Abstract
STUDY QUESTION
What is the impact of administration of the selective progesterone receptor modulator (SPRM), ulipristal acetate (UPA) on the endometrium of women with fibroids?
SUMMARY ANSWER
UPA administration altered expression of sex-steroid receptors and progesterone-regulated genes and was associated with low levels of glandular and stromal cell proliferation.
WHAT IS KNOWN ALREADY
Administration of all SPRM class members results in PAEC (progesterone receptor modulator associated endometrial changes). Data on the impact of the SPRM UPA administration on endometrial sex-steroid receptor expression, progesterone (P)-regulated genes and cell proliferation are currently lacking.
STUDY DESIGN SIZE, DURATION
Observational study with histological and molecular analyses to delineate impact of treatment with UPA on endometrium. Endometrial samples (n = 9) were collected at hysterectomy from women aged 39 to 49 with uterine fibroids treated with UPA (oral 5 mg daily) for 9–12 weeks. Control proliferative (n = 9) and secretory (n = 9) endometrium from women aged 38–52 with fibroids were derived from institutional tissue archives.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Study setting was a University Research Institute. Endometrial biopsies were collected with institutional ethical approval and written informed consent. Concentrations of mRNAs encoded by steroid receptors, P-regulated genes and factors in decidualised endometrium were quantified with qRT-PCR. Immunohistochemistry was employed for localization of progesterone (PR, PRB), androgen (AR), estrogen (ERα) receptors and expression of FOXO1, HAND2, HOXA10, PTEN homologue. Endometrial glandular and stromal cell proliferation was objectively quantified using Ki67.
MAIN RESULTS AND THE ROLE OF CHANCE
UPA induced morphological changes in endometrial tissue consistent with PAEC. A striking change in expression patterns of PR and AR was detected compared with either proliferative or secretory phase samples. There were significant changes in pattern of expression of mRNAs encoded by IGFBP-1, FOXO1, IL-15, HAND2, IHH and HOXA10 compared with secretory phase samples consistent with low agonist activity in endometrium. Expression of mRNA encoded by FOXM1, a transcription factor implicated in cell cycle progression, was low in UPA-treated samples. Cell proliferation (Ki67 positive nuclei) was lower in samples from women treated with UPA compared with those in the proliferative phase.
LARGE SCALE DATA
N/A.
LIMITATIONS REASONS FOR CAUTION
A small number of well-characterized patients were studied in-depth. The impacts on morphology, molecular and cellular changes with SPRM, UPA administration on symptom control remains to be determined.
WIDER IMPLICATIONS OF THE FINDINGS
P plays a pivotal role in endometrial function. P-action is mediated through interaction with the PR. These data provide support for onward development of the SPRM class of compounds as effective long-term medical therapy for heavy menstrual bleeding.
STUDY FUNDING/COMPETING INTEREST(S)
H.O.D.C. received has clinical research support for laboratory consumables and staff from Bayer Pharma Ag and provides consultancy advice (no personal remuneration) for Bayer Pharma Ag, PregLem SA, Gedeon Richter, Vifor Pharma UK Ltd, AbbVie Inc.; A.R.W.W. has received consultancy payments from Bayer, Gedeon Richter, Preglem SA, HRA Pharma; L.H.R.W., A.A.M., R.M., G.S. and P.T.K.S. have no conflicts of interest. Study funded in part from each of: Medical Research Council (G1002033; G1100356/1; MR/N022556/1); National Health Institute for Health Research (12/206/520) and TENOVUS Scotland.
Keywords: selective progesterone receptor modulator (SPRM), endometrium, proliferation, androgen receptor, progesterone receptor, estrogen receptor, progesterone-regulated genes
Introduction
Heavy menstrual bleeding (HMB) is a common condition affecting up to one in four women of reproductive age (Shapley et al., 2004). This condition has significant impact upon the physical, social, emotional and material quality of life of women (NICE, 2007). In the USA, conservative estimates of annual direct and indirect costs are $1 and $12 billion, respectively (Liu et al., 2007). In the UK, it is estimated that 1 million women annually seek help for HMB (NICE, 2007). There are multiple etiologies that are associated with HMB including fibroids (leiomyomas) (Munro et al., 2011). In women with symptomatic fibroids, HMB is the major complaint for which treatment is sought and is a leading indication of hysterectomy in pre-menopausal women (Merrill, 2008) resulting in sterility, an unacceptable side effect for many women. The mechanisms responsible for the development of these benign tumours is not fully understood but it is well established that fibroid growth is regulated by the actions of the sex-steroids estrogen (E) and progesterone (P) (Yin et al., 2007, 2010; Bulun, 2013).
Progesterone is a critical regulator of female reproduction and plays a pivotal role in endometrial differentiation and its withdrawal following demise of the corpus luteum during a non-pregnant cycle precipitates endometrial shedding during menstruation (Maybin and Critchley, 2015). Progesterone responses are mediated through interaction of the steroid with the progesterone receptor (PR); a ligand-activated transcription factor. It has been demonstrated that two main isoforms of the receptor (termed PR-A and PR-B) are present within the endometrium (Patel et al., 2015). Expression and relative abundance of these isoforms within the tissue contribute to normal uterine function and, if dysregulated, uterine pathophysiology. Notably, activation of P-responsive genes has been linked with reduced apoptosis and enhanced proliferation of fibroid cells (Yin et al., 2007, 2010).
Administration of synthetic progestins is a medical therapy currently used for managing HMB, however, these therapies often either fail to fully resolve symptoms or are associated with unacceptable side effects (Roberts et al., 2011). The development of a new class of synthetic compounds the ‘selective progesterone receptor modulators’ (SPRMs) exhibiting mixed agonist and antagonist activity appears to offer a new approach to medical management of hormone dependent uterine disorders such as HMB and fibroids (Wagenfeld et al., 2016). Class members include mifepristone, asoprisnil and ulipristal acetate (UPA).
Administration of the SPRMs mifepristone and asoprisnil has been reported to reduce both fibroid volume and menstrual blood loss (Wilkens et al., 2008; Engman et al., 2009). Currently the SPRM, UPA is the only member of this class of drug licensed in Europe for short-term treatment of fibroids prior to hysterectomy and more recently (in Europe) for intermittent treatment of moderate to severe symptoms of uterine fibroids in adult women of reproductive age. In clinical studies, utilizing a regime of repeated doses of UPA it has been shown that median fibroid volume was reduced by 45% of women receiving treatment and nearly 90% of patients reported a significant reduction in menstrual bleeding (Donnez et al., 2014).
UPA administration, in common with that of other SPRMs, is associated with morphological changes of the endometrium. Characteristics of these effects include large cystic glands, changes within the stromal compartment including the fibroblasts and vasculature and are now recognized as a distinct histological entity, termed progesterone receptor modulator associated endometrial changes (PAEC) (Williams et al., 2012).
Studies have demonstrated that the SPRMs mifepristone and UPA both exert anti-proliferative and pro-apoptotic effects on leiomyoma cells in vitro (Luo et al., 2010; Yin et al., 2010), which may explain mechanisms by which fibroid volume is reduced but there are very limited data as to the mechanism of SPRM action within the intact endometrium.
The mechanism by which control of bleeding is achieved is unclear. Clinical trials report bleeding control in up to 98% of women but only around 70–74% exhibit PAEC (Donnez et al., 2012a,b; Williams et al., 2012). Furthermore, some patients who continue to ovulate still achieve amenorrhoea (Chabbert-Buffet et al., 2007). Hence it is essential to delineate potential endometrial causes for the mitigation of menstrual bleeding.
In this study, we have used histological and molecular analyses to delineate the impact of treatment with UPA for 9–12 weeks on endometrial tissue focusing on expression of sex-steroid receptors, the products of genes known to be progesterone-regulated during the normal cycle and the impact upon endometrial cell proliferation.
