Abstract
Objectives: Urinary tract infections (UTIs) are a common reason for empirical treatment with broad-spectrum antibiotics worldwide. However, population-based antimicrobial resistance (AMR) prevalence data to inform empirical treatment choice are lacking in many regions, because of limited surveillance capacity. We aimed to assess the prevalence of AMR to commonly used antimicrobial drugs in Escherichia coli and Klebsiella pneumoniae isolated from patients with community- or healthcare-associated UTIs on two islands of Indonesia.
Methods: We performed a cross-sectional patient-based study in public and private hospitals and clinics between April 2014 and May 2015. We screened patients for symptoms of UTIs and through urine dipstick analysis. Urine culture and susceptibility testing were supported by telemicrobiology and interactive virtual laboratory rounds. Surveillance data were entered in forms on mobile phones.
Results: Of 3424 eligible patients, 3380 (98.7%) were included in the final analysis, and yielded 840 positive cultures and antimicrobial susceptibility data for 657 E. coli and K. pneumoniae isolates. Fosfomycin was the single oral treatment option with resistance prevalence <20% in both E. coli and K. pneumoniae in community settings. Tigecycline and fosfomycin were the only options for treatment of catheter-associated UTIs with resistance prevalence <20%, whilst the prevalence of resistance to meropenem was 21.3% in K. pneumoniae.
Conclusions: Patient-based surveillance of AMR in E. coli and K. pneumoniae causing UTIs indicates that resistance to the commonly available empirical treatment options is high in Indonesia. Smart AMR surveillance strategies are needed to inform policy makers and to guide interventions.
Introduction
Antimicrobial resistance (AMR) in key pathogens is now widespread in most parts of the world and is recognized as a serious global health threat.1,2 The balance between easy access to affordable antimicrobials that save lives and their excess use, resulting in the rapid selection of AMR, is a difficult one to strike, particularly in low- and middle-income countries (LMICs).3 Enhanced surveillance of antimicrobial usage and AMR is one of the tools required for managing this balance, as it can inform antimicrobial stewardship programmes, including guidelines for empirical treatment, as well as allowing the monitoring of trends in AMR and the potential impact of interventions in reducing AMR.4 However, unbiased, high-quality AMR surveillance has been shown to be difficult in LMICs due to limited financial and human resources and the poor quality and capacity of microbiology laboratories.5,6
Urinary tract infections (UTIs) are among the most prevalent bacterial infectious diseases in the general population and a common reason for empirical treatment with broad-spectrum antibiotics in the community worldwide.7,8 Since a urine specimen is easy to obtain and urine culture is relatively straightforward, population-based surveillance of AMR in UTI pathogens may provide opportunities to gain insight into local clinically relevant AMR prevalence and into resistance determinants and clones circulating in the community.9
AMR is a particularly pressing problem in the Asia–Pacific region, where up to 50% of urinary Escherichiacoli isolates from patients with upper UTIs were resistant to fluoroquinolones and third-generation cephalosporins in the Study for Monitoring Antimicrobial Resistance Trends (SMART), a laboratory-based surveillance programme between 2009 and 2010.10 Indonesia is the country with the highest population in the Asia–Pacific region and antimicrobial drug usage is common and often inappropriate.11 Therefore, the Ministry of Health of Indonesia recognizes that surveillance of AMR is crucial to obtaining representative data that can serve as a basis for guideline development and policy planning, as well as steering clinical practice (http://www.lshk.or.id/uu). Whilst guidelines for the treatment of UTIs, which are fully based on the international guidelines,12,13 are currently available from the Indonesian Society of Urology,14 opportunities for microbiological examination of clinical specimens are lacking in many Indonesian hospitals, precluding participation in national and international surveillance activities.15 In addition, even if hospitals were to engage in AMR surveillance, data obtained through laboratory-based surveillance of urine specimens submitted as part of standard hospital care would not inform the clinician dealing with community-based patients who require empirical treatment.16,17
We have previously reported the application of telemicrobiology, using digital imaging and so-called virtual laboratory rounds, as a tool for capacity building and monitoring of quality in clinical bacteriology in Vietnam.18 Here, we aimed to perform state-of-the-art surveillance of AMR in UTIs based on high-quality microbiology supported by telemicrobiology, to assess the prevalence of AMR to commonly used antimicrobial drugs in E. coli and Klebsiellapneumoniae strains isolated from patients with community- or healthcare-associated UTI, on two islands of Indonesia. We performed a cross-sectional patient-based study to assess the prevalence of AMR as well as appropriateness of empirical therapy for treatment of UTIs, including both public and private hospitals and clinics.
Patients and methods
Study population
Hospital patients were included in two public tertiary facilities in Indonesia: Dr Hasan Sadikin General Hospital (Internal Medicine Ward) in Bandung and H. Adam Malik Hospital (Internal Medicine Ward, Surgery Ward, Obstetric Gynecology Ward and Neurology Ward) in Medan. Community-based patients were recruited from the outpatient urology clinic of the Hasan Sadikin General Hospital and the emergency department and outpatient urology clinic of the Dr Salamun Hospital, both in Bandung, and the outpatient urology clinics of the Marta Friska Hospital, the private Murni Teguh Hospital, and the Bunda Clinic, together with a private obstetrics and gynaecology clinic, all in Medan. These public and private outpatient clinics, emergency departments and clinics provide primary and secondary care to patients in Bandung and Medan, all within the catchment area of one of the two tertiary hospitals.
