Abstract
OBJECTIVES: Virtual-assisted lung mapping (VAL-MAP) is a preoperative bronchoscopic multispot dye-marking technique using virtual images. The purpose of this study was to evaluate the safety, efficacy and reproducibility of VAL-MAP among multiple centres.
METHODS: Selection criteria included patients with pulmonary lesions anticipated to be difficult to identify at thoracoscopy and/or those undergoing sub-lobar lung resections requiring careful determination of resection margins. Data were collected prospectively and, if needed, compared between the centre that originally developed VAL-MAP and 16 other centres.
RESULTS: Five hundred patients underwent VAL-MAP with 1781 markings (3.6 ± 1.2 marks/patient). Complications associated with VAL-MAP necessitating additional management occurred in four patients (0.8%) including pneumonia, fever and temporary exacerbation of pre-existing cerebral ischaemia. Minor complications included pneumothorax (3.6%), pneumomediastinum (1.2%) and alveolar haemorrhage (1.2%), with similar incidences between the original centre and other centres. Marks were identifiable during operation in approximately 90%, whereas the successful resection rate was approximately 99% in both groups, partly due to the mutually complementary marks. The contribution of VAL-MAP to surgical success was highly rated by surgeons resecting pure ground glass nodules (P < 0.0001), tumours ≤ 5 mm (P = 0.0016), and performing complex segmentectomy and wedge resection (P = 0.0072).
CONCLUSIONS: VAL-MAP was found to be safe and reproducible among multiple centres with variable settings. Patients with pure ground glass nodules, small tumours and resections beyond conventional anatomical boundaries are considered the best candidates for VAL-MAP.
Clinical Trial Registration Number: UMIN 000008031. University Hospital Medical Information Network Clinical Trial Registry (http://www.umin.ac.jp/ctr/).
Keywords: Lung cancer , Metastatic pulmonary tumour , Marking , Ground glass opacity , Segmentectomy
INTRODUCTION
Virtual-assisted lung mapping (VAL-MAP) is a new technique to achieve simultaneous multiple markings (lung mapping) to localize a pulmonary lesion using 3D images and bronchoscopic dye injection under regular fluoroscopy [1]. The technique was originally developed as a bronchoscopic lung marking method to avoid the complications of computed tomography (CT)-guided percutaneous needle-mediated lung marking techniques, especially air embolism [2–5], which has been reported to occur in 1–2% of cases [6, 7], and pneumothorax (30–50%) [8, 9]. VAL-MAP has a lower complication rate, and the ability to make multiple marks under the lung surface provides geometric information for localizing a lesion in 3D space for reliable intraoperative guidance for sub-lobar lung resection [10–12].
Previous single-centre studies have shown high efficacy and safety of VAL-MAP in sub-lobar lung resection [1, 11–13]. However, it remains unclear how reproducible the technique is among multiple centres with different imaging and bronchoscopic equipment. Moreover, it is important to identify the cases most suitable for VAL-MAP before operation. The purpose of this study was to examine the safety and reproducibility of VAL-MAP and to identify patients who benefit most from VAL-MAP.
MATERIALS AND METHODS
Patients
Five hundred patients scheduled to undergo VAL-MAP were registered for this study between July 2012 and April 2016. The inclusion criteria in the study were the same as our previous report [12] including a requirement for sub-lobar lung resection (i.e. wedge resection or segmentectomy) to resect a pulmonary lesion and/or lesions that were anticipated either to be hardly palpable intraoperatively or in which the resection margin needed to be carefully selected regardless of the palpability of the lesion. Patients with past or present bronchial asthma were excluded because of concern about the potential for allergic reaction to indigo carmine (blue dye). Further details of the inclusion criteria were summarized in Table 1.
Table 1:
Inclusion criteria for virtual-assisted lung mapping
| Patients with a suspected or diagnosed pulmonary malignant tumour for which thoracoscopic sub-lobar lung resection (wedge resection or segmentectoya) is indicated |
and
|
The surgical procedure was selected by the surgeon(s), taking multiple factors into consideration including, but not limited to: suspected diagnosis (primary or metastatic), estimated degree of malignancy (e.g. % of ground glass components in CT scan), the location of the tumour (close to the pleural surface or not) and patient’s comorbidities.
The study was approved by the research ethics boards of the Kyoto University Hospital (the initial representative centre of the study; the approval number, #C626), the University of Tokyo Hospital (the representative centre after July 2015; #2013027-11Y) and all the other participating centres.
