Abstract
OBJECTIVES: Availability of donor lungs suitable for transplant falls short of current demand and contributes to waiting list mortality. Ex vivo lung perfusion (EVLP) offers the opportunity to objectively assess and recondition organs unsuitable for immediate transplant. Identifying robust biomarkers that can stratify donor lungs during EVLP to use or non-use or for specific interventions could further improve its clinical impact.
METHODS: In this pilot study, 16 consecutive donor lungs unsuitable for immediate transplant were assessed by EVLP. Key inflammatory mediators and tissue injury markers were measured in serial perfusate samples collected hourly and in bronchoalveolar lavage fluid (BALF) collected before and after EVLP. Levels were compared between donor lungs that met criteria for transplant and those that did not.
RESULTS: Seven of the 16 donor lungs (44%) improved during EVLP and were transplanted with uniformly good outcomes. Tissue and vascular injury markers lactate dehydrogenase, HMGB-1 and Syndecan-1 were significantly lower in perfusate from transplanted lungs. A model combining IL-1β and IL-8 concentrations in perfusate could predict final EVLP outcome after 2 h assessment. In addition, perfusate IL-1β concentrations showed an inverse correlation to recipient oxygenation 24 h post-transplant.
CONCLUSIONS: This study confirms the feasibility of using inflammation and tissue injury markers in perfusate and BALF to identify donor lungs most likely to improve for successful transplant during clinical EVLP. These results support examining this issue in a larger study.
Keywords: Lung transplant , EVLP, Lung injury, Inflammation, Biomarkers
INTRODUCTION
Normothermic ex vivo lung perfusion (EVLP) has emerged as a promising technique to expand the donor pool by assessing and reconditioning donor lungs previously considered unsuitable for transplant [1]. The lung is highly susceptible to injury in the critical care environment, and in the hours or days leading up to the donor’s demise it may be exposed to the sequelae of brain-stem death together with infection, aspiration, barotrauma, fluid overload or multiple transfusions [2–4]. Because the extent of lung injury is difficult to assess at the time of organ procurement, donor acceptance criteria are therefore conservative and poor discriminators of injury and infection in the donor lung [5]. The increased use of extended-criteria donors may further elevate the risk of primary graft dysfunction (PGD) and other more severe postoperative complications [6–8].
The use of ex vivo reconditioned donor lungs is steadily growing and now accounts for about 20% of the activity in some established centres [9–11]. The decision to accept organs for transplant after EVLP has, however, been based on the same questionable physiological measures and organ appearance used during standard procurement. Reported discard rates of 10–60% of perfused lungs suggest that some donor lungs may be inappropriately used or declined for transplant after EVLP [1].
In donor lungs transplanted without exvivo evaluation, there is an established relationship between their inflammatory burden and early outcome [12–15]. However, this phenomenon has not been as extensively investigated during EVLP.
In this proof-of-concept study, we evaluated a panel of inflammatory mediators and tissue injury-associated proteins in both perfusate and bronchoalveolar lavage fluid (BALF) from human donor lungs undergoing clinical EVLP with intent for transplant. The panel was based on our own previous work and on available studies of biological markers in standard lung transplant and preclinical and clinical observations during EVLP. The aim was to assess the potential for specific protein markers in perfusate and BALF to distinguish which donor lungs, initially deemed unsuitable for immediate transplantation are most likely to successfully recondition during EVLP and thereby provide a basis for further investigations in a larger validation cohort.
MATERIALS AND METHODS
Study design
We conducted a prospective observational study investigating protein expression in donor lungs exposed to a standardized EVLP protocol. Approval was granted by our local research ethics committee, and informed consent for research was obtained from donor families and lung transplant recipients (REC 09/H0905/10).
Ex vivo lung perfusion protocol
Adult donor lungs deemed unsuitable for lung transplant by all five UK lung transplant centres but meeting strict EVLP criteria were included in the study (Supplementary Table S1), the results of which were previously published by our group [16]. Lungs were procured in a routine fashion and transported to our institution. The EVLP assessment followed a standardized acellular protocol using the Toronto technique with a closed left atrium and reduced perfusate flow, previously described in detail [3, 16]. Transplant suitability was assessed hourly during perfusion. Lungs meeting transplant criteria (Supplementary Table S2) at two consecutive time points were cooled and transplanted. Lungs deemed to have futile prospects for improvement were taken off the circuit and discarded. Two transplanted and five non-transplanted lungs were perfused ≥5h before a transplant decision was made.
Sample collection and processing
A research BAL was performed for all lungs by wedging an adult bronchoscope in a subsegmental bronchus of the right or left lower lobe [17]. Saline (40 ml) was instilled through the suction channel followed by gentle aspiration and sample collection prior to commencing ventilation at the beginning of EVLP. This process was repeated in the same lobe but in a different subsegmental bronchus before disconnecting the ventilation at the end of perfusion. In addition, hourly perfusate samples of 2.5 ml were collected until the assessment was stopped.
Protein expression analysis
All protein expressions measured in perfusate were adjusted to the predicted total lung capacity (pTLC) of the donor as an estimate of perfused donor lung volume and were reported as corrected perfusate concentrations (pg/ml). The pTLC was calculated in a routine fashion based on donor gender and height [18]. If one lung was deemed unusable due to severe consolidation or extensive contusion on inspection, or if the intended recipient required a single lung transplant on a specific side, only one lung was procured. For single-lung perfusions, the pTLC was adjusted to a factor of 0.55 for right lung and 0.45 for left lung perfusion [19].
Lactate dehydrogenase assay
Lactate dehydrogenase (LDH) levels were measured in perfusate and BALF with a colorimetric LDH cytotoxicity assay kit according to manufacturer’s instructions and reported as arbitrary units (U) (Thermo Fisher Scientific Inc., Rockford, IL, USA).