The data presented here offer evidence that UPA, a licensed SPRM alters expression of sex-steroid receptors and PR regulated genes without increasing endometrial cell proliferation.
Materials and Methods
Patients and samples
After ethical approval (Research Ethics Committee 12/SS/0238; 10/S1402/59) and written informed consent, endometrial biopsies (n = 9) were collected at the time of surgery from women aged 39 to 49 years with symptomatic uterine fibroids treated with UPA (oral 5 mg daily) for 9–12 weeks (63–84 days) up to the time of hysterectomy (Table I). All subjects with one exception described excellent control of bleeding whilst receiving UPA with either complete amenorrhoea or only occasional spotting. Control proliferative (n = 9) and secretory (n = 9) endometrium from pre-menopausal women aged 38–52 undergoing hysterectomy for symptomatic fibroids were utilized from tissue archives (REC: 10/S1402/59). All control patients were women with fibroids who reported regular menstrual cycles and no preoperative hormone use. Median estradiol and progesterone serum levels were 497 (range 12.5–1272) and <3 (<3–5.7) in the proliferative group and 323 (range 77.4–530) and 27.1 (14.8–45.7) in the secretory group. Age and BMI were not significantly different from the UPA-treated group. Control endometrial tissues were staged based on standard histological criteria (Noyes et al., 1950), the patient's reported last menstrual period, and circulating estradiol and progesterone levels at the time of collection.
Table I.
Subject characteristics and endometrial histology.
| Subject | Age (Year) | Symptoms | Submucosal component of fibroid | Duration of UPA (days) | Bleeding control | Histology |
|---|---|---|---|---|---|---|
| A | 43 | HMB pain | No | 83 | Amenorrhoea | Extensive features of PAEC with widespread cystic glandular dilatation. Occasional thick-walled vessels |
| B | 48 | HMB pain | No | 63 | Amenorrhoea | Extensive features of PAEC with widespread cystic glandular dilatation. No abnormal vessels |
| C | 39 | HMB | Yes | 84 | Amenorrhoea | Extensive features of PAEC with widespread cystic glandular dilatation. No abnormal vessels |
| D | 39 | HMB pressure | Yes | 76 | Occasional spotting only | Some features of PAEC with occasional dilated cystic glands with tortuous morphology |
| E | 49 | HMB pressure | No | 77 | Amenorrhoea | Some features of PAEC with occasional dilated cystic glands with tortuous morphology |
| F | 45 | HMB | Yes | 81 | Increased HMB | Some features of PAEC with occasional dilated cystic glands with tortuous morphology |
| G | 45 | HMB pressure | Yes | 84 | Amenorrhoea | Some features of PAEC with occasional dilated cystic glands with tortuous morphology. Several thick-walled vessels |
| H | 47 | HMB pressure | Yes | 75 | Irregular spotting | Some features of PAEC with occasional dilated cystic glands with tortuous morphology |
| I | 46 | HMB pressure | No | 63 | Amenorrhoea | Minimal features of PAEC with occasional partially dilated cystic glands |
UPA, ulipristal acetate; HMB, heavy menstrual bleeding; PAEC, progesterone receptor modulator associated endometrial changes.
Gene expression analyses
RNA was isolated from endometrial samples using Qiagen RNAeasy mini kit as per manufacturer's protocol (Qiagen, UK). Quality of RNA was analyzed using the Agilent RNA 600 nano kit according to manufacturer's protocol (Agilent Technologies, USA). RNA integrity numbers were all greater than 7.7. cDNA was prepared using Superscript Vilo cDNA kit (Invitrogen, UK) using 100 ng RNA as template. Taqman RT-qPCR was performed in triplicate reactions using primers designed using the online Universal Probe Assay (Roche Diagnostics, USA) and synthesized by Eurofins Genomics (Germany) (Table II) on an ABI Prism Cycler (Applied Biosystems USA). Indian hedgehog (IHH) and phosphatase and tensin homologue (PTEN) primers were bought as pre-validated sets (Applied Biosystems, UK) and used according to the manufacturer's protocol. Relative quantification of target genes were analyzed after normalization to the geometric mean of endogenous ATP synthase H+ transporting mitochondrial F1 complex, Beta polypeptide (ATPB5) and 18s and a control sample by the comparative ΔΔCT method.
Table II.
Primers and probes for quantitative PCR.
| Target gene | Forward primer | Reverse primer | Roche probe |
|---|---|---|---|
| PR | tttaagagggcaatggaagg | cggattttatcaacgatgcag | 11 |
| PRB | aatgggctgtaccgagaggt | tctcagtccctcgctgagtt | 45 |
| AR | gctgatcataggcctctctc | tgccctgaaagcagtcctct | 14 |
| ESR1 | aaccagtgcaccattgataaaa | tcctcttcggtcttttcgtatc | 68 |
| IGFBP-1 | aatggattttatcacagcagacag | ggtagacgcaccagcagagt | 58 |
| FOXO1 | aagggtgacagcaacagctc | ttccttcattctgcacacga | 11 |
| IL-15 | cagatagccagcccatacaag | ggctatggcaaggggttt | 46 |
| HAND2 | tcaagaagaccgacgtgaaa | gttgctgctcactgtgcttt | 35 |
| HOXA10 | ccttccgagagcagcaaa | ttggctgcgttttcacct | 61 |
| FOXM1 | actttaagcacattgccaagc | cgtgcagggaaaggttgt | 11 |
| COUP TFII | ccatagtcctgttcacctcaga | aatctcgtcggctggttg | 36 |
| BMP2 | cggactgcggtctcctaa | ggaagcagcaacgctagaag | 49 |
Immunohistochemistry
Tissue samples were fixed in 4% neutral buffered formalin, sectioned, processed and stained with Haematoxylin and Eosin by standard methods. For immunohistochemistry 5 μm sections were subjected to antigen retrieval (Table III); non-specific activity was blocked sequentially with 3% hydrogen peroxide and appropriate serum before overnight incubation at 4°C with antibodies specific to: PR, PRB, androgen receptor (AR), estrogen receptor alpha (ERα), FOXO1, HAND2, HOXA10, PTEN and Ki67 (Table III). Appropriate matched IgG was applied as a negative control. Sections were incubated with ImmPRESS™ Ig reagents (Table III); bound antibodies were visualized using 3,3′-diaminobenzidine (Vector Laboratories, UK). Sections were counterstained with haemotoxylin and mounted in Pertex (Cellpath Technologies, UK). Representative images were captured using an Olympus BX51 microscope equipped with a Nikon DSFi1 camera (Olympus Optical Co. and Nikon Ltd, UK).
Table III.
Antibodies for immunohistochemistry.
| Protein | Supplier | Reference | Antibody type | Host | Dilution (normal horse serum) |
Retrieval Buffer |
ImmPRESS™ kit |
|---|---|---|---|---|---|---|---|
| PR | Dako | A0098 | Polyclonal | Rabbit | 1:200 | Citrate | Rabbit MP-7401 |
| PRB | Cell signalling | 3157S | Monoclonal | Rabbit | 1:800 | Citrate | Rabbit MP-7401 |
| AR | Spring bioscientific | M4070 | Monoclonal | Rabbit | 1:200 | Citrate | Rabbit MP-7401 |
| ERα | Vector | VP-E614 | Monoclonal | Mouse | 1:5000 | Citrate | Mouse MP-7402 |
| FOXO1 | Cell signalling | 2880 | Monoclonal | Rabbit | 1:250 | Citrate | Rabbit MP-7401 |
| PTEN | Dako | M3627 | Monoclonal | Mouse | 1:750 | Citrate | Mouse MP-7402 |
| HAND2 | Santa Cruz | SC9409 | Polyclonal | Goat | 1:200 | Citrate | Goat MP-7405 |
| HOXA10 | Santa Cruz | SC17159 | Polyclonal | Goat | 1:200 | Citrate | Goat MP-7405 |
| Ki67 | NovaCastra | NCL-Ki67-MM1 | Monoclonal | Mouse | 1:500 | TRIS | Mouse MP-7402 |
Analysis and scoring of proliferation marker expression
Ki67 stained sections (n = 6/group) were scanned using a Zeiss Imager A1microscope, fitted with a Prior Proscan II automatic stage (Zeiss, UK). For each scanned image 20 random fields were automatically selected by the programme and a 28 × 20 point grid was applied using Image Pro Plus 7.0 software. There were a total of 560 points (intersections of the grid) per field categorized as: (i) stained (positive) stromal cells, (ii) stained (positive) glandular cells, (iii) unstained (negative) stromal cells, (iv) unstained (negative) glandular cells and (v) all other points not overlaying any cells (e.g. empty slide space, lumen, connective tissue or blood). Points not overlaying a cell were excluded from analysis. The proportion of tissue in which each of the categorized cell type occupied was expressed as a percentage of total calculated area; cell proliferation index.