Virtual-assisted lung mapping planning
As described previously [1, 12], lung map was designed by the surgical team of each centre for the purpose of tumour identification and/or indication of the resection lines in wedge resection or segmentectomy. The number of markings was determined depending on the purpose and characteristics of operation. Virtual bronchoscopy based on thin-slice CT images was used to identify target bronchi reaching the designed marking points in all the centres (Fig. 1). Workstations and software utilized to generate virtual bronchoscopy and/or 3D images included Ziostation® (Ziosoft, Tokyo, Japan), Zioterm 2009® (Ziosoft), Synapse Vincent® (Fujifilm Medical, Tokyo, Japan), LungPoint® (Broncus Technologies, Mountain View, CA, USA), Bf-NAVI® (Cybernet Systems, Tokyo, Japan), Aze VirtualPlace® (Aze, Tokyo, Japan) and Aquarius iNtuition Client Viewer® (TeraRecon, Tokyo, Japan).
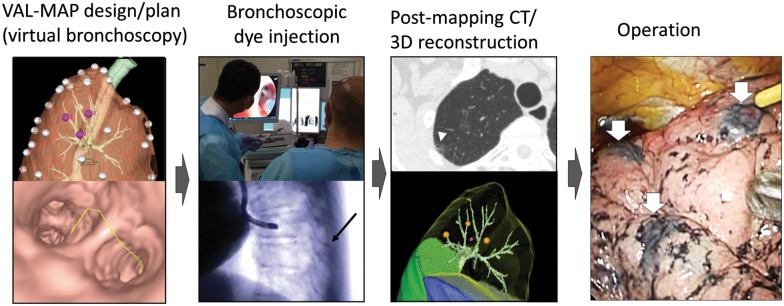
Figure 1:
Steps of VAL-MAP. The lung ‘map’ was designed using radiology workstations and virtual bronchoscopy. Bronchoscopic dye injection was conducted within 3 days before surgery under fluoroscopic guidance to confirm the location of the metal-tip injection catheter (black arrow). After mapping, CT scan was taken within a few hours–days after VAL-MAP to visualize actual locations of markings (arrowhead). Using a radiology workstation, 3D images were further reconstructed, reflecting actual locations of markings. The operation was conducted using the 3D image for guidance. The white arrows indicate dye marks. VAL-MAP: virtual-assisted lung mapping.
Virtual-assisted lung mapping procedure
Up to 3 days before surgery, the patient was brought to the bronchoscopy suite for dye injection with local anaesthesia and mild sedation (Fig. 1). The technique of dye injection in VAL-MAP has been described in detail previously [1, 10, 12]. In short, a metal-tip catheter (PW-6C-1; Olympus, Tokyo, Japan) is inserted through the working channel of the bronchoscope into the predetermined target bronchus utilizing the virtual bronchoscopy images as guidance. The tip of the catheter is confirmed to have reached the visceral pleura using fluoroscopy, and then 1 ml of indigo carmine that has been preloaded in the catheter is injected. This manoeuvre is repeated for all target bronchi. After completing the bronchoscopic procedure, a CT scan is then performed to confirm the location of markings no more than 2 h after the mapping procedure; although indigo carmine is not radiopaque, the water density and the associated bronchial dilation are usually visible in CT scan [1, 10, 12]. The post-VAL-MAP CT images are reconstructed into 3D images for final surgical planning.
Grading of marks and contribution of virtual-assisted lung mapping to operation
The quality of each mark was graded using the system described previously [12]: Grade 0, unidentifiable; Grade 1, identifiable, but faint and hardly visible; Grade 2, easily identifiable without a central red spot or target-like shape; Grade 3, easily identifiable with a central red spot; Grade 4, target-like appearance with or without a central red spot; and Grade 5, bulla formation.
The operation types included wedge resection; simple segmentectomy (i.e. conventional anatomical segmentectomy); and complex segmentectomy, which included single or combined sub-segmentectomy, and extended segmentectomy that extended beyond the anatomical segment into an adjacent segment [14].
As described previously [12], the contribution of VAL-MAP to the operation was graded by the surgeons as follows: Grade A, the same level of operative precision was judged to be impossible without VAL-MAP; Grade B, a similar level of precision was judged to be possible, but VAL-MAP enabled confident performance of the operation; and Grade C, the same operation was judged to be possible without VAL-MAP.