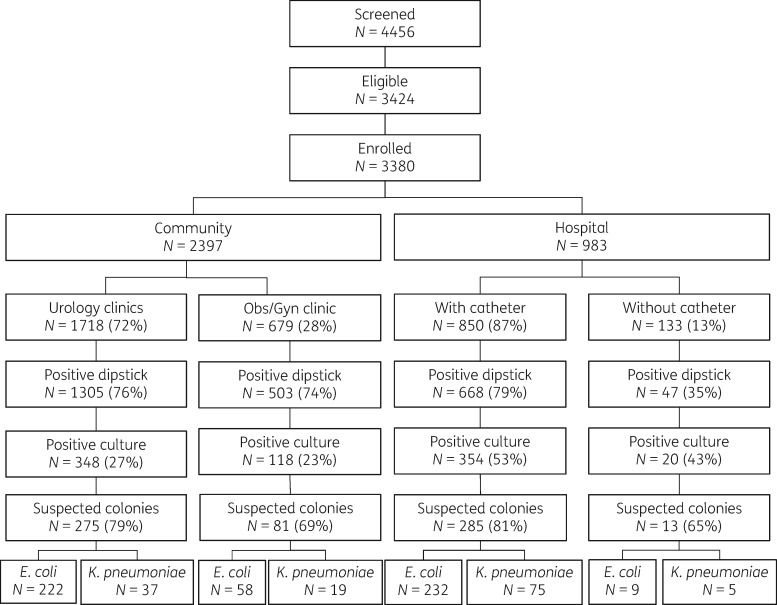
The study population consisted of all adult patients (age ≥18 years) with a clinical suspicion of community-acquired or healthcare-associated (HA) lower or upper UTI, as defined by the US CDC (Supplementary data available at JAC Online).19 Study nurses screened patients for eligibility and included consecutive patients with suspected UTIs. For patients with HA UTI, the study nurse visited participating wards on a daily basis to screen admitted patients for eligibility. All patients were enrolled according to the flowchart presented in Figure 1. Patients were screened for eligibility using a checklist that was designed on the basis of diagnostic criteria from the CDC for community- and hospital-associated UTIs (Supplementary data). Inpatients were identified on the basis of the information provided in their medical charts and were included after an interview using a questionnaire. Eligible outpatients were included after data were obtained using a questionnaire. All subsequent data, including microbiology results, were collected as part of the surveillance activities. Eligible patients were asked for informed consent prior to inclusion.
Figure 1.
Screening eligibility and enrolment of study population. All numbers refer to unique participants, except for the number of strains depicted in the last row. Suspected colonies turned out not to be E. coli or K. pneumoniae, and some participants had multiple unique strains of E. coli and/or K. pneumoniae.
We targeted a sample size of 220 E. coli and/or K. pneumoniae isolates in any of the four settings (community/hospital, Bandung/Medan). This number enabled us to estimate the prevalence of resistance with an absolute precision of ±3%–5.5% if the point estimate of the prevalence lay between 5% and 25%. Inclusion continued for all four strata until 220 isolates were reached or until the end of the data collection period was reached. Participants were followed up by phone 6–8 days after inclusion to assess clinical status and actual treatment taken. Study staff were trained in data collection procedures detailed in standard operating procedures, and participated in a pilot study before the commencement of actual study activities.
Specimen collection
In patients without a catheter, a clean-catch midstream urine specimen was collected. From patients with an indwelling catheter, a 5–10 mL urine specimen was collected through the proximal end of the catheter using an aseptic technique. All urine specimens were immediately stored at 2–8°C, and transferred to the laboratory in one of the tertiary hospitals for culture and susceptibility testing within 24 h.
