Abstract
STUDY QUESTION
Is oral dydrogesterone 30 mg daily (10 mg three times daily [TID]) non-inferior to micronized vaginal progesterone (MVP) 600 mg daily (200 mg TID) for luteal support in in vitro fertilization (IVF), assessed by the presence of fetal heartbeats determined by transvaginal ultrasound at 12 weeks of gestation?
SUMMARY ANSWER
Non-inferiority of oral dydrogesterone versus MVP was demonstrated at 12 weeks of gestation, with a difference in pregnancy rate and an associated confidence interval (CI) that were both within the non-inferiority margin.
WHAT IS KNOWN ALREADY
MVP is routinely used in most clinics for luteal support in IVF, but it is associated with side effects, such as vaginal irritation and discharge, as well as poor patient acceptance. Dydrogesterone may be an alternative treatment due to its patient-friendly oral administration.
STUDY DESIGN, SIZE, DURATION
Lotus I was an international Phase III randomized controlled trial, performed across 38 sites, from August 2013 to March 2016. Subjects were premenopausal women (>18 to <42 years of age; body mass index (BMI) ≥18 to ≤30 kg/m2) with a documented history of infertility who were planning to undergo IVF. A centralized electronic system was used for randomization, and the study investigators, sponsor's study team, and subjects remained blinded throughout the study.
PARTICIPANTS/MATERIALS, SETTING, METHODS
In total, 1031 subjects were randomized to receive either oral dydrogesterone (n = 520) or MVP (n = 511). Luteal support was started on the day of oocyte retrieval and continued until 12 weeks of gestation (Week 10), if a positive pregnancy test was obtained at 2 weeks after embryo transfer.
MAIN RESULTS AND THE ROLE OF CHANCE
In the full analysis set (FAS), 497 and 477 subjects in the oral dydrogesterone and MVP groups, respectively, had an embryo transfer. Non-inferiority of oral dydrogesterone was demonstrated, with pregnancy rates at 12 weeks of gestation of 37.6% and 33.1% in the oral dydrogesterone and MVP treatment groups, respectively (difference 4.7%; 95% CI: −1.2–10.6%). Live birth rates of 34.6% (172 mothers with 213 newborns) and 29.8% (142 mothers with 158 newborns) were obtained in the dydrogesterone and MVP groups, respectively (difference 4.9%; 95% CI: −0.8–10.7%). Oral dydrogesterone was well tolerated and had a similar safety profile to MVP.
LIMITATIONS, REASONS FOR CAUTION
The analysis of the results was powered to consider the clinical pregnancy rate, but the live birth rate may be of greater clinical interest. Conclusions relating to the differences between treatments in live birth rate, observed in this study, should therefore be made with caution.
WIDER IMPLICATIONS OF THE FINDINGS
Oral dydrogesterone may replace MVP as the standard of care for luteal phase support in IVF, owing to the oral route being more patient-friendly than intravaginal administration, as well as it being a well tolerated and efficacious treatment.
STUDY FUNDING/COMPETING INTEREST(S)
Sponsored and supported by Abbott Established Pharmaceuticals Division. H.T.’s institution has received grants from Merck, MSD, Goodlife, Cook, Roche, Besins, Ferring and Mithra (now Allergan) and H.T. has received consultancy fees from Finox, Ferring, Abbott, ObsEva and Ovascience. G.S. has nothing to disclose. E.K. is an employee of Abbott GmbH. G.G. has received investigator fees from Abbott during the conduct of the study; outside of this submitted work, G.G. has received personal fees and non-financial support from MSD, Ferring, Merck-Serono, Finox, TEVA, Glycotope, as well as personal fees from VitroLife, NMC Healthcare LLC, ReprodWissen LLC and ZIVA LLC.
TRIAL REGISTRATION NUMBER
NCT01850030 (clinicaltrials.gov).
TRIAL REGISTRATION DATE
19 April 2013.
DATE OF FIRST PATIENT'S ENROLLMENT
23 August 2013.
Keywords: in vitro fertilization, live birth rate, luteal phase support, micronized vaginal progesterone, oral dydrogesterone
Introduction
Luteal phase support, in conjunction with gonadotropin-releasing hormone analogs, is routinely performed during in vitro fertilization (IVF) procedures to overcome luteal phase progesterone deficiency induced by ovarian stimulation (Practice Committee of the American Society for Reproductive Medicine, 2008; Palomba et al., 2015). Progesterone supplementation or human chorionic gonadotropin (hCG) are commonly administered, although hCG is associated with a higher risk of ovarian hyperstimulation syndrome than progesterone (van der Linden et al., 2015). A systematic review demonstrated that the use of progestogens in IVF was associated with an improvement in the live birth rate (van der Linden et al., 2015). Progesterone can be administered orally, vaginally, rectally, subcutaneously or intramuscularly, although the vaginal route is preferred in the majority of IVF centers globally (Vaisbuch et al., 2012). Indeed, oral administration of progesterone is associated with low bioavailability (due to a substantial first-pass metabolism) and side effects, such as somnolence (Shapiro et al., 2014), while daily intramuscular administration can lead to pain at the injection site, and local abscess (Ghanem and Al-Boghdady, 2012). In contrast, vaginal administration of progesterone can be associated with vaginal irritation, discharge and bleeding (Ghanem and Al-Boghdady, 2012). Therefore, there is an ongoing need for an effective, well tolerated and safe treatment that could also help improve patient satisfaction, convenience and compliance.