Statistics
Data were analyzed using Graphpad prism software (Graphpad, USA) utilizing non-parametric tests. Kruskal–Wallis test was used to determine differences between sample groups. Results are presented as mean ± SEM. P < 0.05 was considered to be statistically significant.
Results
Impact of UPA administration on endometrial morphology
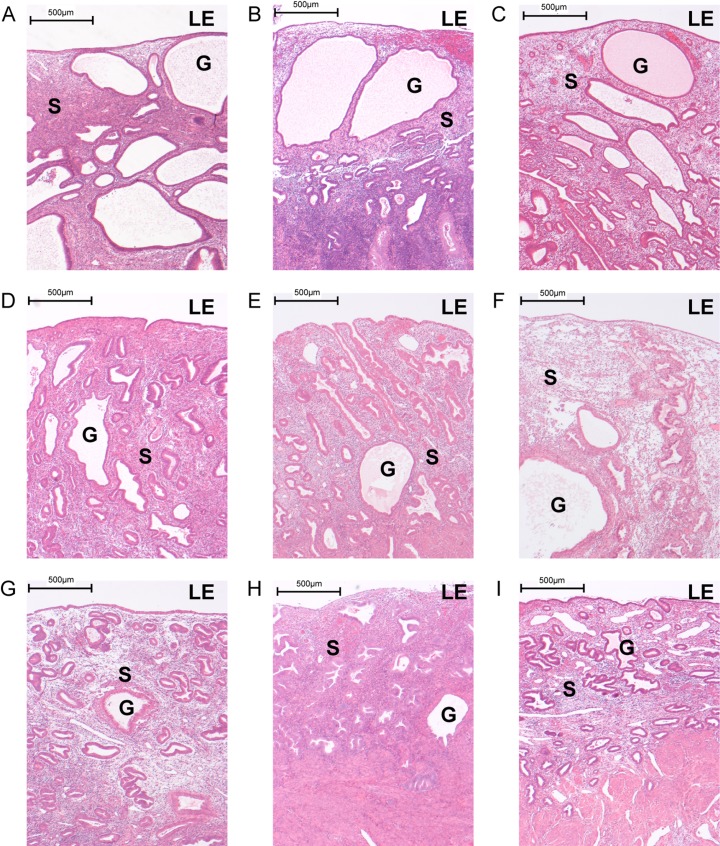
Endometrium from all women treated with UPA showed some histological features of PAEC (Table I). Individual images are illustrated in Fig. 1. Some showed more extensive cystic glandular dilatation than others (Fig. 1A–C); the extent of dilatation did not correlate to the presence of submucosal fibroids, duration of treatment or control of bleeding (Table I). No subjects exhibited histological evidence of inflammation, hyperplasia or neoplasia.
Figure 1.
Selective progesterone receptor modulator (SPRM), ulipristal acetate (UPA) modifies endometrial morphology. Haematoxylin and eosin staining of full thickness endometrium from hysterectomy specimens of nine subjects with uterine fibroids exposed to UPA for up to 12 weeks. All specimens exhibited progesterone receptor modulator associated endometrial changes (PAEC) but with variation in the degree of cystic glandular dilatation, and in overall endometrial thickness. Subjects A–C showed widespread extensive cystic dilatation that was less frequently observed in subjects D–H. Subject I had minimal cystic change. No subjects exhibited histological evidence of inflammation, hyperplasia or neoplasia. ×4 magnification (scale bar = 500 µm); LE, Luminal epithelium; G, Glands; S, Stroma.
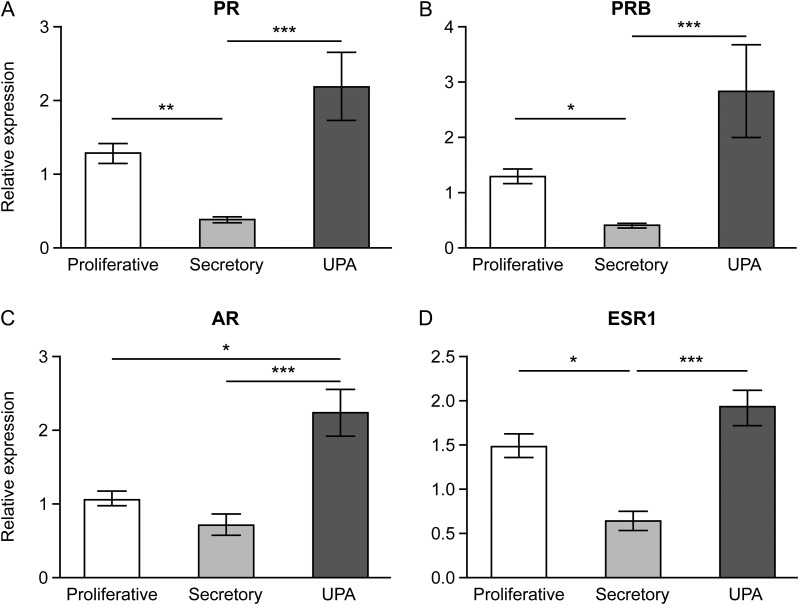
Treatment with UPA increases concentrations of mRNAs encoded by sex-steroid hormone receptors
Concentrations of PR and PRB mRNAs were significantly lower in secretory endometrium compared to proliferative tissue. UPA administration was associated with significantly higher PR and PRB mRNA concentrations than secretory phase endometrium but was not significantly different to proliferative phase samples (Fig. 2A and B). Total AR mRNA levels in UPA-treated samples were significantly increased compared to both proliferative and secretory phase samples. AR mRNA concentrations were not significantly different between proliferative and secretory phase samples (Fig. 2C). Concentrations of ESR1 (ERα) mRNA in secretory phase were significantly lower than proliferative phase and concentrations in UPA-treated samples were significantly higher than secretory phase. There was no significant different in ESR1 concentration between proliferative phase and UPA-treated samples (Fig. 2D)..
Figure 2.
Treatment with SPRM, UPA, increased the concentration of mRNAs encoding sex-steroid receptors in tissue extracts from human endometrium as determined by qRT-PCR. Samples were from women with fibroids obtained during the proliferative and secretory phases or after UPA administration: n = 9 for each group. PR (A), PRB (B), AR (C), ESR1 (D). *P < 0.05, **P < 0.01, ***P < 0.001. Bars: mean ± SEM.
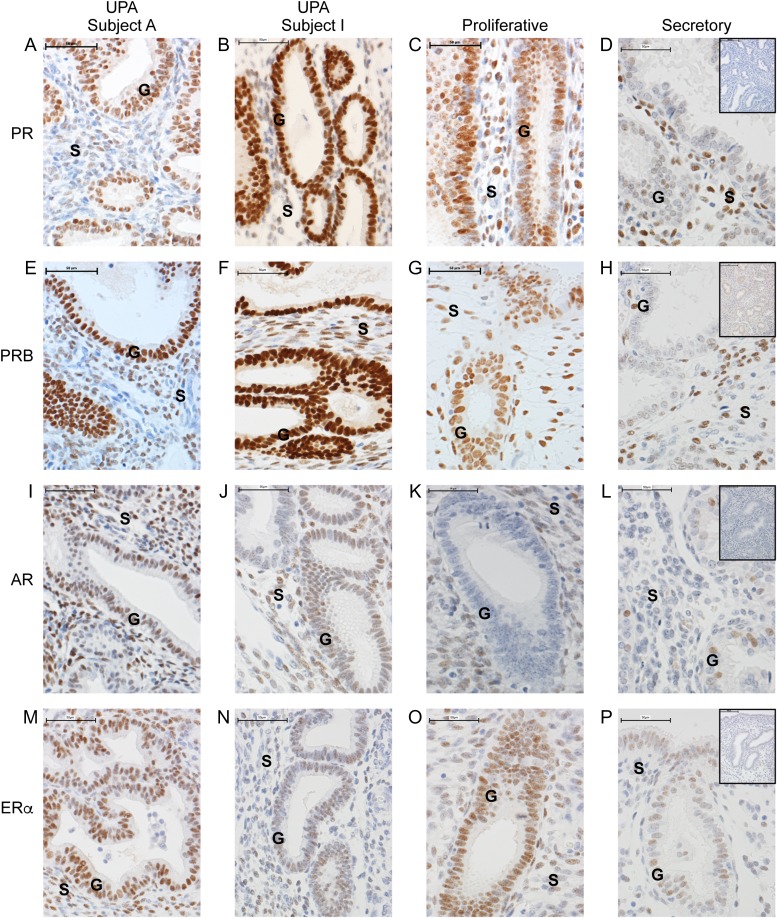
Immunoexpression of endometrial sex-steroid receptors was altered by UPA administration
In agreement with previous studies (Lessey et al., 1988; Wang et al., 1998) intense immunopositive staining for PR (with antibody recognizing both isoforms) was detected in cell nuclei in both glandular epithelial cells and stromal fibroblasts in proliferative endometrium. Intensity was reduced in epithelial cell nuclei in the secretory phase (Fig. 3C versus D). UPA-treated endometrium with marked and minimal cystic glandular dilatation (Fig. 3A and B, respectively) showed a pattern of PR immunopositive staining characterized by intense staining of nuclei in glandular epithelium and weak/negligible immunoexpression in stromal fibroblasts. This pattern did not phenocopy either proliferative or secretory endometrium (Fig. 3C and D). These results were mirrored by results obtained using a PRB-specific antibody (Fig. 3E–H).
Figure 3.
Administration of SPRM, UPA, modulates sex-steroid receptor localization. Representative immuno-localization of progesterone receptor (PR; A–D), PRB (E–H), androgen receptor (AR; I–L) and estrogen receptor alpha (ERα; M–P) in endometrium from woman with fibroids during proliferative and secretory stages and after UPA administration. Subject A shows endometrium in which PAEC are characterized by extensive cystic glandular dilatation; Subject I has PAEC with minimal cystic change. Neither subject had evidence of submucosal fibroids, and both women described amenorrhoea on UPA treatment. Samples from UPA-treated women displayed intense immunopositive (+ = positive and − = negative) glandular nuclei with only a few immunopositive cells in the stroma, a result in contrast with proliferative phase (G+S+) or secretory phase (G−S+). ×40 magnification (scale bar = 50 µm); G, Glands; S, Stroma. Negative controls shown as inserts on secretory endometrium.
Consistent with previous findings in our group (Marshall et al., 2011), intense immunopositive staining for AR was detected in nuclei of stromal fibroblasts in proliferative endometrium (Fig. 3K); AR positive epithelial cells were only detected in secretory phase (Fig. 3L) coincident with a reduction in staining intensity in stromal cells. Immunostaining of UPA-treated endometrial sections revealed a unique pattern characterized by intense immunopositive staining of cell nuclei in both epithelial cells and stromal fibroblasts (Fig. 3I and J). The expression profile of ERα (ESR1) protein differed between proliferative and secretory endometrium with reduced immunoexpression detected in secretory phase tissue in both stromal fibroblasts and glandular epithelium (Fig. 3O and P). ERα immunoexpression in UPA-treated endometrium mirrored that of proliferative endometrium but was less intense in subjects with minimal cystic glandular dilatation (Fig. 3M and N).
The impact of UPA administration on PR responsive genes
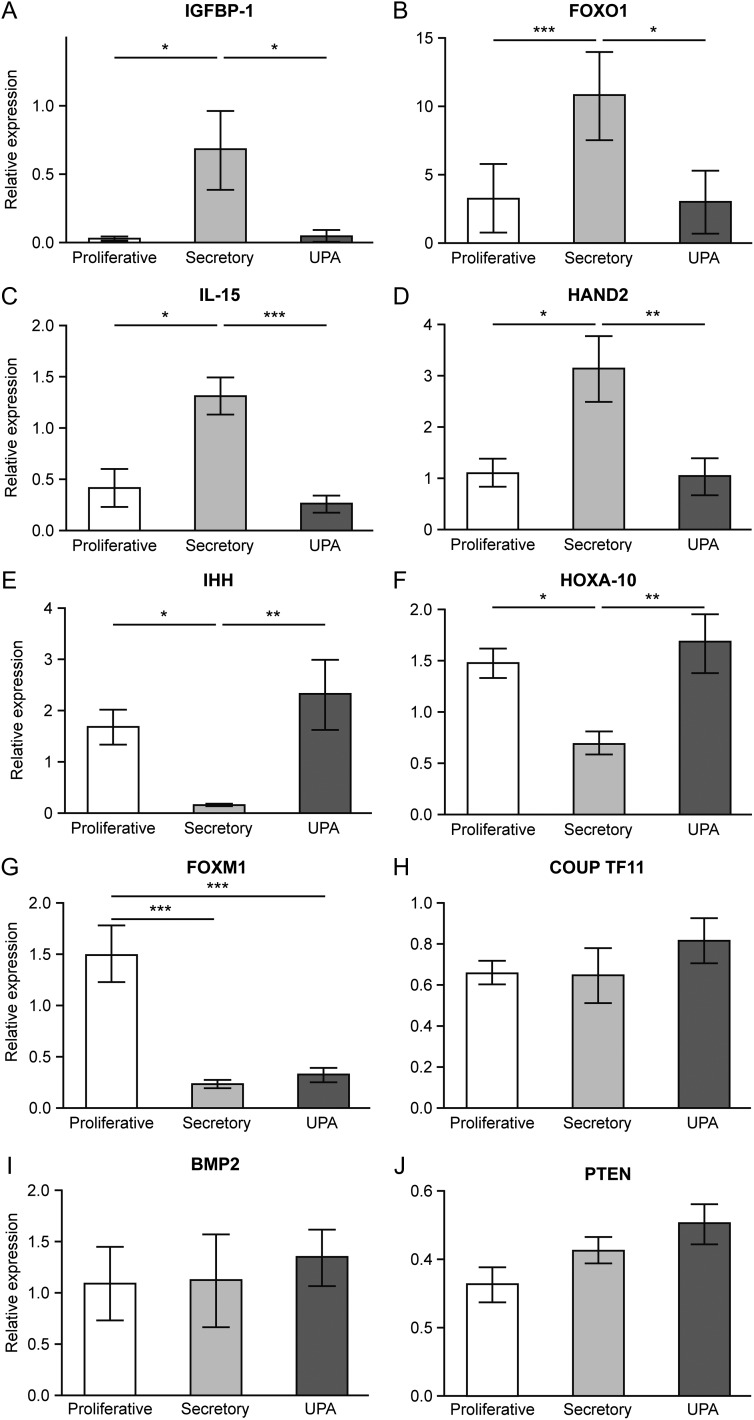
As UPA is a SPRM, further investigations focused on genes known to be regulated during the progesterone-dominated secretory phase of the cycle and associated with decidualisation (Wetendorf and DeMayo, 2012). In contrast to total concentrations of mRNAs in tissues collected during the secretory phase three patterns were detected: a significant increase, a significant decrease and no change. Specifically, treatment with UPA resulted in a significant decrease in mRNAs encoded by IGFBP-1 (Fig. 4A), FOXO1 (Fig. 4B), IL15 (Fig. 4C) and HAND2 (Fig. 4D) compared with secretory phase endometrium. There was no significant difference in mRNA levels of IGFBP-1, FOXO1, IL15 and HAND2 between proliferative and UPA-treated endometrium. In contrast, endometrial mRNA concentrations of IHH and HOXA10 (Fig. 4E and F) were significantly increased between samples from women treated with UPA and those samples collected in the secretory phase. There was no significant difference between proliferative phase and UPA-treated endometrium. Concentrations of FOXM1 mRNA were significantly decreased in both secretory phase and UPA samples compared with proliferative phase (Fig. 4G). There was no significant menstrual cycle or UPA-dependent change in COUP TFII, BMP2 or PTEN although a weak UPA effect was noted (Fig. 4H–J).
