Abstract
While the benefit of oral anticoagulants (OACs) for stroke prevention in patients with atrial fibrillation (AF) is well established, it is not known whether oral anticoagulation is indicated in patients with atrial high-rate episodes (AHRE) recorded on a cardiac implantable electronic device, sometimes also called subclinical AF, and lasting for at least 6 min in the absence of clinically diagnosed AF. Clinical evidence has shown that short episodes of rapid atrial tachycarrhythmias are often detected in patients presenting with stroke and transient ischaemic attack. Patients with AHRE have a higher likelihood of suffering from subsequent strokes, but their stroke rate seems lower than in patients with diagnosed AF, and not all AHRE episodes correspond to AF. The prognostic and pathological significance of AHRE is not yet fully understood. Clinical trials of OAC therapy are being conducted to determine whether therapeutic intervention would be beneficial to patients experiencing AHRE in terms of reducing the risk of stroke.
Keywords: Atrial fibrillation, Stroke, Thromboembolic risk, Atrial high-rate episodes, Subclinical atrial fibrillation, Paroxysmal atrial fibrillation, Anticoagulation, Cardiovascular implantable electronic devices
Introduction
Atrial fibrillation (AF) leads to abnormalities of intracardiac blood flow, endocardium and blood constituents, conferring a prothrombotic state.1 The disorder is associated with a five-fold increased risk of ischaemic stroke and is encountered in ∼40% patients with ischaemic stroke or transient ischaemic attack (TIA).2 Approximately 15 million people worldwide suffer from a stroke each year3 and of these, at least 15% have been related to clinically diagnosed AF.2 Strokes associated with AF have a poor prognosis compared with strokes of other aetiologies.4 One-quarter of ischaemic strokes are of unknown cause, usually designated cryptogenic, and undiagnosed AF may be a potential aetiologic factor.2 While AF typically presents with palpitations, dyspnoea, chest pain, and fatigue, it may also be asymptomatic.5,6 Furthermore, atrial tachycardias (ATs) are heterogeneous in terms of the type of atrial arrhythmias, their duration, resulting ventricular rate and degree of irregularity, and underlying cardiovascular conditions.
Paroxysmal atrial fibrillation (PAF), as opposed to permanent AF, is transient and infrequent, and, like permanent AF, may be asymptomatic. Data suggest that asymptomatic PAF episodes occur with much greater frequency than symptomatic PAF.7 Recent studies have focused on establishing the frequency of short asymptomatic episodes of AF, which have been termed atrial high-rate episodes (AHREs). AHREs are detected in patients with pacemaker or implantable cardioverter defibrillator (ICD) devices and often occur in the absence of AF diagnosed by the usual methods (i.e. electrocardiogram, Holter monitor).8 The definition of AHREs refers to episodes with a duration of >6 min, mainly to reduce the inclusion of electrical artefacts, and is usually confined to patients who do not have clinically detected AF.9 Such patients are at elevated risk of stroke and may have unmet anticoagulation needs. However, it must be stressed that not all studies have used this definition of AHRE. A definition of 5 min' duration has been used in some key studies, based on previously published data that suggested that a 5 min cutoff excludes most episodes of oversensing.10 While the benefits of oral anticoagulation for stroke prevention in patients with clinical AF are well established, it is not known whether the same risk-benefit ratio exists for OAC therapy in patients with AHREs. This article aims to discuss the incidence of AHREs, review the evidence of their association with stroke risk and discuss the need for anticoagulation in these patients.
Current strategies for stroke prevention in atrial fibrillation
In the past, the prescription and persistence with anticoagulation therapy has been suboptimal, largely due to the disadvantages associated with vitamin K antagonists (VKAs) and fear of bleeding among both patients and physicians, although clinical evidence has established that the risk of ischaemic stroke without anticoagulant treatment is higher than the risk of intracranial bleeding.11 In the era before non-vitamin K antagonist oral anticoagulants (NOACs) became available, <50% of at-risk AF patients were effectively anticoagulated, and up to 70% of patients with known AF who had already suffered an ischaemic stroke were not receiving anticoagulant therapy to prevent AF-related stroke at the time of the stroke.12–14 Current AF guidelines recommend the consideration of NOACs in AF patients with a CHA2DS2VASc ≥1.15 However, NOACs are generally underutilized:16 the PREFER in AF registry, a prospective, observational multi-centre registry conducted in seven EU countries, found considerable variation in anticoagulation management strategies. Non-vitamin K antagonist oral anticoagulants were used in ∼6% of patients.17,18 Clinical trial data on the four currently approved NOACs (dabigatran, apixaban, rivaroxaban, and edoxaban) show that NOACs are non-inferior or superior to warfarin with regard to the efficacy outcome of stroke and systemic embolism (SE) and in addition are consistently associated with a reduction of life threatening or critical organ bleeding, such as intracranial bleeds.19–23 A meta-analysis of all trials suggests that NOACs are equal to or superior to warfarin in terms of risk reduction of stroke or systemic embolic events, while NOAC therapy is associated with a lower risk of severe, especially intracranial haemorrhage.19
All available data supporting the use of oral anticoagulation for stroke prevention in AF have been obtained from patients with clinically diagnosed (ECG documented) AF. The effectiveness and safety of NOACs in patients with AHRE/subclinical AF (SCAF) but without ECG documented AF is unknown. If it were demonstrated that NOACs provide a clinical benefit in such patients, potential stroke prevention could be improved, given that these patients represent a high percentage of patients with cardiac rhythm management devices. To optimize NOAC therapy, a greater understanding of stroke risk patients with PAF including SCAF/AHRE is needed. However, it is worth noting that it is very likely that not all strokes seen in patients with either AF or AHRE are of cardio-embolic origin. Atrial fibrillation could be a coincidental finding in many patients, given their burden of underlying cardiovascular disease, which could independently cause ischaemic stroke and AF.
Incidence of atrial high-rate events
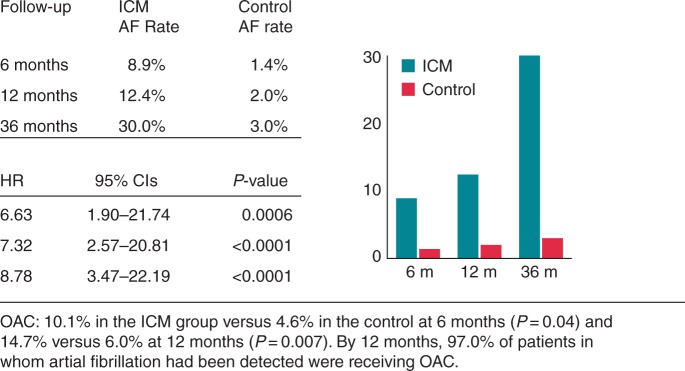
Advances in pacemaker and ICD technology, which allow long-term continuous heart rhythm monitoring, have enabled the continuous assessment of atrial tachyarrhythmias in patients with an atrial lead.24,25 The significance and occurrence of AHRE is increasingly recognized, but these events are often not acted upon in patients presenting with stroke and TIA. An analysis of the AFFIRM study (Atrial Fibrillation Follow-up Investigation of Rhythm Management) found that 12% of patients were asymptomatic at baseline. These patients had a lower incidence of serious heart disease but more cerebrovascular events (CVEs).26 Among patients presenting with an acute stroke, 5% have previously undetected AF on admission. Subsequent intermittent 12-lead ECG or ambulatory Holter ECG monitoring has identified higher incidences of undiagnosed AF in stroke survivors: ∼10% in unselected stroke survivors and 30% in those who presented with cryptogenic stroke (Figure 1).27–31
Figure 1.
Detection of AF among patients with cryptogenic stroke or transient ischaemic attack randomized to an ICM or control (conventional follow-up). Adapted from Sanna et al.29
Atrial high-rate episodes can be detected on a surface ECG, but documentation is less likely since monitoring is either not continuous or relatively short in duration. One study of patients (n = 142) with cryptogenic stroke but with no history of AF, found that long-term heart rhythm monitoring detected silent AF in 46% of patients.32 Another study reviewed the records of 1128 consecutive patients attending a stroke clinic and identified 426 patients with definite stroke and TIA. These patients underwent Holter monitoring for a mean of 22.6 h. Episodes of PAF lasting >30 s in 11 patients (2.5%) and nonsustained atrial arrhythmias in another 28 patients (6.5%).33 A study of a consecutive series of 56 patients with cryptogenic TIA/stroke used 21-day monitoring with mobile cardiac output telemetry. Results showed that 5.3% of cryptogenic stroke patients had episodes of PAF lasting >30 s, and 23% had episodes lasting <30 s.34
Most of the data on AHRE has been obtained from patients with pacemakers or ICDs. Many of these patients have sinus node disease and/or ventricular pacing which are associated with a higher incidence of AF; therefore, the prevalence of AHRE may be lower in the general population.35,36
Atrial high-rate episode and stroke risk
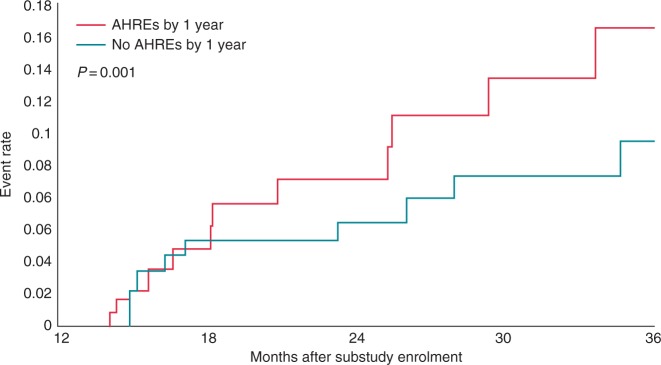
A growing body of clinical data support the hypothesis that AHRE are associated with an elevated risk of stroke (Table 1). Atrial diagnostics ancillary study of the MOde Selection Trial (MOST) found that patients (n = 312) with sinus node dysfunction who experienced AHRE (>220 beats per minute [bpm] lasting at least 5 min) were more likely to have adverse clinical outcomes, including a higher incidence of stroke, death, and subsequent AF than patients without AHRE (Figure 2). Over 6 years, the incidence of AHREs was associated with an increased total mortality (hazard ratio [HR] 2.48; 95% confidence interval [CI] 1.25–4.91, P = 0.0092), death/non-fatal stroke (HR 2.79; 95% CI 1.51–5.15, P = 0.0011), and the development of clinical AF (HR 5.93; 95% CI 2.88–12.2, P = 0.0001). However, there was insufficient evidence to stimulate significant discussion about whether an atrial arrhythmia of 5 min in duration warrants anticoagulation treatment.37
Table 1.
Summary of studies investigating the association between AHREs and stroke risk
| Trial | Study type and duration | Study population | Criteria for the diagnosis of AHRE | Outcomes |
|---|---|---|---|---|
| MOST29 | Subgroup analysis of RCT, 6 years | n = 312, median age 74 years, 55% female, and 60% had a history of SND | Atrial rate >220 bpm for 10 consecutive beats | Compared with control, AHREs were associated with increased total mortality (HR 2.48 95% CI 1.25–4.91, P = 0.0092), death or non-fatal stroke (HR 2.79; 95% CI 1.51–5.15, P = 0.0011), and AF (HR 5.93; 95% CI 2.88–12.2, P = 0.0001) |
| TRENDS30 | Prospective observational study, mean follow-up 1.4 years | n = 2486 with ≥ 1 risk factor for stroke | AT/AF burden = longest total AT/AF duration on any given day during the prior 30-day period and classified as subsets: zero, low (<5.5 h [median duration]), and high (≥ 5.5 h) | Compared with zero burden, AF burden was associated with increased TE: HR 0.98; 95% CI 0.34–2.82, P = 0.97) and 2.20; 95% CI 0.96–5.05, P = 0.06), for low and high, respectively |
| ASSERT31 | Prospective observational study, mean follow-up 2.5 years | n = 2580, age ≥ 65 years, with hypertension and no history of AF | Atrial rate >190 bpm for >6 min | By 3 months, AHREs occurred in 10.1%. AHREs were associated with an increased risk of clinical AF (HR 5.56; 95% CI 3.78–8.17; P < 0.001) and of ischaemic stroke or SE (HR 2.49; 95% CI 1.28–4.85; P = 0.007). After adjustment for predictors of stroke AHREs remained associated with stroke/SE (HR 2.50; 95% CI 1.28–4.89; P = 0.008) |
| Carelink/VA34 | Case crossover study, analysis of data 30 days preceding a stroke | n = 9850, median age 68 years, 99% male, and 98% had a defibrillator | ≥5.5 h of AF on ≥1 day in the preceding 30 days | AHREs was associated with a four-fold increased risk of stroke within 30 days (OR = 4.33. 95% CI 1.19–23.7) Risk was highest in the 5–10 days after AHRE and rapidly declined after 10 days |
| Belgrade Atrial Fibrillation Study35 | Single-centre registry study and mean follow-up 9.9 ± 6.1 years | n = 1100, mean age 52.7 ± 12.2 years, 13.3%) had asymptomatic AF | Asymptomatic presentation of first diagnosed AF | Ischaemic stroke risk (log-rank test = 6.2, P = 0.013) was significantly worse for patients with asymptomatic AF compared with those with symptomatic AF |
| SOS AF project36 | Pooled analysis of individual patient data from five prospective studies | n = 10 016, median age 70 years. Pts without permanent AF with ICDs were included if they had at least 3 months of follow-up | Device-detected AF. Cutoff points of AF burden defined as: 5 min, 1, 6, 12, and 23 h | AF burden 1 h was associated with the risk of ischaemic stroke (HR 2.11, 95% CI 1.22–3.64, P= 0.008) |
AF, atrial fibrillation; AHRE, atrial high-rate event; AT, atrial tachycardia; bpm, beats per minute; RCT, randomized controlled trial; SE, systemic embolism; SND, sinus node; TE, thromboembolic event.
Figure 2.
The MOST study: Kaplan–Meier plot of death or non-fatal stroke after 1 year of the atrial diagnostics ancillary study in patients with atrial high-rate episodes vs. those without atrial high-rate episodes. Source: Glotzer et al.37
The Relationship between daily atrial tachyarrhythmia burdEN from Implantable device Diagnostics and Stroke risk (TRENDS) was a prospective, observational study enrolling patients (n = 2486) with ≥1 stroke risk factor (heart failure, hypertension, age ≥65 years, diabetes, or prior thromboembolic event [TE]) receiving pacemakers or defibrillators that monitor AT. During a mean follow-up of 1.4 years, the rate of TEs was low compared with that in patients with AF diagnosed by traditional modalities and similar CHADS2 scores, but the risk of TEs was doubled in patients with an atrial tachyarrhythmia/AF burden >5.5 h compared with those with no atrial tachyarrhythmia/AF. The annualized TE risk (including TIA) was 1.1% for zero, 1.1% for low, and 2.4% for high AT burden. Compared with zero burden, adjusted HRs (95% CIs) in the low and high burden subsets were 0.98 (0.34–2.82, P = 0.97) and 2.20 (0.96–5.05, respectively, P = 0.06).38
The ASSERT trial (ASymptomatic atrial fibrillation and Stroke Evaluation in pacemaker patients and the atrial fibrillation Reduction atrial pacing Trial) monitored patients (n = 2580, hypertensive, aged ≥ 65 years, with a recently implanted ICD and no history of AF) for 3 months to detect AHRE (episode of atrial rate >190 bpm for >6 min, termed SCAF in this study) and followed them for a mean of 2.5 years.9 Subclinical AF was detected in 261 (10.1%) patients within 3 months of device implantation (see Table 2).9 Subclinical AF was associated with an increased risk of clinical AF (HR 5.56; 95% CI 3.78–8.17; P < 0.001) and of ischaemic stroke or SE (HR 2.49; 95% CI 1.28–4.85; P = 0.007). Of 51 patients who had an ischaemic stroke or SE, SCAF was detected in 11 (21.6%) by 3 months, and none had clinical AF by 3 months. The population attributable risk of stroke or SE associated with SCAF was 13%. The presence of SCAF was predictive of stroke or SE even after adjustment for predictors of stroke (HR 2.50; 95% CI 1.28–4.89; P = 0.008).9
Table 2.
Results of the ASSERT clinical trial
| Event | Device-detected atrial tachyarrhythmia |
Device-detected atrial tachyarrhythmia present vs. absent |
|||||
|---|---|---|---|---|---|---|---|
| Absent n = 2319 |
Present n = 261 |
||||||
| Events | %/year | Events | %/year | RC | 95% CI | P | |
| All patients | |||||||
| Ischaemic stroke or SE | 40 | 0.69 | 11 | 1.69 | 2.49 | 1.28–4.85 | 0.007 |
| Vascular death | 153 | 2.62 | 19 | 2.92 | 1.11 | 0.69–1.79 | 0.67 |
| Stroke/MI/vascular death | 206 | 3.53 | 29 | 4.45 | 1.25 | 0.85–1.84 | 0.27 |
| Clinical AF or flutter | 71 | 1.22 | 41 | 6.29 | 5.56 | 3.76–8.17 | <0.001 |
| Event | Device-detected atrial tachyarrhythmia |
Device-detected atrial tachyarrhythmia present vs. absent |
|||||
| Absent n = 1786 |
Present n = 191 |
||||||
| Events | %/year | Events | %/year | RC | 95% CI | P | |
| Patients with CHADS score ≥2 | |||||||
| Ischaemic stroke or SE | 40 | 0.70 | 10 | 2.14 | 2.67 | 1.32–5.38 | 0.006 |
| Vascular death | 153 | 2.67 | 16 | 3.42 | 1.08 | 0.64–1.82 | 0.77 |
| Stroke/MI/vascular death | 206 | 3.59 | 25 | 5.35 | 1.25 | 0.82–1.90 | 0.30 |
| Clinical AF or flutter | 58 | 1.31 | 30 | 6.42 | 5.29 | 3.40–8.24 | <0.001 |
Source: Healey et al.9
Surprisingly, no correlation was found in ASSERT between AHRE episodes and a history of stroke. This highlights the possible limitations of restricting the sampling period to the first 3 months of the study, and it has been suggested that AHRE may be a transient phenomenon related to lead implantation.39 This phenomenon was noted in the Silent Atrial Fibrillation detection with stored EGMs registry study, which examined the incidence, duration, and predictors of AHRE in patients without previous clinically diagnosed AF after dual-chamber pacemaker implantation. At 6-month follow-up, 10% of patients experienced one or more AHRE (defined as an atrial tachyarrhythmia with a rate ≥180 beats/min lasting ≥5 min) usually lasting 5–60 min. Some patients had AHRE only within the first 30 days after implantation, suggesting that these AHRE may have been related to lead insertion.40
The Carelink/VA study was a case crossover study that examined the records of patients (n = 9850) with ICDs. The study defined ‘significant’ AF as ≥5.5 h of AF on ≥1 day in the preceding 30 days (TRENDS criteria), and compared its presence during the period 30 days prior to the stroke with the control period (91–120 days prior to the stroke). Among 187 eligible strokes, significant AF was associated with a four-fold increased risk of stroke (HR = 4.33. 95% CI 1.19–23.7). The stroke risk was highest in the 5–10 days after AF episode and rapidly declined after 10 days.41
Other studies have also added to the body of evidence for the association between AHREs and stroke. The EURObservational Research Programme Pilot survey on Atrial Fibrillation found that asymptomatic AF is associated with high thromboembolic risk.42 In the Belgrade Atrial Fibrillation Study, a single-centre registry study (n = 1010), 10-year estimates of survival free of progression of AF (log-rank test = 33.4, P < 0.001) and ischaemic stroke (log-rank test = 6.2, P = 0.013) were significantly worse for patients with asymptomatic AF at diagnosis compared with those with symptomatic AF. In the multivariable Cox regression analysis, intermittent asymptomatic AF was significantly associated with progression to permanent AF (HR 1.6; 95% CI 1.1–2.2; P = 0.009).43 An analysis of >10 000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation, information from implanted devices), which included patients without permanent AF, found that 43% of patients experienced at least 1 day with at least 5 min of AF burden and that AF burden was an independent predictor of ischaemic stroke. A threshold of 1 h of AF burden was associated with the highest risk of ischaemic stroke (HR 2.11; 95% CI 1.22–3.64, P = 0.008).44
These data have established a clear association between AHRE and increased risk of stroke, but a high proportion of ischaemic brain lesions could be subclinical and therefore the embolic risk underestimated. A recent study (n = 75) found an association between AHRE and ischaemic brain lesions detected by CT scan. Multivariable analyses showed that the presence of AHRE >5 min was an independent predictor for silent ischaemic brain lesions (HR 9.64 [1.86–50.02; P < 0.05]).45
The health risk conferred by AHRE may extend beyond increased risk of stroke: a study of patients (n = 224) with no history of AF who underwent dual-chamber pacemaker implantation found that AHRE were associated with a significant increase in cardiovascular mortality (HR 2.80; 95% CI 1.24–6.31; P = 0.013) and stroke mortality (HR 9.65; 95% CI 1.56–59.9; P = 0.015), with a trend towards increased all-cause mortality (HR 1.79; 95% CI 0.98–3.26; P = 0.059).46
Implications of study findings on atrial high-rate episodes and stroke risk
Mechanisms of thrombogenesis
Atrial fibrillation may trigger chronic changes in the atria and has been linked with changes in atrial structure and endothelial function,47,48 inflammation,49,50 and prothrombotic activity.51,52 Abnormal changes shown in the vessel and atrial wall, in flow and in blood constituents contribute to thrombogenesis.52,53 However, despite evidence of a temporal association and possible mechanistic link between AHRE and stroke risk, the role of AHRE in these processes has not been established. It is possible than rather than causing embolic events, AHRE are simply a marker for cardio-embolic risk.54 Thromboembolic risk likely involves a complex interplay of atrial arrhythmia, atrial myopathy, endothelial dysfunction related to comorbidity and abnormal haemostasis.55 In AF, the majority of left atrial (LA) thrombi involve the LA appendage. In a recent study (n = 201), a quarter of patients with PAF demonstrated a prothrombotic AF left atrial appendage (LAA) pulse wave Doppler phenotype, i.e. a discordance between the existence of sinus rhythm on ECG and the impaired mechanical LAA function revealed by Doppler that promotes blood clotting, despite concurrent ECGs showing sinus rhythm. This study demonstrates that prolonged ECG monitoring may not detect the patients at risk and may provide a mechanistic explanation of the thrombotic risk in PAF patients.56 The occurrence of AHRE may trigger chronic changes in the atria that result in thrombus formation after the occurrence of SCAF.
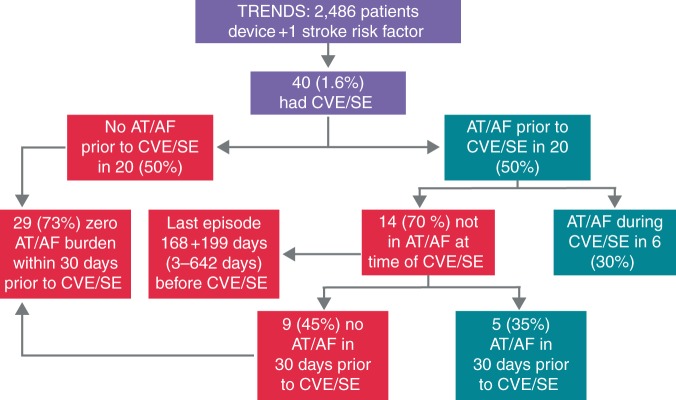
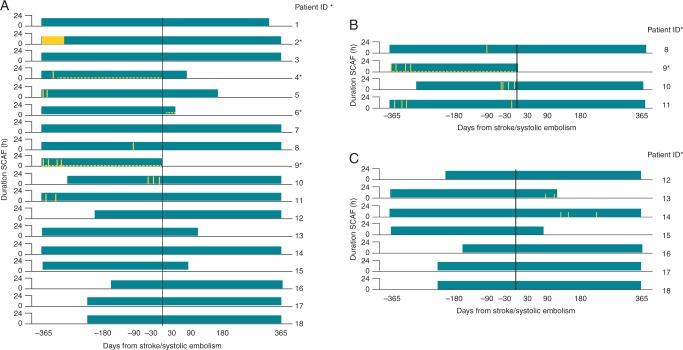
In an attempt to better understand the relationship between AHRE and stroke pathogenesis, a sub-analysis of the ASSERT trial focused on the 51 patients that experienced stroke or SE after 3 months. Of these, 26 (51%) had SCAF and 18 patients (35%) had SCAF before stroke/SE. Only four patients (8%) had SCAF detected within 30 days before stroke/SE, among whom one patient experienced SCAF at the time of the stroke. Eight patients (16%) had SCAF detected only after their stroke, despite continuous monitoring for a median duration of 228 days (25th to 75th percentile, 202–719) before their event.54 Most SCAF occurring before embolic events was considerably <48 h in duration, which is conventionally (but with little or no scientific justification) believed to be the minimum duration required for thrombus to form in the LAA before cardioversion.57,58 In addition, a subgroup analysis of TRENDS found that the majority of CVEs/SE in this population did not occur within 30 days of AT/AF episodes, suggesting either that the thrombus formation or embolization may be delayed or that the mechanisms of CVE/SE in patients with implantable devices may involve mechanisms other than cardio-embolism due to AT (Figures 3 and 4).59 These findings have led to a reassessment of the mechanisms by which AF causes embolic events. If a causal relationship exists, it is likely to be more complex than first believed.1,52
Figure 3.
The TRENDS study: baseline characteristics of study participants. Adapted from Daoud et al.59
Figure 4.
The TRENDS study: relationship of atrial tachyarrhythmias, cerebrovascular events, and systemic emboli. Source: Brambatti et al.54
An analysis of two multi-centre studies, involving 560 heart failure patients using home-monitoring technology, found that patients with device-detected detected AHRE lasting >3.8 h over 24 h were nine times more likely to develop TEs (P= 0.006).60 In addition, data from the Registry of Atrial Tachycardia And Atrial Fibrillation Episodes In The Cardiac Rhythm Management Device Population RATE registry showed that among patients with ICDs, those who experienced long episodes of AHREs were at greater risk of all adverse events than patients with no episodes (OR 1.56). Long episodes of AT/AF were also associated with a greater risk of AF hospitalization (OR 5.86). Short episodes of AT/AF were not associated with increased risk.61 It is noteworthy that in both studies, there were wide variations in timing between the AHRE and the occurrence of TEs.
Implications for clinical practice
Study findings have indicated that patients experiencing AHRE are at elevated risk for stroke, TIA, and SE and raise the question: should these patients receive oral anticoagulation therapy for stroke prevention? Currently, the majority of patients with AHRE do not receive OACs, suggesting that physicians require clarification of the risks associated with AHRE before routinely prescribing treatment.62,63 These clinically silent atrial tachyarrhythmias may not have the same prognosis as AF and the impact of OAC therapy may be significantly different in this population. The ASSERT trial suggested that the stroke risk associated with AHREs is lower than that associated with AF, although study populations differed significantly.9,64 While the data from MOST, TRENDS, and ASSERT support a link between pacemaker-AHRE and the occurrence of stroke or SE, these studies are limited by their small sample size and limited number of clinical events. A systematic literature review and meta-analysis investigated the detection rate of different ECG-monitoring techniques to detect AF in patients who had a TIA or an ischaemic stroke. A total of 32 observational or randomized studies were identified, with 5038 participants. The detection rate for AF was 11.5%, but a wide variation was reported in methods, observation time, and minimal duration of arrhythmia to be defined as AF. The review was also limited by small sample sizes.65
In a retrospective analysis of patients from a single academic hospital (n= 445), pacemaker-detected AF was present in 55.3% patients. Anticoagulants were used in 35.3% of patients with pacemaker-detected AF. Among these patients, OACs were used more frequently in those who also had clinical AF (58.9%) compared with those without (23.7%, P < 0.001).62 Patients with AHRE/SCAF are usually excluded from clinical trials of OACs. In the RE-LY trial, patients with PAF were included if they had at least two documented episodes of AF, but data from pacemakers or ICDs could only be used to record one of these episodes.66 In other major NOAC trials, patients with SCAF were not excluded; inclusion criteria were two or more episodes of AF or flutter, as documented by electrocardiography in ARISTOTLE,21 AF documented on electrocardiography in ROCKET AF22 and AF documented by means of an electrical tracing within the preceding 12 months in ENGAGE AF-TIMI 48.23 However, no clinical trial to date has investigated the use of OACs in patients with AHREs and no clinical AF, and this represents a substantial unmet need.
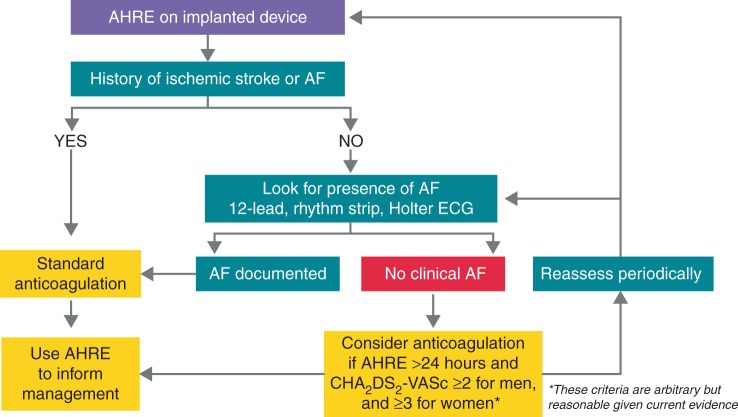
There is a need for high-quality evidence to inform updated guidelines. Expert opinions suggest that anticoagulation therapy should be individualized and promoted in SCAF according to the CHADS2 score.67–69 At the third joint consensus conference of the German Atrial Fibrillation Network (AFNET) and the European Heart Rhythm Association on AF, an algorithm for management of patients with AHREs was proposed and a modified version is shown in Figure 5.71
Figure 5.
Suggested treatment algorithm for management of patients with AHREs. Adapted from Kirchhof et al.70
The results of the Carelink/VA and other studies support a strategy of transient use of rapidly acting OACs linked to the onset of AHREs.41 However, prescription of OAC therapy on this basis in patients with pacemakers/ICDs requires continuous monitoring of device data. Remote home monitoring of pacemaker/ICD data with automatic daily surveillance has proved effective and safe.72 IMPACT (The IMPACT of Biotronik Home Monitoring Guided Anticoagulation on Stroke Risk in Patients with ICD and CRT-D Devices) was an interventional therapy trial (n = 2718) designed to test the hypothesis that initiation and withdrawal of OAC therapy based on occurrence of AHRE detected by continuous ambulatory monitoring of pacemaker/ICD data may reduce the combined rate of stroke, SE, and major bleeding compared with conventional clinical management. In the intervention group, OAC was administered based on patient-specific risk stratification following detection of AHRE. The OAC was withdrawn if AHREs did not recur within a predefined period. Recently published data show that 945 patients (34.8%) developed AT, 264 meeting study anticoagulation criteria. Primary events (2.4 vs. 2.3 per 100 patient-years) did not differ between groups (HR 1.06; 95% CI 0.75–1.51; P = 0.732). Although AT burden was associated with thromboembolism, there was no temporal relationship between AT and stroke. The study concluded that the strategy of early initiation and interruption of anticoagulation based on remotely detected AT did not prevent thromboembolism and bleeding. However, in this trial the antithrombotic agent used was mainly a VKA. It is not known whether the results would have been different if NOACs, which have more rapid onset and offset of action and are safer regarding intracranial bleedings, had been used.73
Recently, small, leadless subcutaneous insertable cardiac monitors (ICM) with remote data transmission capabilities (Reveal XT™ and Reveal LINQ™, Medtronic Inc.) have been developed. These allow remote and continuous evaluation of a patient for AF recurrences. The Rhythm Evaluation for AntiCoagulaTion with COntinuous Monitoring (REACT.COM) pilot study evaluated the feasibility of transient ‘pill-in-the-pocket’ anticoagulation based on the results of daily remote transmissions from an ICM. Inclusion criteria were a CHADS2 score of 1 or 2, non-permanent AF, no documented AF lasting over a 1 h on two consecutive months on a previously implanted ICM, and compliance with an NOAC for 30 consecutive days prior to enrolment. Participants were maintained on aspirin therapy and transmitted data daily from their ICM, allowing detection of AF within 24 h of an episode. If an AF episode lasting ≥ 1 h was detected, NOACs were prescribed for 30 days.74
While the ability to detect AHRE in patients with pacemakers/ICDs involves little additional cost, the same is not true of a wider patient population. Long-term monitoring for AF raises questions about cost-effectiveness. A small (n = 24) study of patients aged under 75 years who had undergone cryptogenic stroke found an unexpectedly low incidence of AF and AHRE.75 However, the CRYSTAL-AF (CRYptogenic STroke and underlying. AtriaL Fibrillation) study was a large (n = 441) randomized, controlled study involving long-term monitoring with an ICM. At 12 months follow-up, AF had been detected in six times more patients with ICM compared with conventional follow-up (Figure 3).29 In the EMBRACE (Atrial Fibrillation After a Cerebral Ischaemic Event) trial (n = 572) non-invasive ambulatory ECG monitoring for 30 days significantly improved the detection of AF by a factor of more than five and nearly doubled the rate of anticoagulant treatment, compared with the short-duration ECG monitoring.30 On the other hand, a recent observational study of younger patients (n = 56, mean age 48 ± 9 years) showed a low rate of PAF after cryptogenic stroke or stroke of known cause.
Experts have suggested that screening procedures take into account risk factors for AF including diabetes mellitus,34 and other recently established factors including obesity and obstructive sleep apnoea.76,77 A recent cost-effectiveness analysis found that mass screening of 75/76-year-old individuals for asymptomatic AF, using intermittent ECG recording and a decision analysis simulation model, resulted in 263 fewer patient-years with undetected AF, and therefore 8 fewer strokes, 11 more life-years, and 12 more quality-adjusted life-years per 1000 screened individuals. The screening protocol was therefore considered cost effective.78
To optimize therapy for AHRE, physicians need to identify high-risk patients.79,80 Numerous potential biomarkers have been investigated, including haemostatic markers of coagulation and fibrinolytic activity,81 plasma von Willebrand levels,82 and cardiac biomarkers such as troponin and natriuretic peptides.83,84 However, to date, none have been found to be sufficiently useful and practical to warrant adoption in influencing clinical decision-making.85 In patients with a history AF, the combination of AF duration and CHADS2 score was predictive of ischaemic TEs.86,87
The ARTESiA (Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Sub-Clinical Atrial Fibrillation) trial is currently recruiting.88 The study aims to enrol 4000 high-risk (CHA2DS2VASc score ≥ 3) participants with permanent pacemakers or ICDs, and at least one episode of SCAF ≥6 min in duration (atrial rate >175/min if an atrial lead is present), but no single episode >24 h in duration. Subclinical AF requires electrogram confirmation (at least one episode) unless ≥6 h in duration. Patients will be randomized to receive apixaban or aspirin. The primary efficacy outcome measures are a composite of ischaemic stroke and SE; the primary safety outcome measure is the incidence of major bleeds.
The AFNET association and the European Society of Cardiology are initiating a new trial, NOAH (non-vitamin K antagonist oral anticoagulants in patients with atrial high-rate episodes). Patients aged 65 years or older with documented AHRE and one additional CHA2DS2VASc factor will be randomized to edoxaban or aspirin/placebo, depending on the indications for antiplatelet therapy.89
Summary and concluding remarks
Following advances in cardiac monitoring, it is likely that AHRE will be increasingly reported in the future. A growing body of clinical trial and registry study data has established a link between AHRE and the risk of stroke and SEs, and it is evident that stroke risk should be managed in all patients with AHREs. However, several questions remain unanswered. Future studies require a standardized definition of AHRE, as past studies have used various definitions. Future studies should also consider how AHREs are diagnosed; a recent study found significant variation in diagnostic accuracy among devices and according to level of operator expertise.90 The pathological and prognostic significance of AHRE has not been fully established. There is a need to identify and validate further markers of risk in patients with AHRE. Finally, the use of oral anticoagulation for stroke prevention in patients with AHRE must be evaluated. Given the risks and inconvenience of OAC therapy, there are currently insufficient data to support their routine use in patients with AHRE, but no clinically detected AF.
Future studies should include information on demographics, stroke characteristics (subtypes and severity), measures of AF burden, subclinical ischaemic events, and long-term incidence of recurrent CE events in patients with AHRE. Ongoing studies should address the key question of whether AHRE are a direct cause of stroke or a marker of a population at risk that would benefit from sustained anticoagulation. Cost-effectiveness should be assessed. Future studies should also address the impact of treatment on patient symptoms and quality of life. Such studies will not only help to standardize current clinical approaches, which are variable and poorly informed, but also help elucidate the relationship between ATs and stroke as well as helping inform shared decision-making, clinical guideline development, and health policy.
Acknowledgements
The authors are grateful to the technical editing support provided by Katrina Mountfort of Medical Media Communications (Scientific) Ltd., which was funded by Daiichi-Sankyo.
Conflict of interest: none declared.
References
- 1. Lip GY. Does atrial fibrillation confer a hypercoagulable state? Lancet 1995;346:1313–4. [DOI] [PubMed] [Google Scholar]
- 2. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983–8. [DOI] [PubMed] [Google Scholar]
- 3. WHO. World Health Organanisation: Tha Atlas of Heart Disease and Stroke. Global burden of stroke http://www.who.int/cardiovascular_diseases/en/cvd_atlas_15_burden_stroke.pdf?ua=1 (16 July 2015, date last accessed).
- 4. Lamassa M, Di Carlo A, Pracucci G, Basile AM, Trefoloni G, Vanni P et al. . Characteristics, outcome, and care of stroke associated with atrial fibrillation in Europe: data from a multicenter multinational hospital-based registry (The European Community Stroke Project). Stroke 2001;32:392–8. [DOI] [PubMed] [Google Scholar]
- 5. Patten M, Maas R, Bauer P, Luderitz B, Sonntag F, Dluzniewski M et al. . Suppression of paroxysmal atrial tachyarrhythmias – results of the SOPAT trial. Eur Heart J 2004;25:1395–404. [DOI] [PubMed] [Google Scholar]
- 6. Savelieva I, Camm AJ. Clinical relevance of silent atrial fibrillation: prevalence, prognosis, quality of life, and management. J Intervent Card Electrophysiol 2000;4:369–82. [DOI] [PubMed] [Google Scholar]
- 7. Page RL, Wilkinson WE, Clair WK, McCarthy EA, Pritchett EL. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation 1994;89:224–7. [DOI] [PubMed] [Google Scholar]
- 8. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH et al. . 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation – developed with the special contribution of the European Heart Rhythm Association. Europace 2012;14:1385–413. [DOI] [PubMed] [Google Scholar]
- 9. Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A et al. . Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–9. [DOI] [PubMed] [Google Scholar]
- 10. Pollak WM, Simmons JD, Interian A Jr, Atapattu SA, Castellanos A, Myerburg RJ et al. . Clinical utility of intraatrial pacemaker stored electrograms to diagnose atrial fibrillation and flutter. Pacing Clin Electrophysiol 2001;24(4 Pt 1):424–9. [DOI] [PubMed] [Google Scholar]
- 11. Friberg L, Skeppholm M, Terent A. Benefit of anticoagulation unlikely in patients with atrial fibrillation and a CHA2DS2-VASc score of 1. J Am Coll Cardiol 2015;65:225–32. [DOI] [PubMed] [Google Scholar]
- 12. Ahmad O, Ahmad KE, Dear KB, Harvey I, Hughes A, Lueck CJ. Atrial fibrillation and anticoagulation in a stroke unit population. Intern Med J 2009;39:752–6. [DOI] [PubMed] [Google Scholar]
- 13. Hannon N, Sheehan O, Kelly L, Marnane M, Merwick A, Moore A et al. . Stroke associated with atrial fibrillation – incidence and early outcomes in the north Dublin population stroke study. Cerebrovasc Dis 2010;29:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Palm F, Kleemann T, Dos Santos M, Urbanek C, Buggle F, Safer A et al. . Stroke due to atrial fibrillation in a population-based stroke registry (Ludwigshafen Stroke Study) CHADS(2), CHA(2) DS(2) -VASc score, underuse of oral anticoagulation, and implications for preventive measures. Eur J Neurol 2013;20:117–23. [DOI] [PubMed] [Google Scholar]
- 15. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH et al. . 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719–47. [DOI] [PubMed] [Google Scholar]
- 16. Camm AJ, Pinto FJ, Hankey GJ, Andreotti F, Hobbs FD, Writing Committee of the Action for Stroke Prevention A. Non-vitamin K antagonist oral anticoagulants and atrial fibrillation guidelines in practice: barriers to and strategies for optimal implementation. Europace 2015;17:1007–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kirchhof P, Ammentorp B, Darius H, De Caterina R, Le Heuzey JY, Schilling RJ et al. . Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC Guidelines on atrial fibrillation: primary results of the PREvention oF thromboemolic events – European Registry in Atrial Fibrillation (PREFER in AF). Europace 2014;16:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Savelieva I, Camm AJ, European Society of Cardiology. ‘Preferred’ management of atrial fibrillation in Europe. Europace 2014;16:1–3. [DOI] [PubMed] [Google Scholar]
- 19. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD et al. . Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–62. [DOI] [PubMed] [Google Scholar]
- 20. Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S et al. . Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806–17. [DOI] [PubMed] [Google Scholar]
- 21. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M et al. . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- 22. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W et al. . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 23. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL et al. . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–104. [DOI] [PubMed] [Google Scholar]
- 24. Defaye P, Dournaux F, Mouton E. Prevalence of supraventricular arrhythmias from the automated analysis of data stored in the DDD pacemakers of 617 patients: the AIDA study. The AIDA Multicenter Study Group. Automatic Interpretation for Diagnosis Assistance. Pacing Clin Electrophysiol 1998;21(1 Pt 2):250–5. [DOI] [PubMed] [Google Scholar]
- 25. Todd D, Hernandez-Madrid A, Proclemer A, Bongiorni MG, Estner H, Blomstrom-Lundqvist C et al. . How are arrhythmias detected by implanted cardiac devices managed in Europe? Results of the European Heart Rhythm Association Survey. Europace 2015;17:1449–53. [DOI] [PubMed] [Google Scholar]
- 26. Flaker GC, Belew K, Beckman K, Vidaillet H, Kron J, Safford R et al. . Asymptomatic atrial fibrillation: demographic features and prognostic information from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am Heart J 2005;149:657–63. [DOI] [PubMed] [Google Scholar]
- 27. Rizos T, Guntner J, Jenetzky E, Marquardt L, Reichardt C, Becker R et al. . Continuous stroke unit electrocardiographic monitoring versus 24-hour Holter electrocardiography for detection of paroxysmal atrial fibrillation after stroke. Stroke 2012;43:2689–94. [DOI] [PubMed] [Google Scholar]
- 28. Grond M, Jauss M, Hamann G, Stark E, Veltkamp R, Nabavi D et al. . Improved detection of silent atrial fibrillation using 72-hour Holter ECG in patients with ischemic stroke: a prospective multicenter cohort study. Stroke 2013;44:3357–64. [DOI] [PubMed] [Google Scholar]
- 29. Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA et al. . Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–86. [DOI] [PubMed] [Google Scholar]
- 30. Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J et al. . Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014;370:2467–77. [DOI] [PubMed] [Google Scholar]
- 31. Doliwa Sobocinski P, Anggardh Rooth E, Frykman Kull V, von Arbin M, Wallen H, Rosenqvist M. Improved screening for silent atrial fibrillation after ischaemic stroke. Europace 2012;14:1112–6. [DOI] [PubMed] [Google Scholar]
- 32. Jorfida M, Antolini M, Cerrato E, Caprioli MG, Castagno D, Garrone P et al. . Cryptogenic ischemic stroke and prevalence of asymptomatic atrial fibrillation: a prospective study. J Cardiovasc Med 2014. Epub. DOI:10.2459/JCM.0000000000000181 [DOI] [PubMed] [Google Scholar]
- 33. Alhadramy O, Jeerakathil TJ, Majumdar SR, Najjar E, Choy J, Saqqur M. Prevalence and predictors of paroxysmal atrial fibrillation on Holter monitor in patients with stroke or transient ischemic attack. Stroke 2010;41:2596–600. [DOI] [PubMed] [Google Scholar]
- 34. Tayal AH, Tian M, Kelly KM, Jones SC, Wright DG, Singh D et al. . Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology 2008;71:1696–701. [DOI] [PubMed] [Google Scholar]
- 35. Kerr CR, Connolly SJ, Abdollah H, Roberts RS, Gent M, Yusuf S et al. . Canadian trial of physiological pacing: effects of physiological pacing during long-term follow-up. Circulation 2004;109:357–62. [DOI] [PubMed] [Google Scholar]
- 36. Skanes AC, Krahn AD, Yee R, Klein GJ, Connolly SJ, Kerr CR et al. . Progression to chronic atrial fibrillation after pacing: the Canadian Trial of Physiologic Pacing. CTOPP Investigators. J Am Coll Cardiol 2001;38:167–72. [DOI] [PubMed] [Google Scholar]
- 37. Glotzer TV, Hellkamp AS, Zimmerman J, Sweeney MO, Yee R, Marinchak R et al. . Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation 2003;107:1614–9. [DOI] [PubMed] [Google Scholar]
- 38. Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker C et al. . The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol 2009;2:474–80. [DOI] [PubMed] [Google Scholar]
- 39. Wiesel J, Spinelli M. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:1351; author reply 2–3. [DOI] [PubMed] [Google Scholar]
- 40. Mittal S, Stein K, Gilliam FR III, Kraus SM, Meyer TE, Christman SA. Frequency, duration, and predictors of newly-diagnosed atrial fibrillation following dual-chamber pacemaker implantation in patients without a previous history of atrial fibrillation. Am J Cardiol 2008;102:450–3. [DOI] [PubMed] [Google Scholar]
- 41. Singer D, Ziegler P, Schmitt S, Chang Y, Fan J, Than CT. et al. Paroxysmal atrial fibrillation poses a large but transient increase in ischemic stroke risk: a case crossover study. J Am Coll Cardiol 2015;65(10_S)A314, doi:10.1016/S0735-1097(15)60314-X. [Google Scholar]
- 42. Boriani G, Laroche C, Diemberger I, Fantecchi E, Popescu MI, Rasmussen LH et al. . Asymptomatic atrial fibrillation: clinical correlates, management, and outcomes in the EORP-AF Pilot General Registry. Am J Med 2015;128:509–18.. [DOI] [PubMed] [Google Scholar]
- 43. Potpara TS, Polovina MM, Marinkovic JM, Lip GY. A comparison of clinical characteristics and long-term prognosis in asymptomatic and symptomatic patients with first-diagnosed atrial fibrillation: the Belgrade Atrial Fibrillation Study. Int J Cardiol 2013;168:4744–9. [DOI] [PubMed] [Google Scholar]
- 44. Boriani G, Glotzer TV, Santini M, West TM, De Melis M, Sepsi M et al. . Device-detected atrial fibrillation and risk for stroke: an analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J 2014;35:508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Benezet-Mazuecos J, Rubio JM, Cortes M, Iglesias JA, Calle S, de la Vieja JJ et al. . Silent ischaemic brain lesions related to atrial high rate episodes in patients with cardiac implantable electronic devices. Europace 2015;17:364–9. [DOI] [PubMed] [Google Scholar]
- 46. Gonzalez M, Keating RJ, Markowitz SM, Liu CF, Thomas G, Ip JE et al. . Newly detected atrial high rate episodes predict long-term mortality outcomes in patients with permanent pacemakers. Heart Rhythm 2014;11:2214–21. [DOI] [PubMed] [Google Scholar]
- 47. Freestone B, Lip GY. The endothelium and atrial fibrillation. The prothrombotic state revisited. Hamostaseologie 2008;28:207–12. [PubMed] [Google Scholar]
- 48. Savelieva I, Kakouros N, Kourliouros A, Camm AJ. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part I: primary prevention. Europace 2011;13:308–28. [DOI] [PubMed] [Google Scholar]
- 49. Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol 2012;60:2263–70. [DOI] [PubMed] [Google Scholar]
- 50. Savelieva I, Kakouros N, Kourliouros A, Camm AJ. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part II: secondary prevention. Europace 2011;13:610–25. [DOI] [PubMed] [Google Scholar]
- 51. Inoue H, Nozawa T, Okumura K, Jong-Dae L, Shimizu A, Yano K. Prothrombotic activity is increased in patients with nonvalvular atrial fibrillation and risk factors for embolism. Chest 2004;126:687–92. [DOI] [PubMed] [Google Scholar]
- 52. Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet 2009;373:155–66. [DOI] [PubMed] [Google Scholar]
- 53. Goldsmith I, Kumar P, Carter P, Blann AD, Patel RL, Lip GY. Atrial endocardial changes in mitral valve disease: a scanning electron microscopy study. Am Heart J 2000;140:777–84. [DOI] [PubMed] [Google Scholar]
- 54. Brambatti M, Connolly SJ, Gold MR, Morillo CA, Capucci A, Muto C et al. . Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation 2014;129:2094–9. [DOI] [PubMed] [Google Scholar]
- 55. Botto GL. Atrial fibrillation and stroke: does the AF burden matter? Presented at the ESC Congress, 27–31 August 2011, Paris, France. Available at: http://spo.escardio.org/abstract-book/ Accessed 3 September 2016. [Google Scholar]
- 56. Warraich HJ, Gandhavadi M, Manning WJ. Mechanical discordance of the left atrium and appendage: a novel mechanism of stroke in paroxysmal atrial fibrillation. Stroke 2014;45:1481–4. [DOI] [PubMed] [Google Scholar]
- 57. You JJ, Singer DE, Howard PA, Lane DA, Eckman MH, Fang MC et al. . Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl.):e531S–75S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weigner MJ, Caulfield TA, Danias PG, Silverman DI, Manning WJ. Risk for clinical thromboembolism associated with conversion to sinus rhythm in patients with atrial fibrillation lasting less than 48 hours. Ann Intern Med 1997;126:615–20. [DOI] [PubMed] [Google Scholar]
- 59. Daoud EG, Glotzer TV, Wyse DG, Ezekowitz MD, Hilker C, Koehler J et al. . Temporal relationship of atrial tachyarrhythmias, cerebrovascular events, and systemic emboli based on stored device data: a subgroup analysis of TRENDS. Heart Rhythm 2011;8:1416–23. [DOI] [PubMed] [Google Scholar]
- 60. Shanmugam N, Boerdlein A, Proff J, Ong P, Valencia O, Maier SK et al. . Detection of atrial high-rate events by continuous home monitoring: clinical significance in the heart failure-cardiac resynchronization therapy population. Europace 2012;14:230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Swiryn S, Benditt DG, DiMarco JP, Lloyd-Jones D, Orlov M, Slawsky MT et al. . Clinical implications of device-detected atrial tachyarrhythmias: results from the Registry of Atrial Tachycardia and Atrial Fibrillation Episodes in the Cardiac Rhythm Management Device Population (RATE). Circulation 2012;126:2792. [DOI] [PubMed] [Google Scholar]
- 62. Healey JS, Martin JL, Duncan A, Connolly SJ, Ha AH, Morillo CA et al. . Pacemaker-detected atrial fibrillation in patients with pacemakers: prevalence, predictors, and current use of oral anticoagulation. Can J Cardiol 2013;29:224–8. [DOI] [PubMed] [Google Scholar]
- 63. Anderson K, Benzauquen BS. Do patients with pacemaker-detected atrial fibrillation receive appropriate anticoagulation? 2011. Europace 2011;13:519. [Google Scholar]
- 64. Hart RG, Pearce LA, Rothbart RM, McAnulty JH, Asinger RW, Halperin JL. Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy. Stroke Prevention in Atrial Fibrillation Investigators. J Am Coll Cardiol 2000;35:183–7. [DOI] [PubMed] [Google Scholar]
- 65. Kishore A, Vail A, Majid A, Dawson J, Lees KR, Tyrrell PJ et al. . Detection of atrial fibrillation after ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Stroke 2014;45:520–6. [DOI] [PubMed] [Google Scholar]
- 66. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A et al. . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 67. Benezet-Mazuecos J, Rubio JM, Farre J. Atrial high rate episodes in patients with dual-chamber cardiac implantable electronic devices: unmasking silent atrial fibrillation. Pacing Clin Electrophysiol 2014;37:1080–6. [DOI] [PubMed] [Google Scholar]
- 68. Chen-Scarabelli C, Scarabelli TM, Ellenbogen KA, Halperin JL. Device-detected atrial fibrillation: what to do with asymptomatic patients? J Am Coll Cardiol 2015;65:281–94. [DOI] [PubMed] [Google Scholar]
- 69. Glotzer TV, Ziegler PD. Does atrial fibrillation detected by cardiac implantable electronic devices have clinical relevance? Cardiol Clin 2014;32:271–81. [DOI] [PubMed] [Google Scholar]
- 70. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B et al. . 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS: The Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESCEndorsed by the European Stroke Organisation (ESO). Europace 2016Aug 27 [Epub ahead of print]. [Google Scholar]
- 71. Kirchhof P, Lip GY, Van Gelder IC, Bax J, Hylek E, Kaab S et al. . Comprehensive risk reduction in patients with atrial fibrillation: emerging diagnostic and therapeutic options – a report from the 3rd Atrial Fibrillation Competence NETwork/European Heart Rhythm Association consensus conference. Europace 2012;14:8–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Varma N, Epstein AE, Irimpen A, Schweikert R, Love C, TRUST Investigators. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation 2010;122:325–32. [DOI] [PubMed] [Google Scholar]
- 73. Martin DT, Bersohn MM, Waldo AL, Wathen M, Choucair W, Lip G et al. . Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices. Eur Heart J 2015;36:1660–8. [DOI] [PubMed] [Google Scholar]
- 74. NCT01706146. Rhythm Evaluation for AntiCoagulaTion with COntinuous Monitoring (REACT COM). https://clinicaltrials.gov/ct2/show/NCT01706146 (23 October 2015, date last accessed).
- 75. Dion F, Saudeau D, Bonnaud I, Friocourt P, Bonneau A, Poret P et al. . Unexpected low prevalence of atrial fibrillation in cryptogenic ischemic stroke: a prospective study. J Intervent Card Electrophysiol 2010;28:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rosiak M, Dziuba M, Chudzik M, Cygankiewicz I, Bartczak K, Drozdz J et al. . Risk factors for atrial fibrillation: not always severe heart disease, not always so ‘lonely’. Cardiol J 2010;17:437–42. [PubMed] [Google Scholar]
- 77. Chiti A, Orlandi G. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:1351–2; author reply 2–3. [DOI] [PubMed] [Google Scholar]
- 78. Aronsson M, Svennberg E, Rosenqvist M, Engdahl J, Al-Khalili F, Friberg L et al. . Cost-effectiveness of mass screening for untreated atrial fibrillation using intermittent ECG recording. Europace 2015;17:1023–9. [DOI] [PubMed] [Google Scholar]
- 79. Vilchez JA, Roldan V, Hernandez-Romero D, Valdes M, Lip GY, Marin F. Biomarkers in atrial fibrillation: an overview. Int J Clin Pract 2014;68:434–43. [DOI] [PubMed] [Google Scholar]
- 80. Kirchhof P, Breithardt G, Bax J, Benninger G, Blomstrom-Lundqvist C, Boriani G et al. . A roadmap to improve the quality of atrial fibrillation management: proceedings from the fifth Atrial Fibrillation Network/European Heart Rhythm Association consensus conference. Europace 2016;18:37–50. [DOI] [PubMed] [Google Scholar]
- 81. Ohara K, Inoue H, Nozawa T, Hirai T, Iwasa A, Okumura K et al. . Accumulation of risk factors enhances the prothrombotic state in atrial fibrillation. Int J Cardiol 2008;126:316–21. [DOI] [PubMed] [Google Scholar]
- 82. Lip GY, Lane D, Van Walraven C, Hart RG. Additive role of plasma von Willebrand factor levels to clinical factors for risk stratification of patients with atrial fibrillation. Stroke 2006;37:2294–300. [DOI] [PubMed] [Google Scholar]
- 83. Hijazi Z, Oldgren J, Siegbahn A, Granger CB, Wallentin L. Biomarkers in atrial fibrillation: a clinical review. Eur Heart J 2013;34:1475–80. [DOI] [PubMed] [Google Scholar]
- 84. Hijazi Z, Oldgren J, Andersson U, Connolly SJ, Ezekowitz MD, Hohnloser SH et al. . Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) substudy. Circulation 2012;125:1605–16. [DOI] [PubMed] [Google Scholar]
- 85. Camm AJ, Savelieva I, Potpara T, Hindriks G, Pison L, Blomstrom-Lundqvist C. The changing circumstance of atrial fibrillation – progress towards precision medicine. J Intern Med 2016;279:412–27. [DOI] [PubMed] [Google Scholar]
- 86. Botto GL, Padeletti L, Santini M, Capucci A, Gulizia M, Zolezzi F et al. . Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol 2009;20:241–8. [DOI] [PubMed] [Google Scholar]
- 87. Boriani G, Botto GL, Padeletti L, Santini M, Capucci A, Gulizia M et al. . Improving stroke risk stratification using the CHADS2 and CHA2DS2-VASc risk scores in patients with paroxysmal atrial fibrillation by continuous arrhythmia burden monitoring. Stroke 2011;42:1768–70. [DOI] [PubMed] [Google Scholar]
- 88. NCT01938248. Apixaban for the Reduction of Thrombo-Embolism in Patients with Device-Detected Sub-Clinical Atrial Fibrillation (ARTESiA); https://clinicaltrials.gov/ct2/show/NCT01938248 (23 October 2015, date last accessed). [DOI] [PubMed]
- 89. https://clinicaltrials.gov/ct2/show/NCT02618577 Non-vitamin K Antagonist Oral Anticoagulants in Patients with Atrial High Rate Episodes (NOAH). https://clinicaltrialsgov/ct2/show/NCT02618577 (22 December 2015, date last accessed).
- 90. Orlov MV, Houde-Walter HQ, Qu F, Swiryn S, Waldo AL, Benditt DGwe et al. . Atrial electrograms improve the accuracy of tachycardia interpretation from ICD and pacemaker recordings: The RATE Registry. Heart Rhythm 2016;13:1475–80. [DOI] [PubMed] [Google Scholar]