Dydrogesterone is an established oral retroprogesterone approved for the treatment of threatened and recurrent miscarriage (associated with proven progesterone deficiency), and infertility due to luteal phase insufficiency. It has been extensively used for a variety of indications worldwide in an estimated 113 million women since 1960 (based on sales data), with approximately 20 million fetuses being exposed to dydrogesterone in utero. Compared with progesterone, dydrogesterone has a greater affinity for the progesterone receptors and can be used at lower oral doses to promote endometrial proliferation, owing to its better bioavailability and to the progestogenic activity of its metabolites (Schindler et al., 2008). Dydrogesterone also appears to have no affinity for androgenic, estrogenic, glucocorticoid or mineralocorticoid receptors (Schindler, 2009), demonstrating a favorable safety and tolerability profile in pregnancy, both to the mother and child (Queisser-Luft, 2009; Mirza et al., 2016). Furthermore, data from prospective trials for luteal phase support in IVF show that oral dydrogesterone is as effective as micronized vaginal progesterone (MVP), is well tolerated overall, and has a higher patient satisfaction rate than MVP (Chakravarty et al., 2005; Patki and Pawar, 2007; Ganesh et al., 2011; Salehpour et al., 2013; Tomic et al., 2015; van der Linden et al., 2015; Saharkhiz et al., 2016).
The aim of the Lotus I study was to substantiate the empirical use of dydrogesterone for luteal support in IVF in a Phase III randomized controlled trial (RCT), and to demonstrate non-inferiority to the current standard of care (i.e. MVP).
Materials and Methods
Study design
Lotus I was a double-blind, double-dummy, two-arm, multicenter Phase III RCT conducted at 38 sites in Austria, Belgium, Germany, Finland, Israel, Russia and Spain, from 23 August 2013 to 26 March 2016, and was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Abbott Laboratories GmbH (or an authorized representative) or the Investigator (according to national provisions) obtained written approval for the clinical study protocol (including all substantial protocol amendments), the written subject informed consent form, informed consent updates, subject-recruitment procedures and any other written information provided to subjects by an Institutional Review Board (IRB)/Independent Ethics Committee (IEC) that complied with the local regulatory requirements. This information was reviewed and approved by a qualified IRB/IEC prior to subject enrollment. The study was registered at clinicaltrials.gov with the identifier NCT01850030.
Subjects with infertility who were planning to undergo IVF with or without intracytoplasmic sperm injection (ICSI) were screened for possible study inclusion and enrolled prior to oocyte retrieval (recruitment period was 23 August 2013 to 04 June 2015; the last subject was randomized on 15 June 2015). Subjects were then randomized to receive either oral dydrogesterone 10 mg tablets (Abbott B.V., The Netherlands) with placebo intravaginal capsules (Abbott B.V.) three times daily (TID) (Group 1), or MVP 200 mg capsules (Utrogestan®, Besins Healthcare, Belgium) with oral placebo tablets (Catalent, Germany) TID (Group 2). All of the active and placebo capsules/tablets were identical in appearance, shape, smell and taste, and packaged in the proper proportion to ensure desired dosages and maintenance of the blind. Luteal support was started on the day of oocyte retrieval (Day 1) and continued until 12 weeks of gestation (Week 10) if a positive pregnancy test was obtained 2 weeks after embryo transfer and no miscarriage occurred. The duration of treatment was determined in accordance with the prescribing information of the MVP 200 mg capsules. Follow-up period was 30 days after birth, except for Russia where the follow-up period was 6 months due to regulatory requirements.
A centralized electronic system (Interactive Response Technology; IRT) was used to assign subjects to treatment groups. Subjects were randomized to treatment in a 1:1 ratio in blocks of 4 subject numbers per block stratified by country and age (i.e. ≤35 or >35 years). The electronic system assigned a 5-digit randomization number to each subject according to a randomization scheme, which was provided by Clinical Supply Management (Product Development) of Abbott Healthcare Products B.V. To maintain the blind, the investigator received a treatment allocation number for each subject from the IRT system. The subject received their study-drug package from the study site or the institutional pharmacy. Study investigators, the sponsor's study team and subjects remained blinded during the duration of the study.
Study participants
The study enrolled premenopausal women (>18 to <42 years of age; body mass index [BMI] ≥18 to ≤30 kg/m2) with a documented history of infertility (defined as being unable to conceive for ≥1 year [or 6 months for women ≥38 years of age], or as having bilateral tubal occlusion or absence) who were planning to undergo IVF with a fresh embryo (single or dual embryo transfer). Enrolled women had an early follicular phase (Day 2–4), a follicle-stimulating hormone level of ≤15 IU/L, and levels demonstrating early follicular phase of estradiol (<80 pg/mL), luteinizing hormone, prolactin, testosterone and thyroid-stimulating hormone. Furthermore, a normal transvaginal ultrasound at screening and a negative pregnancy test on the day of pituitary down-regulation were also required. Subjects with >3 unsuccessful IVF attempts or history of ≥3 miscarriages were excluded. Other key exclusion criteria included: evidence of cardiovascular, respiratory, urogenital, gastrointestinal/hepatic, hematologic/immunologic, head, ear, eye, nose, throat, dermatologic/connective tissue, musculoskeletal, metabolic/nutritional, endocrine, neurologic/psychiatric disorders or other relevant diseases; allergy; recent major surgery (within 3 months); current or recent substance abuse, including that of alcohol and tobacco; history of chemotherapy or radiotherapy.
Intake of other progesterone products was not permitted during the study.