Data analysis and statistics
If appropriate, data were compared between Kyoto University (KU), the original institution that developed VAL-MAP, and the other multiple centres (MC). As described previously [12], the relationship between the grade of the contribution of VAL-MAP to the operation and the type of operation or tumour characteristics (size, depth, CT characteristics and palpability during operation) was examined using Pearson’s chi-squared test. In cases where multiple resections were performed or multiple tumours were resected, the most representative operation type and tumour were used for analysis. With respect to tumour characteristics, tumour size was categorized as ≤ 5, >5–10 and >10 mm; tumour depth (distance from the closest visceral pleura) was categorized as ≤ 10 and >10 mm; CT appearance of the tumour was categorized as pure ground glass nodule (GGN), mixed GGN (GGN with solid component), solid nodule and others. Data are expressed as the mean ± SD as appropriate. Statistical analysis was conducted using commercial software (JMP ver 11.0®; SAS Institute, Cary, NC, USA).
RESULTS
Patients, targeted lesions and markings
In total, 500 patients were enrolled in the study and 675 lesions were targeted by VAL-MAP-assisted lung resection, including 272 lesions of 178 patients in the KU group and 409 lesions of 322 patients in the MC group. To resect these lesions, in total 1780 markings were planned for VAL-MAP and 1773 markings were actually conducted (3.6 ± 1.2 marks per patient). Characteristics of patients and targeted lesions are shown in Table 2.
Table 2:
Characteristics of patients and targeted lesions
| Total | KU | MC | P-value | |
|---|---|---|---|---|
| Number of patients | 500 | 178 | 322 | |
| Sex (female/male) | 272/228 | 98/80 | 174/148 | NS |
| Mean age ± SD (range) | 64.7±10.8 | 64.0±11.2 (26–84) | 65.1±10.7 (27–85) | NS |
| Left/right/bilateral lesions | 292/204/4 | 104/71/3 | 188/133/1 | NS |
| Number of lesions | 681 | 272 | 409 | |
| Lesions per patient (range) | 1.43±1.84 | 1.53±1.05 (1–9) | 1.27±0.69 (1–5) | 0.00086 |
| Diameter (mm) | 9.9±5.8 | 9.3±5.8 | 10.4±5.8 | 0.023 |
| Deptha (mm) | 8.2±9.3 | 7.6±9.3 | 8.6±9.2 | NS |
| Appearance on CT scan, n (%) | ||||
| Pure GGN | 257 (38) | 83 (31) | 174 (43) | |
| Mixed GGN | 113 (17) | 43 (16) | 70 (17) | |
| Solid nodule | 294 (43) | 138 (51) | 156 (38) | |
| Other | 17 (3) | 8 (3) | 9 (2) | |
| Number of planned markings | 1780 | 686 | 1094 | |
| Number of actual markings | 1773 | 683 | 1090 | |
| Marks/patient, mean ± SD (range) | 3.55±1.22 | 3.85±1.19 (1–8) | 3.38±1.20 (1–9) | <0.001 |
GGN: ground glass nodule; KU: Kyoto University; MC: multiple centres.
Closest distance to pleura.
Numbers of markings and the selected operations are shown in Table 3. At the time of bronchoscopic marking, eight markings (one KU and seven MC) were conducted additionally to the plan, while 15 markings (4 KU and 11 MC) were not conducted as planned. The reported reasons for additional markings included: additional marking(s) was felt needed on site (n = 3); mistaken injection in a wrong bronchus resulting in an additional, correct injection being added (n = 1); and unknown reasons (n = 4). The reasons for cancelled markings included difficult dye injection (n = 8), insufficient sedation hindering continuation of marking procedures (n = 5) and unknown (n = 2).
Table 3:
Characteristics of markings and selected operations
| Total | KU | MC | P-value | |
|---|---|---|---|---|
| Number of patients | 500 | 178 | 322 | |
| Number of planned markings | 1780 | 686 | 1094 | |
| Number of actual markings | 1773 | 683 | 1090 | |
| Marks/patient, mean ± SD (range) | 3.55±1.22 (1–9) | 3.85±1.19 (1–8) | 3.38±1.20 (1–9) | <0.001 |
| Timing of surgery after VAL-MAP | ||||
| Same day | 114 | 89 | 25 | |
| One day after | 309 | 72 | 237 | |
| Two days after | 72 | 17 | 55 | |
| Three days after | 4 | 0 | 4 | |
| Evaluated markings, n | 1765 | 683 | 1082 | |
| Visible markings, n (%) | 1613 (91) | 630 (92) | 983 (91) | NS |
| Operation types, n (%) | ||||
| Wedge | 340 (56) | 117 (49) | 223 (60) | |
| Simple segmentectomy | 137 (22) | 54 (23) | 83 (22) | |
| Complex segmentectomya | 87 (14) | 61(26) | 26 (7) | |
| Wedge > lobectomy | 26 (4) | 1 (<1) | 25 (7) | |
| Wedge > segmentectomy | 7 (1) | 1 (<1) | 6 (2) | |
| Segmentectomy > lobectomy | 2 (<1) | 1 (<1) | 1 (<1) | |
| Others | 12 (2) | 3 (1) | 9 (2) |
KU: Kyoto University; MC: multiple centres.
