. 2009;1268:154–161. doi: 10.1016/j.brainres.2009.02.063. [DOI] [PubMed] [Google Scholar]
- Foran PG, Fletcher LM, Oatey PB, Mohammed N, Dolly JO, Tavare JM. Protein kinase B stimulates the translocation of GLUT4 but not GLUT1 or transferrin receptors in 3T3-L1 adipocytes by a pathway involving SNAP-23, synaptobrevin-2, and/or cellubrevin. The Journal of Biological Chemistry. 1999;274:28087–28095. doi: 10.1074/jbc.274.40.28087. [DOI] [PubMed] [Google Scholar]
- Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. The New England Journal of Medicine. 2003;348:383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circulation Research. 2010;106:1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AQ, Krishnan S, Finucane FM, Rayman G. Altered C-fiber function as an indicator of early peripheral neuropathy in individuals with impaired glucose tolerance. Diabetes Care. 2010;33:174–176. doi: 10.2337/dc09-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg EW, Sorlie P, Paulose-Ram R, Gu Q, Eberhardt MS, Wolz M, Burt V, Curtin L, Engelgau M, Geiss L, et al. Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999–2000 national health and nutrition examination survey. Diabetes Care. 2004;27:1591–1597. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- Grewal AS, Bhardwaj S, Pandita D, Lather V, Sekhon BS. Updates on Aldose Reductase Inhibitors for Management of Diabetic Complications and Non-diabetic Diseases. Mini Reviews in Medicinal Chemistry. 2015;16:120–162. doi: 10.2174/1389557515666150909143737. [DOI] [PubMed] [Google Scholar]
- Gritsch S, Lu J, Thilemann S, Wortge S, Mobius W, Bruttger J, Karram K, Ruhwedel T, Blanfeld M, Vardeh D, et al. Oligodendrocyte ablation triggers central pain independently of innate or adaptive immune responses in mice. Nature Communications. 2014;5:5472. doi: 10.1038/ncomms6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote CW, Groover AL, Ryals JM, Geiger PC, Feldman EL, Wright DE. Peripheral nervous system insulin resistance in ob/ob mice. Acta Neuropathologica Communications. 2013;1:15. doi: 10.1186/2051-5960-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote CW, Wright DE. A role for insulin in diabetic neuropathy. Frontiers in Neuroscience. 2016;10:581. doi: 10.3389/fnins.2016.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Huang J, Waxman SG. Sodium channel Nav1.8: Emerging links to human disease. Neurology. 2016;86:473–483. doi: 10.1212/WNL.0000000000002333. [DOI] [PubMed] [Google Scholar]
- Hanani M, Blum E, Liu S, Peng L, Liang S. Satellite glial cells in dorsal root ganglia are activated in streptozotocin-treated rodents. Journal of Cellular and Molecular Medicine. 2014;18:2367–2371. doi: 10.1111/jcmm.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CS, Jensen TM, Jensen JS, Nawroth P, Fleming T, Witte DR, Lauritzen T, Sandbaek A, Charles M, Fleischer J, et al. The role of serum methylglyoxal on diabetic peripheral and cardiovascular autonomic neuropathy: the ADDITION Denmark study. Diabetic Medicine. 2015;32:778–785. doi: 10.1111/dme.12753. [DOI] [PubMed] [Google Scholar]
- Hinder LM, Figueroa-Romero C, Pacut C, Hong Y, Vivekanandan-Giri A, Pennathur S, Feldman EL. Long-chain acyl coenzyme A synthetase 1 overexpression in primary cultured Schwann cells prevents long chain fatty acid-induced oxidative stress and mitochondrial dysfunction. Antioxidants & Redox Signaling. 2014;21:588–600. doi: 10.1089/ars.2013.5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho EC, Lam KS, Chen YS, Yip JC, Arvindakshan M, Yamagishi S, Yagihashi S, Oates PJ, Ellery CA, Chung SS, Chung SK. Aldose reductase-deficient mice are protected from delayed motor nerve conduction velocity, increased c-Jun NH2-terminal kinase activation, depletion of reduced glutathione, increased superoxide accumulation, and DNA damage. Diabetes. 2006;55:1946–1953. doi: 10.2337/db05-1497. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers JG, Faber CG, Merkies IS, Waxman SG. Channelopathies, painful neuropathy, and diabetes: which way does the causal arrow point? Trends in Molecular Medicine. 2014;20:544–550. doi: 10.1016/j.molmed.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Holman RR, Dornan TL, Mayon-White V, Howard-Williams J, Orde-Peckar C, Jenkins L, Steemson J, Rolfe R, Smith B, Barbour D, et al. Prevention of deterioration of renal and sensory-nerve function by more intensive management of insulin-dependent diabetic patients. A two-year randomised prospective study. Lancet. 1983;1:204–208. doi: 10.1016/s0140-6736(83)92586-2. [DOI] [PubMed] [Google Scholar]
- Horikoshi M, Beaumont RN, Day FR, Warrington NM, Kooijman MN, Fernandez-Tajes J, Feenstra B, van Zuydam NR, Gaulton KJ, Grarup N, et al. Genome-wide associations for birth weight and correlations with adult disease. Nature. 2016;538:248–252. doi: 10.1038/nature19806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humpert PM, Papadopoulos G, Schaefer K, Djuric Z, Konrade I, Morcos M, Nawroth PP, Bierhaus A. sRAGE and esRAGE are not associated with peripheral or autonomic neuropathy in type 2 diabetes. Hormone and Metabolic Research. 2007;39:899–902. doi: 10.1055/s-2007-993155. [DOI] [PubMed] [Google Scholar]
- Hur J, Dauch JR, Hinder LM, Hayes JM, Backus C, Pennathur S, Kretzler M, Brosius FC, 3rd, Feldman EL. The metabolic syndrome and microvascular complications in a murine model of type 2 diabetes. Diabetes. 2015;64:2294–2304. doi: 10.2337/db15-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur J, Sullivan K, Schuyler A, Hong Y, Pande M, States D, Jagadish H, Feldman E. Literature-based discovery of diabetes- and ROS-related targets. BMC Medical Genomics. 2010;3:49. doi: 10.1186/1755-8794-3-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur J, Sullivan KA, Callaghan BC, Pop-Busui R, Feldman EL. Identification of factors associated with sural nerve regeneration and degeneration in diabetic neuropathy. Diabetes Care. 2013;36:4043–4049. doi: 10.2337/dc12-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur J, Sullivan KA, Pande M, Hong Y, Sima AA, Jagadish HV, Kretzler M, Feldman EL. The identification of gene expression profiles associated with progression of human diabetic neuropathy. Brain. 2011;134:3222–3235. doi: 10.1093/brain/awr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadarola MJ, Max MB, Berman KF, Byas-Smith MG, Coghill RC, Gracely RH, Bennett GJ. Unilateral decrease in thalamic activity observed with positron emission tomography in patients with chronic neuropathic pain. Pain. 1995;63:55–64. doi: 10.1016/0304-3959(95)00015-K. [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation. IDF Diabetes Atlas, 2014 Update. Brussels, Belgium: International Diabetes Federation; 2014. [Google Scholar]
- Islam MS. Animal models of diabetic neuropathy: progress since 1960s. Journal of Diabetes Research. 2013;2013:149452. doi: 10.1155/2013/149452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, Cuddihy R, Cushman WC, Genuth S, Grimm RH, Jr, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom DG, Sridharan V, Baker R, Clement ST, Smalley DM, Dohlman HG. Protons as second messenger regulators of G protein signaling. Molecular Cell. 2013;51:531–538. doi: 10.1016/j.molcel.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurology. 2014;13:924–935. doi: 10.1016/S1474-4422(14)70102-4. [DOI] [PubMed] [Google Scholar]
- Julius D. TRP channels and pain. Annual Review of Cell and Developmental Biology. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- Kim B, Feldman EL. Insulin resistance in the nervous system. Trends in Endocrinology and Metabolism. 2012;23:133–141. doi: 10.1016/j.tem.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiryu-Seo S, Ohno N, Kidd GJ, Komuro H, Trapp BD. Demyelination increases axonal stationary mitochondrial size and the speed of axonal mitochondrial transport. Journal of Neuroscience. 2010;30:6658–6666. doi: 10.1523/JNEUROSCI.5265-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Perkins BA, Kayaniyil S, Harris SB, Retnakaran R, Gerstein HC, Zinman B, Hanley AJ. Peripheral neuropathy and nerve dysfunction in individuals at high risk for type 2 diabetes: The PROMISE cohort. Diabetes Care. 2015;38:793–800. doi: 10.2337/dc14-2585. [DOI] [PubMed] [Google Scholar]
- Lee JH, Park CK, Chen G, Han Q, Xie RG, Liu T, Ji RR, Lee SY. A monoclonal antibody that targets a NaV1.7 channel voltage sensor for pain and itch relief. Cell. 2014;157:1393–1404. doi: 10.1016/j.cell.2014.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QS, Cheng P, Favis R, Wickenden A, Romano G, Wang H. SCN9A variants may be implicated in neuropathic pain associated with diabetic peripheral neuropathy and pain severity. The Clinical Journal of Pain. 2015;31:976–982. doi: 10.1097/AJP.0000000000000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T, Ortac K, Laube H, Federlin K. Intensive therapy in adult insulin-dependent diabetes mellitus is associated with improved insulin sensitivity and reserve: a randomized, controlled, prospective study over 5 years in newly diagnosed patients. Metabolism. 1996;45:1508–1513. doi: 10.1016/s0026-0495(96)90180-8. [DOI] [PubMed] [Google Scholar]
- Low PA, Nickander KK, Tritschler HJ. The roles of oxidative stress and antioxidant treatment in experimental diabetic neuropathy. Diabetes. 1997;46(Suppl 2):S38–42. doi: 10.2337/diab.46.2.s38. [DOI] [PubMed] [Google Scholar]
- Lukic IK, Humpert PM, Nawroth PP, Bierhaus A. The RAGE pathway: activation and perpetuation in the pathogenesis of diabetic neuropathy. Annals of the New York Academy of Sciences. 2008;1126:76–80. doi: 10.1196/annals.1433.059. [DOI] [PubMed] [Google Scholar]
- Lupachyk S, Watcho P, Hasanova N, Julius U, Obrosova IG. Triglyceride, nonesterified fatty acids, and prediabetic neuropathy: role for oxidative-nitrosative stress. Free Radical Biology & Medicine. 2012;52:1255–1263. doi: 10.1016/j.freeradbiomed.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Pan P, Anyika M, Blagg BS, Dobrowsky RT. Modulating molecular chaperones improves mitochondrial bioenergetics and decreases the inflammatory transcriptome in diabetic sensory neurons. ACS Chemical Neuroscience. 2015;6:1637–1648. doi: 10.1021/acschemneuro.5b00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani P, Cherian PV, Gould GW, Greene DA, Sima AA, Brosius FC., 3rd Glucose transporters in rat peripheral nerve: paranodal expression of GLUT1 and GLUT3. Metabolism. 1996;45:1466–1473. doi: 10.1016/s0026-0495(96)90174-2. [DOI] [PubMed] [Google Scholar]
- Maier C, Baron R, Tolle TR, Binder A, Birbaumer N, Birklein F, Gierthmuhlen J, Flor H, Geber C, Huge V, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150:439–450. doi: 10.1016/j.pain.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Malik RA, Tesfaye S, Newrick PG, Walker D, Rajbhandari SM, Siddique I, Sharma AK, Boulton AJ, King RH, Thomas PK, Ward JD. Sural nerve pathology in diabetic patients with minimal but progressive neuropathy. Diabetologia. 2005;48:578–585. doi: 10.1007/s00125-004-1663-5. [DOI] [PubMed] [Google Scholar]
- Martin CL, Albers JW, Pop-Busui R, Group DER. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37:31–38. doi: 10.2337/dc13-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoin CS, Knotts TA, Adams SH. Acylcarnitines--old actors auditioning for new roles in metabolic physiology. Nature Reviews Endocrinology. 2015;11:617–625. doi: 10.1038/nrendo.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack K, Santos S, Chapman ML, Krafte DS, Marron BE, West CW, Krambis MJ, Antonio BM, Zellmer SG, Printzenhoff D, et al. Voltage sensor interaction site for selective small molecule inhibitors of voltage-gated sodium channels. Proceedings of the National Academy of Sciences. 2013;110:E2724–2732. doi: 10.1073/pnas.1220844110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng W, Deshmukh HA, Donnelly LA, Torrance N, Colhoun HM, Palmer CN, Smith BH Wellcome Trust Case Control C, Surrogate markers for M, Macro-vascular hard endpoints for Innovative diabetes Tools study g. A genome-wide association study provides evidence of sex-specific involvement of Chr1p35.1 (ZSCAN20-TLR12P) and Chr8p23.1 (HMGB1P46) with diabetic neuropathic pain. EBioMedicine. 2015;2:1386–1393. doi: 10.1016/j.ebiom.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314:1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- Messinger RB, Naik AK, Jagodic MM, Nelson MT, Lee WY, Choe WJ, Orestes P, Latham JR, Todorovic SM, Jevtovic-Todorovic V. In vivo silencing of the Ca(V)3.2 T-type calcium channels in sensory neurons alleviates hyperalgesia in rats with streptozocin-induced diabetic neuropathy. Pain. 2009;145:184–195. doi: 10.1016/j.pain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke JG, Wang YT. Insulin, synaptic function, and opportunities for neuroprotection. Prog Mol Biol Transl Sci. 2011;98:133–186. doi: 10.1016/B978-0-12-385506-0.00004-1. [DOI] [PubMed] [Google Scholar]
- Misur I, Zarkovic K, Barada A, Batelja L, Milicevic Z, Turk Z. Advanced glycation endproducts in peripheral nerve in type 2 diabetes with neuropathy. Acta Diabetologica. 2004;41:158–166. doi: 10.1007/s00592-004-0160-0. [DOI] [PubMed] [Google Scholar]
- Miyauchi Y, Shikama H, Takasu T, Okamiya H, Umeda M, Hirasaki E, Ohhata I, Nakayama H, Nakagawa S. Slowing of peripheral motor nerve conduction was ameliorated by aminoguanidine in streptozocin-induced diabetic rats. European Journal of Endocrinology. 1996;134:467–473. doi: 10.1530/eje.0.1340467. [DOI] [PubMed] [Google Scholar]
- Mizisin AP. Mechanisms of diabetic neuropathy: Schwann cells. Handbook of Clinical Neurology. 2014;126:401–428. doi: 10.1016/B978-0-444-53480-4.00029-1. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D, Das K, Grimes KV. Protein kinase C, an elusive therapeutic target? Nature Reviews. 2012;11:937–957. doi: 10.1038/nrd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk KR, Feltri ML, Taveggia C. New insights on Schwann cell development. Glia. 2015;63:1376–1393. doi: 10.1002/glia.22852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison BM, Tsingalia A, Vidensky S, Lee Y, Jin L, Farah MH, Lengacher S, Magistretti PJ, Pellerin L, Rothstein JD. Deficiency in monocarboxylate transporter 1 (MCT1) in mice delays regeneration of peripheral nerves following sciatic nerve crush. Experimental Neurology. 2015;263:325–338. doi: 10.1016/j.expneurol.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468:244–252. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- Nave KA, Werner HB. Myelination of the nervous system: mechanisms and functions. Annual Reviews Cellular and Developmental Biology. 2014;30:503–533. doi: 10.1146/annurev-cellbio-100913-013101. [DOI] [PubMed] [Google Scholar]
- Norbury TA, MacGregor AJ, Urwin J, Spector TD, McMahon SB. Heritability of responses to painful stimuli in women: a classical twin study. Brain. 2007;130:3041–3049. doi: 10.1093/brain/awm233. [DOI] [PubMed] [Google Scholar]
- O’Brien PD, Hur J, Hayes JM, Backus C, Sakowski SA, Feldman EL. BTBR ob/ob mice as a novel diabetic neuropathy model: Neurological characterization and gene expression analyses. Neurobiology of Disease. 2014a;73C:348–355. doi: 10.1016/j.nbd.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien PD, Hur J, Robell NJ, Hayes JM, Sakowski SA, Feldman EL. Gender-specific differences in diabetic neuropathy in BTBR ob/ob mice. Journal of Diabetes and its Complications. 2016;30:30–37. doi: 10.1016/j.jdiacomp.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien PD, Sakowski SA, Feldman EL. Mouse models of diabetic neuropathy. ILAR Journal. 2014b;54:259–272. doi: 10.1093/ilar/ilt052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates PJ. Aldose reductase, still a compelling target for diabetic neuropathy. Current Drug Targets. 2008;9:14–36. doi: 10.2174/138945008783431781. [DOI] [PubMed] [Google Scholar]
