Abstract
Aims
The effect of macitentan on haemodynamic parameters and NT-proBNP levels was evaluated in pulmonary arterial hypertension (PAH) patients in the SERAPHIN study. Association between these parameters and disease progression, assessed by the primary endpoint (time to first morbidity/mortality event), was explored.
Methods and results
Of the 742 randomized patients, 187 with right heart catheterization at baseline and month 6 participated in a haemodynamic sub-study. Prespecified endpoints included change from baseline to month 6 in cardiac index (CI), right atrial pressure (RAP), mean pulmonary arterial pressure (mPAP), pulmonary vascular resistance (PVR), mixed-venous oxygen saturation, and NT-proBNP. Exploratory analyses examined associations between CI, RAP, and NT-proBNP and disease progression using the Kaplan-Meier method and Cox regression models. Macitentan improved CI, RAP, mPAP, PVR and NT-proBNP vs. placebo at month 6. Absolute levels of CI, RAP and NT-proBNP at baseline and month 6, but not their changes, were associated with morbidity/mortality events. Patients with CI > 2.5 L/min/m2, RAP < 8 mmHg, or NT-proBNP < 750 fmol/ml at month 6 had a lower risk of morbidity/mortality than those not meeting these thresholds (HR 0.49, 95% CL 0.28–0.86; HR 0.72, 95% CL 0.42–1.22; and HR 0.22, 95% CL 0.15–0.33, respectively).
Conclusions
For all treatment groups, baseline and month 6 values of CI, RAP, and NT-proBNP, but not their changes, were associated with morbidity/mortality events, confirming their relevance in predicting disease progression in patients with PAH. By improving those parameters, macitentan increased the likelihood of reaching threshold values associated with lower risk of morbidity/mortality.
Keywords: Haemodynamics, Prognosis , Macitentan, Pulmonary hypertension, Right ventricular function
Introduction
Pulmonary arterial hypertension (PAH) is a chronic disease characterized by progressive symptomatic and cardiopulmonary haemodynamic deterioration. Elevated pulmonary vascular resistance (PVR) and the resulting increased right ventricular (RV) afterload contribute to the maladaptive processes of RV hypertrophy and dilatation, which eventually result in RV failure and premature death.1,2 Other hallmarks of RV impairment include reduced cardiac index (CI), increased right atrial pressure (RAP), and elevated levels of N-terminal pro-brain natriuretic peptide (NT-proBNP).1,2 Recommendations1–3 for the management of PAH advocate treatment strategies targeted to improvements in RV function as assessed by CI, RAP, and NT-proBNP. The ESC/ERS Guidelines outline risk categories for these parameters based on their association with survival in observational studies.4–9
Macitentan is a dual endothelin receptor antagonist (ERA) approved for the long-term treatment of PAH. In the randomized controlled trial SERAPHIN (Study with an Endothelin Receptor Antagonist in Pulmonary arterial Hypertension to Improve cliNical outcome), macitentan 10 mg significantly reduced the risk for the primary endpoint of time to first morbidity/mortality event by 45% (P < 0.001).10 The trial included a haemodynamic sub-study; a subset of the 742 enrolled patients (n = 187) underwent right heart catheterization (RHC) at baseline and at month 6. Treatment with macitentan 10 mg resulted in significant improvements in PVR and CI at month 6 compared with placebo.10 Here, we describe the effect of macitentan on a broader range of haemodynamic parameters and NT-proBNP. In addition, the study allowed us to evaluate in a randomized controlled trial with a prospectively defined morbidity/mortality endpoint the association between risk thresholds for CI, RAP and NT-proBNP outlined in the guidelines and disease progression.1,2
Methods
Study population
SERAPHIN was a multicentre, randomized, double-blind, event-driven trial (NCT00660179) and is described in detail elsewhere.10 Eligible patients were aged > 12 years with a confirmed PAH diagnosis. Patients were required to have a 6MWD of ≥ 50m and be in WHO functional class II, III, or IV. Patients naive to PAH treatment or those receiving phosphodiesterase type 5 inhibitors, oral or inhaled prostanoids, calcium channel blockers, or L-arginine at stable doses for at least 3 months prior to randomization were eligible. Patients receiving intravenous/subcutaneous prostanoids or ERAs were excluded.
Study design
After a screening period of ≤ 28 days, patients were randomly assigned in a 1:1:1 ratio to receive macitentan 3 mg or macitentan 10 mg, or placebo, once daily. Patients received double-blind treatment until they experienced a primary endpoint event, or until the end of the study (when 285 events had occurred). The primary endpoint was the time from study treatment initiation to the first PAH-related morbidity event (worsening of PAH, initiation of treatment with intravenous/subcutaneous prostanoids, lung transplantation, or atrial septostomy) or death from any cause up to the end of treatment.10 All primary endpoint events were adjudicated by a blinded independent committee. The trial adhered to the Declaration of Helsinki and the research protocol was approved by local institutional review boards/independent ethics committees. Written informed consent was obtained from all patients.
Haemodynamic sub-study and outcomes
A haemodynamic sub-study was conducted at 44 centres in 16 countries (Supplementary material online, Table S1). Centres were selected if they regularly followed patients by RHC. Patients were eligible if their baseline RHC was assessed within 3 months before randomization. The sub-study included a second RHC performed at month 6. Pre-specified outcomes included changes from baseline to month 6 in CI, RAP, mean pulmonary arterial pressure (mPAP), PVR, and SvO2. Changes from baseline to month 6 in NT-proBNP, measured in plasma using the BNP Fragment Enzyme ImmunoAssay BI-208528 (Biomedica Medizinprodukte GmbH, Germany), was analysed in the sub-study patients and in the overall SERAPHIN population. Safety was assessed throughout.