Figure 4.
Selective progesterone (P) receptor modulator, UPA, administration impacts on concentrations of P-responsive genes. Relative quantification of P-responsive genes in tissue extracts from human endometrium as determined by qRT-PCR. Samples were from women with fibroids obtained during the proliferative and secretory phases or after UPA treatment: n = 9 for each group. IGFBP-1 (A), FOXO1 (B), IL-15 (C), HAND2 (D), IHH (E), HOXA-10 (F), FOXM1 (G), COUP TF11 (H), BMP2 (I), PTEN (J). *P < 0.05, **P < 0.01, ***P < 0.001. Bars: mean ± SEM.
Impact of UPA on immunoexpression of proteins normally present in the progesterone-dependent secretory phase of the cycle
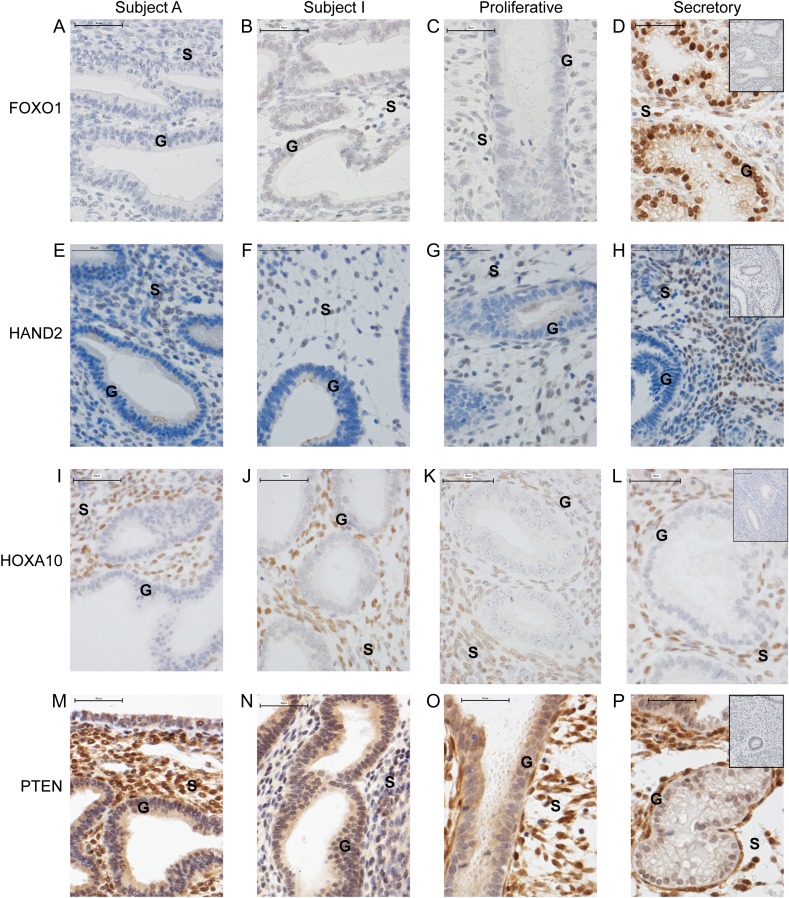
In keeping with the impact of UPA on concentrations of mRNA; immunolocalisation of FOXO1 was intense in the nuclei of the glandular epithelium in secretory phase endometrium; immunostaining was minimal in both proliferative phase and following UPA treatment (Fig. 5A–D). HAND2 immunolocalisation was most dense in the nuclei of stromal fibroblasts of secretory phase endometrium with less intense staining in proliferative and UPA-treated endometrium (Fig. 5E–H). HOXA10 immunolocalisation was present in stromal cells of both control and UPA-treated samples, with less intense staining observed in proliferative phase compared with either secretory or UPA-treated samples (Fig. 5I–L). PTEN immunolocalisation paralleled that of mRNA concentrations with minimal alteration between groups (Fig. 5M–P).
Figure 5.
Effects of SPRM, UPA, administration on mRNA levels are reflected in modulation of protein localization. Representative images showing immuno-localization of FOXO1 (A–D), HAND2 (E–H), HOXA10 (I–L) and PTEN (M–P) in endometrium from woman with fibroids at proliferative and secretory stages and after UPA treatment. Subject A shows endometrium in which PAEC are characterized by extensive cystic glandular dilatation; Subject I has PAEC with minimal cystic glandular change. ×40 magnification (scale bar = 50 µm); G: Glands, S: Stroma. Negative controls shown as inserts on secretory endometrium.
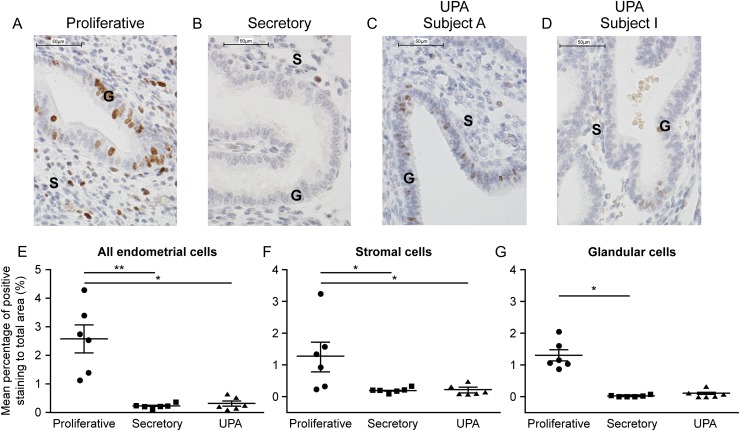
UPA administration does not increase endometrial cell proliferation
Immunoexpression of Ki67 was used to assess cell proliferation within the stromal and glandular compartments of the endometrium. As expected immunopositive staining for Ki67 was detected in nuclei of both stromal cells and glandular epithelium in endometrium during proliferative phase and appeared more numerous than in secretory phase tissue (Fig. 6A and B). In glandular cells UPA treatment appeared to reduce Ki67 immunoexpression compared to samples from proliferative phase (Fig. 6C or D versus A). Quantification of proliferating cells confirmed that there were significantly more Ki67 positive cells in proliferative phase tissue compared to both secretory phase and UPA-treated endometrium (Fig. 6E). Analysis of stromal cells showed a significant reduction in Ki67 positive cells in secretory phase and in UPA-treated samples. In glandular epithelium Ki67 positive cells were significantly decreased in secretory compared to proliferative phase: UPA-treated tissue showed a similar trend although this did not reach significance (P = 0.069) (Fig. 6E–G).