Study objectives
The primary objective of the study was to demonstrate non-inferiority of oral dydrogesterone 10 mg tablets TID versus MVP 200 mg capsules TID. This objective was assessed by the presence of fetal heartbeats at 12 weeks of gestation (Week 10 of treatment) determined by transvaginal ultrasound. A non-inferiority margin of 10% was used in accordance with recent licensing studies for products indicated for luteal support in IVF (Doody et al., 2009; Food and Drug Administration (FDA), 2009; Baker et al., 2014; Lockwood et al., 2014). This was pre-specified in the study protocol and agreed with the Regulatory Authority.
Secondary objectives included: positive pregnancy test at treatment day 15 after embryo transfer and the incidence of live births. Newborn assessments included the following variables: gender, appearance, pulse, grimace, activity, respiration (APGAR) score, weight, height, head circumference, abnormal findings of physical examination and any malformations.
The study also obtained safety and tolerability data by means of documentation of treatment-emergent adverse events (TEAEs), including serious TEAEs, as well as routine laboratory findings, vital signs and physical examinations.
Study procedures
During subject screening and enrollment, informed consent, demographic data and a medical history were collected. Vital signs, physical examination and blood analysis for routine laboratory values were measured during the subject-screening period and at the end of treatment. On the day of oocyte retrieval (Day 1), subjects were randomly allocated to treatment following their stratification to country and age group, and luteal support was started. Embryo transfer was performed between Days 2–5 after oocyte retrieval according to local clinical practice. On Day 15 ± 3 (2 weeks after embryo transfer), a pregnancy test (serum β-hCG and urine strip test) was performed to determine whether treatment should be continued in cases of ongoing pregnancy until Day 71 ± 3 (12 weeks of gestation), at which point a transvaginal ultrasound was used to establish the presence of fetal heartbeats.
TEAEs, including miscarriage and pre-term delivery, and the use of concomitant medications were recorded throughout the study. Time of delivery and newborn parameters were obtained at delivery. Post-treatment, two visits/phone calls were made approximately 2 months apart, during which the mother's and newborn's safety and wellbeing were recorded. A study termination form was completed for all subjects who entered the study.
Sample size
An evaluation of clinical studies using oral dydrogesterone, micronized progesterone capsules or gel for treatment in IVF predicted a ~35% pregnancy rate at 12 weeks of gestation (Ludwig et al., 2002; Kleinstein, 2005; Ganesh et al., 2011). With a non-inferiority margin of 10%, it was estimated that a sample size of 479 subjects per treatment arm would provide 90% power to demonstrate non-inferiority, should there be no difference in pregnancy rate at 12 weeks of gestation between the two treatment groups. With the addition of a dropout rate of 10%, a total sample size of 533 subjects per group would be required, or a total sample size of 1066 study subjects.
Statistical analyses
The safety population included all subjects who were randomly allocated to treatment and received at least one administration of study drug(s). The full analysis sample (FAS) consisted of all subjects who were included in the safety population and had a successful embryo transfer or did not discontinue due to non-study-drug-related issues prior to embryo transfer. The per protocol sample (PPS) consisted of all subjects who were included in the FAS, did not have any major protocol deviations unrelated to treatment, and had a single or dual embryo transfer. Only seven subjects were omitted from the FAS for the PPS.
Disposition of subjects, demographics, concomitant medication and safety data were summarized by treatment groups. The primary efficacy analysis used a two-sided 95% confidence interval (CI) with a non-inferiority margin of 10% for the difference in pregnancy rates between dydrogesterone and MVP, whereby dydrogesterone was declared non-inferior if the lower bound of the 95% CI excluded a difference greater than 10%. A Cochran–Mantel–Haenszel statistical test stratified for country and age group was used for this analysis.
Results
Study population
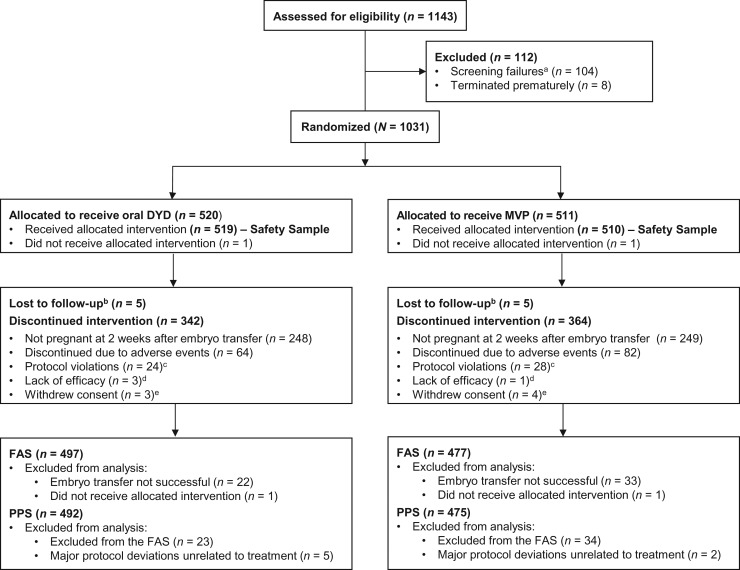
A total of 1143 subjects were screened and 1031 randomized to treatment (Fig. 1). In total, 520 and 511 subjects were randomized in the dydrogesterone and MVP groups, with 497 and 477 included in the FAS analysis, and 492 and 475 in the PPS analysis, respectively. Overall, 30.5% (315/1031) of subjects completed the study. The primary reason for study discontinuation was a non-confirmed pregnancy at 4 weeks of gestation (48.2% [497/1031]).
Figure 1.