Statistical analyses
Changes in haemodynamic parameters and NT-proBNP at month 6
Mean changes from baseline in CI, RAP, mPAP, SvO2, and NT-proBNP were summarized descriptively by treatment group. Changes in PVR were calculated as the percentage change from baseline and summarized using geometric means and two-sided 95% CLs. This methodology was prespecified to account for the high variability in baseline PVR levels and assumed a normal distribution of the log-transformed fold change from baseline to month 6. Changes from baseline to month 6 were also analysed post hoc by WHO functional class and background PAH-specific therapy at baseline. Treatment effects between macitentan 3 mg and macitentan 10 mg vs. placebo are presented as a difference in mean changes with 95% CL, using the observed data at month 6.
Associations of CI, RAP, and NT-proBNP with disease progression
We investigated the prognostic relevance of the continuous variables for CI, RAP and NT-proBNP at baseline and month 6, and the changes in these levels from baseline to month 6, on disease progression as assessed by the primary endpoint. We also analysed whether being above or below a specific CI, RAP and NT-proBNP threshold at month 6 was associated with the risk of a morbidity/mortality event. Thresholds of 2.5 L/min/m2 for CI and of 8 mmHg for RAP were applied based on the low-risk profile described in the ESC/ERS 2015 PAH guidelines.1,2 For NT-proBNP, the threshold of 300 ng/L outlined in the ESC/ERS 2015 guidelines could not be applied because the assay in our study was not compatible with the assay used to determine the 300 ng/L threshold.11 We used a threshold of 750 fmol/ml, the median NT-proBNP level at month 6 among all randomized patients with available data (n = 502).
All associations were analysed using Kaplan–Meier estimates and the log-rank test. Hazard ratios (HRs) and 95% CL were primarily calculated using univariate Cox’s proportional hazard models. The baseline analyses were conducted on patients with available baseline values. The month 6 analyses were conducted on patients who had available data at baseline and month 6, with exclusion of patients who had experienced a morbidity/mortality event before month 6. All analyses were performed on observed data. All models were also adjusted for study treatment, baseline PAH-therapy and time from RHC to randomization. Consistency of the associations between the month 6 levels and disease progression across treatment groups was assessed using interaction tests (significance level 5%).
Finally, in patients with available data at baseline and at month 6, we evaluated the proportions of patients with CI > 2.5 L/min/m2, RAP < 8 mmHg, or NT-proBNP < 750 fmol/ml in each treatment group (placebo, macitentan 3 mg and macitentan 10 mg). Odds ratios, 95% CL and P-values for macitentan 3 mg vs. placebo and macitentan 10 mg vs. placebo were calculated using logistic regression models, adjusted for baseline values.
Results
Patient characteristics
Of the 187 haemodynamic sub-study patients, 68 were randomized to placebo, 62 to macitentan 3 mg and 57 to macitentan 10 mg. The majority were in WHO functional class II and III, and half of the patients were on background PAH-specific therapy, mainly sildenafil (Table 1). The baseline characteristics were consistent with the overall SERAPHIN population and were comparable across treatment groups. The mean duration between RHC and study enrolment was 0.6 (±1.1 SD) months. At month 6, RHC was performed in 147 patients. Reasons for missing data are provided in Supplementary material online, Table S2, and cohorts with complete data are indicated in the footnotes of each table.
Table 1.
Demographics and baseline characteristics
| SERAPHIN [Pulido 2013a] | SERAPHIN haemodynamic substudy |
||||
|---|---|---|---|---|---|
| Overall patients (n = 742) | All patients (n = 187) | Placebo (n = 68) | Macitentan 3 mg (n = 62) | Macitentan 10 mg (n = 57) | |
| Female sex, % | 77 | 76 | 74 | 74 | 81 |
| Age, years (mean ± SD) | 46 ± 16 | 47 ± 16 | 48 ± 16 | 47 ± 16 | 47 ± 15 |
| Time from diagnosis, weeks (mean ± SD) | 142 ± 208 | 143 ± 249 | 158 ± 282 | 137 ± 232 | 132 ± 229 |
| Time from RHC to randomization, months (mean ± SD) | 1.8 ± 2.9a | 0.6 ± 1.1 | 0.5 ± 0.9 | 0.6 ± 1.1 | 0.7 ± 1.3 |
| 6MWD, m (mean ± SD) | 360 ± 100 | 353 ± 103 | 360 ± 112 | 341 ± 100 | 359 ± 94 |
| WHO FC, n (%) | |||||
| Ib | 1 (0.1) | 1 (0.5) | – | – | 1 (1.8) |
| II | 387 (52.4) | 81 (43.3) | 32 (47.1) | 25 (40.3) | 24 (42.1) |
| III | 337 (45.6) | 100 (53.5) | 35 (51.5) | 35 (56.5) | 30 (52.6) |
| IV | 14 (1.9) | 5 (2.7) | 1 (1.5) | 2 (3.2) | 2 (3.5) |
| Background PAH-specific therapyc, % | 64 | 49 | 50 | 55 | 42 |
| CI, L/min/m2 (mean ± SD) | 2.4 ± 0.8 | 2.5 ± 0.8 | 2.5 ± 0.7 | 2.4 ± 0.8 | 2.6 ± 0.9 |
| RAP, mmHg (mean ± SD) | 9 ± 6 | 8 ± 5 | 8 ± 4 | 9 ± 5 | 8 ± 6 |
| mPAP, mmHg (mean ± SD) | 54 ± 18 | 53 ± 18 | 53 ± 20 | 53 ± 18 | 54 ± 17 |
| PVR, dyn·sec/cm5 (mean ± SD) | 1026 ± 696.7 | 919 ± 548.6 | 900 ± 556.3 | 934 ± 564.0 | 924 ± 531.5 |
| SvO2, % (mean ± SD) | 65 ± 10a | 65 ± 10 | 65 ± 8 | 63 ± 10 | 65 ± 11 |
| NT-proBNP, fmol/ml (mean ± SD) | n = 501 | n = 142 | n = 50 | n = 46 | n = 46 |
| 1070 ± 825a | 1294 ± 960 | 1264 ± 1001 | 1447 ± 1055 | 1173 ± 800 | |
In the haemodynamic sub-study, baseline RAP, mPAP, and CI values were missing for 1 patient in the placebo group; baseline PVR was missing for 3 patients (1 in each treatment group); baseline SvO2 was missing in 15 patients (5 in the placebo group, 7 in the macitentan 3 mg group and 3 in the macitentan 10 mg group).