- Orestes P, Osuru HP, McIntire WE, Jacus MO, Salajegheh R, Jagodic MM, Choe W, Lee J, Lee SS, Rose KE, et al. Reversal of neuropathic pain in diabetes by targeting glycosylation of Ca(V)3.2 T-type calcium channels. Diabetes. 2013;62:3828–3838. doi: 10.2337/db13-0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orstavik K, Namer B, Schmidt R, Schmelz M, Hilliges M, Weidner C, Carr RW, Handwerker H, Jorum E, Torebjork HE. Abnormal function of C-fibers in patients with diabetic neuropathy. The Journal of Neuroscience. 2006;26:11287–11294. doi: 10.1523/JNEUROSCI.2659-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande M, Hur J, Hong Y, Backus C, Hayes JM, Oh SS, Kretzler M, Feldman EL. Transcriptional profiling of diabetic neuropathy in the BKS db/db mouse: a model of type 2 diabetes. Diabetes. 2011;60:1981–1989. doi: 10.2337/db10-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanas N, Ziegler D. Efficacy of alpha-lipoic acid in diabetic neuropathy. Expert Opinions in Pharmacotherapy. 2014;15:2721–2731. doi: 10.1517/14656566.2014.972935. [DOI] [PubMed] [Google Scholar]
- Payne CE, Brown AR, Theile JW, Loucif AJ, Alexandrou AJ, Fuller MD, Mahoney JH, Antonio BM, Gerlach AC, Printzenhoff DM, et al. A novel selective and orally bioavailable Nav 1.8 channel blocker, PF-01247324, attenuates nociception and sensory neuron excitability. Brittish Journal of Pharmacology. 2015;172:2654–2670. doi: 10.1111/bph.13092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, Sosenko JM, Ziegler D. Diabetic neuropathy: A position statement by the American Diabetes Association. Diabetes Care. 2017;40:136–154. doi: 10.2337/dc16-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachana KS, Manu MS, Advirao GM. Insulin influenced expression of myelin proteins in diabetic peripheral neuropathy. Neurosci Letters. 2016;629:110–115. doi: 10.1016/j.neulet.2016.06.067. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Peles E. The nodes of Ranvier: Molecular assembly and maintenance. Cold Spring Harbor Perspectives in Biology. 2015;8:a020495. doi: 10.1101/cshperspect.a020495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard P, Nilsson BY, Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. The New England Journal of Medicine. 1993;329:304–309. doi: 10.1056/NEJM199307293290502. [DOI] [PubMed] [Google Scholar]
- Rizzo MA, Kocsis JD, Waxman SG. Slow sodium conductances of dorsal root ganglion neurons: intraneuronal homogeneity and interneuronal heterogeneity. Journal of Neurophysiology. 1994;72:2796–2815. doi: 10.1152/jn.1994.72.6.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolyan H, Liu S, Hoeijmakers JGJ, Faber CG, Merkies ISJ, Lauria G, Black JA, Waxman SG. Painful neuropathy-associated G856D mutation of Nav1.7 produces time dependent decrease in ATP and increase in ROS in small-diameter axons of DRG neurons in vitro. Molecular Pain. 2016;12:1744806916674472. doi: 10.1177/1744806916674472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab AS, Tzvetanova ID, Nave KA. The role of myelin and oligodendrocytes in axonal energy metabolism. Current Opinion in Neurobiology. 2013;23:1065–1072. doi: 10.1016/j.conb.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Saab AS, Tzvetavona ID, Trevisiol A, Baltan S, Dibaj P, Kusch K, Mobius W, Goetze B, Jahn HM, Huang W, et al. Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron. 2016;91:119–132. doi: 10.1016/j.neuron.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer JL, Brophy PJ, Peles E. Molecular domains of myelinated axons in the peripheral nervous system. Glia. 2008;56:1532–1540. doi: 10.1002/glia.20750. [DOI] [PubMed] [Google Scholar]
- Schemmel KE, Padiyara RS, D’Souza JJ. Aldose reductase inhibitors in the treatment of diabetic peripheral neuropathy: a review. Journal of Diabetes and its Complications. 2010;24:354–360. doi: 10.1016/j.jdiacomp.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Scherer SS. Finding the causes of inherited neuropathies. Archives of Neurology. 2006;63:812–816. doi: 10.1001/archneur.63.6.812. [DOI] [PubMed] [Google Scholar]
- Scholz J, Rathmell JP, David WS, Chad DA, Broderick AC, Perros SG, Shin NS, Wells JL, Davis JB, DiMaggio CJ, et al. A standardized clinical evaluation of phenotypic diversity in diabetic polyneuropathy. Pain. 2016;157:2297–2308. doi: 10.1097/j.pain.0000000000000648. [DOI] [PubMed] [Google Scholar]
- Selvarajah D, Wilkinson ID, Maxwell M, Davies J, Sankar A, Boland E, Gandhi R, Tracey I, Tesfaye S. Magnetic resonance neuroimaging study of brain structural differences in diabetic peripheral neuropathy. Diabetes Care. 2014;37:1681–1688. doi: 10.2337/dc13-2610. [DOI] [PubMed] [Google Scholar]
- Shettar A, Muttagi G. Developmental regulation of insulin receptor gene in sciatic nerves and role of insulin on glycoprotein P0 in the Schwann cells. Peptides. 2012;36:46–53. doi: 10.1016/j.peptides.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Singh B, Xu Y, McLaughlin T, Singh V, Martinez JA, Krishnan A, Zochodne DW. Resistance to trophic neurite outgrowth of sensory neurons exposed to insulin. Journal of Neurochemistry. 2012;121:263–276. doi: 10.1111/j.1471-4159.2012.07681.x. [DOI] [PubMed] [Google Scholar]
- Sorensen L, Siddall PJ, Trenell MI, Yue DK. Differences in metabolites in pain-processing brain regions in patients with diabetes and painful neuropathy. Diabetes Care. 2008;31:980–981. doi: 10.2337/dc07-2088. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nature Neuroscience. 2015;18:1081–1083. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavniichuk R, Shevalye H, Lupachyk S, Obrosov A, Groves JT, Obrosova IG, Yorek MA. Peroxynitrite and protein nitration in the pathogenesis of diabetic peripheral neuropathy. Diabetes/Metabolism Research and Reviews. 2014;30:669–678. doi: 10.1002/dmrr.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart WF, Ricci JA, Chee E, Hirsch AG, Brandenburg NA. Lost productive time and costs due to diabetes and diabetic neuropathic pain in the US workforce. Journal of Occupational and Environmental Medicine. 2007;49:672–679. doi: 10.1097/JOM.0b013e318065b83a. [DOI] [PubMed] [Google Scholar]
- Sun W, Miao B, Wang XC, Duan JH, Wang WT, Kuang F, Xie RG, Xing JL, Xu H, Song XJ, et al. Reduced conduction failure of the main axon of polymodal nociceptive C-fibres contributes to painful diabetic neuropathy in rats. Brain. 2012;135:359–375. doi: 10.1093/brain/awr345. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Sato J, Kawanishi M, Mizumura K. Tissue glucose level modulates the mechanical responses of cutaneous nociceptors in streptozotocin-diabetic rats but not normal rats in vitro. Pain. 2002;99:475–484. doi: 10.1016/S0304-3959(02)00244-0. [DOI] [PubMed] [Google Scholar]
- Tan AM, Samad OA, Fischer TZ, Zhao P, Persson AK, Waxman SG. Maladaptive dendritic spine remodeling contributes to diabetic neuropathic pain. The Journal of Neuroscience. 2012;32:6795–6807. doi: 10.1523/JNEUROSCI.1017-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Themistocleous AC, Ramirez JD, Shillo PR, Lees JG, Selvarajah D, Orengo C, Tesfaye S, Rice AS, Bennett DL. The Pain in Neuropathy Study (PiNS): A cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain. 2016;157:1132–1145. doi: 10.1097/j.pain.0000000000000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MC, Woodward M, Neal B, Li Q, Pickering R, Marre M, Williams B, Perkovic V, Cooper ME, Zoungas S, et al. Relationship between levels of advanced glycation end products and their soluble receptor and adverse outcomes in adults with type 2 diabetes. Diabetes Care. 2015;38:1891–1897. doi: 10.2337/dc15-0925. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Archives of Biochemistry and Biophysics. 2003;419:31–40. doi: 10.1016/j.abb.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Ueno H, Kataoka A, Tozaki-Saitoh H, Inoue K. Activation of dorsal horn microglia contributes to diabetes-induced tactile allodynia via extracellular signal-regulated protein kinase signaling. Glia. 2008;56:378–386. doi: 10.1002/glia.20623. [DOI] [PubMed] [Google Scholar]
- Tzvetanova ID, Nave KA. Axons hooked to Schwann cell metabolism. Nature Neuroscience. 2014;17:1293–1295. doi: 10.1038/nn.3825. [DOI] [PubMed] [Google Scholar]
- UKPDS Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- Ursino G, Alberti MA, Grandis M, Reni L, Pareyson D, Bellone E, Gemelli C, Sabatelli M, Pisciotta C, Luigetti M, et al. Influence of comorbidities on the phenotype of patients affected by Charcot-Marie-Tooth neuropathy type 1A. Neuromuscular Disorders. 2013;23:902–906. doi: 10.1016/j.nmd.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Vas PR, Edmonds ME. Early recognition of diabetic peripheral neuropathy and the need for one-stop microvascular assessment. The Lancet Diabetes & Endocrinology. 2016;4:723–725. doi: 10.1016/S2213-8587(16)30063-8. [DOI] [PubMed] [Google Scholar]
- Verheijen MH, Chrast R, Burrola P, Lemke G. Local regulation of fat metabolism in peripheral nerves. Genes & Development. 2003;17:2450–2464. doi: 10.1101/gad.1116203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viader A, Sasaki Y, Kim S, Strickland A, Workman CS, Yang K, Gross RW, Milbrandt J. Aberrant Schwann cell lipid metabolism linked to mitochondrial deficits leads to axon degeneration and neuropathy. Neuron. 2013;77:886–898. doi: 10.1016/j.neuron.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AM, Calabek B, Roberts L, Feldman EL. Biology of diabetic neuropathy. Handbook of Clinical Neurology. 2013;115:591–606. doi: 10.1016/B978-0-444-52902-2.00034-5. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Callaghan BC, Smith AL, Feldman EL. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nature Reviews Neurology. 2011;7:573–583. doi: 10.1038/nrneurol.2011.137. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Edwards JL, McLean LL, Hong Y, Cerri F, Lopez I, Quattrini A, Feldman EL. Mitochondrial biogenesis and fission in axons in cell culture and animal models of diabetic neuropathy. Acta Neuropathologica. 2010;120:477–489. doi: 10.1007/s00401-010-0697-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinik AI, Bril V, Kempler P, Litchy WJ, Tesfaye S, Price KL, Bastyr EJ, 3rd, Group MS. Treatment of symptomatic diabetic peripheral neuropathy with the protein kinase C beta-inhibitor ruboxistaurin mesylate during a 1-year, randomized, placebo-controlled, double-blind clinical trial. Clinical Therapeutics. 2005;27:1164–1180. doi: 10.1016/j.clinthera.2005.08.001. [DOI] [PubMed] [Google Scholar]
- von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron. 2012;73:638–652. doi: 10.1016/j.neuron.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J, Foyt H, Daniels M, Arezzo JC. Long-acting C-peptide and neuropathy in type 1 diabetes: A 12-month clinical trial. Diabetes Care. 2016;39:596–602. doi: 10.2337/dc15-2068. [DOI] [PubMed] [Google Scholar]
- Waldbillig RJ, LeRoith D. Insulin receptors in the peripheral nervous system: a structural and functional analysis. Brain Research. 1987;409:215–220. doi: 10.1016/0006-8993(87)90704-9. [DOI] [PubMed] [Google Scholar]
- Wang JT, Medress ZA, Barres BA. Axon degeneration: molecular mechanisms of a self-destruction pathway. The Journal of Cell Biology. 2012;196:7–18. doi: 10.1083/jcb.201108111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YZ, Xu TL. Acidosis, acid-sensing ion channels, and neuronal cell death. Molecular Neurobiology. 2011;44:350–358. doi: 10.1007/s12035-011-8204-2. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Merkies IS, Gerrits MM, Dib-Hajj SD, Lauria G, Cox JJ, Wood JN, Woods CG, Drenth JP, Faber CG. Sodium channel genes in pain-related disorders: phenotype-genotype associations and recommendations for clinical use. The Lancet Neurology. 2014;13:1152–1160. doi: 10.1016/S1474-4422(14)70150-4. [DOI] [PubMed] [Google Scholar]
- Wei H, Hamalainen MM, Saarnilehto M, Koivisto A, Pertovaara A. Attenuation of mechanical hypersensitivity by an antagonist of the TRPA1 ion channel in diabetic animals. Anesthesiology. 2009;111:147–154. doi: 10.1097/ALN.0b013e3181a1642b. [DOI] [PubMed] [Google Scholar]
- West SJ, Bannister K, Dickenson AH, Bennett DL. Circuitry and plasticity of the dorsal horn--toward a better understanding of neuropathic pain. Neuroscience. 2015;300:254–275. doi: 10.1016/j.neuroscience.2015.05.020. [DOI] [PubMed] [Google Scholar]
- Wiggin TD, Kretzler M, Pennathur S, Sullivan KA, Brosius FC, Feldman EL. Rosiglitazone treatment reduces diabetic neuropathy in streptozotocin-treated DBA/2J mice. Endocrinology. 2008;149:4928–4937. doi: 10.1210/en.2008-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzel II, Jelinek HF, Khalaf K, Lee S, Khandoker AH, Alsafar H. Identifying common genetic risk factors of diabetic neuropathies. Frontiers in Endocrinology. 2015;6:88. doi: 10.3389/fendo.2015.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagihashi S, Yamagishi SI, Wada Ri R, Baba M, Hohman TC, Yabe-Nishimura C, Kokai Y. Neuropathy in diabetic mice overexpressing human aldose reductase and effects of aldose reductase inhibitor. Brain. 2001;124:2448–2458. doi: 10.1093/brain/124.12.2448. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain. 2012;153:1193–1198. doi: 10.1016/j.pain.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Yorek MA. Alternatives to the streptozotocin-diabetic rodent. International Review of Neurobiology. 2016;127:89–112. doi: 10.1016/bs.irn.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorek MS, Obrosov A, Shevalye H, Coppey LJ, Kardon RH, Yorek MA. Early vs. late intervention of high fat/low dose streptozotocin treated C57Bl/6J mice with enalapril, alpha-lipoic acid, menhaden oil or their combination: Effect on diabetic neuropathy related endpoints. Neuropharmacology. 2016;116:122–131. doi: 10.1016/j.neuropharm.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewska JM, Palmer J, Ettlin DA, Obermann M, Giblin GM, Morisset V, Tate S, Gunn K. Novel design for a phase IIa placebo-controlled, double-blind randomized withdrawal study to evaluate the safety and efficacy of CNV1014802 in patients with trigeminal neuralgia. Trials. 2013;14:402. doi: 10.1186/1745-6215-14-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenker J, Poirot O, de Preux Charles AS, Arnaud E, Medard JJ, Lacroix C, Kuntzer T, Chrast R. Altered distribution of juxtaparanodal kv1.2 subunits mediates peripheral nerve hyperexcitability in type 2 diabetes mellitus. The Journal of Neuroscience. 2012;32:7493–7498. doi: 10.1523/JNEUROSCI.0719-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenker J, Ziegler D, Chrast R. Novel pathogenic pathways in diabetic neuropathy. Trends in Neuroscience. 2013;36:439–449. doi: 10.1016/j.tins.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Zochodne DW. Mechanisms of diabetic neuron damage: Molecular pathways. Handbook of Clinical Neurology. 2014;126:379–399. doi: 10.1016/B978-0-444-53480-4.00028-X. [DOI] [PubMed] [Google Scholar]