Figure 6.
SPRM, UPA, administration does not increase endometrial cell proliferation. Proliferation assessed by Ki67 immunohistochemistry (A–D) and stereological quantification (E–G). Subject A shows endometrium in which PAEC are characterized by extensive cystic glandular dilatation; Subject I has PAEC with minimal cystic change. ×40 magnification (scale bar = 50 µm); G: Glands, S: Stroma. Kruskal–Wallis statistical test *P < 0.05; **P < 0.01.
Discussion
UPA is a SPRM which, like other members of this class of compound, exhibits both agonist and antagonist activities in vitro (Wagenfeld et al., 2016). The impact of SPRMs is tissue-dependent and may be influenced both by bioavailabilty of different PR isoforms and the concentrations of different co-repressor and co-activator proteins in different cell types (Wagenfeld et al., 2016). In this study, we have demonstrated that UPA administration alters the pattern of expression of both PR and AR and mimics the anti-proliferative impact of progesterone in secretory phase endometrium.
We have conducted a comprehensive analysis of the pattern of gene expression in endometrium from women with fibroids treated with UPA prior to hysterectomy and compared this to untreated endometrium from women who also had fibroids. In keeping with the established literature (Williams et al., 2012) the extent of morphological change within the endometrium varied. Larger studies have demonstrated PAEC rates of around 60% following short but repeated courses of UPA administration although there is an evidence demonstrating that PAEC rapidly regresses on cessation of treatment (Donnez et al., 2014). Why not all subjects develop PAEC is uncertain and the impact of this morphological observation upon symptom control is unknown; in our study the degree of PAEC was not correlated with duration of treatment.
Two isoforms of PR are expressed in a differential manner within the endometrium (Mote et al., 1999). Our data herein demonstrate downregulation of both PR isoforms within the stroma and upregulation within the glandular epithelium following UPA administration. This finding is consistent with previous reports studying the effect of the SPRM asoprisnil on PR protein localization in human endometrium (Wilkens et al., 2013). The pattern of expression of ESR1 (ERα) mRNA and protein after treatment with UPA was similar to that of the proliferative phase showing this SPRM did not induce PR-dependent downregulation of ERα gene expression. This finding is similar to findings for the PR-antagonist mifepristone when administered to Rhesus macaques (Slayden and Brenner, 1994).
One of the most striking and consistent findings in this study using the SPRM UPA was a significant increase in concentrations of AR mRNA, which was accompanied by a unique pattern of AR protein expression, distinct to that reported during the normal menstrual cycle (Marshall et al., 2011). These findings in human are consistent with reports from analysis of endometrium from Rhesus macaques following treatment with an intrauterine device containing UPA for three artificial menstrual cycles (Brenner et al., 2010). A similar effect has been reported following treatment with mifepristone, which increased stromal and glandular AR immunoexpression in Rhesus macaques (Slayden et al., 2001) and in women treated with a single post-ovulatory dose of the SPRM (Slayden et al., 2001).
To explore whether UPA was acting as an agonist or antagonist with respect to progesterone-dependent gene expression we measured mRNAs encoded by genes that have been identified in rodent studies where PR and PRB have been conditionally knocked-out or increased during the progesterone-dominated secretory phase of the cycle (Wetendorf and DeMayo, 2012). Consistent with previous reports, mRNAs encoded by IGFBP-1, FOXO1, IL-15 and HAND2 were all significantly increased in secretory phase control samples compared with those in proliferative phase. Treatment with UPA resulted in mRNA concentrations similar to proliferative phase and significantly lower than secretory phase, consistent with lack of morphological differentiation and suggesting UPA exhibiting limited PR-dependent agonism in endometrium. Previous work using human endometrial stromal cells treated with a decidualisation stimulus has suggested that HAND2 may regulate FOXO1 and IGFBP-1 expression (Huyen and Bany, 2011) and thus the reduction in HAND2 by UPA may be implicated in the reduction of FOXO1 and IGFBP-1. Other studies have shown expression of FOXO1 in endometrial stromal cells is upregulated by cAMP and progesterone (Labied et al., 2006) and a genomic screen of human endometrial stromal cells treated with a decidualisation protocol showed 15% of the genes induced were aberrantly expressed if FOXO1 was knocked down with key targets including IGFBP-1 (Vasquez et al., 2015). As FOXO1 binding sites are present in the majority of DNA regions associated with PR binding (Vasquez et al., 2015) our finding of reduced expression of FOXO1 in UPA-treated women may explain some of the changes in PR-dependent genes.
In the normal menstrual cycle IL-15 rises in response to progesterone (Dunn et al., 2002). Suppression of genes in the IL-15 pathway have been reported following asoprisnil administration. This observation was corroborated with reduced expression of IL-15 mRNA and the absence of endometrial CD-56 positive cells on immunolocalisation (Wilkens et al., 2013). In our current data set, IL-15 expression in UPA-treated endometrium was reduced compared to secretory phase endometrium consistent with UPA lacking agonist activity; however, the impact upon the CD-56 positive immune-cell population was not investigated.
IHH has been identified as a rapidly induced mediator of PR activation that localizes to the luminal and glandular epithelium (Takamoto et al., 2002) and acts in a paracrine fashion to initiate a cascade of gene expression in the stromal cell compartment (Takamoto et al., 2002). Whilst one genome wide molecular phenotyping study indicated that IHH was downregulated during the progression of the endometrium through the secretory phase (Talbi et al., 2006), Wei et al. (2010) reported that IHH protein expression was upregulated in secretory when compared to proliferative endometrium. The same authors, also reported an upregulation of IHH mRNA of 12 women in response to UPA in proliferative endometrium suggesting that UPA administration may have had an agonistic PR effect. In our study, IHH mRNA was reduced in secretory phase samples compared with those in the proliferative phase. Treatment with UPA was associated with mRNA concentrations similar to those in the proliferative phase. There are a number of reasons that may explain these contrasting findings. The dose of UPA administered in our study was lower (5 mg daily) compared with 10–20 mg. In our study, all UPA patients exhibited PAEC to a greater or lesser degree in contrast to low levels of PAEC reported by Wei et al. It is unknown what impact the development of endometrial PAEC may have had on our data.
HOXA10 is an important transcription factor for endometrial development including decidualisation. HOXA10 is expressed in both glandular and stromal compartments and expression is regulated by both estradiol and progesterone (Eun Kwon and Taylor, 2004). In healthy women expression peaks during mid-secretory phase and its expression is considered an important factor in endometrial receptivity (Kulp et al., 2016). In women with uterine fibroids secretory phase upregulation may be impaired (Makker et al., 2016). As all our subjects had fibroids this may explain why we demonstrated a reduced expression of HOXA10 mRNA from the secretory endometrium compared with proliferative phase at the PCR level although this was not reflected in protein expression. In those subjects exposed to UPA, HOXA10 mRNA expression was similar to proliferative phase. Evidence regarding HOXA10 gene expression in women with uterine pathology is beginning to emerge with evidence of alterations in gene methylation suggesting this may be a common molecular mechanism that becomes aberrant in some conditions (Kulp et al., 2016). Future studies may shed light on this by exploring the impact of UPA on HOXA10 promoter methylation but this was outside the scope of the current study.
In this study, COUP TFII and BMP2 mRNA levels were constant irrespective of stage of cycle or PR modulation or UPA treatment. PTEN acts as a tumour suppressor. Inactivation of this is a common feature of endometrial cancer, particularly endometrioid subtypes, and often predates morphological evidence of malignancy and pre-malignant precursors. Exogenous progestins may play an important role in elimination of PTEN-null glands (Orbo et al., 2006). Here we have shown that administration of UPA had little effect on PTEN, consistent with observations after asoprisnil administration (Wilkens et al., 2009).