Time from RHC to randomization, SvO2 and NT-proBNP not presented in the Pulido et al. publication.
1 patient in WHO FC I was incorrectly included in the study.
Phosphodiesterase-5 inhibitors and oral/inhaled prostacyclin therapy.
6MWD, 6-min walk distance; CI, cardiac index; mPAP, mean pulmonary arterial pressure; RAP, right atrial pressure; NT-proBNP, N-terminal pro-brain natriuretic peptide; PAH, pulmonary arterial hypertension; PVR, pulmonary vascular resistance; RHC, right heart catheterization; SD, standard deviation; SvO2, mixed venous oxygen saturation; WHO FC, World Health Organization functional class.
Changes in cardiopulmonary haemodynamic parameters and NT-proBNP at month 6
Cardiopulmonary haemodynamic parameters and NT-proBNP levels worsened from baseline to month 6 in placebo-treated patients and improved in patients treated with macitentan 3 mg and macitentan 10 mg (Table 2). When compared with placebo, treatment effects in favour of macitentan 3 mg and macitentan 10 mg were observed for mPAP, CI, PVR, and NT-proBNP. Macitentan improved CI, mPAP, PVR, and NT-proBNP irrespective of WHO functional class and background PAH-specific therapy (Tables 3 and 4). In the overall SERAPHIN population, NT-proBNP levels were available for 495 patients at both baseline and month 6; macitentan reduced NT-proBNP levels to a similar extent in this larger population (Supplementary material online, Table S3).
Table 2.
Changes from baseline to month 6 and values at month 6 for haemodynamic parameters and NT-proBNP
| Placebo |
Macitentan 3 mg |
Macitentan 10 mg |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean change from baseline ±SDa | Mean ± SD at month 6 | n | Mean change from baseline ± SDa | Mean ± SD at month 6 | Mean treatment effect vs. placebo (95% CL)b | n | Mean change from baseline ±SDa | Mean±SD at month 6 | Mean treatment effect vs. placebo (95% CL)b | |
| CI, L/min/m2 | 50 | −0.33 ± 0.65 | 2.21 ± 0.63 | 47 | 0.36 ± 0.57 | 2.69 ± 0.83 | 0.69 (0.44, 0.93)* | 48 | 0.30 ± 0.85 | 2.93 ± 0.73 | 0.63 (0.33,0.93)* |
| RAP, mmHg | 50 | 0.2 ± 4.5 | 8.1 ± 5.7 | 47 | −0.3 ± 5.5 | 8.8 ± 5.2 | −0.5 (−2.5, 1.5) | 49 | −0.6 ± 4.8 | 7.7 ± 5.5 | −0.8 (−2.7, 1.0) |
| mPAP, mmHg | 50 | 1.0 ± 7.4 | 54.3 ± 21.4 | 47 | −3.4 ± 7.9 | 49.3 ± 15.9 | −4.4 (−7.5, −1.3)* | 49 | −5.3 ± 11.4 | 47.5 ± 19.9 | −6.4 (−10.2, −2.5)* |
| PVR, dyn·sec/cm5 | 50 | 115.8 (104.7, 128.1) | 1042 ± 656 | 47 | 76.9 (69.8, 84.6) | 736 ± 434 | −33.6 (−42.2, −23.8)* | 48 | 71.3 (62.4, 81.4) | 680 ± 497 | −38.5 (−47.8, −27.5)* |
| SvO2, % | 48 | −2.0 ± 6.2 | 64.8 ± 8.6 | 40 | 0.8 ± 7.2 | 64.8 ± 7.2 | 2.7 (−0.1, 5.6) | 45 | 0.1 ± 6.9 | 66.0 ± 8.2 | 2.0 (−0.7, 4.7) |
| NT−proBNP, fmol/ml | 49 | 194 ± 575 | 1451 ± 1258 | 45 | −168 ± 633 | 1280 ± 958 | −362 (−610, −115)* | 46 | −109 ± 552 | 1064 ± 891 | −303 (−533, −73)* |
P-value < 0.05 for the comparison between macitentan and placebo.
PVR data are the geometric mean of month 6/baseline (%) (95% CL), data for all other variables are mean ± SD.
PVR data are expressed as a percent change (%) between macitentan and placebo: (ratio of geometric means − 1) × 100, data for all other variables are the placebo-corrected mean ± SD.
n represents the number of patients in the haemodynamic sub-study with non-missing values for change from baseline to month 6. Results are based on observed data with no imputation rules applied for missing values.
CI, cardiac index; CL, confidence limits; mPAP, mean pulmonary arterial pressure; RAP, right atrial pressure; NT-proBNP, N-terminal pro-brain natriuretic peptide; PVR, pulmonary vascular resistance; SD, standard deviation; SvO2, mixed venous oxygen saturation.
Table 3.
Changes from baseline to month 6 for haemodynamic parameters and NT-proBNP by WHO functional class
| Placebo (n = 68) |
Macitentan 3 mg (n = 62) |
Macitentan 10 mg (n = 57) |
||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean change ± SDa | n | Mean change ± SDa | Mean treatment effect vs. placebo (95% CL)b | n | Mean change ± SDa | Mean treatment effect vs. placebo (95% CL)b | |
| WHO functional class I/II | ||||||||
| CI, L/min/m2 | 26 | −0.34 ± 0.77 | 18 | 0.35 ± 0.47 | 0.69 (0.28, 1.10)* | 21 | 0.35 ± 0.80 | 0.69 (0.22, 1.15)* |
| mPAP, mmHg | 26 | 1.0 ± 6.92 | 18 | −6.0 ± 7.50 | −7.0 (−11.4, −2.5)* | 22 | −7.3 ± 13.30 | −8.3 (−14.3, −2.2)* |
| PVR, % | 26 | 118.7 (102.9, 137.0) | 18 | 70.2 (58.2, 84.7) | −40.9 (−52.8, −25.9)* | 21 | 65.6 (52.3, 82.4) | −44.7 (−57.0, −29.0)* |
| NT−proBNP, fmol/ml | 25 | 194.8 ± 530.9 | 16 | −118.6 ± 433.1 | −313 (−634, 7.5) | 20 | −67.3 ± 316.3 | −262 (−534, 9.5) |
| WHO functional class III/IV | ||||||||
| CI, L/min/m2 | 24 | −0.32 ± 0.51 | 29 | 0.36 ± 0.64 | 0.68 (0.36, 1.00)* | 27 | 0.25 ± 0.89 | 0.58 (0.16, 0.99)* |
| mPAP, mmHg | 24 | 1.1 ± 8.09 | 29 | −1.8 ± 7.77 | −2.9 (−7.3, 1.5) | 27 | −3.8 ± 9.63 | −4.8 (−9.9, 0.2) |
| PVR, % | 24 | 112.7 (96.7, 131.3) | 29 | 81.3 (73.0, 90.6) | −27.9 (−39.7, −13.8)* | 27 | 76.0 (64.3, 89.7) | −32.6 (−46.0, −15.8)* |
| NT−proBNP, fmol/ml | 24 | 193.4 ± 629.5 | 29 | −195.4 ± 726.5 | −389 (−768, −9.6)* | 26 | −141 ± 685.8 | −334 (−710, 41) |
P-value <0.05 for the comparison between macitentan and placebo.
PVR data are the geometric mean of month 6/baseline (%) (95% CL), data for all other variables are mean ± SD.
PVR data are expressed as a percent change (%) between macitentan and placebo: (ratio of geometric means − 1) × 100 , data for all other variables are the placebo-corrected mean ± SD.
n represents the number of patients in the haemodynamic sub-study at baseline; n represents the number of patients with non-missing values for the change from baseline to month 6.
Of the 68 placebo patients, 32 were in WHO FC I/II and 36 were in WHO FC III/IV; of the 62 macitentan 3 mg patients, 25 were in WHO FC I/II and 37 were in WHO FC III/IV; of the 57 macitentan 10 mg patients, 25 were in WHO FC I/II and 32 were in WHO FC III/IV.
Results are based on observed data with no imputation rules applied for missing values.
CL, confidence limits; CI, cardiac index; NT-proBNP, N-terminal pro-brain natriuretic peptide; mPAP, mean pulmonary arterial pressure; PVR, pulmonary vascular resistance; WHO FC, World Health Organization functional class.
Table 4.
Changes from baseline to month 6 for haemodynamic parameters and NT-proBNP by background PAH-specific therapy
| Placebo (n = 68) |
Macitentan 3 mg (n = 62) |
Macitentan 10 mg (n = 57) |
||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean change ±SDa | n | Mean change ±SDa | Mean treatment effect vs. placebo (95% CL)b | n | Mean change ±SDa | Mean treatment effect vs. placebo (95% CL)b | |
| With background PAH-specific therapy | ||||||||
| CI, L/min/m2 | 29 | −0.34 ± 0.52 | 30 | 0.40 ± 0.64 | 0.74 (0.43, 1.04)* | 22 | 0.28 ± 0.79 | 0.61 (0.24, 0.98)* |
| mPAP, mmHg | 29 | 1.1 ± 6.7 | 30 | −3.7 ± 8.1 | −4.8 (−8.7, −0.9)* | 22 | −3.3 ± 7.9 | −4.4 (−8.5, −0.3)* |
| PVR, % | 29 | 119.7 (105.4, 135.8) | 30 | 74.9 (67.5, 83.0) | −37.4 (−46.6, −26.7)* | 22 | 75.5 (63.7, 89.6) | −36.9 (−48.5, −22.7)* |
| NT-proBNP, fmol/ml | 28 | 37.5 ± 321.9 | 26 | −245.1 ± 738.3 | −283 (−590, 25) | 21 | −228.8 ± 501 | −266 (−503, −29)* |
| Without background PAH-specific therapy | ||||||||
| CI, L/min/m2 | 21 | −0.32 ± 0.81 | 17 | 0.28 ± 0.44 | 0.60 (0.15, 1.04)* | 26 | 0.32 ± 0.91 | 0.64 (0.12, 1.15)* |
| mPAP, mmHg | 21 | 0.9 ± 8.5 | 17 | −3.0 ± 7.7 | −3.8 (−9.2, 1.6) | 27 | −7.0 ± 13.6 | −7.9 (−14.7, −1.1)* |
| PVR, % | 21 | 110.7 (92.7, 132.1) | 17 | 80.5 (65.3, 99.3) | −27.2 (−44.1, −5.4)* | 26 | 67.8 (55.2, 83.4) | −38.7 (−53.3, −19.5)* |
| NT-proBNP, fmol/ml | 21 | 402.9 ± 757.6 | 19 | −62.6 ± 450.9 | −466 (−870, −61)* | 25 | −8.2 ± 582.5 | −411 (−810, −13)* |
P-value < 0.05 for the comparison between macitentan and placebo.
PVR data are the geometric mean of month 6/baseline (%) (95% CL), data for all other variables are mean ± SD.
PVR data are expressed as a percent change (%) between macitentan and placebo: (ratio of geometric means − 1) × 100, data for all other variables are the placebo corrected mean ± SD.
n represents the number of patients in the haemodynamic sub-study at baseline; n represents the number of patients with non-missing values for the change from baseline to month 6.
Of the 68 placebo patients, 34 were on background PAH-specific therapy and 34 were not; of the 62 macitentan 3 mg patients, 34 were on background PAH-specific therapy and 28 were not; of the 57 macitentan 10 mg patients, 24 were on background PAH-specific therapy and 33 were not.
Results are based on observed data with no imputation rules applied for missing values.
CL, confidence limits; CI, cardiac index; NT-proBNP, N-terminal pro-brain natriuretic peptide; mPAP, mean pulmonary arterial pressure; PVR pulmonary vascular resistance.
Associations of CI, RAP, and NT-proBNP with disease progression
CI, RAP, and NT-proBNP at baseline and at month 6
CI, RAP and NT-proBNP at baseline and at month 6 were associated with the risk of a morbidity/mortality event. For every 0.5 L/min/m2 increase in CI at baseline there was a 24% lower risk (HR 0.76, 95% CL 0.70–0.83) and at month 6 a 25% lower risk (HR 0.75, 95% CL 0.62–0.92) of experiencing a morbidity/mortality event. For every 1 mmHg increase in RAP at both baseline and month 6, there was a 6% greater risk (HR 1.06, 95% CL 1.04–1.08 and HR 1.06, 95% CL 1.01–1.11) of experiencing a morbidity/mortality event. For every 300 fmol/ml increase in NT-proBNP at baseline there was a 15% greater risk (HR 1.15, 95% CL 1.11–1.20) and at month 6 a 19% greater risk (HR 1.19, 95% CL 1.15–1.24) of morbidity/mortality. Similar results were obtained when adjusted for study treatment, background PAH-specific therapy and time from RHC to randomization (Supplementary material online, Table S4).
CI, RAP, and NT-proBNP changes from baseline to month 6
For every increase of 0.5 L/min/m2 in CI from baseline to month 6, there was a non-significant 16% reduction in the risk of morbidity/mortality events [HR = 0.84, 95% CL 0.69–1.03]. Changes in RAP at month 6 were not associated with the risk of morbidity/mortality [HR = 1.01, 95% CL 0.95–1.08; for every increase of 1 mmHg from baseline to month 6]. For every increase of 300 fmol/ml in NT-proBNP from baseline to month 6, there was a 15% higher risk of morbidity/mortality [HR 1.15, 95% CL 1.04–1.27]. Similar results were obtained when adjusted for study treatment, background PAH-specific therapy and time from RHC to randomization (Supplementary material online, Table S4).
CI, RAP, and NT-proBNP thresholds at month 6
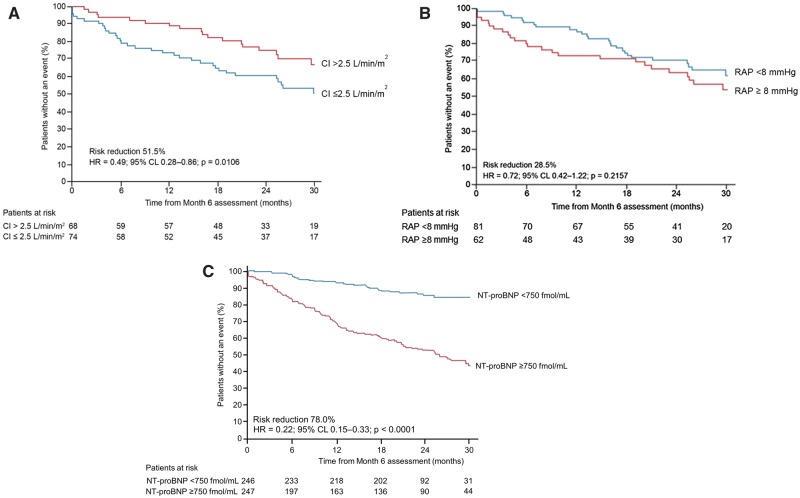
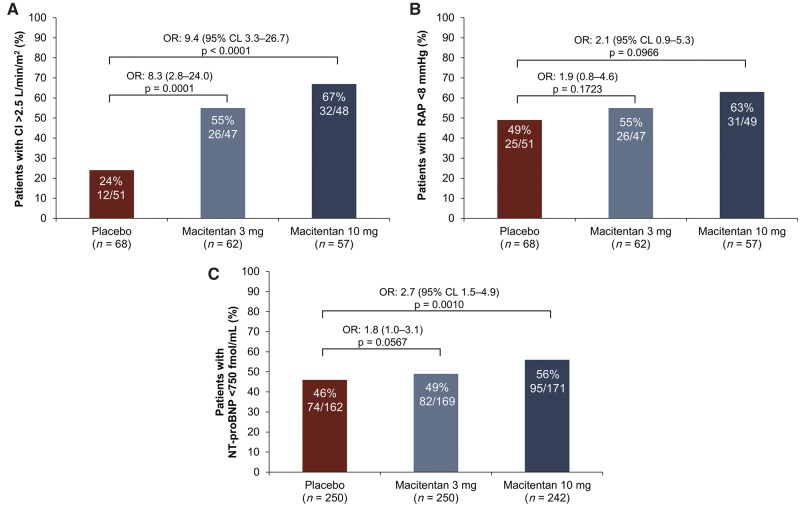
After 6 months, patients with a CI > 2.5 L/min/m2 vs. a CI ≤ 2.5 L/min/m2 had a 51% reduction in the risk of morbidity/mortality events (HR 0.49, 95% CL 0.28–0.86; Figure 1A). Patients with RAP < 8 mmHg vs. RAP ≥ 8 mmHg at month 6 had a 28% reduction in the risk of morbidity/mortality (HR 0.72, 95% CL 0.42–1.22; Figure 1B) and patients with NT-proBNP < 750 fmol/ml vs. ≥ 750 fmol/ml at month 6 had a 78% reduction in the risk of morbidity/mortality (HR 0.22, 95% CL 0.15–0.33; Figure 1C). We observed a consistent reduction in the risk of morbidity/mortality after the adjustment for study treatment, background PAH-specific therapy and time from RHC to randomization (Supplementary material online, Table S5). At month 6, patients treated with macitentan were more likely to have a CI > 2.5 L/min/m2, RAP < 8 mmHg and NT-proBNP < 750 fmol/ml compared with placebo-treated patients (Figure 2).
Figure 1.
Risk of a morbidity/mortality event according to (A) CI threshold of 2.5 L/min/m2(B) RAP threshold of 8 mmHg and (C) NT-proBNP threshold of 750 fmol/ml at month 6. Patients at risk at time zero are those with non-missing values at baseline and month 6 who had not already experienced a morbidity/mortality event prior to month 6. CL, confidence limit; HR, hazard ratio; CI, cardiac index; RAP, right atrial pressure; NT-proBNP, N-terminal pro-brain natriuretic peptide.
Figure 2.
Proportion of patients with (A) CI > 2.5 L/min/m2(B) RAP of < 8 mmHg or (C) NT-proBNP < 750 fmol/ml at month 6. Adjusted for values at baseline. For CI and RAP, n denotes the number of patients who participated in the haemodynamic sub-study; for NT-proBNP, n denotes all randomized patients in the SERAPHIN study. The percentages are proportions of patients with values at month 6 who reached the respective thresholds. OR, odds ratio; CL, confidence limit; CI, cardiac index; RAP, right atrial pressure; NT-proBNP, N-terminal pro-brain natriuretic peptide.
Consistency of findings across study arms
The associations were generally consistent across study arms (placebo, macitentan 3 mg and macitentan 10 mg), with P-values for treatment interaction indicating no statistical differences (Supplementary material online, Table S6). Differences in the associations across the study arms were observed only for changes in NT-proBNP at month 6 (treatment interaction P-value = 0.0101).
Safety and tolerability
The most frequently reported adverse events in haemodynamic sub-study patients are reported in Supplementary material online, Table S7. Similar proportions of placebo-, macitentan 3 mg-, and macitentan 10 mg-treated patients experienced at least one adverse event. Macitentan 3 mg and macitentan 10 mg were associated with increased rates of headache compared with placebo. Macitentan 10 mg was associated with increased rates of viral respiratory tract infections and bronchitis compared with placebo. No adverse events related to RHC were reported.
Discussion
In the SERAPHIN sub-study, macitentan improved haemodynamic parameters and NT-proBNP levels after 6 months of treatment in patients with PAH irrespective of WHO functional class and background PAH-specific therapy (predominantly sildenafil) at baseline, whereas these parameters worsened in patients who received placebo. These data are consistent with the favourable effects of macitentan on long-term (median duration of treatment 115 weeks) morbidity/mortality in patients with PAH.10 In fact, haemodynamics and NT-proBNP plasma levels, which are indirect indicators of RV function, represent a major determinant of the PAH patients’ prognosis.12–15
The prognostic relevance of baseline CI, RAP and NT-proBNP on disease progression has been confirmed for the first time in a randomized controlled trial using a prospectively defined composite primary endpoint of morbidity/mortality. This finding highlights the importance of starting PAH medications as soon as possible, before advanced changes of RV function. In addition, the prognostic relevance of CI, RAP, and NT-proBNP after 6 months of treatment provides evidence for their contribution to the overall evaluation of the treatment response.1,2
In clinical practice, the goal of the PAH physician is to maintain or bring their patient to a low-risk profile, part of which includes achieving and/or maintaining good RV function.1,2 In this sub-study, patients who at month 6 had a CI > 2.5 L/min/m2, RAP < 8 mmHg and NT-proBNP < 750 fmol/ml experienced a lower risk of disease progression compared with patients who did not. We also observed that patients treated with macitentan 10 mg were more likely to reach these thresholds at month 6 compared with placebo. These results do not demonstrate that improving haemodynamic parameters results in improved long-term outcomes. Nevertheless, they offer an indirect confirmation of the recommendations proposed in the ESC/ERS guidelines1,2 for the initial risk stratification and evaluation of treatment response. These findings deserve to be highlighted as they may strengthen the current level of evidence for these recommendations (level C: consensus of opinion of the experts and/or small studies, retrospective studies, registries).
The low-risk profile of the ESC/ERS guidelines includes a threshold of NT-proBNP that is based on data from observational studies.4,5 An NT-proBNP threshold of 300 ng/L (equivalent to 35 fmol/ml) is considered appropriate to identify a better prognosis.1,2 This threshold was derived from NT-proBNP levels measured by the Roche Diagnostics Elecsys NT-proBNP immunoassay. In the SERAPHIN study, the Biomedica BNP Fragment Enzyme ImmunoAssay was used. The two assays detect different epitopes; the Roche assay detects larger fragments of NT-proBNP than the Biomedica assay. Because the differences between the assays are non-linear, plasma levels derived from these assays cannot be compared.11 Although the data of this study are not comparable with the NT-proBNP threshold proposed in the guidelines, they support the use of NT-proBNP for the evaluation of treatment response.
In this study, CI, RAP and NT-proBNP values, at baseline and at month 6, were associated with disease progression, while there was no consistent evidence for an association between the changes in these parameters and long-term outcomes. In previous studies, improvement or deterioration in certain indicators of RV function were sometimes but not always associated with survival.16–18 Our study suggests that an increase in NT-proBNP was indicative of disease progression. This association was observed in the placebo arm, but was less apparent in patients treated with macitentan. The inconsistency in associations observed between changes in the parameters evaluated and disease progression may be influenced by patients' baseline condition. Patients with characteristics indicative of low risk at baseline (high CI, low RAP, or low NT-proBNP) who experience small improvements with treatment may have better outcomes than patients with indicators of high risk at baseline who experience comparatively large improvements but nevertheless remain with measures indicative of poor prognosis. Theoretically, to avoid this apparent discrepancy, changes in a given parameter should not be correlated with outcome per se, but rather with the change in risk associated with that outcome between two time points (e.g. baseline and month 6). In practice, however, only the long-term outcome can be observed, and changes in risk can only be indirectly estimated.
A limitation of these analyses is that they include only a subset of the SERAPHIN population. However, the baseline characteristics of the sub-study patients were similar to those of the overall SERAPHIN population and balanced between treatment arms, hence mitigating a potential selection bias in this non-randomized sample of the study population. Nevertheless, the results should be interpreted with caution; they are based on a small sample size, are exploratory in nature and are not adjusted for multiple comparisons. In addition, the associations found in our analysis between haemodynamic parameters and NT-proBNP and disease progression are achieved with a patient population selected for a clinical trial. Different results may be possible with diverse PAH populations and/or treatment strategies. The mechanism of action by which macitentan affects these parameters is not explained by these data and is beyond the scope of this study. This may be addressed in the on-going REPAIR (Right vEntricular remodelling in Pulmonary ArterIal hypeRtension) study (NCT02310672), which will evaluate the effects of macitentan on RV remodelling in PAH assessed by cardiac magnetic resonance imaging.
Conclusions
In summary, for all treatment groups in the SERAPHIN haemodynamic substudy, baseline and month 6 values of CI, RAP, and NT-proBNP, but not their changes, were associated with morbidity/mortality events. These results confirm the relevance of these parameters in predicting disease progression in patients with PAH and highlight that monitoring these parameters can provide useful prognostic information. By improving haemodynamic parameters and reducing NT-proBNP levels, macitentan increased the chance of reaching threshold values associated with lower risk of morbidity/mortality, irrespective of WHO functional class, and background PAH-specific therapy.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
The authors thank Dr Mariana Thomson from nspm ltd for medical writing assistance, funded by Actelion Pharmaceuticals Ltd. The authors do hereby declare that all illustrations and figures in the article are entirely original and do not require reprint permission.
Funding
This work was sponsored by Actelion Pharmaceuticals Ltd.
Conflict of interest: Dr Galiè has financial relationships with Actelion Pharmaceuticals Ltd, Bayer HealthCare, Pfizer, and GlaxoSmithKline. Dr Jansa has received fees and grants from Actelion Pharmaceuticals Ltd during the conduct of this study and from United Therapeutics, AOP Orphan, Bayer HealthCare, and GlaxoSmithKline. Dr Pulido has served on advisory boards of Actelion Pharmaceuticals Ltd and Bayer HealthCare, and has received grant support through institutional funds from Actelion Pharmaceuticals Ltd, Bayer HealthCare, Gilead, United Therapeutics, GlaxoSmithKline, and Eli Lilly & Company; and has received consultancy honoraria from Actelion Pharmaceuticals Ltd and Bayer HealthCare. Dr Channick has consulted for Actelion Pharmaceuticals Ltd and Bayer HealthCare and has received a research grant from Bayer HealthCare. Dr Delcroix has received grants from Actelion Pharmaceuticals Ltd, GlaxoSmithKline, and Pfizer; has received speaker fees from Actelion Pharmaceuticals Ltd, Bayer HealthCare, and GlaxoSmithKline; and has consulted for Actelion Pharmaceuticals Ltd, GlaxoSmithKline, Pfizer, United Therapeutics, and Bayer HealthCare. Dr Ghofrani has financial relationships with Actelion Pharmaceuticals Ltd, Bayer HealthCare Ergonex, Pfizer, GlaxoSmithKline, Novartis, Gilead, and Merck & Co. Dr Le Brun is an employee, stock owner, and shareholder of Actelion Pharmaceuticals Ltd. Dr Mehta has received grant support from Actelion Pharmaceuticals Ltd; speaker’s fees from Actelion Pharmaceuticals Ltd, Bayer HealthCare, and GlaxoSmithKline; and fees as a participant in pharmaceutical clinical trials through institutional support from Actelion Pharmaceuticals Ltd, Bayer HealthCare, Gilead, GlaxoSmithKline, Ikaria, Eli Lilly & Co., and United Therapeutics. Dr Perchenet is an employee and stock holder of Actelion Pharmaceuticals Ltd. Dr Rubin has consulted for Actelion Pharmaceuticals Ltd, United Therapeutics, Lung LLC, GeNO, and Gilead. Dr Sastry has received grants from Actelion Pharmaceuticals Ltd and Pfizer. Dr Simonneau consults for and has received grants from Actelion Pharmaceuticals Ltd, Bayer, GlaxoSmithKline, and Pfizer. Dr Sitbon has consulted for Actelion Pharmaceuticals Ltd, GlaxoSmithKline, Pfizer, Eli Lilly, United Therapeutics, and Bayer and received grants from Actelion Pharmaceuticals Ltd, GlaxoSmithKline, Pfizer, Eli Lilly, and Bayer. Dr Souza has received lecture and advisory fees from Actelion Pharmaceuticals Ltd, Bayer HealthCare, GlaxoSmithKline, and Bristol-Meyers Squibb. Dr Torbicki has received speaker fees from Actelion Pharmaceuticals Ltd, Bayer, Novartis, GlaxoSmithKline, MSD, and AOP; and consultancy and investigator honoraria from Actelion Pharmaceuticals Ltd, Bayer HealthCare, MSD, and United Therapeutics.
References
- 1. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H, Luis Zamorano J.. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119.26320113 [Google Scholar]
- 2. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M.. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015;46:903–975. [DOI] [PubMed] [Google Scholar]
- 3. McLaughlin VV, Gaine S, Howard LS, Leuchte H, Mathier M, Mehta S, Palazzini M, Park M, Tapson V, Sitbon O.. Treatment Goals of Pulmonary Hypertension. J Am Coll Cardiol 2013;62:D73–D81. [DOI] [PubMed] [Google Scholar]
- 4. Fijalkowska A, Kurzyna M, Torbicki A, Szewczyk G, Florczyk M, Pruszczyk P, Szturmowicz M.. Serum N-terminal brain natriuretic peptide as a prognostic parameter in patients with pulmonary hypertension. Chest 2006;129:1313–1321. [DOI] [PubMed] [Google Scholar]
- 5. Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG, McGoon MD.. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010;122:164–172. [DOI] [PubMed] [Google Scholar]
- 6. Escribano-Subias P, Blanco I, Lopez-Meseguer M, Lopez-Guarch CJ, Roman A, Morales P, Castillo-Palma MJ, Segovia J, Gomez-Sanchez MA, Barbera JA, Investigators R. Survival in pulmonary hypertension in Spain: insights from the Spanish registry. Eur Respir J 2012;40:596–603. [DOI] [PubMed] [Google Scholar]
- 7. Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Cottin V, Degano B, Jais X, Montani D, Souza R, Simonneau G.. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010;122:156–163. [DOI] [PubMed] [Google Scholar]
- 8. Lee WT, Ling Y, Sheares KK, Pepke-Zaba J, Peacock AJ, Johnson MK.. Predicting survival in pulmonary arterial hypertension in the UK. Eur Respir J 2012;40:604–611. [DOI] [PubMed] [Google Scholar]
- 9. Thenappan T, Shah SJ, Rich S, Tian L, Archer SL, Gomberg-Maitland M.. Survival in pulmonary arterial hypertension: a reappraisal of the NIH risk stratification equation. Eur Respir J 2010;35:1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pulido T, Adzerikho I, Channick R, Delcroix M, Galiè N, Ghofrani A, Jansa P, Jing Z-C, Le Brun F, Mehta S, Mittelholzer C, Perchenet L, Sastry BKSSitbon O, Souza R, Torbicki A, Zeng X, Rubin L, Simonneau G.. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013;369: 809–818. [DOI] [PubMed] [Google Scholar]
- 11. Mueller T, Gegenhuber A, Poelz W, Haltmayer M.. Comparison of the Biomedica NT-proBNP enzyme immunoassay and the Roche NT-proBNP chemiluminescence immunoassay: implications for the prediction of symptomatic and asymptomatic structural heart disease. Clin Chem 2003;49:976–979. [DOI] [PubMed] [Google Scholar]
- 12. Chin KM, Kim NH, Rubin LJ.. The right ventricle in pulmonary hypertension. Coron Artery Dis 2005;16:13–18. [DOI] [PubMed] [Google Scholar]
- 13. Gan CT, McCann GP, Marcus JT, van Wolferen SA, Twisk JW, Boonstra A, Postmus PE, Vonk-Noordegraaf A.. NT-proBNP reflects right ventricular structure and function in pulmonary hypertension. Eur Respir J 2006;28:1190–1194. [DOI] [PubMed] [Google Scholar]
- 14. Nagaya N, Nishikimi T, Uematsu M, Satoh T, Kyotani S, Sakamaki F, Kakishita M, Fukushima K, Okano Y, Nakanishi N, Miyatake K, Kangawa K.. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation 2000;102:865–870. [DOI] [PubMed] [Google Scholar]
- 15. Bernus A, Wagner BD, Accurso F, Doran A, Kaess H, Ivy DD.. Brain natriuretic peptide levels in managing pediatric patients with pulmonary arterial hypertension. Chest 2009;135:745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sitbon O, Humbert M, Nunes H, Parent F, Garcia G, Herve P, Rainisio M, Simonneau G.. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol 2002;40:780–788. [DOI] [PubMed] [Google Scholar]
- 17. Tiede H, Sommer N, Milger K, Voswinckel R, Bandorski D, Schermuly RT, Weissmann N, Grimminger F, Seeger W, Ghofrani HA.. Short-term improvement in pulmonary hemodynamics is strongly predictive of long-term survival in patients with pulmonary arterial hypertension. Pulm Circ 2013;3:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nickel N, Golpon H, Greer M, Knudsen L, Olsson K, Westerkamp V, Welte T, Hoeper MM.. The prognostic impact of follow-up assessments in patients with idiopathic pulmonary arterial hypertension. Eur Respir J 2012;39:589–596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.