Multiplex inflammatory cytokine array
Interleukin (IL)-1β, IL-6, IL-8, TNF-α, and IL-10 were analysed with an MSD Multi-Array® (Meso Scale Diagnostics, LLC, Rockville, MD, USA). The assay was performed according to the manufacturer’s instructions. Technical issues prevented reading of perfusate samples from donor lung EVLP03; therefore, these samples were excluded from the analysis.
Enzyme-linked immunosorbent assays
Syndecan-1, IL-33, S100A9 (all R&D Systems, Inc., Minneapolis, MN, USA) and high-mobility group box-1 (HMGB-1) (Shino-Test Corporation, Kanagawa, Japan) were measured with commercially available ELISA kits according to the manufacturers’ instructions.
Statistical analysis
Donor characteristics and physiological parameters are expressed as medians with interquartile ranges and were compared between transplanted and non-transplanted lungs using Mann–Whitney U tests. Paired samples, start-end of perfusion, were compared with Wilcoxon signed-ranks tests. Log protein expressions were compared between transplanted and non-transplanted lungs with multiple t-tests. Correlations between IL-8 and IL-1β expressions and seven post-transplant outcomes (PaO2:FiO2 24-h post-transplant; PGD3 at 72 h post-transplant; ventilation time; intensive care unit stay hospital stay‘; percent of predicted FEV1 at 6 months post-transplant; and percent of predicted FVC at 6 months post-transplant) were analysed by Pearson’s correlation tests. The data were transformed back into non-logged values for reporting as mean (T) for transplanted and mean (NT) for non-transplanted lungs with standard deviations (SD). Multiple testing of donor parameters, protein analyses, and correlations was corrected using the Benjamini-Hochberg false discovery rate (FDR) controlling procedure [20]. Because this was a feasibility study aiming to identify potential markers for further investigation in a validation cohort, an FDR corrected P-value <0.1 was deemed significant. Corrected P-values are reported.
A multiple logistic regression model was fitted using the 11 log transformed protein covariates and their squared counterparts as independent variables and the EVLP outcome as the dependent variable. The optimal model was established by leave-one-out cross-validation using the software package R from R Core Team (2014) (R Foundation for Statistical Computing, Vienna, Austria) [21].
RESULTS
Study group and donor characteristics
Sixteen consecutive clinical EVLP assessments of lungs deemed unacceptable for standard transplant were included in the study (Table 1). Seven (44%) were accepted for transplant after EVLP assessment and were implanted as four bilateral and three single-lung procedures. Nine (56%) failed to achieve transplant criteria and were excluded. The characteristics of the donor groups of transplanted and non-transplanted lungs were not significantly different and are shown in Tables 1 and 2.
Table 1:
Donor characteristics
| Donor no. | Age/ gender | Smoker | Donor cause of death | Donor type | TLC (L) | Micro on offer | Radiographic infiltrates | Secretions | EVLP indication |
|---|---|---|---|---|---|---|---|---|---|
| EVLP 01 | 18 m | No | Diabetic coma | DL DBD | 7.30 | Nil | Bilateral | Nil | PaO2 <300 mmHg |
| EVLP 02 | 51 f | Yes | ICH | DL DBD | 4.31 | Nil | Clear | Nil | PaO2 <300 mmHg |
| EVLP 03 | 33 m | Yes | ICH | DL DBD | 7.14 | Nil | Left diffuse | Purulent | PaO2 <300 mmHg |
| EVLP 04 | 15 f | No | Hanging | DL DCD | 5.10 | Nil | Bilateral | Nil | PaO2 <300 mmHg |
| EVLP 05 | 52 m | No | ICH | DL DBD | 5.78 | Nil | Clear | Mucopurulent | PaO2 <300 mmHg |
| EVLP 06 | 57 m | Yes | ICH | DL DBD | 7.54 | Nil | Right basal | Mucopurulent | oedema |
| EVLP 07 | 52 f | Yes | ICH | SL DBD | 2.70 | Nil | Bibasal | Purulent | PaO2 <300 mmHg |
| EVLP 08 | 20 m | Yes | Hanging | SL DBD | 3.18 | Nil | Right basal | Nil | PV PaO2 <225 mmHg + oedema |
| EVLP 09 | 36 m | No | Brain tumour | SL DBD | 3.11 | Yesa | Left diffuse | Nil | PaO2 <300 mmHg |
| EVLP 10 | 47 m | No | HBI | DL DBD | 6.74 | Nil | Clear | Nil | PaO2 <300 mmHg |
| EVLP 11 | 45 f | No | TBI | DL DBD | 6.18 | Nil | Clear | Nil | PV PaO2 <225 mmHg + mild contusion RLL |
| EVLP 12 | 56 m | No | ICH | DL DBD | 6.50 | Nil | Clear | Purulent | PaO2 <300 mmHg |
| EVLP 13 | 18 f | Yes | ICH | DL DCD | 5.03 | Nil | Bilateral | Mucopurulent | oedema |
| EVLP 14 | 53 m | Yes | ICH | DL DBD | 7.06 | Nil | Clear | Purulent | PaO2 <300 mmHg |
| EVLP 15 | 48 f | No | ICH | DL DBD | 4.57 | Nil | Bibasal | Purulent | PaO2 <300 mmHg |
| EVLP 16 | 38 m | Yes | Hanging | DL DCD | 6.98 | Nil | Left basal | Nil | oedema |
Shaded donor lungs were not transplanted after EVLP .
BALF: bronchoalveolar lavage fluid; EVLP : ex vivo lung perfusion; ICH: intracranial haemorrhage; HBI: hypoxic brain injury secondary to variceal bleed; TBI: traumatic brain injury following a road traffic accident; DL: double lung perfusion; SL: single lung perfusion; DBD: organ donation after brain death; DCD: organ donation after circulatory death; TLC: predicted total lung capacity of perfused lung; PaO2: partial pressure of arterial oxygen; PV: selective pulmonary vein blood gas analyses; RLL: right lower lobe.
Sputum culture with moderate growth of Staphylococcus aureus, Haemophilus influenzae, and Streptococcus pneumoniae.
Table 2:
EVLP and transplant outcomes
| Donor no. | Optimized donor PaO2:FiO2 (mmHg) | PaO2:FiO2 after EVLP (mmHg) | Ischaemic time (min)a | EVLP time (min)b | Total time ex vivo (min)c | Transplant | PGD 3 at 72 h | 90-day survival |
|---|---|---|---|---|---|---|---|---|
| EVLP 01 | 149 | 521 | 345 | 290 | 1020 | Yes–BL | Yes | Yes |
| EVLP 02 | 248 | 342 | 360 | 360 | N/A | No–emphysema | ||
| EVLP 03 | 209 | 167 | 330 | 270 | N/A | No–gross oedema | ||
| EVLP 04 | 222 | 426 | 450 | 360 | 870 | Yes–SL | No | Yes |
| EVLP 05 | 167 | 440 | 320 | 330 | N/A | No–heavy gram-stain in BALF | ||
| EVLP 06 | 357 | 525 | 395 | 240 | 582 | Yes–SL | No | Yes |
| EVLP 07 | 178 | 557 | 315 | 255 | 1048 | Yes–SL | Yes | Yes |
| EVLP 08 | 372 | 392 | 340 | 240 | N/A | No–emphysema | ||
| EVLP 09 | 293 | 330 | 430 | 300 | N/A | No–gross oedema | ||
| EVLP 10 | 171 | 291 | 305 | 300 | N/A | No–gross oedema | ||
| EVLP 11 | 526 | 452 | 340 | 330 | N/A | No–gross oedema | ||
| EVLP 12 | 191 | 512 | 395 | 220 | 785 | Yes–BL | No | Yesd |
| EVLP 13 | 360 | 352 | 345 | 180 | N/A | No–gross oedema | ||
| EVLP 14 | 165 | 516 | 270 | 190 | 496 | Yes–BL | No | Yes |
| EVLP 15 | 293 | 443 | 360 | 165 | 713 | Yes–BL | No | Yes |
| EVLP 16 | 527 | 322 | 335 | 180 | N/A | No–gross oedema |
Shaded donor lungs were not transplanted after EVLP.
PaO2:FiO2: the ratio of partial pressure of arterial oxygen to fraction of inspired oxygen; EVLP: ex vivo lung perfusion; PGD: primary graft dysfunction at 72 h; SL: single lung transplant; BL: bilateral lung transplant; N/A: not applicable; BALF: bronchoalveolar lavage fluid.
Start of donor lung flush at procurement to start of pulmonary artery (PA) perfusion on EVLP circuit.
Start of PA perfusion to disconnection from EVLP circuit.
Start of donor lung flush at procurement to reperfusion in recipient.
Death due to H1N1 infection 11 months post-transplant.
Ex vivo lung perfusion outcomes
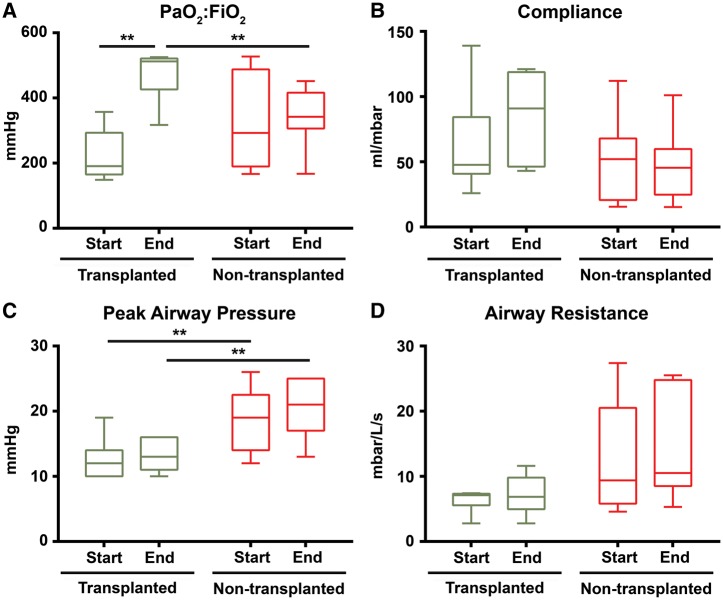
The median partial pressure of oxygen on 100% inspired oxygen (PaO2:FiO2) at the end of EVLP was 516 (478–523) mmHg for transplanted and 342 (322–392) mmHg for non-transplanted donor lungs (Mann–Whitney U = 4, P = 0.02). The median PaO2:FiO2 improvement after EVLP was 321 (186–362) mmHg in transplanted donor lungs (Wilcoxon Z = −2.37, P = 0.03). No improvement was seen in lungs that failed assessment; the median PaO2:FiO2 change was 20 (−42–94) mmHg, (Wilcoxon Z = −0.18, P = 0.91) (Fig. 1).
Figure 1:
Physiological characteristics of transplanted and non-transplanted EVLP donor lungs. Box and whisker plots with boxes showing medians with interquartile range and whiskers at max and min. **P<0.05. EVLP; start time: point when the lung had been successfully rewarmed to 37 °C in the beginning of the EVLP; end time: point before start of cooling at the end of the EVLP.
Of the nine lungs that failed EVLP reconditioning, six displayed worsening pulmonary oedema and two showed signs of persistent hyperinflation and raised PVR, suggesting diffuse injury and a degree of intrinsic emphysema. This suggestion was later verified by a pathologist from pulmonary biopsies. Lastly, one set of lungs was turned down due to heavy microbial contamination with multiple gram-negative organisms identified in the tracheal aspirate.
Transplant outcomes
In the seven donor lungs that were transplanted, perfusion and ventilation parameters remained stable or improved during assessment. The data on changes in lung compliance, airway resistance, PVR, perfusate flow, and ventilation and perfusion pressures are shown in Fig. 1.
None of the recipients required extracorporeal life support. Two patients (29%) suffered from PGD (Grade 3) at 72 h post-transplant. However, both patients responded well to standard management. All seven recipients survived to hospital discharge. One recipient died of severe pneumonia at 11 months post-transplant due to an influenza infection unrelated to the EVLP procedure. The other six (86%) remain alive and well >2 years after transplant (Table 2).
Analysis of perfusate and bronchoalveolar lavage fluid
Assessed lungs were divided into two groups based on decision after EVLP: transplanted (n = 7) or non-transplanted (n = 9). Decision on transplant suitability was based on strict criteria summarized in Supplementary Table S2. Samples were analysed retrospectively for protein expressions.
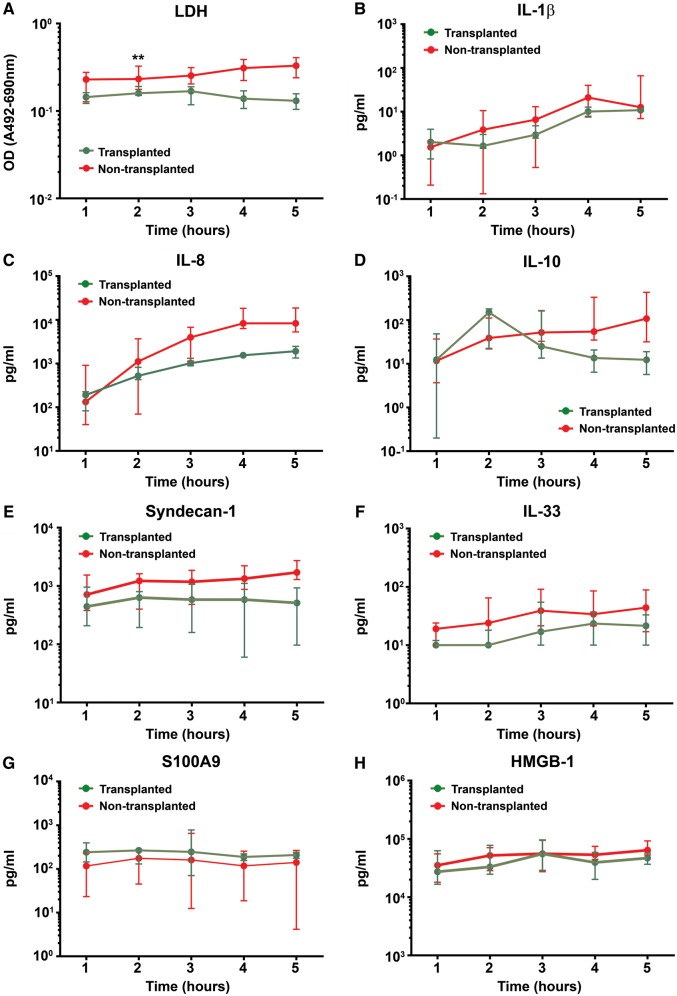
For an initial general assessment of tissue injury, we measured LDH levels. LDH was found in substantially lower levels in perfusate from lungs meeting criteria for transplant (T) after EVLP compared to those that did not meet the criteria for transplant (NT) lungs. Suitability for transplant was evident after 2 h of perfusion: mean (T) = 0.150 U (SD 0.030, n = 14) and mean (NT) = 0.223 U (SD 0.082, n = 18) [t(30) = 2.77, P = 0.03] (Fig. 2). The LDH perfusate levels remained significantly different when assessed over all sample time points: mean (T) = 0.149 U (SD 0.033, n = 24) and mean (NT) = 0.259 U (SD 0.088, n = 40) [t(62) = 5.45, P < 0.001]. No difference in LDH levels was seen in BALF samples collected pre- and post-perfusion.
Figure 2:
Protein expressions during EVLP in perfusate from transplanted and non-transplanted donor lungs. Protein levels expressed as medians with interquartile ranges. **P<0.05. EVLP: ex vivo lung perfusion; LDH: lactate dehydrogenase; IL: interleukin; TNF-α: tumour necrosis factor alpha; HMGB-1: high-mobility group box-1; Time: hours of ex vivo lung perfusion after start of pulmonary artery perfusion.
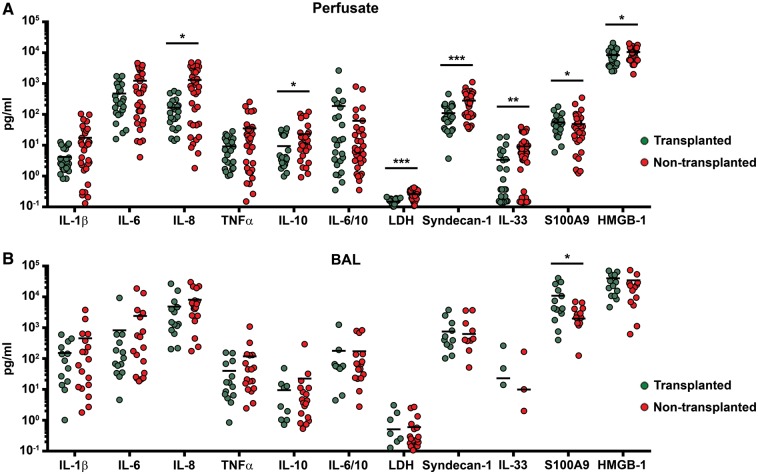
To assess the integrity of the pulmonary vascular compartment, we measured Syndecan-1 levels as a marker of endothelial glycocaylx disruption. Syndecan-1 levels were significantly lower in perfusate samples from transplanted donor lungs: mean (T) = 111 (pg/ml) (SD 98, n = 24) compared to mean (NT) = 281 (pg/ml) (SD 219, n = 40) [t(62) = 4.14, P < 0.001] (Fig. 3).
Figure 3:
Protein expressions in perfusate and BALF from transplanted and non-transplanted EVLP donor lungs. Interleaved scatter plots with molecular marker levels expressed in (pg/ml) and lines representing means. *P<0.1. **P<0.05. ***P<0.001. BALF: bronchoalveolar lavage fluid; EVLP: ex vivo lung perfusion; IL: interleukin; TNF-α: tumour necrosis factor alpha; LDH: lactate dehydrogenase; HMGB-1: high-mobility group box-1.
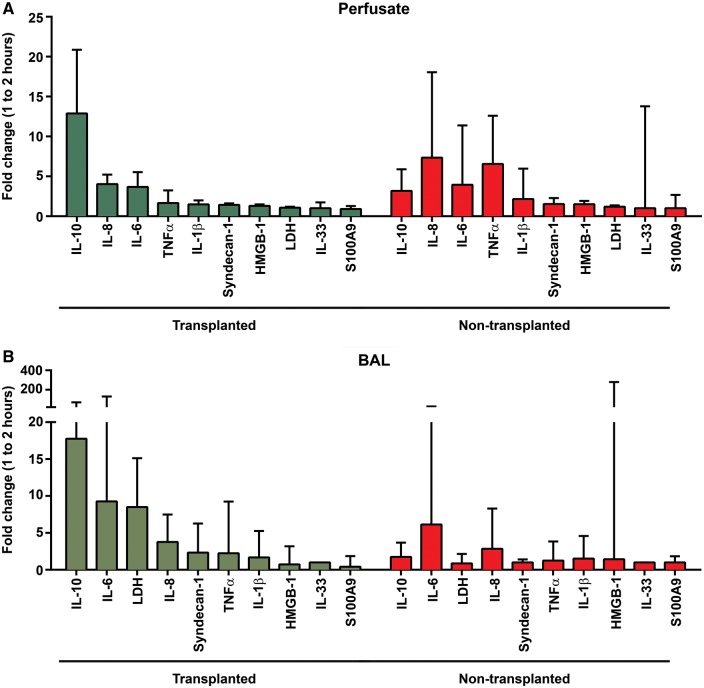
We noted a similarly consistent pattern towards lower release of proinflammatory cytokines into the circulating perfusate from transplanted compared to non-transplanted lungs. The average level of IL-8 was nearly eight times higher in perfusate samples from non-transplanted lungs: mean (T) = 165 (pg/ml) (SD 155, n = 24) compared to mean (NT) = 1310 (pg/ml) (SD 1510, n = 35) [t(57) = 2.27, P = 0.06]. Levels of the anti-inflammatory cytokine IL-10 increased noticeably over the first 2 h of perfusion in the transplanted group, with high initial fold changes in both perfusate and BALF (Fig. 4). Perfusate levels then diminished and were less than half those in non-transplanted lungs when measured over the full assessment: mean (T) = 9 (pg/ml) (SD 11, n = 24) compared to mean (NT) = 23 (pg/ml) (SD 32, n = 40) [t(57) = 2.02, P = 0.07]. No difference in levels of IL-10 in BALF was seen between the groups.
Figure 4:
Protein median fold changes in perfusate and BALF from EVLP donor lungs. Bar graphs showing median fold changes with interquartile range. BALF: bronchoalveolar lavage fluid; EVLP: ex vivo lung perfusion; IL: interleukin; TNF-α: tumour necrosis factor alpha; LDH: lactate dehydrogenase; HMGB-1: high-mobility group box-1.
A panel of three damage-associated molecular patterns, IL-33, HMGB-1 and S100A9, was used to assess the extent of cellular injury in the donor lung. Lower levels of IL-33 and HMGB-1 were found in perfusate from transplanted donor lungs. The average IL-33 levels in perfusate were mean (T) = 3 (pg/ml) (SD 5, n = 24) and mean (NT) = 9 (pg/ml) (SD 10, n = 40) [t(62) = 2.93, P = 0.02]. HMGB-1 was highly expressed in both perfusate and BALF. The HMGB-1 levels in perfusate were mean (T) = 8318 (pg/ml) (SD 4474, n = 24) and mean (NT) = 10 545 (pg/ml) (SD 4,313, n = 40) [t(62) = 2.10, P = 0.07]. No difference in lavage fluid levels of IL-33 or HMGB-1 was seen between the groups.
We noted a consistent pattern towards increasing perfusate protein levels and a more pronounced separation between transplanted and non-transplanted lungs over time of perfusion (Fig. 2). This pattern was most noticeable for the investigated inflammatory cytokines. The only protein marker not showing this pattern was S100A9, which appeared lower in the non-transplanted group.
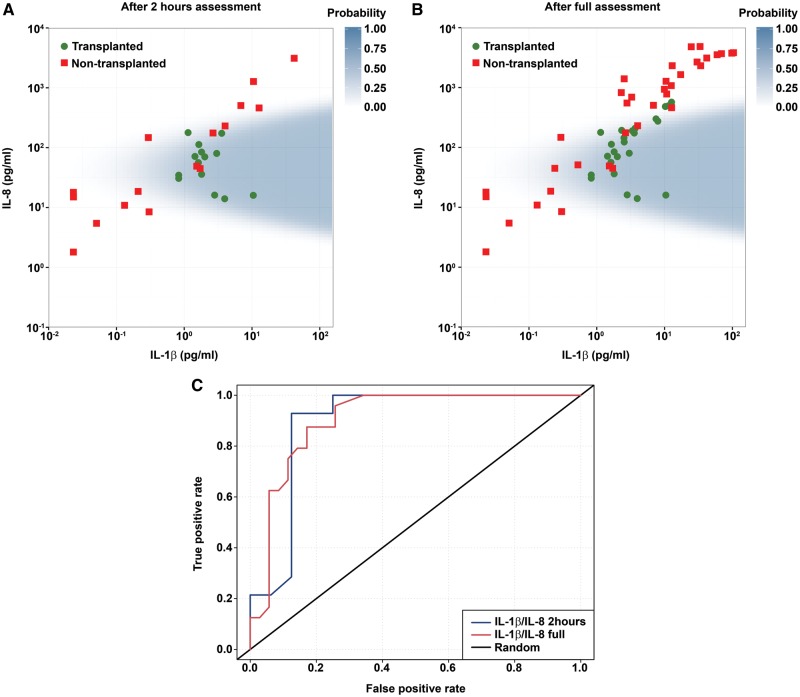
A multivariate analysis model was fitted to assess the predictive value of combining two or more protein markers. The optimal model was established by leave-one-out cross-validation and is shown in Fig. 5 as a scatterplot of IL-1β and IL-8 perfusate levels from the 16 assessments. The shaded prediction region was derived from the regression analysis with covariates IL-1β, IL-8 and (IL-8)2 after 2 h of perfusion. The model suggests that the closer to the centre of the area, the higher was the probability of a donor lung being found suitable for transplant after EVLP assessment. This early IL-1β–IL-8 signature remained equally as robust when applied to the full set of samples (Fig. 5B). An ROC curve showing the potential benefit of the model in predicting EVLP outcome compared to random is shown in Fig. 5C, with a sensitivity of 0.81 and specificity of 0.92 at 2 h of perfusion.
Figure 5:
(A) Early IL-1β-IL-8 signature in EVLP perfusate after 2 h of perfusion. (B) IL-1β-IL-8 signature in EVLP perfusate after full assessment. (C) ROC curve. (A) and (B) Scatter plots of IL-1β and IL-8 levels in perfusate samples from transplanted and non-transplanted EVLP donor lungs. The shaded area is the prediction region from the optimal logistic regression model with covariates IL-1β, IL-8 and (IL-8)2. (C) Receiver operating characteristic (ROC) curve of the model as a predictor of EVLP outcome. Calculated sensitivity (y axis) is plotted against the 1-specificity formula (x axis) of the logistic regression function 2 h into perfusion and after full assessment.
EVLP: ex vivo lung perfusion; IL: interleukin.
The modelled proteins were lastly assessed for correlations to post-transplant outcome measures. Donor IL-1β levels in the perfusate at time of transplant decision showed a significant negative correlation to recipient PaO2:FiO2 24 h post-transplant (r = −093, P = 0.03) (Fig. 6). No other correlations remained significant after corrections for multiple testing in this cohort.
Figure 6:
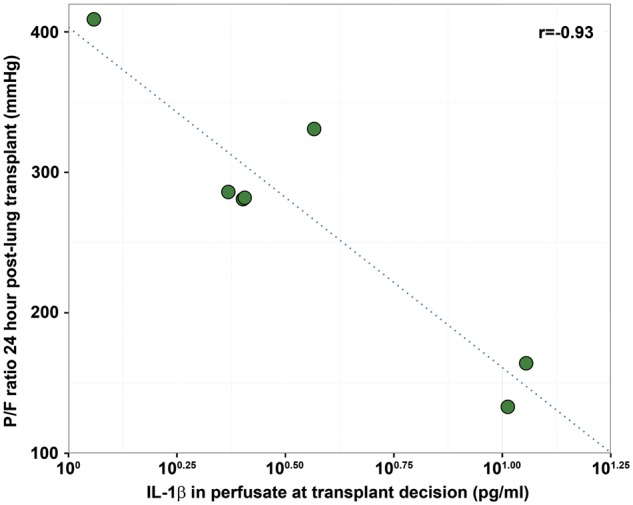
Association between donor IL-1β level in EVLP perfusate at the time of transplant decision and recipient P/F ratio 24 h post lung transplantation. Pearson correlation plot. **P<0.05. EVLP: ex vivo lung perfusion; IL: interleukin; PaO2:FiO2: partial pressure of oxygen on 100% inspired oxygen.
DISCUSSION
In this proof-of-concept study, the feasibility of using inflammation and tissue injury markers in both perfusate and BALF from human donor lungs undergoing EVLP to validate the decision to use or decline them for clinical transplant was evaluated. All 16 donor lungs in this study were assessed consecutively with intent for transplant following a strict protocol with predefined criteria for EVLP and transplant suitability to reduce the risk of bias in decision making. All protein analyses were done retrospectively and had no impact on the transplant decision. Our results demonstrate a difference in protein expression in perfusate from lungs that were transplanted compared with those that were declined. This difference became more apparent for each hour of perfusion, and BALF samples showed a comparable but weaker signal from the airway compartment.
The difference in donor lungs was effectively demonstrated by a logistic regression model combining inflammatory cytokines IL-1β and IL-8 after 2 h of perfusion. The model revealed a clear separation of the transplanted lungs from those declined. Importantly, the model remained equally as robust when applied to perfusate samples from all time points. Furthermore, perfusate IL-1β concentrations in transplanted lungs demonstrated a clear negative correlation to early recipient oxygenation post-transplant.
Even though our results are noteworthy, overinterpretation of this regression model should be avoided at this stage. Further investigations are required to assess its biological plausibility. A few declined lungs displayed a very low IL-1β/IL-8 signal—below that of lungs deemed reconditioned and transplanted with good outcomes after the EVLP assessment. If this finding indicates that those lungs were wrongfully declined and could safely have been transplanted, that EVLP-assessed lungs deemed suitable for transplant truly express an intermediate level of inflammation, or that this is a signal specific to this relatively small cohort needs further assessment in follow-up studies.
The measurement of LDH levels in perfusate had the potential to significantly predict EVLP outcome as early as 2 h into perfusion. Although real-time ligand binding assays for inflammatory cytokines and other tissue injury markers may soon become accessible [22], LDH has the benefit of being a widely recognized marker of tissue injury in serum and BALF and rapidly available as a point-of-care test [23, 24].
The Brisbane lung transplant group have described measures of endothelial dysfunction as potentially useful in EVLP [25, 26]. Our results strengthen that belief. Syndecan-1, released by disruption of the endothelial glycocalyx, may be a discriminatory marker in EVLP perfusate and should be further evaluated.
Increasing levels of inflammatory cytokines in perfusate and tissue have been shown during experimental lung perfusion in porcine models and a small preclinical human lung study, which might reflect procedure-mediated inflammation [27–29]. Our findings during full clinical EVLP support recent observations made by Machuca and colleagues [30] from Toronto and demonstrate the feasibility of identifying biomarkers that might improve EVLP assessment.
IL-8, proposed as one of the best markers of EVLP transplant outcome in the Machuca study, has consistently shown potential in previous studies of donor lung injury from our group [12, 13]. The potential of IL-8 to discriminate successful EVLP in our study was enhanced by combining it with IL-1β.
Our observations add significantly to previous observations because we have included an assessment of BALF inflammatory and tissue injury markers compared to EVLP perfusate measures. Even though BALF samples in this study did not add any apparent clinical value to perfusate sampling, we feel it is too early to draw any firm conclusions, and we will continue to assess a broad spectrum of markers and lung compartment samples in future studies. In addition, because protein levels are measured in a fixed volume of 2 litres of perfusate solution, we corrected protein levels for the size of lung tissue perfused. This potential confounder has been disregarded in previous studies. The pTLC of the donor lung ranged from 4.31 to 7.54 litres among the double lungs perfused, and the smallest single lung had a pTLC of only 2.70 litres. Unadjusted marker levels are therefore likely to differ widely between donor lungs regardless of the degree of lung injury present.
A limitation of our study is its relatively small sample size, with 16 human EVLP assessments included, yet this number is sufficient to demonstrate the feasibility of using perfusate markers to discriminate successful EVLP. Outcomes were almost universally good, and this study does not identify predictors of early lung injury. Larger studies, with a cohort of patients doing less well, are needed to address that particular question. Caution is, however, required in overinterpretation of moderate to severe PGD (Grade 2–3) after transplant as a reason for donor lungs to be declined after EVLP, because many recipients with PGD 2–3 will recover and have satisfactory early outcomes.
Truly marginal donor lungs subjected to EVLP for reconditioning purposes form by nature a subpopulation that is likely to have a higher inflammatory burden than standard donor lungs. We believe that biomarker profiles of these lungs could help support the clinical decision to use or decline the donor lungs after EVLP and allow continued safe transplant activity. Furthermore, this approach offers the potential to attenuate the inflammatory response or encourage recovery of vascular integrity in the donor lung before implantation in a stratified medicine approach, which may improve early outcomes after lung transplant and help to safely maximize lung use from the existing donor pool.
The results of this study need further evaluation in a larger validation cohort of lungs exposed to ex vivo perfusion but demonstrate the feasibility of identifying an early perfusate signature potentially predictive of successful EVLP reconditioning.
SUPPLEMENTARY MATERIAL
Supplementary material (Tables 1 and 2) is available at EJCTS online.
Fundi ng
This work was also partly funded by the National Institute for Health Research Blood and Transplant Research Unit (NIHR BTRU) in Organ Donation and Transplantation at the University of Cambridge in collaboration with Newcastle University and in partnership with NHS Blood and Transplant (NHSBT). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health or NHSBT. The funding organizations had no role in the collection of data, its analysis or interpretation, and had no right to approve or disapprove publication of the finished manuscript. This study was supported by a research grant awarded by the United Kingdom Cystic Fibrosis Trust as a result of a donation made by the Robert Luff Foundation for research on lung transplants.
Conflict of interest: none declared.