In women treated with UPA circulating estrogen levels are maintained and it is therefore important to determine whether rates of endometrial cell proliferation increase with treatment. The Ki67 antigen is expressed during several phases of the cell cycle (G1, S, G2 and M), and is a well established prognostic and predictive biomarker and cell proliferation marker in clinical assessment of endometrium (Sivalingam et al., 2016). We thus considered Ki67 a reasonable choice for assessment of cell proliferation in endometrium in the present study.
In the current study, there was no evidence that rates of cell proliferation were increased compared with secretory phase and the number of ki67 immunopositive cells in both stromal and epithelial compartments was significantly lower than in proliferative phase. This is consistent with effects observed with other SPRMs (Wilkens et al., 2009) but such data have not previously been demonstrated in a quantitative manner following UPA administration. The mechanisms responsible for the low level of proliferation in UPA-treated women have not been explored. AR regulation has been implicated in the endometrial anti-proliferative effect observed with mifepristone administration (Slayden and Brenner, 1994). It is notable that AR is present in both stromal and epithelial cell nuclei and androgens are known to have anti-proliferative effects on endometrial tissue (Miller et al., 1986). In primates co-administration of mifepristone with the anti-androgen flutamide abolished the reduction in mitotic indices and endometrial atrophy (Brenner et al., 2003). The overexpression of AR in epithelial cells may be implicated in the low epithelial cell proliferation after UPA treatment. The impact of androgens on development of endometrial malignancy is complex (Gibson et al., 2014) and further studies on the relationship between UPA and AR are merited. UPA-dependent changes in expression of other factors may also play a role in the low levels of cell proliferation. For example, mRNAs encoded by FOXM1, a transcription factor that plays crucial roles in cell proliferation (Gao et al., 2015), were highest in the proliferative phase samples consistent with previous reports. FOXM1 was reduced in both secretory phase and UPA-treated samples and whether the low level of this transcription factor contributes to reduced cell proliferation merits further investigation.
Conclusions
New medical therapies for HMB are required and UPA has shown promise as a treatment for fibroids but its direct impacts on endometrium have received less attention. In this study, we have focused on an in-depth analysis of a cohort of women treated with UPA for 9–12 weeks prior to hysterectomy. The treatment resulted in changes in endometrial morphology and gene expression consistent with UPA acting as a PR antagonist in the absence of an increased rate of endometrial cell proliferation. These novel data indicate that UPA has the potential to fill an unmet clinical need in treatment of HMB.
Acknowledgements
We thank Ronnie Grant for assistance with figure preparation, Sheila Milne for assistance with manuscript preparation, Catherine Murray and Sharon McPherson for help with patient recruitment and Moira Nicol, Reena Murgai and Mike Millar for technical support.
Authors’ roles
H.O.D.C., A.R.W.W., L.H.R.W., A.A.M. and P.T.K.S. conceived the study design. Experiments were conducted and data collected by L.H.R.W., A.A.M., R.M. and G.S. Histopathological assessments were performed by A.R.W.W., L.H.R.W., A.A.M., A.R.W.W., P.T.K.S. and H.O.D.C. were involved in data analysis and interpretation, and manuscript preparation. All authors approved the submitted final version of the manuscript.
Funding
TENOVUS Scotland to H.O.D.C., A.R.W.W. and P.T.K.S.; Medical Research Council/National Institute for Health Research Efficacy and Mechanism Evaluation Grant (12/206/520) to H.O.D.C. and A.R.W.W.; MRC Programme Grant (G1100356/1) to P.T.K.S. and MRC Centre Grant (G111002033, MR/N022556/1).
Conflict of interest
H.O.D.C. has received clinical research support for laboratory consumables and staff from Bayer Pharma Ag and provides consultancy advice (but with no personal remuneration) for Bayer Pharma Ag, PregLem SA, Gedeon Richter, Vifor Pharma UK Ltd, AbbVie Inc.; A.R.W.W. has received consultancy payments from Bayer, Gedeon Richter, Preglem SA, HRA Pharma; L.H.R.W., A.A.M., R.M., G.S. and P.T.K.S. have no conflicts of interest.
References
- Brenner RM, Slayden OD, Nayak NR, Baird DT, Critchley HO. A role for the androgen receptor in the endometrial antiproliferative effects of progesterone antagonists. Steroids 2003;68:1033–1039. [DOI] [PubMed] [Google Scholar]
- Brenner RM, Slayden OD, Nath A, Tsong YY, Sitruk-Ware R. Intrauterine administration of CDB-2914 (Ulipristal) suppresses the endometrium of rhesus macaques. Contraception 2010;81:336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE. Uterine fibroids. N Engl J Med 2013;369:1344–1355. [DOI] [PubMed] [Google Scholar]
- Chabbert-Buffet N, Pintiaux-Kairis A, Bouchard P. Effects of the progesterone receptor modulator VA2914 in a continuous low dose on the hypothalamic-pituitary-ovarian axis and endometrium in normal women: a prospective, randomized, placebo-controlled trial. J Clin Endocrinol Metab 2007;92:3582–3589. [DOI] [PubMed] [Google Scholar]
- Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T, Ugocsai G, Mara M, Jilla MP, Bestel E et al. . Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med 2012. a;366:409–420. [DOI] [PubMed] [Google Scholar]
- Donnez J, Tomaszewski J, Vazquez F, Bouchard P, Lemieszczuk B, Baro F, Nouri K, Selvaggi L, Sodowski K, Bestel E et al. . Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med 2012. b;366:421–432. [DOI] [PubMed] [Google Scholar]
- Donnez J, Vazquez F, Tomaszewski J, Nouri K, Bouchard P, Fauser BC, Barlow DH, Palacios S, Donnez O, Bestel E et al. . Long-term treatment of uterine fibroids with ulipristal acetate. Fertil Steril 2014;101:1565–1573. e1561–1518. [DOI] [PubMed] [Google Scholar]
- Dunn CL, Critchley HO, Kelly RW. IL-15 regulation in human endometrial stromal cells. J Clin Endocrinol Metab 2002;87:1898–1901. [DOI] [PubMed] [Google Scholar]
- Engman M, Granberg S, Williams AR, Meng CX, Lalitkumar PG, Gemzell-Danielsson K. Mifepristone for treatment of uterine leiomyoma. A prospective randomized placebo controlled trial. Hum Reprod 2009;24:1870–1879. [DOI] [PubMed] [Google Scholar]
- Eun Kwon H, Taylor HS. The role of HOX genes in human implantation. Ann N Y Acad Sci 2004;1034:1–18. [DOI] [PubMed] [Google Scholar]
- Gao F, Bian F, Ma X, Kalinichenko VV, Das SK. Control of regional decidualization in implantation: Role of FoxM1 downstream of Hoxa10 and cyclin D3. Sci Rep 2015;5:13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DA, Simitsidellis I, Collins F, Saunders PT. Evidence of androgen action in endometrial and ovarian cancers. Endocr Relat Cancer 2014;21:T203–T218. [DOI] [PubMed] [Google Scholar]
- Huyen DV, Bany BM. Evidence for a conserved function of heart and neural crest derivatives expressed transcript 2 in mouse and human decidualization. Reproduction 2011;142:353–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulp JL, Mamillapalli R, Taylor HS. Aberrant HOXA10 Methylation in Patients With Common Gynecologic Disorders: Implications for Reproductive Outcomes. Reprod Sci 2016;23:455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labied S, Kajihara T, Madureira PA, Fusi L, Jones MC, Higham JM, Varshochi R, Francis JM, Zoumpoulidou G, Essafi A et al. . Progestins regulate the expression and activity of the forkhead transcription factor FOXO1 in differentiating human endometrium. Mol Endocrinol 2006;20:35–44. [DOI] [PubMed] [Google Scholar]
- Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KS Jr. Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab 1988;67:334–340. [DOI] [PubMed] [Google Scholar]
- Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and health-care costs and utilization in abnormal uterine bleeding. Value Health 2007;10:183–194. [DOI] [PubMed] [Google Scholar]
- Luo X, Yin P, Coon VJ, Cheng YH, Wiehle RD, Bulun SE. The selective progesterone receptor modulator CDB4124 inhibits proliferation and induces apoptosis in uterine leiomyoma cells. Fertil Steril 2010;93:2668–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makker A, Goel MM, Nigam D, Bhatia V, Mahdi AA, Das V, Pandey A. Endometrial Expression of Homeobox Genes and Cell Adhesion Molecules in Infertile Women With Intramural Fibroids During Window of Implantation. Reprod Sci 2016. [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Marshall E, Lowrey J, MacPherson S, Maybin JA, Collins F, Critchley HO, Saunders PT. In silico analysis identifies a novel role for androgens in the regulation of human endometrial apoptosis. J Clin Endocrinol Metab 2011;96:E1746–E1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maybin JA, Critchley HO. Menstrual physiology: implications for endometrial pathology and beyond. Hum Reprod Update 2015;21:748–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill RM. Hysterectomy surveillance in the United States, 1997 through 2005. Med Sci Monit 2008;14:CR24–CR31. [PubMed] [Google Scholar]
- Miller N, Bedard YC, Cooter NB, Shaul DL. Histological changes in the genital tract in transsexual women following androgen therapy. Histopathology 1986;10:661–669. [DOI] [PubMed] [Google Scholar]
- Mote PA, Balleine RL, McGowan EM, Clarke CL. Colocalization of progesterone receptors A and B by dual immunofluorescent histochemistry in human endometrium during the menstrual cycle. J Clin Endocrinol Metab 1999;84:2963–2971. [DOI] [PubMed] [Google Scholar]
- Munro MG, Critchley HO, Fraser IS. The FIGO classification of causes of abnormal uterine bleeding: Malcolm G. Munro, Hilary O.D. Crithcley, Ian S. Fraser, for the FIGO Working Group on Menstrual Disorders. Int J Gynaecol Obstet 2011;113:1–2. [DOI] [PubMed] [Google Scholar]
- NICE Clinical Guideline 44; Heavy Menstrual Bleeding 2007; Available from http://www.nice.org.uk/nicemedia/pdf/CG44FullGuideline.pdf.
- Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril 1950;1:3–25. [DOI] [PubMed] [Google Scholar]
- Orbo A, Rise CE, Mutter GL. Regression of latent endometrial precancers by progestin infiltrated intrauterine device. Cancer Res 2006;66:5613–5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel B, Elguero S, Thakore S, Dahoud W, Bedaiwy M, Mesiano S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum Reprod Update 2015;21:155–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TE, Tsourapas A, Middleton LJ, Champaneria R, Daniels JP, Cooper KG, Bhattacharya S, Barton PM. Hysterectomy, endometrial ablation, and levonorgestrel releasing intrauterine system (Mirena) for treatment of heavy menstrual bleeding: cost effectiveness analysis. BMJ 2011;342:d2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley M, Jordan K, Croft PR. An epidemiological survey of symptoms of menstrual loss in the community. Br J Gen Pract 2004;54:359–363. [PMC free article] [PubMed] [Google Scholar]
- Sivalingam VN, Kitson S, McVey R, Roberts C, Pemberton P, Gilmour K, Ali S, Renehan AG, Kitchener HC, Crosbie EJ. Measuring the biological effect of presurgical metformin treatment in endometrial cancer. Br J Cancer 2016;114:281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayden OD, Brenner RM. RU 486 action after estrogen priming in the endometrium and oviducts of rhesus monkeys (Macaca mulatta). J Clin Endocrinol Metab 1994;78:440–448. [DOI] [PubMed] [Google Scholar]
- Slayden OD, Nayak NR, Burton KA, Chwalisz K, Cameron ST, Critchley HO, Baird DT, Brenner RM. Progesterone antagonists increase androgen receptor expression in the rhesus macaque and human endometrium. J Clin Endocrinol Metab 2001;86:2668–2679. [DOI] [PubMed] [Google Scholar]
- Takamoto N, Zhao B, Tsai SY, DeMayo FJ. Identification of Indian hedgehog as a progesterone-responsive gene in the murine uterus. Mol Endocrinol 2002;16:2338–2348. [DOI] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA et al. . Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 2006;147:1097–1121. [DOI] [PubMed] [Google Scholar]
- Vasquez YM, Mazur EC, Li X, Kommagani R, Jiang L, Chen R, Lanz RB, Kovanci E, Gibbons WE, DeMayo FJ. FOXO1 is required for binding of PR on IRF4, novel transcriptional regulator of endometrial stromal decidualization. Mol Endocrinol 2015;29:421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenfeld A, Saunders PT, Whitaker L, Critchley HO. Selective progesterone receptor modulators (SPRMs): progesterone receptor action, mode of action on the endometrium and treatment options in gynecological therapies. Expert Opin Ther Targets 2016;20:1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Critchley HO, Kelly RW, Shen D, Baird DT. Progesterone receptor subtype B is differentially regulated in human endometrial stroma. Mol Hum Reprod 1998;4:407–412. [DOI] [PubMed] [Google Scholar]
- Wei Q, Levens ED, Stefansson L, Nieman LK. Indian Hedgehog and its targets in human endometrium: menstrual cycle expression and response to CDB-2914. J Clin Endocrinol Metab 2010;95:5330–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetendorf M, DeMayo FJ. The progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network. Mol Cell Endocrinol 2012;357:108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkens J, Chwalisz K, Han C, Walker J, Cameron IT, Ingamells S, Lawrence AC, Lumsden MA, Hapangama D, Williams AR et al. . Effects of the selective progesterone receptor modulator asoprisnil on uterine artery blood flow, ovarian activity, and clinical symptoms in patients with uterine leiomyomata scheduled for hysterectomy. J Clin Endocrinol Metab 2008;93:4664–4671. [DOI] [PubMed] [Google Scholar]
- Wilkens J, Williams AR, Chwalisz K, Han C, Cameron IT, Critchley HO. Effect of asoprisnil on uterine proliferation markers and endometrial expression of the tumour suppressor gene, PTEN. Hum Reprod 2009;24:1036–1044. [DOI] [PubMed] [Google Scholar]
- Wilkens J, Male V, Ghazal P, Forster T, Gibson DA, Williams AR, Brito-Mutunayagam SL, Craigon M, Lourenco P, Cameron IT et al. . Uterine NK cells regulate endometrial bleeding in women and are suppressed by the progesterone receptor modulator asoprisnil. J Immunol 2013;191:2226–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AR, Bergeron C, Barlow DH, Ferenczy A. Endometrial morphology after treatment of uterine fibroids with the selective progesterone receptor modulator, ulipristal acetate. Int J Gynecol Pathol 2012;31:556–569. [DOI] [PubMed] [Google Scholar]
- Yin P, Lin Z, Cheng YH, Marsh EE, Utsunomiya H, Ishikawa H, Xue Q, Reierstad S, Innes J, Thung S et al. . Progesterone receptor regulates Bcl-2 gene expression through direct binding to its promoter region in uterine leiomyoma cells. J Clin Endocrinol Metab 2007;92:4459–4466. [DOI] [PubMed] [Google Scholar]
- Yin P, Lin Z, Reierstad S, Wu J, Ishikawa H, Marsh EE, Innes J, Cheng Y, Pearson K, Coon JS 5th et al. . Transcription factor KLF11 integrates progesterone receptor signaling and proliferation in uterine leiomyoma cells. Cancer Res 2010;70:1722–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]