Laboratory procedures
Urine analysis and culture
Urine specimens were assessed by dipstick analysis (Combur 10, Roche Diagnostic, Germany) according to the manufacturer’s instructions. All specimens with a positive dipstick (defined as at least one positive nitrite reaction or leucocyte esterase reaction) were processed for bacterial culture. Urine was inoculated onto a MacConkey agar plate (Oxoid, Thermo Scientific, UK) using calibrated loops (10 μL of urine) and incubated at 37°C for 18 h. Identification of colonies suspected as being E. coli or K. pneumoniae was performed using a combination of Gram staining and a set of biochemical reactions (IMViC tests: indole, methyl red, Voges-Proskauer and citrate) (Merck, Germany). Biochemical tests were prepared according to the manufacturer’s instructions and, for each new batch, sterility, positive- and negative controls were tested. Quality controls for IMViC tests were performed for each batch using reference strains E.coli ATCC 25922 and K.pneumoniae ATCC 700603, as recommended by WHO.20
Antimicrobial susceptibility testing and reporting
E. coli and K. pneumoniae isolates were subjected to antibiotic susceptibility testing using the modified Kirby–Bauer disc diffusion method as recommended by the CLSI.21 The antibiotics used were ampicillin (10 μg), amoxicillin/clavulanic acid (20/10 μg), ampicillin/sulbactam (10/10 μg), piperacillin/tazobactam (100/10 μg), cefepime (30 μg), ceftriaxone (30 μg), cefuroxime (30 μg), ceftazidime (30 μg), ertapenem (10 μg), meropenem (10 μg), gentamicin (10 μg), amikacin (30 μg), ciprofloxacin (5 μg), levofloxacin (5 μg), trimethoprim/sulfamethoxazole (1.25/23.75 μg), nitrofurantoin (300 μg), tigecycline (15 μg) (all Oxoid) and fosfomycin (200 μg) (Becton Dickinson). Zone diameters were measured and captured in a standardized way using a purpose-built, dedicated camera (ImagA, BD-Kiestra, the Netherlands). Zone diameters were measured manually and compared with the results obtained with the ImagA on a weekly basis as a quality control. Results were interpreted as susceptible, intermediate or resistant according to breakpoints as defined by CLSI 2012.21 If the breakpoints were not defined by CLSI, the EUCAST breakpoints were used (http://www.eucast.org/clinical_breakpoints/). For tigecycline, only breakpoints for the categories susceptible or resistant were defined. Quality controls for each batch of agar culture media were performed using E.coli ATCC 25922, Proteus mirabilis and Enterococcus faecalis clinical isolates. Quality controls for susceptibility testing were performed on a weekly basis according to CLSI guidelines, using reference strains E.coli ATCC 25922, E.coli ATCC 35218 and K.pneumoniae ATCC 700603. All procedures were as described in dedicated standard operating procedures. Quality of microbiology procedures and culture results was monitored during weekly virtual laboratory rounds between the study sites in Medan, Bandung and Amsterdam, using the telemicrobiology approach. This approach allows interactive exchange of information and images of cultures and susceptibility test results through images generated by the ImagA, using the accompanying imaging and sharing software (Kiestra browser) and the freely available applications Skype® and Team Viewer®.18
Culture results were not reported to clinicians on a routine individual patient basis, but clinicians were informed that they could request the results if they considered it useful for patient management. Feedback of surveillance results to clinicians was provided after completion of the study.
Data entry and analysis
All data were entered directly in a formatted electronic questionnaire on mobile phones using Open DataKit (opendatakit.org) and were uploaded daily to the database. The susceptibility test results were captured as images by the ImagA, after which the inhibition zones for each of the discs were measured within the same application, exported into Excel files and merged with the clinical data. Data were analysed using Stata version 12.1 (Stata Corp, TX, USA).
Because of the expected high frequency of antimicrobial pre-treatment in patients presenting with UTI symptoms to a clinic or on the hospital ward, a culture was considered positive if E. coli and/or K. pneumoniae was present in colony counts of at least 103 cfu/mL.
Intermediate susceptible strains were considered resistant in this surveillance study, allowing reporting of the prevalence of resistance, as well as of multidrug resistance, at population level. Multidrug resistance was defined as resistance to at least three different classes of antimicrobial drugs.
The primary outcome was the prevalence of resistance to selected antibiotics, which is presented as the percentage of the number of strains tested, stratified by clinic type and the presence or absence of a urinary catheter, for E.coli and K. pneumoniae separately. In addition, we present the prevalence of resistance combining these two species, given the fact that in an empirical treatment setting the causative microorganism is not known. Finally, we assessed appropriateness of empirical treatment on an individual patient basis for those patients who had an antimicrobial prescribed during the presenting visit and whose urinary specimen yielded E. coli and/or K. pneumoniae. For patients with polymicrobial infection, we considered all isolates for assessment of appropriateness of therapy. Appropriateness of therapy was expressed as the percentage of patients with a culture-positive urinary specimen who had an antimicrobial treatment prescription for which the cultured isolates were tested as susceptible. Duration and dosage of treatment were not included in this definition.
Ethics
The study was conducted in accordance with the ethics standards of the Helsinki Declaration of 1975, as revised in 1983. This study was approved by the University of Sumatera Utara Faculty of Medicine Ethics Committee, H. Adam Malik General Hospital Research Committee, Universitas Padjadjaran Faculty of Medicine Ethics Committee (286/KOMET/FK USU/2013) and Dr Hasan Sadikin General Hospital Research Committee (LB.04.01/A05/EC/013/11/2013). Written informed consent was obtained from each participant.
Results
Data were collected from April 2014 to May 2015. In total, 4456 participants were screened, of whom 3424 (76.8%) were eligible for the study. Costovertebral and/or suprapubic pain and frequency were the most commonly reported symptoms at screening (Table S1). Among those eligible, 3380 (98.7%) were included in the final analysis (Figure 1). Eligible patients who were not included did not consent (n = 6), did not provide urine (n = 25) or did not have information on the location of enrolment, precluding adequate classification (n = 13). Of all included participants, 1461 (43.2%) used medication in the 3 months preceding the current consultation/hospitalization. Six-hundred and twenty two patients (18.4%) took this medication for their presenting symptoms of UTI, and 432 (12.7%) reported that the medication included at least one antimicrobial drug. Demographic and clinical characteristics of the study population are described in Table 1.