Supplementary Material
REFERENCES
- 1. Andreasson AS, Dark JH, Fisher AJ.. Ex vivo lung perfusion in clinical lung transplantation–state of the art. Eur J Cardiothorac Surg 2014;46:779–88. [DOI] [PubMed] [Google Scholar]
- 2. Avlonitis VS, Fisher AJ, Kirby JA, Dark JH.. Pulmonary transplantation: the role of brain death in donor lung injury. Transplantation 2003;75:1928–33. [DOI] [PubMed] [Google Scholar]
- 3. Cypel M, Yeung JC, Hirayama S, Rubacha M, Fischer S, Anraku M. et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant 2008;27:1319–25. [DOI] [PubMed] [Google Scholar]
- 4. Reyes KG, Mason DP, Thuita L, Nowicki ER, Murthy SC, Pettersson GB. et al. Guidelines for donor lung selection: time for revision? Ann Thorac Surg 2010;89:1756–64. [DOI] [PubMed] [Google Scholar]
- 5. Fisher AJ, Donnelly SC, Pritchard G, Dark JH, Corris PA.. Objective assessment of criteria for selection of donor lungs suitable for transplantation. Thorax 2004;59:434–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Perrot M, Sekine Y, Fischer S, Waddell TK, McRae K, Liu M. et al. Interleukin-8 release during early reperfusion predicts graft function in human lung transplantation. Am J Respir Crit Care Med 2002;165:211–5. [DOI] [PubMed] [Google Scholar]
- 7. Christie JD, Kotloff RM, Ahya VN, Tino G, Pochettino A, Gaughan C. et al. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med 2005;171:1312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diamond JM, Lee JC, Kawut SM, Shah RJ, Localio AR, Bellamy SL. et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 2013;187:527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cypel M, Yeung JC, Liu M, Anraku M, Chen F, Karolak W. et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 2011;364:1431–40. [DOI] [PubMed] [Google Scholar]
- 10. Boffini M, Ricci D, Barbero C, Bonato R, Ribezzo M, Mancuso E. et al. Ex vivo lung perfusion increases the pool of lung grafts: analysis of its potential and real impact on a lung transplant program. Transplant Proc 2013;45:2624–6. [DOI] [PubMed] [Google Scholar]
- 11. Sage E, Mussot S, Trebbia G, Puyo P, Stern M, Dartevelle P. et al. Lung transplantation from initially rejected donors after ex vivo lung reconditioning: the French experience. Eur J Cardiothorac Surg 2014;46:794–9. [DOI] [PubMed] [Google Scholar]
- 12. Fisher AJ, Donnelly SC, Hirani N, Haslett C, Strieter RM, Dark JH. et al. Elevated levels of interleukin-8 in donor lungs is associated with early graft failure after lung transplantation. Am J Respir Crit Care Med 2001;163:259–65. [DOI] [PubMed] [Google Scholar]
- 13. Fisher AJ, Donnelly SC, Hirani N, Burdick MD, Strieter RM, Dark JH. et al. Enhanced pulmonary inflammation in organ donors following fatal non-traumatic brain injury. Lancet 1999;353:1412–3. [DOI] [PubMed] [Google Scholar]
- 14. Kaneda H, Waddell TK, de Perrot M, Bai XH, Gutierrez C, Arenovich T. et al. Pre-implantation multiple cytokine mRNA expression analysis of donor lung grafts predicts survival after lung transplantation in humans. Am J Transplant 2006;6:544–51. [DOI] [PubMed] [Google Scholar]
- 15. Saito T, Takahashi H, Kaneda H, Binnie M, Azad S, Sato M. et al. Impact of cytokine expression in the pre-implanted donor lung on the development of chronic lung allograft dysfunction subtypes. Am J Transplant 2013;13:3192–201. [DOI] [PubMed] [Google Scholar]
- 16. Andreasson A, Karamanou DM, Perry JD, Perry A, Oezalp F, Butt T. et al. The effect of ex vivo lung perfusion on microbial load in human donor lungs. J Heart Lung Transplant 2014;33:910–16. [DOI] [PubMed] [Google Scholar]
- 17. Haslam PL, Baughman RP.. Report of ERS Task Force: guidelines for measurement of acellular components and standardization of BAL. Eur Respir J 1999;14:245–8. [DOI] [PubMed] [Google Scholar]
- 18. Stocks J, Quanjer PH.. Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on lung volume measurements. Official statement of the European Respiratory Society. Eur Respir J 1995;8:492–506. [DOI] [PubMed] [Google Scholar]
- 19. Johansen B, Bjortuft O, Boe J.. Static lung volumes in healthy subjects assessed by helium dilution during occlusion of one mainstem bronchus. Thorax 1993;48:381.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Benjamini Y, Hochberg Y.. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 1995;57:289–300. [Google Scholar]
- 21. Picard RR, Cook RD.. Cross-validation of regression-models. J Am Stat Assoc 1984;79:575–83. [Google Scholar]
- 22. Fraser S, Cameron M, O'connor E, Schwickart M, Tanen M, Ware M.. Next generation ligand binding assays-review of emerging real-time measurement technologies. AAPS J 2014;16:914–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Drent M, Cobben NA, Henderson RF, Wouters EF, van Dieijen-Visser M.. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J 1996;9:1736–42. [DOI] [PubMed] [Google Scholar]
- 24. Londeree W, Davis K, Helman D, Abadie J.. Bodily fluid analysis of non-serum samples using point-of-care testing with iSTAT and Piccolo analyzers versus a fixed hospital chemistry analytical platform. Hawaii J Med Public Health 2014;73:3–8. [PMC free article] [PubMed] [Google Scholar]
- 25. Chambers DC, Hunt W, Smith IJ, Samson L, Sladden TM, Yerkovich S. et al. Endothelial glycocalyx integrity is critical to organ function during human ex-vivo lung perfusion. J Heart Lung Transplant 2014;33:S17. [Google Scholar]
- 26. Falconnet D, She J, Tornay R, Leimgruber E, Bernasconi D, Lagopoulos L. et al. Rapid, sensitive and real-time multiplexing platform for the analysis of protein and nucleic-acid biomarkers. Anal Chem 2015;87: 1582–9. [DOI] [PubMed] [Google Scholar]
- 27. Adrian K, Skogby M, Gatzinsky V, Friberg LG, Mellgren K.. Procedure-induced inflammation and endothelial cell activation in an artificially ventilated and circulated porcine double-lung model. Artif Organs 2006;30:922–28. [DOI] [PubMed] [Google Scholar]
- 28. Kakishita T, Oto T, Hori S, Miyoshi K, Otani S, Yamamoto S. et al. Suppression of inflammatory cytokines during ex vivo lung perfusion with an adsorbent membrane. Ann Thorac Surg 2010;89:1773–9. [DOI] [PubMed] [Google Scholar]
- 29. Sadaria MR, Smith PD, Fullerton DA, Justison GA, Lee JH, Puskas F. et al. Cytokine expression profile in human lungs undergoing normothermic ex-vivo lung perfusion. Ann Thorac Surg 2011;92:478–84. [DOI] [PubMed] [Google Scholar]
- 30. Machuca TN, Cypel M, Yeung JC, Bonato R, Zamel R, Chen M. et al. Protein expression profiling predicts graft performance in clinical ex vivo lung perfusion. Ann Surg 2015;261:591–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.