Patient disposition (CONSORT flow-diagram).
aDetermined by inclusion/exclusion criteria. bSubject failed to return to the study site for scheduled visits and did not respond to telephone or written attempts to contact. cAnything which was in direct violation of the Clinical Study Protocol (e.g. inclusion/exclusion violation). dSubject failed to respond to the study drug at an acceptable level where the subject or the Investigator felt it was in the best interests of the subject to seek another treatment. eSubject decided to stop her participation in the study for any reason other than an adverse event, or was unable to complete the study as described in the Clinical Study Protocol (e.g. subject was relocating to another location). DYD, dydrogesterone; FAS, full analysis sample; MVP, micronized vaginal progesterone; PPS, per protocol sample.
The demographics and baseline characteristics of each group are summarized in Table I and were similar between the two treatment groups. The majority (71.9%) of subjects in the FAS were ≤35 years of age, had a BMI of less than 24 kg/m2 (63.8%) and were Caucasian (96.3%).
Table I.
Demographics and baseline characteristics (full analysis sample).
| Oral DYD (n = 497) | MVP (n = 477) | All (N = 974) | |
|---|---|---|---|
| Demographics | |||
| Mean age, years (SD) | 32.5 (4.5) | 32.5 (4.4) | 32.5 (4.4) |
| Age category, n (%) | |||
| ≤35 years of age | 352 (70.8) | 348 (73.0) | 700 (71.9) |
| >35 years of age | 145 (29.2) | 129 (27.0) | 274 (28.1) |
| Race or ethnicity, n (%) | |||
| Caucasian | 485 (97.6) | 453 (95.0) | 938 (96.3) |
| Black or African American | 9 (1.8) | 14 (2.9) | 23 (2.4) |
| Asian | 4 (0.8) | 9 (1.9) | 13 (1.3) |
| Other | 0 (0.0) | 2 (0.4) | 2 (0.2) |
| Mean BMI, kg/m2 (SD) | 23.3 (3.1)a | 23.2 (3.1)b | 23.2 (3.1)c |
| Prior treatment, n (%) | 30 (6.0) | 25 (5.2) | 55 (5.6) |
Note: Percentages are based on the number of subjects in the full analysis sample with data available. Body mass index (BMI) values were calculated from the following populations: an = 496; bn = 476; cn = 972.
DYD, dydrogesterone; MVP, micronized vaginal progesterone; SD, standard deviation.
Efficacy
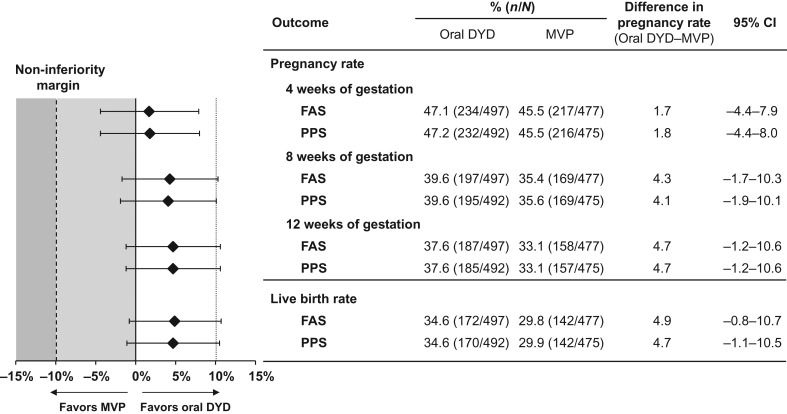
The pregnancy status post-treatment is shown in Fig. 2. The primary objective of this study was met, with dydrogesterone demonstrating non-inferiority to MVP at 12 weeks of gestation. Results were similar between the FAS and PPS for all measures of efficacy. In the PPS, the crude pregnancy rates at 12 weeks were 37.6% and 33.1% in the dydrogesterone and MVP treatment groups, respectively (difference 4.7%; 95% CI: −1.2–10.6%). Thus, non-inferiority of oral dydrogesterone versus MVP was demonstrated as the lower-bound CI was greater than −10%. From these results, the number of subjects needed to treat (NNT) with oral dydrogesterone to obtain a benefit versus MVP would be 22 (95% CI for absolute risk reduction of NNT [benefit] 9.4 to NNT [harm] 83). The primary analysis was adjusted for country and age (as pre-specified in the protocol). For these pre-specified factors, subgroup analyses were conducted and there was no relevant difference of effects observed between the different levels of the factor (no interaction between the factors country or age group and treatment). In the FAS, live birth rates of 34.6% (172 mothers with 213 newborns) and 29.9% (142 mothers with 158 newborns) were observed for the dydrogesterone and MVP groups, respectively (difference 4.9%; 95% CI: −0.8–10.7%) (Fig. 2). Thus, the NNT with oral dydrogesterone to obtain a benefit versus MVP would be 21 (95% CI for absolute risk reduction of NNT [benefit] 9.3 to NNT [harm] 125).
Figure 2.
Pregnancy status post-treatment. Positive pregnancy rates at 4, 8 and 12 weeks of gestation, and the live birth rates are shown for both the FAS and PPS. A non-inferiority margin of 10% was used, whereby the test drug is non-inferior if the lower bound of the 95% CI excludes a difference greater than 10% in favor of the comparator. CI, confidence interval; DYD, dydrogesterone; FAS, full analysis sample; MVP, micronized vaginal progesterone; PPS, per protocol sample.
Course and outcomes of treatment and pregnancy are summarized in Table II. The number of embryos transferred was similar between the two treatment groups. Pregnancy loss after 8 weeks of gestation, which included spontaneous abortions, induced abortion due to illness of the fetus and loss to follow-up (with last information available that the patient was pregnant but no information on births reported), was similar between groups, with rates of 5.0% and 5.6% being observed in the dydrogesterone and MVP groups, respectively.
Table II.
Course and outcomes of treatment/pregnancy.
| Oral DYD (30 mg) | MVP (600 mg) | All | |
|---|---|---|---|
| Number of subjects who underwent embryo transfer, n | 497 | 477 | 974 |
| Subjects who underwent embryo transfer after ICSI, n (%)a | 368 (74.0) | 338 (70.9) | 706 (72.5) |
| Day of embryo transfer after oocyte retrieval, n (%)a | |||
| <5 days | 350 (70.4) | 328 (68.8) | 678 (69.6) |
| ≥5 days | 147 (29.6) | 149 (31.2) | 296 (30.4) |
| Number of embryos transferred, n (%)a | |||
| 1 | 212 (42.7) | 217 (45.5) | 429 (44.1) |
| 2 | 278 (55.9) | 252 (52.8) | 530 (54.4) |
| >2 | 7 (1.4) | 8 (1.7) | 15 (1.5) |
| Number of subjects who had at least one newborn, n (%)a | 172 (34.6) | 142 (29.8) | 314 (32.2) |
| Total number of newborns, n | 213 | 158 | 371 |
| One newborn infant, n (%)b | 132 (76.7) | 126 (88.7) | 258 (82.2) |
| Two newborn infants, n (%)b | 39 (22.7) | 16 (11.3) | 55 (17.5) |
| More than two newborn infants, n (%)b | 1 (0.6) | 0 (0.0) | 1 (0.3) |
aPercentages calculated according to the number of subjects in the full analysis sample who received embryo transfer in the respective oral DYD and MVP groups.
bPercentages calculated according to the number of subjects who had at least one newborn in the respective oral DYD and MVP groups.
DYD, dydrogesterone; ICSI, intracytoplasmic sperm injection; MVP, micronized vaginal progesterone.
Safety and tolerability
Maternal and fetal/neonatal TEAEs are summarized in Table III. Overall, dydrogesterone was well tolerated as indicated by the reported TEAEs being in line with its known safety and tolerability profile and as expected in this patient population. TEAEs leading to study termination were reported by 12.4% of subjects in the dydrogesterone group and 16.0% of subjects in the MVP group. The majority of TEAEs leading to study termination or discontinuation of study drug were reported by no more than one subject in either treatment group.
Table III.
Maternal and fetal/neonatal TEAEs.
| Oral DYD (30 mg) | MVP (600 mg) | All | |
|---|---|---|---|
| (n = 518) | (n = 511) | (n = 1029) | |
| Maternal population, n (%)a | |||
| All TEAEs | 290 (56.0) | 276 (54.0) | 566 (55.0) |
| At least one serious TEAE | 56 (10.8) | 68 (13.3) | 124 (12.1) |
| At least one severe TEAE | 37 (7.1) | 54 (10.6) | 91 (8.8) |
| TEAEs leading to study discontinuation | 64 (12.4) | 82 (16.0) | 146 (14.2) |
| Deaths (maternal) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Liver enzyme analysis | 1 (0.2) | 2 (0.4) | 3 (0.3) |
| Alanine aminotransferase increased | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| Hepatic enzyme increased | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Vascular disorders | 18 (3.5) | 18 (3.5) | 36 (3.5) |
| Peripheral embolism and thrombosis | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| Reproductive system and breast disorders | 113 (21.8) | 94 (18.4) | 207 (20.1) |
| Vaginal hemorrhage | 60 (11.6) | 47 (9.2) | 107 (10.4) |
| Gastrointestinal disorders | 99 (19.1) | 88 (17.2) | 187 (18.2) |
| Nervous system disorders | 40 (7.7) | 42 (8.2) | 82 (8.0) |
| Fetal/neonatal population, n (%)b | |||
| At least one serious AE | 9 (4.2) | 9 (5.7) | 18 (4.9) |
| TEAEs of special interest relating to congenital, familial and genetic disorders, n (%)c | |||
| Congenital, familial and genetic disorders | 5 (1.0) | 6 (1.2) | 11 (1.1) |
| Congenital hand malformation | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Congenital hydrocephalus | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Congenital tricuspid valve atresia | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Interruption of aortic arch | 1 (0.2) | 0 (0.0) | 1 (0.1) |
| Kidney malformation | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Pulmonary artery atresia | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Spina bifida | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Talipes | 1 (0.2) | 0 (0.0) | 1 (0.1) |
| Tracheo-esophageal fistula | 1 (0.5) | 0 (0.0) | 1 (0.1) |
| Univentricular heart | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Ventricular septal defect | 2 (0.4) | 0 (0.0) | 2 (0.2) |
| Trisomy 21 | 1 (0.2) | 2 (0.4) | 3 (0.3) |
| Trisomy 13 | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Turner's syndrome | 1 (0.2) | 0 (0.0) | 1 (0.1) |
aPercentages are calculated based on the Safety Sample.
bPercentages are calculated based on the infant population (i.e. N = 212 for the oral DYD group and N = 159 for the MVP group).
cPercentages are calculated based on the Safety Sample. Detection and reporting of the congenital, familial, and genetic disorders occurred during with the pre- or post-natal period; some fetuses/neonates had more than one disorder.
AE, adverse event; DYD, dydrogesterone; MVP, micronized vaginal progesterone; TEAE, treatment-emergent adverse event.
The proportion of subjects reporting TEAEs was similar between treatment groups, with 56.0% of those receiving dydrogesterone and 54.0% receiving MVP. Of these, 13.1% and 12.9%, respectively, reported TEAEs that were related to treatment (as assessed by the investigator). The incidence of common TEAEs (reported by at least 5% of subjects in either treatment group) was similar between groups. The most common TEAE for both treatment groups was vaginal bleeding that codes to vaginal hemorrhage (11.6% in the dydrogesterone group and 9.2% in the MVP group).
The proportion of subjects experiencing serious TEAEs was similar between the dydrogesterone (10.8%) and MVP groups (13.3%). The following severe TEAEs were reported by ≥1% of subjects in either treatment group: spontaneous abortion (1.5% in the dydrogesterone group and 2.0% in the MVP group), missed abortion (1.4% and 1.4%, respectively) and ovarian hyperstimulation syndrome (0.6% and 1.0%, respectively). The only TEAEs of special interest reported by at least 2% of subjects in either treatment group were spontaneous abortion (2.3% in the dydrogesterone group and 4.1% in the MVP group) and missed abortion (2.5% and 2.0%, respectively). The incidence of congenital, familial and genetic disorders in the fetus or infant was limited, with five subjects (1.0%) in the dydrogesterone group and six subjects (1.2%) in the MVP group (Table III). There was no notable difference in the incidence of TEAEs of special interest between the two treatment groups. Nor was there a difference in the incidence of the most common TEAEs between the BMI subgroups <24 kg/m2 and ≥24 to <28 kg/m2, or between the subgroups containing subjects with one or two embryos transferred. There were too few subjects with a BMI of ≥28 kg/m2, or with zero and more than two embryos transferred to draw any meaningful comparisons from these subgroup analyses.
Infant safety data collected at delivery were similar between the two treatment groups, with the majority of infants being born with no abnormal findings at physical examination (93.4% in the dydrogesterone group and 92.4% in the MVP group). The gender, APGAR score, height, weight, head circumference and findings of physical examination of newborns are summarized in Table IV. The incidence of infants experiencing at least one serious adverse event was also similar between groups, with 4.2% in the dydrogesterone group and 5.7% in the MVP group. The most common TEAE in infants was prematurity, with seven cases in the dydrogesterone group and nine in the MVP group.
Table IV.
Newborn characteristics.
| Oral DYD (30 mg) | MVP (600 mg) | |
|---|---|---|
| (n = 497) | (n = 477) | |
| Gender, n (%)a | ||
| Male | 120 (56.3) | 88 (55.7) |
| Female | 93 (43.7) | 70 (44.3) |
| Abnormal findings of physical examination, n (%)a | ||
| Yes | 14 (6.6) | 12 (7.6) |
| No | 199 (93.4) | 146 (92.4) |
| Height, cm (mean ± SD) | 48.8 ± 3.9 | 49.4 ± 2.8 |
| Weight, kg (mean ± SD) | 2.9 ± 0.7 | 3.0 ± 0.6 |
| Head circumference, cm (mean ± SD) | 33.4 ± 2.4 | 33.8 ± 1.9 |
| APGAR score (mean ± SD) | ||
| 1 min postpartal | 8.1 ± 1.5 | 8.2 ± 1.5 |
| 5 min postpartal | 9.0 ± 1.3 | 9.2 ± 1.1 |
aPercentages are calculated based on the full analysis sample.
APGAR, appearance, pulse, grimace, activity, respiration; DYD, dydrogesterone; MVP, micronized vaginal progesterone; SD, standard deviation.
Discussion
The Lotus I study demonstrated that oral dydrogesterone was non-inferior to MVP for the primary objective, which was the presence of fetal heartbeats at 12 weeks of gestation. The results for the secondary objectives supported the primary efficacy results, with similar findings observed for pregnancy rates (2 weeks after embryo transfer and at 8 weeks of gestation) and live birth rates in both treatment groups. Dydrogesterone treatment had a similar safety profile to MVP for luteal support as part of an Assisted Reproductive Technology (ART) treatment. Note that possible differences in TEAEs related to the route of administration of oral dydrogesterone versus MVP (e.g. vaginal discharge) could not be observed in this study due to the double-dummy study design. Rates of TEAEs relating to congenital, familial and genetic disorders identified in the Lotus I study (dydrogesterone [1.0%], MVP [1.2%]) were lower than those identified in a previous analysis of 308 974 births, which reported an 8.3% birth defect rate in pregnancies involving assisted conception compared with 5.8% in those not involving assisted conception (Davies et al., 2012). As such, dydrogesterone has a well-established safety profile, and no new safety concerns were identified in this study.
The results obtained in this study provide robust evidence supporting the benefits of using dydrogesterone for luteal support as part of ART treatment, supporting findings previously reported in smaller published studies (Kupferminc et al., 1990; Chakravarty et al., 2005; Patki and Pawar, 2007; Ganesh et al., 2011; Salehpour et al., 2013; Tomic et al., 2015; van der Linden et al., 2015; Barbosa et al., 2015; Saharkhiz et al., 2016). Of note, in previous studies conducted in luteal phase support for IVF, the daily dose of dydrogesterone ranged from 20 to 30 mg; however, 10 mg twice daily was shown to have reduced endometrial development compared with 200 mg MVP TID (Fatemi et al., 2007). The dose of 10 mg dydrogesterone TID used herein was chosen based on recommendations by IVF specialists and previous studies.
The Lotus I study had some limitations. The analysis of the results was powered to consider the clinical pregnancy rate, but a primary objective of greater clinical interest may have been the live birth rate. Differences in live birth rate were observed between the oral dydrogesterone and MVP groups; however, conclusions relating to the significance of this result cannot be made. The findings of the present study are strengthened by the selection of an appropriate sample size of 1031 randomized subjects, the fact that both treatment arms were well balanced, and the use of broad eligibility criteria.
The results presented add to the body of evidence and are supported by the 2015 Cochrane systematic review of 94 randomized trials comparing different luteal phase support regimens, which concluded that synthetic progesterone (i.e. dydrogesterone) was associated with a higher clinical pregnancy rate than micronized progesterone (vaginal and oral) (van der Linden et al., 2015). More recently, a meta-analysis and systematic review of eight randomized clinical trials comparing dydrogesterone with MVP found no significant differences between treatments for ongoing pregnancy, clinical pregnancy or miscarriage rate (Barbosa et al., 2015).
Lotus I was a robust study which provides appropriate evidence that dydrogesterone is as effective as the current standard of care in women undergoing IVF. Taking into account the safety data collected in this study, dydrogesterone displayed a favorable benefit/risk profile. The results of this study, in addition to those of previous studies indicating that oral administration is preferred over intravaginal application, have the potential to induce a paradigm shift for the treatment of the estimated 1.5 million women worldwide undergoing IVF each year (Dyer et al., 2016; Chambers et al., 2012) who could benefit from this oral form of luteal phase support.
Acknowledgements
Abbott study support: Darline Cheatham-Seitz, Elke Kahler and Erik van Leeuwen. Manuscript support: John Timney (Alpharmaxim Medical Communications). We would like to thank the following study investigators from their respective countries. Austria: Katharina Walch (Vienna); Belgium: Dirk Coeman (Brasschaat), Tom Coetsier (Gent), Wim Decleer (Gent), Caroline Lecocq (Brussels), Willem Ombelet (Genk), Karen Peeraer (Leuven), Jean-François Simon (Mons) and Christine Wyns (Brussels); Finland: Kirsimarja Kestilä (Turku), Anna Kivijärvi (Jyväskylä) and Niklas Simberg (Helsinki); Germany: Maren Goeckenjan-Festag (Dresden), Anette Siemann (Berlin), Thomas Strowitzki (Heidelberg) and Peter Sydow (Berlin); Israel: Ilan Calderon (Haifa), Adrian Ellenbogen (Hadera), Eitan Lunenfeld (Beer Sheva), Daniel Seidman (Tel Aviv) and Alex Simon (Jerusalem); Russia: Nadezhda Bashmakova (Ekaterinburg), Irina Dankova (Ekaterinburg), Tatiana Gurskaya (Moscow), Vladimir Kuzmin (Moscow), Galina Melnichenko (Moscow), Vera Prilepskaya (Moscow) and Nina Tatarova (Saint-Petersburg); Spain: Ernesto Bosch Aparicio (Valencia), Ana Belen Casas Balazote (Barcelona), Marcos Ferrando Serrano (Bilboa), Antonio Gosalvez (Pozuelo de Alarcon) and Roberto Matorras (Barakaldo).
Authors’ roles
All authors contributed to the conception, design, data interpretation, and writing and revision of the manuscript. E.K. contributed to the analysis of the data. G.G., G.S. and H.T. contributed to the acquisition of the data. The final manuscript and order of authorship has been approved by all authors.
Funding
Sponsored and supported by Abbott Established Pharmaceuticals.
Conflict of interest
H.T.’s institution has received grants from Merck, MSD, Goodlife, Cook, Roche, Besins, Ferring and Mithra (now Allergan), and H.T. has received consultancy fees from Finox, Ferring, Abbott, ObsEva and Ovascience. G.S. has nothing to disclose. E.K. is an employee of Abbott GmbH. G.G. has received investigator fees from Abbott during the conduct of the study; outside of this submitted work, G.G. has received personal fees and non-financial support from MSD, Ferring, Merck-Serono, Finox, TEVA, Glycotope, as well as personal fees from VitroLife, NMC Healthcare LLC, ReprodWissen LLC and ZIVA LLC.
References
- Baker VL, Jones CA, Doody K, Foulk R, Yee B, Adamson GD, Cometti B, DeVane G, Hubert G, Trevisan S et al. . A randomized, controlled trial comparing the efficacy and safety of aqueous subcutaneous progesterone with vaginal progesterone for luteal phase support of in vitro fertilization. Hum Reprod 2014;29:2212–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa MW, Silva LR, Navarro PA, Ferriani RA, Nastri CO, Martins WP. Dydrogesterone versus progesterone for luteal-phase support: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol 2015;48:161–170. [DOI] [PubMed] [Google Scholar]
- Chakravarty BN, Shirazee HH, Dam P, Goswami SK, Chatterjee R, Ghosh S. Oral dydrogesterone versus intravaginal micronised progesterone as luteal phase support in assisted reproductive technology (ART) cycles: results of a randomised study. J Steroid Biochem Mol Biol 2005;97:416–420. [DOI] [PubMed] [Google Scholar]
- Chambers GM, Hoang VP, Zhu R, Illingworth PJ. A reduction in public funding for fertility treatment - an econometric analysis of access to treatment and savings to government. BMC Health Serv Res 2012;12:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MJ, Moore VM, Willson KJ, Van EP, Priest K, Scott H, Haan EA, Chan A. Reproductive technologies and the risk of birth defects. N Engl J Med 2012;366:1803–1813. [DOI] [PubMed] [Google Scholar]
- Doody KJ, Schnell VL, Foulk RA, Miller CE, Kolb BA, Blake EJ, Yankov VI. Endometrin for luteal phase support in a randomized, controlled, open-label, prospective in-vitro fertilization trial using a combination of Menopur and Bravelle for controlled ovarian hyperstimulation. Fertil Steril 2009;91:1012–1017. [DOI] [PubMed] [Google Scholar]
- Dyer S, Chambers GM, de Mouzon J, Nygren KG, Zegers-Hochschild F, Mansour R, Ishihara O, Banker M, Adamson GD. International Committee for Monitoring Assisted Reproductive Technologies world report: Assisted Reproductive Technology 2008, 2009 and 2010. Hum Reprod 2016;31:1588–1609. [DOI] [PubMed] [Google Scholar]
- Fatemi HM, Bourgain C, Donoso P, Blockeel C, Papanikolaou EG, Popovic-Todorovic B, Devroey P. Effect of oral administration of dydrogestrone versus vaginal administration of natural micronized progesterone on the secretory transformation of endometrium and luteal endocrine profile in patients with premature ovarian failure: a proof of concept. Hum Reprod 2007;22:1260–1263. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration (FDA) Drug Approval Package: Endometrin (Progesterone) Vaginal Insert 100 mg 2009. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022057_endometrin_toc.cfm (21 October 2016, date last accessed).
- Ganesh A, Chakravorty N, Mukherjee R, Goswami S, Chaudhury K, Chakravarty B. Comparison of oral dydrogestrone with progesterone gel and micronized progesterone for luteal support in 1,373 women undergoing in vitro fertilization: a randomized clinical study. Fertil Steril 2011;95:1961–1965. [DOI] [PubMed] [Google Scholar]
- Ghanem ME, Al-Boghdady LA. Luteal Phase Support in ART: an Update 2012. http://cdn.intechopen.com/pdfs-wm/41085.pdf (21 October 2016, date last accessed).
- Kleinstein J. Efficacy and tolerability of vaginal progesterone capsules (Utrogest 200) compared with progesterone gel (Crinone 8%) for luteal phase support during assisted reproduction. Fertil Steril 2005;83:1641–1649. [DOI] [PubMed] [Google Scholar]
- Kupferminc MJ, Lessing JB, Amit A, Yovel I, David MP, Peyser MR. A prospective randomized trial of human chorionic gonadotrophin or dydrogesterone support following in-vitro fertilization and embryo transfer. Hum Reprod 1990;5:271–273. [DOI] [PubMed] [Google Scholar]
- Lockwood G, Griesinger G, Cometti B. Subcutaneous progesterone versus vaginal progesterone gel for luteal phase support in in vitro fertilization: a noninferiority randomized controlled study. Fertil Steril 2014;101:112–119. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Schwartz P, Babahan B, Katalinic A, Weiss JM, Felberbaum R, Al-Hasani S, Diedrich K. Luteal phase support using either Crinone 8% or Utrogest: results of a prospective, randomized study. Eur J Obstet Gynecol Reprod Biol 2002;103:48–52. [DOI] [PubMed] [Google Scholar]
- Mirza FG, Patki A, Pexman-Fieth C. Dydrogesterone use in early pregnancy. Gynecol Endocrinol 2016;32:97–106. [DOI] [PubMed] [Google Scholar]
- Palomba S, Santagni S, La Sala GB. Progesterone administration for luteal phase deficiency in human reproduction: an old or new issue. J Ovarian Res 2015;8:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patki A, Pawar VC. Modulating fertility outcome in assisted reproductive technologies by the use of dydrogesterone. Gynecol Endocrinol 2007;23:68–72. [DOI] [PubMed] [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine Progesterone supplementation during the luteal phase and in early pregnancy in the treatment of infertility: an educational bulletin. Fertil Steril 2008;90:S150–S153. [DOI] [PubMed] [Google Scholar]
- Queisser-Luft A. Dydrogesterone use during pregnancy: overview of birth defects reported since 1977. Early Hum Dev 2009;85:375–377. [DOI] [PubMed] [Google Scholar]
- Saharkhiz N, Zamaniyan M, Salehpour S, Zadehmodarres S, Hoseini S, Cheraghi L, Seif S, Baheiraei N. A comparative study of dydrogesterone and micronized progesterone for luteal phase support during in vitro fertilization (IVF) cycles. Gynecol Endocrinol 2016;32:213–217. [DOI] [PubMed] [Google Scholar]
- Salehpour S, Tamimi M, Saharkhiz N. Comparison of oral dydrogesterone with suppository vaginal progesterone for luteal-phase support in in vitro fertilization (IVF): a randomized clinical trial. Iran J Reprod Med 2013;11:913–918. [PMC free article] [PubMed] [Google Scholar]
- Schindler AE. Progestational effects of dydrogesterone in vitro, in vivo and on the human endometrium. Maturitas 2009;65:S3–S11. [DOI] [PubMed] [Google Scholar]
- Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH. Classification and pharmacology of progestins. Maturitas 2008;61:171–180. [DOI] [PubMed] [Google Scholar]
- Shapiro D, Boostanfar R, Silverberg K, Yanushpolsky EH. Examining the evidence: progesterone supplementation during fresh and frozen embryo transfer. Reprod Biomed Online 2014;29:S1–14. [DOI] [PubMed] [Google Scholar]
- Tomic V, Tomic J, Klaic DZ, Kasum M, Kuna K. Oral dydrogesterone versus vaginal progesterone gel in the luteal phase support: randomized controlled trial. Eur J Obstet Gynecol Reprod Biol 2015;186:49–53. [DOI] [PubMed] [Google Scholar]
- Vaisbuch E, Leong M, Shoham Z. Progesterone support in IVF: is evidence-based medicine translated to clinical practice? A worldwide web-based survey. Reprod Biomed Online 2012;25:139–145. [DOI] [PubMed] [Google Scholar]
- van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev 2015;7:CD009154. [DOI] [PMC free article] [PubMed] [Google Scholar]