Table 1.
Patient characteristics
| Community |
Hospital |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| urology |
obstetrics/gynaecology |
catheter + |
catheter − |
Total |
||||||
|
N = 1718 |
N = 679 |
N = 850 |
N = 133 |
N = 3380 |
||||||
| Characteristic | n | % | n | % | n | % | n | % | n | % |
| Sex | ||||||||||
| female | 664 | 38.6 | 679 | 100 | 489 | 57.5 | 64 | 48.1 | 1896 | 56.1 |
| male | 1029 | 59.9 | 0 | 0.0 | 361 | 42.5 | 69 | 51.9 | 1459 | 43.2 |
| missing | 25 | 1.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 25 | 0.7 |
| Age group (years) | ||||||||||
| 18–24 | 69 | 4.0 | 73 | 10.5 | 71 | 8.4 | 19 | 14.3 | 232 | 6.8 |
| 25–34 | 194 | 11.3 | 374 | 55.1 | 105 | 12.4 | 27 | 20.3 | 700 | 20.7 |
| 35–44 | 276 | 16.1 | 161 | 23.7 | 131 | 15.4 | 24 | 18.0 | 592 | 17.5 |
| 45–54 | 440 | 25.6 | 55 | 8.1 | 222 | 26.1 | 36 | 27.1 | 753 | 22.3 |
| 55–64 | 411 | 23.9 | 10 | 1.5 | 178 | 20.9 | 20 | 15.0 | 619 | 18.3 |
| ≥65 | 303 | 17.6 | 2 | 0.3 | 143 | 16.8 | 7 | 5.3 | 455 | 13.5 |
| missing | 25 | 1.5 | 4 | 0.6 | 0 | 0.0 | 0 | 0.0 | 29 | 0.9 |
| Diabetes | ||||||||||
| no | 1390 | 80.9 | 596 | 87.8 | 681 | 80.1 | 118 | 88.7 | 2785 | 82.4 |
| yes | 180 | 10.5 | 2 | 0.3 | 141 | 16.6 | 7 | 5.3 | 330 | 9.8 |
| do not know | 104 | 6.1 | 79 | 11.6 | 16 | 1.9 | 1 | 0.8 | 200 | 5.9 |
| missing | 44 | 2.6 | 2 | 0.3 | 12 | 1.4 | 7 | 5.3 | 65 | 1.9 |
| Malignancy | ||||||||||
| no | 1601 | 93.2 | 549 | 80.9 | 656 | 77.2 | 84 | 63.2 | 2890 | 85.5 |
| yes | 29 | 1.7 | 19 | 2.8 | 175 | 20.6 | 39 | 29.3 | 262 | 7.8 |
| do not know | 44 | 2.6 | 109 | 16.1 | 7 | 0.8 | 3 | 2.3 | 163 | 4.8 |
| missing | 44 | 2.6 | 2 | 0.3 | 12 | 1.4 | 7 | 5.3 | 65 | 1.9 |
| Medication in past 3 months | ||||||||||
| no | 924 | 53.8 | 485 | 71.4 | 381 | 44.8 | 51 | 38.3 | 1841 | 54.5 |
| yes | 749 | 43.6 | 191 | 28.1 | 447 | 52.6 | 74 | 55.6 | 1461 | 43.2 |
| do not know | 1 | 0.1 | 1 | 0.1 | 10 | 1.2 | 1 | 0.8 | 13 | 0.4 |
| missing | 44 | 2.6 | 2 | 0.3 | 12 | 1.4 | 7 | 5.3 | 65 | 1.9 |
| Treatment for presenting symptomsa | ||||||||||
| no | 470 | 62.8 | 133 | 69.3 | 101 | 22.6 | 23 | 31.1 | 727 | 49.7 |
| yes | 237 | 31.6 | 48 | 25.0 | 298 | 66.7 | 39 | 52.7 | 622 | 42.5 |
| do not know | 42 | 5.6 | 11 | 5.7 | 48 | 10.7 | 12 | 16.2 | 113 | 7.7 |
| Antibiotic useb | ||||||||||
| no | 15 | 6.3 | 2 | 4.2 | 17 | 5.7 | 1 | 2.6 | 35 | 5.6 |
| yes | 162 | 68.4 | 8 | 16.7 | 231 | 77.5 | 31 | 79.5 | 432 | 69.5 |
| do not know | 60 | 25.3 | 38 | 79.2 | 50 | 16.8 | 7 | 17.9 | 155 | 24.9 |
Of those reporting medication use in past 3 months.
Of those reporting treatment for presenting symptoms.
Positive dipsticks were obtained from 1305 (76%) patients attending the urology outpatient clinics, yielding 348 (27%) positive cultures; 503 (74%) patients attending the obstetrics/gynaecology outpatient clinics, yielding 118 (23%) positive cultures; 668 (79%) hospitalized patients with a catheter, yielding 354 (53%) positive cultures; and 47 (35%) hospitalized patients without a catheter, yielding 20 (43%) positive cultures (Figure 1). Of the 840 positive cultures, 288 (34%) contained an estimated 103 cfu/mL, 103 (12%) 104 cfu/mL and 439 (52%) 105 cfu/mL (information missing for 10 cultures). Final identification revealed 280 E. coli and 56 K. pneumoniae isolates in urine samples from community-based patients, and 241 E. coli and 80 K. pneumoniae isolates in urine samples from hospital-based patients (Figure 1).
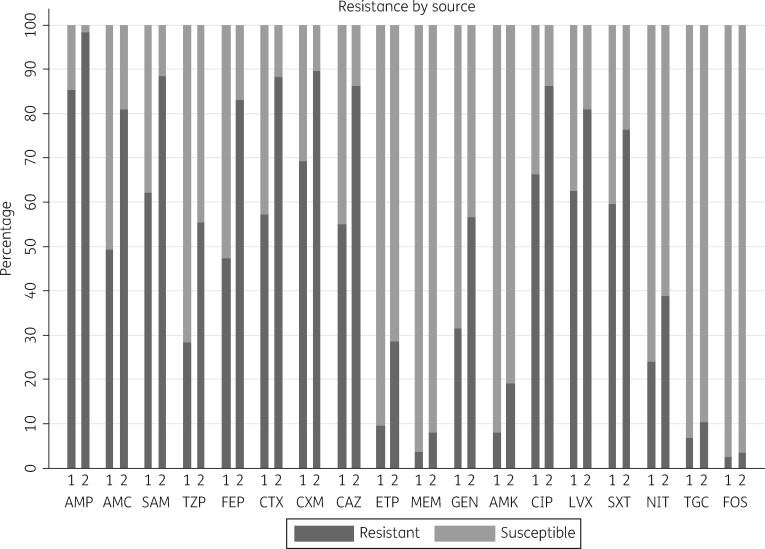
Fluoroquinolone resistance prevalence in E. coli ranged from 41.4% for levofloxacin resistance in isolates from women attending the obstetrics/gynaecology clinic to 71.6% for ciprofloxacin resistance in isolates from patients attending the urology clinics (Figure 2 and Table 2). Fluoroquinolone resistance of K. pneumoniae was high in isolates from community participants attending the urology clinics (78.4% resistant to levofloxacin) but moderate in K. pneumoniae isolated from participants attending the obstetrics/gynaecology clinic (15.8%) (Figure 2 and Table 2). The prevalence of resistance to other key oral antimicrobial drugs used in the treatment of uncomplicated UTIs showed acceptable levels for nitrofurantoin in E. coli isolated from community-based patients, and for fosfomycin in both E. coli and K. pneumoniae. In the hospital setting, the prevalence of resistance to fosfomycin was <5% for E. coli and <20% for K. pneumoniae in patients with a urinary catheter, whilst high resistance of E. coli to all other oral and intravenous antibiotics was observed. Tigecycline and fosfomycin were the only options for treatment of catheter-associated UTIs, with a resistance prevalence <20%, and a prevalence of resistance to meropenem of 21.3% in K. pneumoniae.
Figure 2.
Percentage of E. coli and K. pneumoniae isolates resistant by antimicrobial drug and patient characteristics. 1, community based; 2, hospital associated; AMP, ampicillin; AMC, amoxicillin/clavulanic acid; SAM, ampicillin/sulbactam; TZP, piperacillin/tazobactam; FEP, cefepime; CTX, ceftriaxone; CXM, cefuroxime; CAZ, ceftazidime; ETP, ertapenem; MEM, meropenem; GEN, gentamicin; AMK, amikacin; CIP, ciprofloxacin; LVX, levofloxacin; SXT, trimethoprim/sulfamethoxazole; NIT, nitrofurantoin; TGC, tigecycline; FOS, fosfomycin.
Table 2.
Resistance to selected antibiotics by setting and species
| Community |
Hospital |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| urology |
obstetrics/gynaecology |
catheter + |
catheter − |
|||||||||||||
| E. coli | K. pneumoniae | E. coli | K. pneumoniae | E. coli | K. pneumoniae | E. coli | K. pneumoniae | |||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Ampicillina | 221 | 85.5 | NA | NA | 58 | 84.5 | NA | NA | 223 | 99.1 | NA | NA | 9 | 77.8 | NA | NA |
| Amoxicillin/clavulanic acid | 222 | 50.5 | 37 | 67.6 | 58 | 36.2 | 19 | 42.1 | 232 | 81.0 | 75 | 85.4 | 9 | 44.4 | 5 | 80.0 |
| Ampicillin/sulbactam | 222 | 63.5 | 37 | 86.5 | 58 | 46.6 | 19 | 47.4 | 232 | 88.8 | 75 | 90.7 | 9 | 55.6 | 5 | 100 |
| Piperacillin/tazobactam | 222 | 24.3 | 37 | 73.0 | 58 | 15.5 | 19 | 26.3 | 232 | 56.0 | 75 | 61.3 | 9 | 0.0 | 5 | 40.0 |
| Cefepime | 222 | 47.7 | 37 | 73.0 | 58 | 34.5 | 19 | 31.6 | 232 | 84.9 | 75 | 81.3 | 9 | 44.4 | 5 | 100 |
| Ceftriaxone | 222 | 58.1 | 37 | 78.4 | 58 | 43.1 | 19 | 47.4 | 232 | 90.5 | 75 | 85.3 | 9 | 44.4 | 5 | 100 |
| Cefuroxime | 222 | 68.9 | 37 | 83.8 | 58 | 62.1 | 19 | 68.4 | 232 | 91.8 | 75 | 86.7 | 9 | 55.6 | 5 | 100 |
| Ceftazidime | 222 | 55.4 | 37 | 73.0 | 58 | 43.1 | 19 | 52.6 | 232 | 87.5 | 75 | 85.3 | 9 | 55.6 | 5 | 100 |
| Ertapenem | 222 | 10.8 | 37 | 16.2 | 58 | 3.4 | 19 | 0.0 | 232 | 28.9 | 75 | 28.0 | 9 | 11.1 | 5 | 40.0 |
| Meropenem | 222 | 4.1 | 37 | 5.4 | 58 | 1.7 | 19 | 0.0 | 232 | 3.9 | 75 | 21.3 | 9 | 0.0 | 5 | 20.0 |
| Gentamicin | 222 | 32.0 | 37 | 40.5 | 58 | 29.3 | 19 | 15.8 | 232 | 57.3 | 75 | 57.3 | 9 | 22.2 | 5 | 80.0 |
| Amikacin | 222 | 10.8 | 37 | 2.7 | 58 | 3.4 | 19 | 0.0 | 232 | 16.8 | 75 | 28.0 | 9 | 0.0 | 5 | 20.0 |
| Ciprofloxacin | 222 | 71.6 | 37 | 83.8 | 58 | 48.3 | 19 | 26.3 | 232 | 88.8 | 75 | 82.7 | 9 | 66.7 | 5 | 60.0 |
| Levofloxacin | 222 | 69.4 | 37 | 78.4 | 58 | 41.4 | 19 | 15.8 | 232 | 87.5 | 75 | 66.7 | 9 | 55.6 | 5 | 40.0 |
| Trimethoprim/sulfamethoxazole | 222 | 56.8 | 37 | 86.5 | 58 | 53.4 | 19 | 57.9 | 232 | 74.1 | 75 | 85.3 | 9 | 55.6 | 5 | 80.0 |
| Nitrofurantoin | 222 | 13.1 | 37 | 83.8 | 58 | 15.5 | 19 | 63.2 | 232 | 27.6 | 75 | 74.7 | 9 | 0.0 | 5 | 100 |
| Tigecycline | 222 | 2.3 | 37 | 24.3 | 58 | 0.0 | 19 | 15.8 | 232 | 1.7 | 75 | 26.7 | 9 | 0.0 | 5 | 40.0 |
| Fosfomycina | 219 | 0.9 | 15 | 6.7 | 55 | 7.3 | 3 | 0.0 | 217 | 1.4 | 34 | 17.6 | 9 | 0.0 | 1 | 0.0 |
NA, not applicable (not tested).
Missing results due to unavailable subcultures.
Figure 2 shows the percentage resistance to all antibiotics when the two causative organisms were combined. Multidrug resistance was observed in 536 (81.6%) of 657 strains, of which 419/521 (80.4%) were E. coli and 117/136 (86.0%) were K. pneumoniae.
Appropriateness of empirical therapy for fluoroquinolones, the empirical treatment recommended by urologists in Indonesia, prescribed at the time of the study visit for patients visiting the outpatient clinics is depicted in Table 3. Just under 25% of the patients presenting at an urology outpatient clinic with a positive culture for E. coli and/or K. pneumoniae were prescribed at least one antibiotic during the visit, of which more than two-thirds were deemed inappropriate given resistance to this drug. Out of 66 women presenting at the obstetrics/gynaecology clinic with signs and symptoms compatible with UTI and E. coli or K. pneumoniae cultured from the urine, only 2 were prescribed antibiotics. Inpatients were often prescribed antibiotics, but with poor recording of clinical indications. Therefore, appropriateness of therapy could not be assessed in inpatients.
Table 3.
Appropriateness of fluoroquinolone treatment prescribed to outpatients at urology outpatient clinics
| Fluoroquinolone treatment (n = 222)a |
||||
|---|---|---|---|---|
| prescribed |
appropriate |
|||
| Fluoroquinolone | n | % | n | % |
| Any | 53 | 23.9 | ||
| Levofloxacin | 22 | 9.9 | 6 | 27.3 |
| Ciprofloxacin | 22 | 9.9 | 5 | 22.7 |
| Amoxicillin | 3 | 1.4 | 1 | 33.3 |
| Ceftriaxone | 3 | 1.4 | 1 | 33.3 |
Number of patients with at least one E. coli or K. pneumoniae strain in urine culture.
A follow-up assessment 6–8 days after inclusion was available for 1926 (57.0%) participants, while the remaining participants could not be reached by phone despite several attempts. Of those assessed, 178 (9.2%) reported full recovery, 650 (33.8%) partial reduction of symptoms and 1098 (57.0%) no improvement at all. Use of antimicrobial drugs (as prescribed during consultation or obtained otherwise after consultation) did not differ markedly between patients who reported full or partial recovery [182 (38.7%) reported using antimicrobial drugs] and patients who did not report recovery [639 (43.9%) reported using antimicrobial drugs].
Discussion
Surveillance of AMR is required to combat AMR and to inform empirical treatment guidelines, but has been shown to be difficult in LMICs due to limited capacities. Here, we demonstrate that high-quality surveillance of AMR is feasible in LMICs. To our knowledge, this study is the first to determine the prevalence of resistance to the main antimicrobial drugs commonly used for the treatment of UTIs, using an unbiased patient-based approach in Indonesia. We show a high prevalence of resistance, including multidrug resistance, adding even more alarming data to earlier reports describing hospital-based surveillance results from the Asia-Pacific region.10,22 Our findings indicate that appropriate empirical treatment of UTIs with affordable oral or systemic antimicrobial drugs is extremely difficult in Indonesia. Given the observed prevalence of resistance, the carbapenems (ertapenem, meropenem) and amikacin are the only antimicrobial drugs tested that could be used to treat UTIs that require intravenous therapy. Similar findings have been reported from India.23 The current drug of first choice to treat both lower and upper UTIs in adults in Indonesia empirically is a fluoroquinolone, in particular levofloxacin, because it is a cheap broad-spectrum antibiotic that is readily available. With at least 70% of the isolates in the outpatient urology clinics resistant to levofloxacin or ciprofloxacin, this practice appears inadequate. Unfortunately, alternatives are difficult to find. Nitrofurantoin is a longstanding choice for the treatment of uncomplicated UTIs in primary care settings because it is relatively cheap and the prevalence of resistance is generally acceptable.24 The prevalence of resistance to nitrofurantoin in community-based patients was <15% for E. coli, making it a possible treatment option.12,13 Fosfomycin is a second alternative for the empirical treatment of uncomplicated lower UTIs.7,25,26 However, both nitrofurantoin and fosfomycin are difficult treatment alternatives in Indonesia given their restricted accessibility. Nitrofurantoin is poorly distributed throughout the health system, whilst fosfomycin is not included as a first-choice treatment option in the recently modified national health insurance scheme, precluding its use in empirical treatment in Indonesia.
One of the strengths of our study is the patient-based study sample, in both community and hospital settings, rather than relying on a laboratory-based study sample. The latter is often biased given the potential barriers to and selection processes of submission of clinical specimens to the laboratory for culture and susceptibility testing, particularly in resource-constrained settings. In our strategy, selection bias is highly unlikely given the consecutive enrolment of patients and the study nurse-independent patient flow in the facility. However, in this study, the population of patients who presented at the urology outpatient clinics and the population of inpatients were more likely to suffer from underlying problems, complicated UTIs and recurrent UTI symptoms than a more healthy population, such as pregnant women presenting to an obstetrics/gynaecology clinic, resulting in a higher prevalence of resistance in E. coli and K. pneumoniae. A second strength of the study is the high quality of the microbiology procedures. Previous studies have shown the difficulties in obtaining quality data associated with surveillance in resource-constrained settings.5,6 Not only were quality control procedures initiated and followed, but weekly virtual laboratory rounds, which took place throughout the entire study period, allowed the close monitoring of laboratory practices and adherence to procedures, and provided opportunities for capacity building. A limitation of the study is the incomplete follow-up by phone. However, a bias with respect to the clinical effect of treatment in those responding and those not responding to phone calls is unlikely. The absence of an effect of antibiotic use on clinical recovery is therefore likely to be valid and potentially a result of the high prevalence of AMR. A second limitation is the potential underreporting of prior antibiotic usage and of subsequent antibiotic prescriptions at outpatient clinics. Patients were asked about prior medicine usage, including the use of antibiotics, but since many patients typically lack knowledge of the nature of the medicines they have used, these data are likely to suffer from ascertainment bias. Study nurses did go through medical records at the participating clinics on the day of study inclusion, making the underreporting of prescribed antibiotics likely a result of selective recording by the physician rather than biased data collection during the study. Finally, inpatients could be prescribed antimicrobial treatment for conditions other than UTIs, and medical record keeping did not allow disentanglement of the different indications for antimicrobial treatment, precluding assessment of its appropriateness. Despite this limitation, the data show a large potential for inappropriate prescription of antibiotics given the underlying resistance patterns.
A review of antibiotic prescribing practices by healthcare providers, dispensing of antibiotics by pharmacists and the use of antibiotics in the community in low-income countries showed lack of knowledge, economic motivations and marketing of antibiotics as important determinants for inappropriate use of antimicrobial drugs.27 Prescribers lack access to quality diagnostics and fear withholding a potentially adequate therapy, while dispensers can take advantage of the lack of regulation and enforcement of guidelines. Inappropriate sources of information and beliefs about infectious diseases and antibiotics add further to inappropriate antibiotic use. Many of these determinants are present in Indonesia, resulting in widespread inappropriate antimicrobial drug use and contributing to the ensuing high prevalence of antimicrobial drug resistance as observed in our study. In conclusion, we show an extremely high prevalence of antimicrobial resistance in E. coli and K. pneumoniae isolated from urinary samples of patients with suspected UTIs in Indonesia, threatening rational choices for empirical treatment of both community-associated and HA UTIs. Smart strategies for rapid and frequent surveillance are urgently needed to monitor antimicrobial resistance prevalence in low- and middle-income settings with limited resources, where antimicrobial resistance is reaching unmanageable scales. Surveillance strategies that can assist in the design of appropriate empirical treatment guidelines may need to adopt alternative approaches to achieve scalability and sustainability, and to reach relevant patient populations. We recently described lot quality assurance sampling (LQAS) as one such potential strategy.28 This study clearly demonstrates the urgent need for rapid policy actions to reduce inappropriate use of antimicrobial drugs and to provide up-to-date guidelines for empirical treatment of infectious diseases in Indonesia. Studies on the optimal approach to the introduction of antimicrobial stewardship programmes and improvement of quality of care in LMICs will be essential to achieve this.
Supplementary Material
Acknowledgements
We thank all clinicians who participated in the study roll-out. We thank Thomas Osinga for his tireless technical support of the telemicrobiology.
Funding
This study was funded through a grant of the Royal Netherlands Academy of Arts and Sciences as part of the Scientific Program Indonesia-the Netherlands (SPIN). The funding source did not have any role in the writing of the manuscript or the decision to submit it for publication.
Transparency declarations
None to declare.
Author contributions
A. K. S., F. G., S. G., M. D. dJ., I. P., F. vL. and C. S. designed the study. A. K. S., F. G., R. L. K., E. H. P., A. P. P., F. G., F. vL. and C. S. contributed to data collection and data analysis. A. K. S., F. G., F. vL. and C. S. drafted the manuscript. All authors contributed to the writing of the manuscript and have seen the manuscript before submission. A. K. S. and F. G., and F. vL. and C. S. contributed equally.
Supplementary data
The inclusion and exclusion criteria and Table S1 are available as Supplementary data at JAC Online (https://academic.oup.com/jac/advance-access).
References
- 1. Global Union for Antibiotics Research and Development (GUARD). Berlin Declaration on Antimicrobial Resistance.2015. http://www.bundesgesundheitsministerium.de/fileadmin/dateien/Downloads/G/G7-Ges.Minister_2015/G7_Health_Ministers_Declaration_AMR_and_EBOLA.pdf.
- 2. WHO. Antimicrobial Resistance: Global Report on Surveillance2014. http://www.who.int/drugresistance/documents/surveillancereport/en/.
- 3. Laxminarayan R, Matsoso P, Pant S. et al. Access to effective antimicrobials: a worldwide challenge. Lancet 2016; 387: 168–75. [DOI] [PubMed] [Google Scholar]
- 4. WHO. Global Action Plan on Antimicrobial Resistance http://www.who.int/drugresistance/global_action_plan/en/.
- 5. Holloway K, Mathai E, Gray A. et al. Surveillance of community antimicrobial use in resource-constrained settings – experience from five pilot projects. Trop Med Int Health 2011; 16: 152–61. [DOI] [PubMed] [Google Scholar]
- 6. Holloway K, Mathai E, Gray A. et al. Surveillance of antimicrobial resistance in resource-constrained settings – experience from five pilot projects. Trop Med Int Health 2011; 16: 368–74. [DOI] [PubMed] [Google Scholar]
- 7. Grigoryan L, Trautner BW, Gupta K.. Diagnosis and management of urinary tract infections in the outpatient setting: a review. JAMA 2014; 312: 1677–84. [DOI] [PubMed] [Google Scholar]
- 8. Tandogdu Z, Wagenlehner FME.. Global epidemiology of urinary tract infections. Curr Opin Infect Dis 2016; 29: 73–9. [DOI] [PubMed] [Google Scholar]
- 9. Chin TL, McNulty C, Beck C. et al. Antimicrobial resistance surveillance in urinary tract infections in primary care. J Antimicrob Chemother 2016; 71: 2723–8. [DOI] [PubMed] [Google Scholar]
- 10. Hsueh P-R, Hoban DJ, Carmeli Y. et al. Consensus review of the epidemiology and appropriate antimicrobial therapy of complicated urinary tract infections in Asia-Pacific region. J Infect 2011; 63: 114–23. [DOI] [PubMed] [Google Scholar]
- 11. Hadi U, van den Broek P, Kolopaking EP. et al. Cross-sectional study of availability and pharmaceutical quality of antibiotics requested with or without prescription (over the counter) in Surabaya, Indonesia. BMC Infect Dis 2010; 10: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grabe M, