Abstract
Aims
The differentiation between idiopathic right ventricular outflow tract (RVOT) arrhythmias and early arrhythmogenic right ventricular cardiomyopathy (ARVC) can be challenging. We aimed to assess whether QRS morphological features and coupling interval of ventricular ectopic beats (VEBs) can improve differentiation between the two conditions.
Methods and Results
Twenty desmosomal-gene mutation carriers (13 females, mean age 43 years) with no or mild ARVC phenotypic expression and 33 age- and sex-matched subjects with idiopathic RVOT arrhythmias were studied. All patients exhibited isolated monomorphic VEBs with left bundle branch block/inferior axis morphology. The predictive value of ectopic QRS morphology and coupling interval was evaluated. Five ectopic QRS features were significantly more common in desmosomal-gene mutation carriers than in idiopathic RVOT-ventricular arrhythmia patients: maximal QRS duration >160 ms (60 vs. 27%, P = 0.02), intrinsicoid deflection time >80 ms (65 vs. 24%, P = 0.01), initial QRS slurring (40 vs. 12%, P = 0.04), QS pattern in lead V1 (90 vs. 36%, P < 0.001), and QRS axis >90° in limb leads (60 vs. 24%, P = 0.01). In the multivariate analysis, intrinsicoid deflection time >80 ms [odds ratio (OR) = 9.9], QS pattern in lead V1 (OR = 28), and QRS axis >90° (OR = 5.7) remained independent predictors of early ARVC. The coupling interval did not differ between the two groups.
Conclusions
In patients with RVOT VEBs and no major electrocardiographic or echocardiographic abnormalities, the ectopic QRS morphology aids in the differential diagnosis between idiopathic RVOT arrhythmias and early ARVC.
Keywords: Arrhythmogenic cardiomyopathy, Cardiomyopathy, ECG, Ventricular tachycardia, Ventricular ectopic beat
What’s new.
The long-term (8.5 years) arrhythmic risk of DS-gene mutation carriers, with no prior sustained ventricular tachycardia or fibrillation, is strongly related to the ARVC phenotypic expression and the presence of major risk factors such as syncope, ventricular dysfunction, or non-sustained ventricular tachycardia.
The study results do not support the concept that DS-gene carriers may experience life-threatening arrhythmic events before and/or regardless of the development of ARVC phenotypic features (‘concealed phase’), as postulated on the basis of experimental studies showing that lethal arrhythmogenic mechanisms may arise at a molecular/cellular level as a result of a cross-talk of altered desmosomes with sodium channel and gap junction proteins.
Our findings also suggest that the ‘concealed phase’ of the disease may be the result of the low sensitivity of routine clinical tests (i.e. ECG and echocardiography) for the detection of early/minor disease phenotypic manifestations such as isolated epicardial scar involvement of the left ventricle, rather than expression of a subcellular arrhythmogenic mechanism.
Introduction
In the majority of patients, ventricular ectopic beats (VEBs) with a left bundle branch block/inferior axis configuration, suggesting a right ventricular outflow tract (RVOT) origin, are benign and idiopathic.1 In a minority of cases, however, those VEBs may be the expression of an underlying arrhythmogenic right ventricular cardiomyopathy (ARVC), a genetically determined heart muscle disease predisposing to ventricular arrhythmias (VA) and sudden death.2,3
Previous studies focusing on electrocardiographic criteria for differential diagnosis between cardiomyopathy-related and idiopathic RV arrhythmias included patients with definite ARVC and overt structural cardiomyopathic changes.4–13 Therefore, this differentiation from patients with idiopathic RVOT-VAs, who demonstrate a structurally normal heart, is usually straightforward without the need for complex analysis of the ectopic QRS morphology.14 However, genetically affected patients with early ARVC phenotypic expression may show VEBs with a left bundle branch block/inferior axis configuration and no or only minor abnormalities on ECG/echocardiography, thus mimicking idiopathic RVOT arrhythmias.15 Differentiation between the two conditions is particularly important because electrical instability and sudden cardiac death may occur in ARVC patients before the disease becomes fully manifest.
The aim of the study was to compare the QRS morphology and the coupling interval of VEBs with a left bundle branch block/inferior axis morphology between pathogenic desmosomal-gene mutation carriers with early phenotypic expression (i.e. no or minor ARVC features at ECG and echocardiography) and subjects with idiopathic RVOT arrhythmias and structurally normal hearts. We hypothesized that the QRS morphology can differentiate between the two subgroups of patients.