In operation type, ‘Wedge > lobectomy’ means wedge resection followed by lobectomy, usually after frozen-section diagnosis.
Single or combined subsegmentectomy.
Complications
Complications associated with VAL-MAP are summarized in Table 4. Four patients experienced major complications that needed additional medical management, including fever necessitating the postponement of surgery (two patients), pneumonia and self-limited hemiplegia attributable to underlying cerebrovascular disease.
Table 4:
Complications associated with virtual-assisted lung mapping
| Total | MC | KU | |
|---|---|---|---|
| Number of patients | 500 | 178 | 322 |
| Major complicationsa, n (%) | 4 (1) | 1 (<1) | 3 (1) |
| Minor complications detected by CT scan, n (%) | |||
| Pneumothorax | 18 (4) | 7 (4) | 11 (3) |
| Pneumomediastinum | 6 (1) | 1 (<1) | 5 (2) |
| Alveolar haemorrhage | 6 (1) | 1 (<1) | 5 (2) |
KU: Kyoto University; MC: multiple centres.
Major complications are defined as those requiring management, including pneumonia, transient ischaemic attack secondary to pre-existing vascular stenosis and postponed operation due to fever. See details in the text.
CT scans taken after VAL-MAP to confirm the markings revealed asymptomatic complications including bulla formation (7%), minor pneumothorax (4%), pneumomediastinum (1%) and minor intra-alveolar haemorrhage (1%). None of these asymptomatic complications needed treatment.
Intraoperative findings
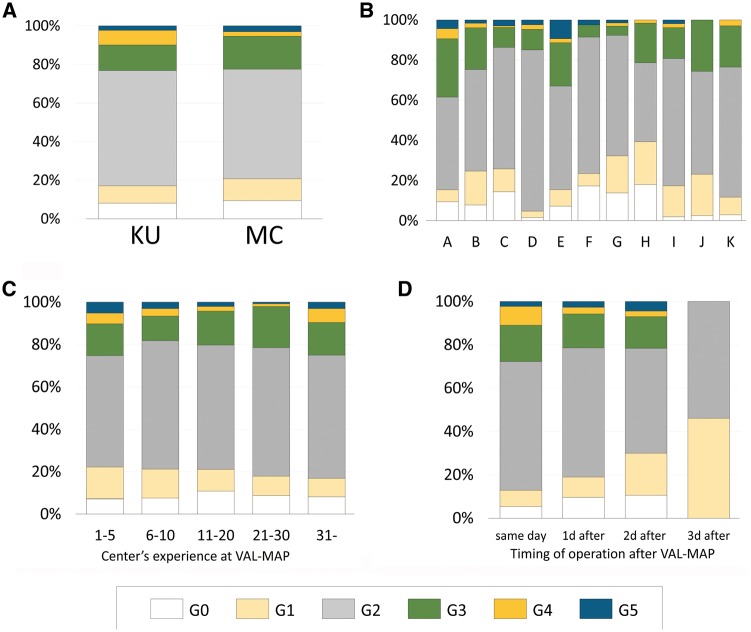
After VAL-MAP, surgery was conducted most frequently on the next day. Surgery on the same day as VAL-MAP (Table 3) was next most common. As described above, operations of two febrile patients in the MC group were postponed and eight markings of these two patients were not evaluated. Consequently, 1765 markings were evaluated during operation. Figure 2 shows the intensity grade of each mark. In total, 1613 markings (91%) were graded ≥G1 (i.e. identifiable during operation). There was no statistical difference in the success ratio between groups (Fig. 2A; 92% in KU vs 91% in MC, P > 0.05). The result was also similar among different centres in the MC group (Fig. 2B). The degree of the centres’ experience had little impact on visibility of markings and grading (Fig. 2C), whereas a longer duration between VAL-MAP and surgery was significantly associated with decreased intensity of markings (Fig. 2D). The explanations for unidentified (G0) or faint (G1) markings were reported (Table 5). The most frequent was central injection, a technical issue of VAL-MAP described previously [12], followed by severe anthracosis, and the presence of pulmonary emphysema.
Figure 2:
Visibility of VAL-MAP marks during surgery. (A) Visibility of marks was graded similarly between the leading institution (KU) and other multiple centres (MC). (B) Among the MC group, visibility of marks was graded similarly. (C) Visibility of marks according to the centre’s case number, showing almost no change throughout the experience. (D) Visibility of marks according to the time from VAL-MAP to surgery, demonstrating a decrease in visibility. VAL-MAP: virtual-assisted lung mapping.
Table 5:
Reported explanations for unidentifiable (G0) or faint (G1) marking
| G0 | G1 | Total | |
|---|---|---|---|
| Central injection | 75 | 56 | 131 |
| Anthracosis | 31 | 33 | 64 |
| Pulmonary emphysema | 11 | 28 | 39 |
| Pleural thickening | 9 | 11 | 20 |
| Time lapse (dilution) | 2 | 13 | 15 |
| Insufficient injection force | 4 | 9 | 13 |
| Intrapulmonary scar/septum | 3 | 2 | 5 |
| Overlapped markings | 4 | 0 | 4 |
| Other | 4 | 7 | 11 |
| Unknown | 4 | 9 | 13 |
Selected operation types are shown in Table 3. Two lesions of two patients in the MC group did not undergo surgery after VAL-MAP as described above; failure to remove the targeted lesions or loss of the lesion despite careful examination of the specimen was experienced in five lesions in five patients. Thus, the successful resection ratio was 674 of 681 lesions, or 99%.
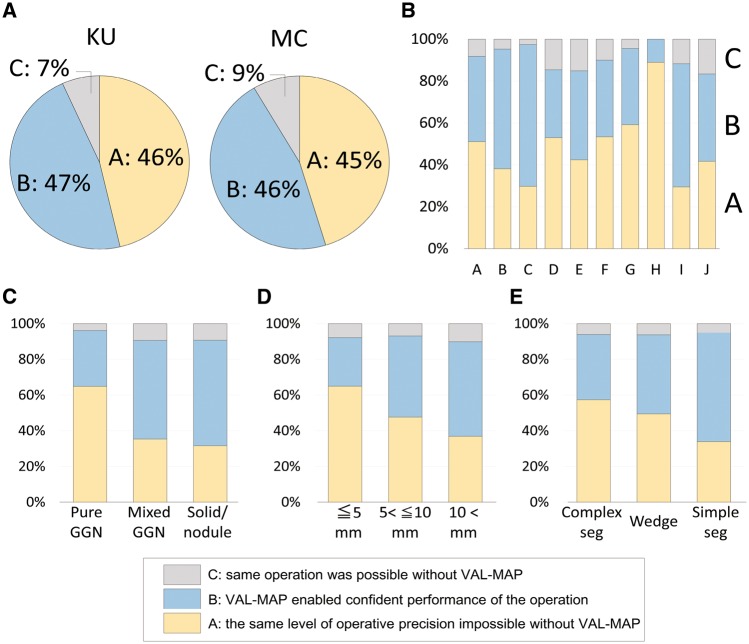
Contribution of virtual-assisted lung mapping to surgical success
After surgery, the contribution of VAL-MAP to operation was evaluated for each case. The results were similar between the KU and MC groups (Fig. 3A). Among the multiple centres, the degree of contribution was also similar (Fig. 3B). The radiological features of a targeted lesion were significantly associated with the degree of contribution, with the more essential contribution associated with pure GGN (Fig. 3C, P < 0.0001). The size of a targeted lesion was significantly associated with the degree of contribution (Fig. 3D, P = 0.0016). Regarding operation types, complex segmentectomy was most associated with essential contribution of VAL-MAP followed by wedge resection, and simple segmentectomy (Fig. 3E, P = 0.0073).
Figure 3:
Contribution of VAL-MAP to surgery. (A) VAL-MAP’s contribution to surgery in the leading institution (KU) and other multiple centres (MC). There was no statistical difference between the groups. As described previously [12], the contribution was evaluated for each case by surgeons as Grade A, the same level of operative precision was judged to be impossible without VAL-MAP; Grade B, a similar level of precision was judged to be possible, but VAL-MAP enabled confident performance of the operation; and Grade C, the same operation was judged to be possible without VAL-MAP. (B) VAL-MAP’s contribution to surgery among the MC group. The centres that enrolled more than 10 patients are shown. VAL-MAP’s contribution was analysed by (C) radiological characteristics of the targeted lesion on CT scan (P < 0.0001), (D) the largest diameter of the targeted lesion measured on CT scan (P = 0.0016) and (E) selected operation types (P = 0.0072). VAL-MAP: virtual-assisted lung mapping.
DISCUSSION
In this multicentre study, we demonstrated safety, efficacy and reproducibility of VAL-MAP. Previous single-centre studies have made similar findings [12, 13]. This study further demonstrated the reproducibility of VAL-MAP accuracy among different institutions utilizing various radiology workstations and settings, suggesting VAL-MAP is a versatile technique.
We found that VAL-MAP is a relatively safe procedure compared with conventional CT-guided needle-mediated marking methods such as using a hookwire, in which the incidences of pneumothorax and haemorrhage have been reported to be 30–50% and 15–30%, respectively [2, 8, 9]. In contrast, the incidences of these complications in VAL-MAP were only 4% and 1%, respectively. The degree of pneumothorax in VAL-MAP was as small as that barely identifiable in post-VAL-MAP CT [12] and none of them needed chest tube drainage. Moreover, VAL-MAP has not been associated with air embolism, the most concerning complication of CT-guided needle-mediated marking techniques [3–7]. In this study, patients with bronchial asthma were excluded because of the concern about possible allergic reactions to indigo carmine. However, such a reaction has not been experienced so far.
Notably, however, VAL-MAP was not free of major complications necessitating additional management. Fever was the most frequent cause of postponement of operation after VAL-MAP. In general, fever is a common complication of bronchoscopy, reportedly occurring in 5–10% of cases; the cause is usually an inflammatory response rather than infection [15]. Although it might be difficult to completely avoid this complication, attention should be paid to conduct as sterile a procedure as possible. Although prophylactic treatment with antibiotics is not generally recommended for bronchoscopy [15], we have recently begun using routine antibiotic prophylaxis and no longer encounter post-VAL-MAP fever. In addition, unexpected hypertension or hypoxaemia could cause serious medical problems. Appropriate sedation and topical anaesthesia as well as careful monitoring during the procedure including blood pressure, heart rate, electrocardiogram and oxygenation are strongly recommended. Fragile elderly patients may benefit from VAL-MAP conducted on the same day as surgery, considering possible pneumothorax and other complications due to fragility of the lung tissue. For patients with present or past history of bronchial asthma, VAL-MAP should be conducted taking the risk–benefit balance into consideration. Prophylactic treatment with bronchodilators or steroids may be considered, following general guidelines for bronchoscopic procedures [15].
Consistent with previous studies [12, 13], the success ratio of marking (i.e. intraoperative visibility) in this study was approximately 90%. The difference in the success ratio among centres or between early and late experience was very small, suggesting excellent reproducibility of the technique. Furthermore, the successful resection ratio of up to 99% was considered to be achieved by the multiple marks that are mutually complementary and the post-VAL-MAP CT scan that allows adjustment of dislocated markings [10].
The failed marks were attributed mostly to ‘central injection’ (dye injection without reaching the pleura), a technical issue associated with VAL-MAP. The result is consistent with a previous single-centre study [12]. Despite the generally good reproducibility of VAL-MAP, it is also true that there is certainly a technical aspect that determines the visibility of marks. A bronchoscopist with abundant experience with trans-bronchial lung biopsies may have advantages in the technique of VAL-MAP [13].
This study also elucidated which patients or lesions benefit from VAL-MAP. Consistent with a previous study [12], surgeons felt VAL-MAP the most valuable for the resection of pure GGNs and/or small nodules <5 mm in diameter, suggesting that palpability strongly affects the necessity of VAL-MAP. In addition, regarding surgical procedures, the contribution of VAL-MAP to complex segmentectomy and wedge resection was greater than that to simple segmentectomy, indicating that resections beyond conventional anatomical borders benefit most from the contribution of VAL-MAP. VAL-MAP contributes to complex segmentectomy by enabling marking close to an inter-segmental line; marking can be made from a bronchus in the involved segment or from a bronchus in an adjacent segment. The technical details of VAL-MAP-assisted segmentectomy have recently been reported [16].
Importantly, however, even if VAL-MAP was not rated as ‘essential’, it is important to note that most operations were still rated as ‘confident resection was made possible by VAL-MAP’. For example, VAL-MAP has been described as useful for inter-segmental demarcation even in simple anatomical segmentectomy [10]. Inter-segmental resection lines could also be determined by conventional or devised inflation–deflation lines [17], or intraoperative bronchial dye injection [18], while VAL-MAP may have potential advantages. There is no need for lung inflation that may interfere with thoracoscopy during operation; the lung map predicts an accurate distance from the tumour to resection lines in the preoperative CT scan [10]. Conversely, in most cases where VAL-MAP was rated as ‘useless’, the markings were invisible for various reasons. Thus, as long as the markings were visible, VAL-MAP conducted with the current criteria for patient selection was felt helpful in most of the operations.
Current techniques of VAL-MAP have several limitations. Dye markings were hardly visible with anthracotic or emphysematous lungs, although moderate emphysema may not be a significant challenge [13]. We also found a clear negative relationship between the marking intensity and duration from VAL-MAP to surgery (Fig. 1C), most likely due to dye absorption. Taken together, these findings suggest that a patient with risk factors for poor visibility, such as Brinkman Index > 500 [13], may need to undergo surgery immediately following the VAL-MAP procedure. Obtaining deep margins is another limitation of the present technique. Although segmentectomy is a reliable option in such a case, marking by placing a radiopaque device may overcome these challenges in the near future [10].
One of the limitations of this study design was the lack of evaluation of surgical margins despite the fact that one of the most important features of VAL-MAP is believed to be sufficient resection margins [10]. It is thought that sufficient resection margins may have a significant impact on the incidence of local recurrence and, ultimately, patients’ prognosis [19, 20], which used to be the major drawback for sub-lobar resection [21]; however, appropriate patient selection and selection of appropriate procedures are likely to result in a loco-regional recurrence rate equivalent to that of lobectomy [22]. A challenge is to standardize the measurement of surgical margins, for example, the resection margin of deflated versus inflated lungs and the management of staple lines. We are currently preparing the next national multicentre study to address the question.
In conclusion, the present multicentre study demonstrated safety, reproducibility and efficacy of VAL-MAP. Further studies are under preparation.
ACKNOWLEDGEMENTS
The authors thank all the participating centres and their staff represented by Dr Takuji Fujinaga (Nagara Medical Center, Gifu, Japan), Dr Seijiro Sato, Dr Masanori Tsuchida (Niigata University Graduate School of Medical and Dental Sciences, Niigata, Japan), Dr Toshiya Toyazaki, Dr Yasumichi Yamamoto (Shimane Prefectural Central Hospital, Izumo, Japan), Dr Jun-Ichi Nitadori, Dr Hideki Kuwano (The University of Tokyo, Tokyo, Japan), Dr Ryo Okabe, Dr Hideaki Miyamoto (Matsue Red Cross Hospital, Matsue, Japan), Dr Fumihiro Tanaka (University of Occupational and Environmental Health, Kitakyushyu, Japan), Dr Junko Tokuno, Dr Cheng-long Huang (Kitano Hospital, Osaka, Japan), Dr Toru Bando, Dr Fumitsugu Kojima (St. Luke’s International Hospital, Tokyo, Japan), Dr Osamu Mishima (Aizawa Hospital, Matsumoto, Japan), Dr Kenji Suzuki (Juntendo University School of Medicine, Tokyo, Japan), Dr Chihiro Takasaki, Dr Yasuhiro Nakashima, Dr Kenichi Okubo (Tokyo Medical and Dental University, Tokyo, Japan).
Funding
This work was supported by the internal funding of the University of Tokyo Hospital and Kyoto University Hospital.
Conflict of interest: none declared.
REFERENCES
- 1. Sato M, Omasa M, Chen F, Sato T, Sonobe M, Bando T. et al. Use of virtual assisted lung mapping (VAL-MAP), a bronchoscopic multispot dye-marking technique using virtual images, for precise navigation of thoracoscopic sublobar lung resection. J Thorac Cardiovasc Surg 2014;147:1813–9. [DOI] [PubMed] [Google Scholar]
- 2. Sortini D, Feo C, Maravegias K, Carcoforo P, Pozza E, Liboni A. et al. Intrathoracoscopic localization techniques. Review of literature. Surg Endosc 2006;20:1341–7. [DOI] [PubMed] [Google Scholar]
- 3. Kamiyoshihara M, Sakata K, Ishikawa S, Morishita Y.. Cerebral arterial air embolism following CT-guided lung needle marking. Report of a case. J Cardiovasc Surg (Torino) 2001;42:699–700. [PubMed] [Google Scholar]
- 4. Iguchi T, Yoshioka T, Muro M, Miyasho K, Inoue D, Hiraki T. et al. Systemic air embolism during preoperative pulmonary marking with a short hook wire and suture system under CT fluoroscopy guidance. Jpn J Radiol 2009;27:385–8. [DOI] [PubMed] [Google Scholar]
- 5. Sakiyama S, Kondo K, Matsuoka H, Yoshida M, Miyoshi T, Yoshida S. et al. Fatal air embolism during computed tomography-guided pulmonary marking with a hook-type marker. J Thorac Cardiovasc Surg 2003;126:1207–9. [DOI] [PubMed] [Google Scholar]
- 6. Matsuura Y, Watari M.. Two cases of air bubble in intracardiac cavity after computed tomography-guided lung puncture. JPN J Chest Surg 2010;24:967–71. [Google Scholar]
- 7. Kondo T, Tokunaga Y, Saito M, Nakagawa T.. Two cases of air embolism during percutaneous pulmonary marking under computed tomography guidance. JPN J Chest Surg 2012;26:31–5. [Google Scholar]
- 8. Dendo S, Kanazawa S, Ando A, Hyodo T, Kouno Y, Yasui K. et al. Preoperative localization of small pulmonary lesions with a short hook wire and suture system: experience with 168 procedures. Radiology 2002;225:511–8. [DOI] [PubMed] [Google Scholar]
- 9. Yoshida Y, Inoh S, Murakawa T, Ota S, Fukayama M, Nakajima J.. Preoperative localization of small peripheral pulmonary nodules by percutaneous marking under computed tomography guidance. Interact CardioVasc Thorac Surg 2011;13:25–8. [DOI] [PubMed] [Google Scholar]
- 10. Sato M. Virtual assisted lung mapping: navigational thoracoscopic lung resection. Cancer Res Front 2016;2:85–104. [Google Scholar]
- 11. Sato M, Aoyama A, Yamada T, Menjyu T, Chen F, Sato T. et al. Thoracoscopic wedge lung resection using virtual-assisted lung mapping. Asian Cardiovasc Thorac Ann 2015;23:46–54. [DOI] [PubMed] [Google Scholar]
- 12. Sato M, Yamada T, Menju T, Aoyama A, Sato T, Chen F. et al. Virtual-assisted lung mapping: outcome of 100 consecutive cases in a single institute. Eur J Cardiothorac Surg 2015;47:e131–9. [DOI] [PubMed] [Google Scholar]
- 13. Yamanashi K, Sato M, Marumo S, Fukui T, Sumitomo R, Shoji T. et al. Emphysematous lungs do not affect visibility of virtual-assisted lung mapping. Asian Cardiovasc Thorac Ann 2016;24:152–7. [DOI] [PubMed] [Google Scholar]
- 14. Tsubota N, Ayabe K, Doi O, Mori T, Namikawa S, Taki T. et al. Ongoing prospective study of segmentectomy for small lung tumors. Study group of extended segmentectomy for small lung tumor. Ann Thorac Surg 1998;66:1787–90. [DOI] [PubMed] [Google Scholar]
- 15. Du Rand IA, Blaikley J, Booton R, Chaudhuri N, Gupta V, Khalid S. et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax 2013;68: i1–44. [DOI] [PubMed] [Google Scholar]
- 16. Sato M, Murayama T, Nakajima J.. Techniques of stapler-based navigational thoracoscopic segmentectomy using virtual assisted lung mapping (VAL-MAP). J Thorac Dis 2016;8:S716–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Okada M, Mimura T, Ikegaki J, Katoh H, Itoh H, Tsubota N.. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753–8. [DOI] [PubMed] [Google Scholar]
- 18. Oh S, Suzuki K, Miyasaka Y, Matsunaga T, Tsushima Y, Takamochi K.. New technique for lung segmentectomy using indocyanine green injection. Ann Thorac Surg 2013;95:2188–90. [DOI] [PubMed] [Google Scholar]
- 19. Goldstein NS, Ferkowicz M, Kestin L, Chmielewski GW, Welsh RJ.. Wedge resection margin distances and residual adenocarcinoma in lobectomy specimens. Am J Clin Pathol 2003;120:720–4. [DOI] [PubMed] [Google Scholar]
- 20. Sawabata N, Ohta M, Matsumura A, Nakagawa K, Hirano H, Maeda H. et al. Optimal distance of malignant negative margin in excision of nonsmall cell lung cancer: a multicenter prospective study. Ann Thorac Surg 2004;77:415–20. [DOI] [PubMed] [Google Scholar]
- 21. Ginsberg RJ, Rubinstein LV.. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615–22. [DOI] [PubMed] [Google Scholar]
- 22. De Giacomo T, Di Stasio M, Diso D, Anile M, Venuta F, Furio Coloni G.. Sub-lobar lung resection of peripheral T1N0M0 NSCLC does not affect local recurrence rate. Scand J Surg 2009;98:225–8. [DOI] [PubMed] [Google Scholar]