Methods
Early arrhythmogenic right ventricular cardiomyopathy patients
The early ARVC cohort was recruited at the Heart Hospital, University College of London Trust, UK (N = 12) and the Department of Cardiac, Thoracic, and Vascular Sciences, University of Padova, Italy (N = 8), which are tertiary care hospitals with dedicated inherited arrhythmogenic cardiomyopathy units. Patients were included if they fulfilled all the following criteria: (i) demonstration of a pathogenic desmosomal gene, according to the previously described criteria;14,16,17 (ii) recording of ≥10 isolated monomorphic VEBs with a left bundle branch block/inferior axis at 12-lead 24 h ECG Holter monitoring; and (iii) absence of major diagnostic criteria and two or less minor criteria for ARVC diagnosis, according to the 2010 International Task Force Criteria.14
Idiopathic right ventricular outflow tract arrhythmias
The study enrolled a consecutive series of 33 individuals, matched for age and gender with early ARVC patients, who underwent ablation of monomorphic RVOT VEBs at the Heart Hospital during 2012–13. Subjects were included if they had: (i) negative family history of sudden cardiac death or ARVC; (ii) diagnosis of structurally normal heart after complete clinical evaluation (including contrast-enhanced cardiac magnetic resonance in selected cases); (iii) absence of low-voltage electrogram areas at endocardial voltage mapping; and (iv) successful ablation of ectopics in the RVOT.
Electrocardiogram analysis
Standard 12-lead ECGs were obtained using the traditional lead positioning and recorded at 1 mV/cm and 25 mm/s paper speed. The traces were digitally acquired, and each measurement was performed with digital calipers after magnification. In sinus rhythm, the analysis focused on the following minor ECG abnormalities associated with ARVC: (i) delayed S-wave upstroke in leads V1–V3 (>60 ms from nadir of S wave to baseline) in the absence of complete right bundle branch block; (ii) negative T-waves ≥0.1 mV in depth in V1 and V2; (iii) negative T-waves ≥0.1 mV in depth in two contiguous leads V4–V6; and (iv) low QRS voltage in limb leads (≤0.5 mV). The ectopic beats morphology and coupling interval were analysed on 12-lead 24 h ECG-Holter monitoring. The morphology analysis of the ectopic QRS focused on the following characteristics: (i) presence of QRS notching in one or more leads; (ii) presence of multiple QRS notching (two or more leads); (iii) maximal QRS duration >160 ms; (iv) intrinsicoid deflection time >80 ms in one or more leads, defined as the time between the QRS onset and the peak of the R-wave or the nadir of the Q-wave; (v) initial slurring (‘pseudo-delta’) in one or more leads, defined as the time between the QRS onset and earliest fast QRS deflection; (vi) precordial transition, defined as the precordial lead in which the R-wave amplitude is greater than equal to S-wave amplitude; (vii) QS morphology in leads V1 and V2, defined as the absence of positive QRS deflections; (viii) predominantly negative QRS in lead 1; (ix) presence of Q wave in lead 1, defined as an initial negative QRS deflection; and (x) QRS duration >120 ms in lead 1. To determine the onset and termination of the QRS, when a sharp deflection or offset was not seen, we used the intersection of the downslope or upslope of the QRS morphology with the isoelectric line.
For the coupling interval analysis, we evaluated 10 consecutive monomorphic VEBs recorded during sinus rhythm with a rate between 60 and 80 b.p.m. The ECG interpretation was performed independently by two observers who were blinded for the clinical diagnosis (J.N. and A.Z.); in the case of disagreement, a third observer (S.C.) was consulted.
Echocardiography
Echocardiography, which was performed at the time of enrolment by operators experienced in the evaluation of patients with cardiomyopathies, focused on the RV and included measurements of overall RV size and function, as well as description of regional dilation or wall motion abnormalities. According to the 2010 ITF criteria,14 a combination of regional wall motion abnormalities in one or more RV segments and either mild RVOT dilation (29–32 mm in long-axis view or 26–32 mm in short-axis view) or mild global RV dysfunction (fractional area change 33–40%) was considered a minor diagnostic criterion for ARVC.
Statistical analysis
Continuous variables were reported as median (25–75th percentiles) and compared with the rank-sum test because normality could not be assumed for any variable. Categorical variables were analysed with the χ2 test or the Fisher's exact test, as appropriate. Two-tailed, 95% confidence interval (CI) of sensitivity, specificity, and negative and predictive values were calculated on the basis of the binomial distribution. Variables with a P-value <0.15 at univariate analysis were entered into a multivariate binary logistic regression model. The inter-observer agreement was evaluated by the Cohen kappa statistic test. The ECG parameters, which were independently correlated to the diagnosis of early ARVC, were used to build a score system (one point each variable). This new model was compared with the score model proposed by Hoffmayer et al.,11 which assigned one point to each of the following variables: QRS duration >120 ms in lead 1, multiple QRS notching, and precordial transition in V5 or later. The score system of Hoffmayer et al. also assigned 3 points to the presence of anterior T-wave inversion (TWI) extending to V3, but according to our study design, no patients exhibited this feature as this is a major ARVC diagnostic criterion. The receiver operating characteristic (ROC) curves obtained by the two score models were compared with the method of DeLong et al. A two-tailed P < 0.05 was considered statistically significant. All analyses were performed using SPSS 17 (SPSS Inc., Chicago, IL, USA).
Results
Clinical characteristics of the study population are shown in Table 1. There were no statistically significant differences in the prevalence of ECG abnormalities between early ARVC and idiopathic RVOT arrhythmia patients. The ECG was completely normal in 12 (60%) early ARVC and in 27 (82%) idiopathic RVOT arrhythmia patients (P = 0.08). As per inclusion criteria, echocardiography was normal in all idiopathic RVOT arrhythmia patients, whereas a combination of regional wall motion abnormalities and mild RVOT dilation or mild global RV dysfunction (minor ARVC diagnostic criterion) was present in five (20%) early ARVC patients. Arrhythmogenic right ventricular cardiomyopathy patients showed a mean of 0.8 minor diagnostic criteria and, as per inclusion criteria, no major diagnostic criteria.
Table 1.
Clinical characteristics of patients with early ARVC and with idiopathic RVOT-VT
| Early ARVC (N = 20) | Idiopathic RVOT-VA (N = 33) | P-value | |
|---|---|---|---|
| Age (years) | 43 (26–54) | 39 (30–45) | 0.82 |
| Gender (male) | 13 (70) | 23 (70) | 1.0 |
| History | |||
| Palpitations | 8 (40) | 27 (82) | 0.003 |
| Chest pain | 3 (15) | 2 (6) | 0.35 |
| Pre-syncope | 5 (25) | 8 (24) | 0.95 |
| Syncope | 5 (25) | 8 (24) | 0.95 |
| Baseline ECG findings | |||
| S-wave >60 ms in V1–V3 | 4 (20) | 2 (6) | 0.18 |
| Negative T-waves in V1 and V2 | 4 (20) | 3 (9) | 0.40 |
| Negative T-waves in V4–V6 | 0 | 1 (3) | 1.0 |
| Low (≤0.5 mV) QRS voltages in limb leads | 2 (10) | 0 | 1.0 |
| Echocardiographic findings | |||
| Regional RV WMA | 13 (65) | 0 | <0.001 |
| RVOT WMA | 1 (5) | 0 | 1.0 |
| RVOT long axis 29–32 mm | 6 (30) | 0 | 0.002 |
| RVOT short axis 32–36 mm | 0 | 0 | – |
| RV FAC 33–40% | 2 (10) | 0 | 1.0 |
ARVC, arrhythmogenic right ventricular cardiomyopathy; FAC, fractional area change; RV, right ventricular; RVOT, right ventricular outflow tract; WMA, wall motion abnormalities.
The comparison between ectopic QRS morphology and coupling interval between early ARVC and idiopathic RVOT arrhythmia patients is shown in Table 2. Five ectopic QRS features were significantly more common in early ARVC than in RVOT arrhythmia patients: maximal QRS duration >160 ms (60 vs. 27%, P = 0.02), intrinsicoid deflection time >80 ms (65 vs. 24%, P = 0.01), initial QRS slurring (40 vs. 12%, P = 0.04), QS pattern in lead V1 (90 vs. 36%, P < 0.001), and predominantly negative QRS complex in lead 1 (60 vs. 24%, P = 0.01). At the multivariate analysis (Table 3), intrinsicoid deflection time >80 ms [odds ratio (OR) = 9.9], QS pattern in lead V1 (OR = 28), and predominantly negative QRS complex in lead 1 (OR = 5.7) remained independent predictors of early ARVC.
Table 2.
Comparison between electrocardiographic findings at admission in patients with early ARVC and idiopathic RVOT-VA
| Early ARVC (N=20) | Idiopathic RVOT-VA (N=33) | P-value | |
|---|---|---|---|
| Age (years) | 43 (26–54) | 39 (30–45) | 0.82 |
| Gender (male) | 13 (70) | 23 (70) | 1.0 |
| Ectopic morphology | |||
| Maximal QRS duration >160 ms | 12 (60) | 9 (27) | 0.02 |
| QRS axis >90° | 12 (60) | 8 (24) | 0.01 |
| QRS notching in one or more leads | 14 (70) | 26 (79) | 0.47 |
| QRS notching in two or more leads | 10 (50) | 15 (45) | 0.75 |
| Intrinsicoid deflection time >80 ms | 12 (60) | 8 (24) | 0.01 |
| Initial slurring (pseudo-delta) | 8 (40) | 4 (12) | 0.04 |
| Precordial transition ≥V4 | 11 (55) | 17 (52) | 1.0 |
| Precordial transition ≥V5 | 3 (15) | 4 (12) | 1.0 |
| QS morphology in lead V1 | 18 (90) | 12 (36) | <0.001 |
| QS morphology in lead V2 | 10 (50) | 8 (24) | 0.06 |
| Q-wave in lead 1 | 5 (25) | 10 (30) | 0.76 |
| QRS duration >120 ms in lead 1 | 6 (30) | 7 (21) | 0.52 |
| Ectopic coupling | |||
| Max interval sQRS-eQRS (ms) | 540 (500–600) | 480 (440–560) | 0.12 |
| Min interval sQRS-eQRS (ms) | 420 (360–500) | 400 (380–420) | 0.23 |
| Max–min sQRS-eQRS (ms) | 100 (40–140) | 60 (40–150) | 0.71 |
| Variance (ms) | 32 (15–54) | 24 (15–41) | 0.65 |
ARVC, arrhythmogenic right ventricular cardiomyopathy; eQRS, QRS of the ventricular ectopic beat; sQRS, QRS at sinus rhythm.
Bold values depict characteristics with significant P-values.
Table 3.
Multivariable ECG predictors for the diagnosis of early arrhythmogenic cardiomyopathy
| OR | 95% CI | P-value | |
|---|---|---|---|
| QRS >160 ms | 1.3 | 0.2–8.6 | 0.8 |
| QRS axis >90° | 5.7 | 1.0–32 | 0.048 |
| Intrinsicoid deflection >80 ms | 9.9 | 1.1–90 | 0.037 |
| Initial slurring (pseudo-delta) | 2.9 | 0.3–24 | 0.3 |
| QS morphology in lead V1 | 27.8 | 3.0–255 | 0.003 |
Bold values depict characteristics with significant P-values.
Sensitivity, specificity, and positive and negative predictive values of the three ECG criteria that were found to independently predict ARVC mutation carriage are shown in Table 4. QS morphology in lead V1 showed the highest sensitivity (90%, 95% CI 68–99), whereas intrinsicoid deflection time >80 ms and predominantly negative QRS morphology in lead 1 had the highest specificity (76%, 95% CI 58–89). The combination of all three criteria (Figure 1) showed a sensitivity of 55% (95% CI 32–78), a specificity of 91% (95% CI 76–98), a positive predictive value of 79% (95% CI 49–95), and a negative predictive value of 77% (95% CI 61–89).
Table 4.
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) with 95% CIs of different ECG criteria for the diagnosis of early arrhythmogenic cardiomyopathy
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|
| QRS axis >90° | 60 (36–81) | 76 (58–89) | 60 (36–81) | 76 (58–89) |
| Intrinsicoid deflection time >80 ms | 60 (36–81) | 76 (58–89) | 60 (36–81) | 76 (58–89) |
| QS morphology in lead V1 | 90 (68–99) | 64 (45–80) | 60 (41–77) | 91 (72–99) |
| All three criteria | 55 (32–78) | 91 (76–98) | 79 (49–95) | 77 (61–89) |
Figure 1.
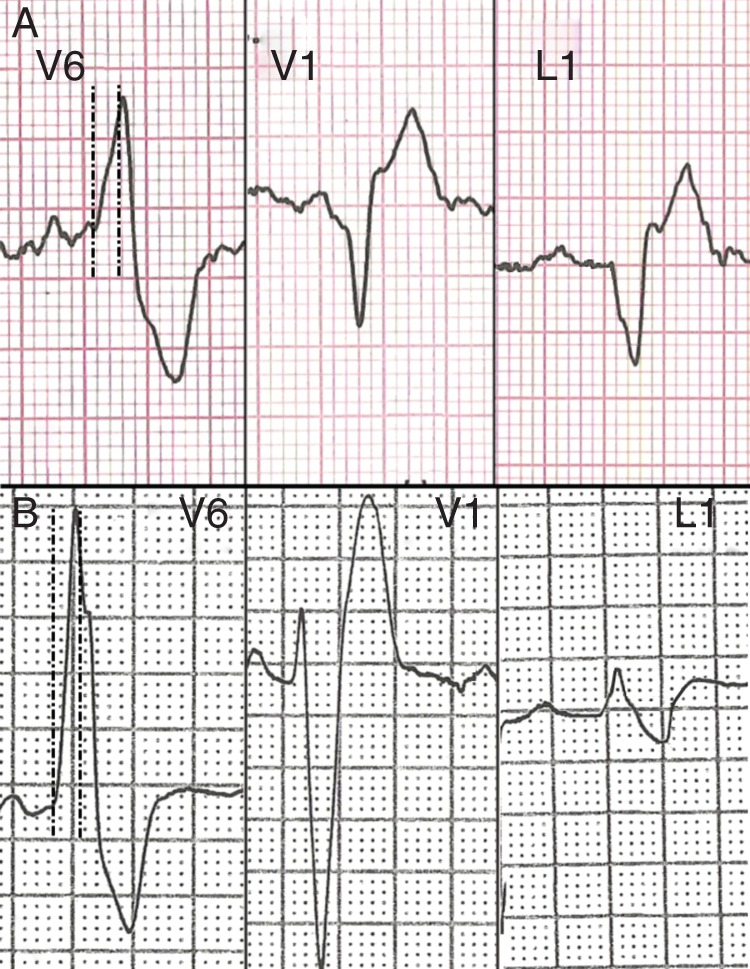
One representative example of ECG leads V6, V1, and 1 in a patient with early ARVC (A) and in a patient with idiopathic RVOT arrhythmia (B). In lead V6, the dotted lines identified an 80 ms interval after the beginning of the QRS. The intrinsicoid deflection time is >80 ms in the early ARVC patient, whereas it is ≤80 ms in the patient with idiopathic RVOT arrhythmia. In lead V1, there is a QS morphology in the early ARVC patient but not in the idiopathic RVOT arrhythmia patient. In lead 1, a Q-wave is observed only in the early ARVC patient. The co-existence of all three factors has a sensitivity of 55% and a specificity of 91% for early ARVC.
Inter-observer agreement was 100% for QRS axis >90° and QS in lead V1, 96% for intrinsicoid deflection time >80 ms (kappa = 0.92, P < 0.001), 94% for maximal QRS duration >160 ms (kappa = 0.88, P < 0.001), and 92% for initial QRS slurring (kappa = 0.80, P < 0.001).
The comparison between the accuracy of the score system proposed by Hoffmayer et al. and our new scoring system including the three variables that were independently associated with early ARVC diagnosis in the present study is shown in Table 5. The number of points was similar between early ARVC and idiopathic RVOT-VA patients [1 (0–2) vs. 1 (0–1), P = 0.63] using the score of Hoffmayer et al.,11 although it was significantly higher in ARVC than in idiopathic RVOT-VA patients [3 (2–3) vs. 0 (0–2), P < 0.001] using the new score model. The area under the ROC curve of the new score model was significantly higher than that under the ROC curve of the score of Hoffmayer et al. [0.84 (0.73–0.95) vs. 0.54 (0.37–0.70), P = 0.007].
Table 5.
Comparison between the score system proposed by Hoffmayer et al. [cit] and a new score system built based on the results of the present study
| Hoffmayer et al.'s score |
New score |
|||||
|---|---|---|---|---|---|---|
| Correctly classified (%) | Sensitivity (%) | Specificity (%) | Correctly classified (%) | Sensitivity (%) | Specificity (%) | |
| One or more criteria | 47 (33–61) | 60 (36–81) | 39 (23–58) | 68 (64–80) | 95(75–100) | 52 (34–69) |
| Two or more criteria | 62 (48–65) | 30 (52–44) | 82 (65–93) | 77 (64–88) | 90 (68–99) | 67 (48–82) |
| Three or more criteria | 64 (50–77) | 5 (0–25) | 100 (89–100) | 75 (63–86) | 55 (32–78) | 91 (76–98) |
| ROC (AUC) | 0.54 (0.37–0.70) | 0.84 (0.73–0.95) | ||||
Discussion
This study was designed to compare the VEBs morphology and coupling interval in a series of patients with early ARVC phenotypic expression, defined as the presence of pathogenic desmosomal-gene mutation and no major electrocardiographic or echocardiographic ARVC features, and in a group of gender- and age-matched patients with idiopathic RVOT arrhythmia patients. The main findings were as follows: (i) patients with early ARVC may have VEBs with a left bundle branch block/inferior axis as the only phenotypic manifestation of the disease, mimicking idiopathic RVOT arrhythmias, and (ii) the combination of QS morphology in lead V1, predominantly negative QRS complex in lead 1, and intrinsicoid deflection time >80 ms showed a high specificity for an underlying cardiomyopathy.
In the general population, RVOT VEBs are usually ‘idiopathic’ (i.e. unrelated to structural heart disease) and carry a benign prognosis,1 but in a minority of cases, they can be the expression of an underlying ARVC, a genetically determined cardiomyopathy characterized by desmosomal-protein dysfunction leading to progressive fibrofatty infiltration, ECG abnormalities, VAs, and regional and/or global ventricular dysfunction.2,3,15 Although in the ‘overt’ phase of ARVC, differentiation with idiopathic RVOT-VT is usually straightforward; as ECG and echocardiographic abnormalities are prominent, desmosomal-gene mutation carriers with early ARVC phenotypic expression may show VEBs with a left bundle branch block/inferior axis configuration as the only feature of the disease, thus mimicking idiopathic RVOT arrhythmias.15
The arrhythmogenic substrate of ARVC-related and idiopathic VEBs is different. Although the characteristic of idiopathic RVOT arrhythmias is the absence of underlying cardiac disease, resulting in focal origin of ectopic beats, caused by a relatively small cluster of cells showing enhanced automaticity in the context of a healthy myocardium, ARVC VEBs are caused by triggered activity of electrically isolated myocytes interspersed among fibrofatty tissue18 or re-entry.6 Recent pre-clinical studies have disclosed novel arrhythmic mechanisms in the early ARVC phase: it has been demonstrated that the genetically determined loss of expression of the desmosomal proteins may alter the amplitude and kinetics of sodium current and lead to a decrease in total content and a significant redistribution of Cx43, thus predisposing to the development of electrical instability unrelated to structural myocardial changes.17,19–21 Desmosomal uncoupling and fibrosis may slow propagation through the myocardium and impact upon surface ECG QRS complex generation. The site of origin is also different in the two conditions: idiopathic RVOT VEBs usually arise from the endocardial and septal portions of the RVOT, whereas the source of ARVC-related VEBs is typically the epicardial layer of the anterior wall.1,2,22,23
The different pathophysiological mechanisms, substrate features, and site of VEBs origin in the two conditions may account for different morphological features of the ectopic QRS complex on surface ECG. Accordingly, several studies investigated whether simple ECG criteria help in the differentiation of VAs in overt ARVC from idiopathic RVOT. Ainsworth et al.8 compared 20 patients with overt ARVC and 24 with idiopathic RVOT ventricular tachycardia (VT) and found that QRS duration >120 ms in lead I coupled with QRS axis <30° was a sensitive and specific marker for the diagnosis of ARVC. Based on a previous retrospective study from the same group,10 Hoffmayer et al.11 developed a scoring system to distinguish QRS morphology in ARVC patients with arrhythmias (either VT or VEBs) with a left bundle branch block/inferior axis and patients with idiopathic RVOT arrhythmias. Criteria for differential diagnosis included anterior TWIs (V1–V3) in sinus rhythm (3 points), lead I ectopic QRS duration >120 ms (2 points), ectopic QRS notching in multiple leads (2 points), and R/S transition of the ectopic QRS in V5 or later (1 point). A score higher than 5 predicted ARVC with a sensitivity of 84% and a specificity of 100%. However, the results of these studies were of limited clinical value because they mostly referred to overt ARVC patients, whose ECG and echocardiographic findings are easily distinguishable from those of patients with idiopathic RVOT arrhythmia without the need for analysing the morphology of the ectopic beats. Furthermore, the majority of ARVC patients presented with VT, a clinical scenario that justifies the use of invasive investigations, such as endocardial voltage mapping, which can differentiate between idiopathic RVOT and early ARVC through the identification of ‘low voltages areas’, corresponding to regions of fibrofatty replacement. In contrast, in the evaluation of patients with isolated VEBs, clinicians often have to rely only on ECG and imaging to exclude an underlying ARVC.
Our study is the first to enrol desmosomal-gene mutation carriers with isolated VEBs with a left bundle branch block/inferior axis configuration and no or minor ARVC electrocardiographic and echocardiographic features of the disease, i.e. the clinical scenario that actually raises problems of differential diagnosis with idiopathic RVOT VEBs. We found that intrinsicoid deflection time (the time between the QRS onset and the peak of the R-wave or the nadir of the Q-wave) >80 ms, QS morphology in lead V1, and axis >90% were VEB features that were independently associated with the diagnosis of ARVC. The delayed intrinsicoid deflection in ARVC patients may be explained by: (i) the delayed conduction because of segmental fibrofatty replacement and/or secondary sodium channel dysfunction and (ii) the epicardial origin of the VEBs, as it has been demonstrated that epicardial VAs typically show delayed intrinsicoid deflection.24 Instead, QS morphology in lead V1 and negative QRS polarity in lead 1 are typical features of arrhythmias arising from the anterior RVOT wall near the pulmonary valve rather than the septal/postero-septal RVOT region.1,23 This finding is consistent with a study on electroanatomical mapping in patients with early ARVC, showing that the majority of patients with an abnormal mapping had electroanatomical scars confined to the antero-infundibular free wall.2
Although no single feature was accurate enough to allow differential diagnosis, the combination of these three criteria was highly specific (91%) for early ARVC. The new score system that we could build using these three criteria demonstrated good diagnostic accuracy (area under ROC curve = 0.84). Instead, we did not confirm the diagnostic values of VEB features such as QRS duration in lead I, multiple QRS notching, and QRS transition ≥V5 transition, and, as a result, we calculated a low diagnostic power of the score proposed by Hoffmayer et al. (area under ROC curve = 0.54). The differences between this previous and the present study results may be explained by the different inclusion criteria: Hoffmayer et al. enrolled ARVC patients with more advanced disease phenotype and only 41% of their ARVC sample demonstrated isolated VEBs.11 Moreover, we specifically excluded from the study ARVC patients with anterior TWI extending to lead V3, i.e. the most important criterion for differential diagnosis in the score proposed by Hoffmayer et al., because this is a major ARVC diagnostic criterion14 that strongly orients towards a ‘cardiomyopathic’ origin of VAs regardless of the ectopic QRS features.25
A significantly larger variance in coupling intervals was noted by Bradfield et al.26 in VEBs arising from the aortic sinus or great cardiac vein as opposed to those arising from the ventricular wall, which was thought to resemble lack of restraining electrotonic coupling effects of the surrounding myocardium, a mechanism that may also be relevant in ARVC. In our study, we found similar VEB coupling intervals between idiopathic RVOT VA and early ARVC patients, possibly because of the limited amount of fibrofatty tissue in the latter. However, in both groups, the coupling interval was variable, suggesting a focal rather than re-entrant mechanism.
Our study has some limitations that should be acknowledged. First, we included only desmosomal-gene mutation carriers who show arrhythmias with a left bundle branch block/inferior axis configuration and no other major ARVC features. As this condition is rare, the study population was relatively small and this limits the statistical power of the multivariate analysis. Secondly, ARVC patients did not undergo invasive electrophysiological study to confirm the RVOT site of origin of the arrhythmia. Thirdly, although idiopathic RVOT-VA patients did not show any ARVC feature or positive family history, the presence of desmosomal-gene mutations could not be definitely excluded because genetic testing was not performed. Finally, the design of our study is retrospective, and validation in a prospective cohort of patients is needed.
In conclusion, our study confirmed that desmosomal-gene mutation carriers with no or minimal ARVC phenotypic expression (no major ECG or echocardiographic abnormality) may show VEBs with a left bundle branch block/inferior axis configuration, mimicking idiopathic and benign RVOT arrhythmias. The morphology of the QRS in the two conditions may help differential diagnosis, and features such as ectopic QRS axis >90° (negative QRS polarity in lead 1), intrinsicoid deflection time >80 ms, and QS morphology in lead V1, particularly when present in combination, should raise high clinical suspicion of an underlying cardiomyopathy when evaluating patients with apparently idiopathic RVOT VEBs to prompt more in-depth clinical evaluation.
Conflict of interest: none declared.
Funding
P.D.L. is funded by UCLH Biomedicine NIHR and British Heart Foundation. Funding to pay the Open Access publication charges for this article was provided by University College London (UCL).
References
- 1. Lerman BB. Mechanism, diagnosis, and treatment of outflow tract tachycardia. Nat Rev Cardiol 2015;12:597–608. [DOI] [PubMed] [Google Scholar]
- 2. Corrado D, Basso C, Leoni L, Tokajuk B, Turrini P, Bauce B et al. . Three-dimensional electroanatomical voltage mapping and histologic evaluation of myocardial substrate in right ventricular outflow tract tachycardia. J Am Coll Cardiol 2008;51:731–9. [DOI] [PubMed] [Google Scholar]
- 3. Thiene G, Nava A, Corrado D, Rossi L, Pennelli N. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med 1988;318:129–33. [DOI] [PubMed] [Google Scholar]
- 4. Kazmierczak J, De Sutter J, Tavernier R, Cuvelier C, Dimmer C, Jordaens L. Electrocardiographic and morphometric features in patients with ventricular tachycardia of right ventricular origin. Heart 1998;79:388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Niroomand F, Carbucicchio C, Tondo C, Riva S, Fassini G, Apostolo A et al. . Electrophysiological characteristics and outcome in patients with idiopathic right ventricular arrhythmia compared with arrhythmogenic right ventricular dysplasia. Heart 2002;87:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O'Donnell D, Cox D, Bourke J, Mitchell L, Furniss S. Clinical and electrophysiological differences between patients with arrhythmogenic right ventricular dysplasia and right ventricular outflow tract tachycardia. Eur Heart J 2003;24:801–10. [DOI] [PubMed] [Google Scholar]
- 7. Miljoen H, State S, de Chillou C, Magnin-Poull I, Dotto P, Andronache M et al. . Electroanatomic mapping characteristics of ventricular tachycardia in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Europace 2005;7:516–24. [DOI] [PubMed] [Google Scholar]
- 8. Ainsworth CD, Skanes AC, Klein GJ, Gula LJ, Yee R, Krahn AD. Differentiating arrhythmogenic right ventricular cardiomyopathy from right ventricular outflow tract ventricular tachycardia using multilead QRS duration and axis. Heart Rhythm 2006;3:416–23. [DOI] [PubMed] [Google Scholar]
- 9. Cox MG, Nelen MR, Wilde AA, Wiesfeld AC, van der Smagt JJ, Loh P et al. . Activation delay and VT parameters in arrhythmogenic right ventricular dysplasia/cardiomyopathy: toward improvement of diagnostic ECG criteria. J Cardiovasc Electrophysiol 2008;19:775–81. [DOI] [PubMed] [Google Scholar]
- 10. Hoffmayer KS, Machado ON, Marcus GM, Yang Y, Johnson CJ, Ermakov S et al. . Electrocardiographic comparison of ventricular arrhythmias in patients with arrhythmogenic right ventricular cardiomyopathy and right ventricular outflow tract tachycardia. J Am Coll Cardiol 2011;58:831–8. [DOI] [PubMed] [Google Scholar]
- 11. Hoffmayer KS, Bhave PD, Marcus GM, James CA, Tichnell C, Chopra N et al. . An electrocardiographic scoring system for distinguishing right ventricular outflow tract arrhythmias in patients with arrhythmogenic right ventricular cardiomyopathy from idiopathic ventricular tachycardia. Heart Rhythm 2013;10:477–82. [DOI] [PubMed] [Google Scholar]
- 12. Samol A, Wollmann C, Vahlhaus C, Gerss J, Bruns HJ, Breithardt G et al. . T-wave integral: an electrocardiographic marker discriminating patients with arrhythmogenic right ventricular cardiomyopathy from patients with right ventricular outflow tract tachycardia. Europace 2013;15:582–9. [DOI] [PubMed] [Google Scholar]
- 13. Batchvarov VN, Bastiaenen R, Postema PG, Clark EN, Macfarlane PW, Wilde AA et al. . Novel electrocardiographic criteria for the diagnosis of arrhythmogenic right ventricular cardiomyopathy. Europace 2015; doi:10.1093/europace/euv379. [DOI] [PubMed] [Google Scholar]
- 14. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA et al. . Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Circulation 2010;121:1533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perrin MJ, Angaran P, Laksman Z, Zhang H, Porepa LF, Rutberg J et al. . Exercise testing in asymptomatic gene carriers exposes a latent electrical substrate of arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol 2013;62:1772–9. [DOI] [PubMed] [Google Scholar]
- 16. Rigato I, Bauce B, Rampazzo A, Zorzi A, Pilichou K, Mazzotti E et al. . Compound and digenic heterozygosity predicts lifetime arrhythmic outcome and sudden cardiac death in desmosomal gene-related arrhythmogenic right ventricular cardiomyopathy. Circ Cardiovasc Genet 2013;6:533–42. [DOI] [PubMed] [Google Scholar]
- 17. Gomes J, Finlay M, Ahmed AK, Ciaccio EJ, Asimaki A, Saffitz JE et al. . Electrophysiological abnormalities precede overt structural changes in arrhythmogenic right ventricular cardiomyopathy due to mutations in desmoplakin-A combined murine and human study. Eur Heart J 2012;33:1942–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Philips B, Madhavan S, James C, Tichnell C, Murray B, Needleman M et al. . High prevalence of catecholamine-facilitated focal ventricular tachycardia in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Arrhythm Electrophysiol 2013;6:160–6. [DOI] [PubMed] [Google Scholar]
- 19. Cerrone M, Lin X, Zhang M, Agullo-Pascual E, Pfenniger A, Chkourko Gusky H et al. . Missense mutations in plakophilin-2 cause sodium current deficit and associate with a Brugada syndrome phenotype. Circulation 2014;129:1092–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rizzo S, Lodder EM, Verkerk AO, Wolswinkel R, Beekman L, Pilichou K et al. . Intercalated disc abnormalities, reduced Na(+) current density, and conduction slowing in desmoglein-2 mutant mice prior to cardiomyopathic changes. Cardiovasc Res 2012;95:409–18. [DOI] [PubMed] [Google Scholar]
- 21. Saffitz JE. Arrhythmogenic cardiomyopathy and abnormalities of cell-to-cell coupling. Heart Rhythm 2009;6(Suppl):S62–5. [DOI] [PubMed] [Google Scholar]
- 22. Migliore F, Zorzi A, Silvano M, Bevilacqua M, Leoni L, Marra MP et al. . Prognostic value of endocardial voltage mapping in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ Arrhythm Electrophysiol 2013;6:167–76. [DOI] [PubMed] [Google Scholar]
- 23. Asirvatham SJ. Correlative anatomy for the invasive electrophysiologist: outflow tract and supravalvar arrhythmia. J Cardiovasc Electrophysiol 2009;20:955–68. [DOI] [PubMed] [Google Scholar]
- 24. Berruezo A, Mont L, Nava S, Chueca E, Bartholomay E, Brugada J. Electrocardiographic recognition of the epicardial origin of ventricular tachycardias. Circulation 2004;109:1842–7. [DOI] [PubMed] [Google Scholar]
- 25. Zorzi A, Migliore F, Elmaghawry M, Silvano M, Marra MP, Niero A et al. . Electrocardiographic predictors of electroanatomic scar size in arrhythmogenic right ventricular cardiomyopathy: implications for arrhythmic risk stratification. J Cardiovasc Electrophysiol 2013;24:1321–7. [DOI] [PubMed] [Google Scholar]
- 26. Bradfield JS, Homsi M, Shivkumar K, Miller JM. Coupling interval variability differentiates ventricular ectopic complexes arising in the aortic sinus of valsalva and great cardiac vein from other sources: mechanistic and arrhythmic risk implications. J Am Coll Cardiol 2014;63:2151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


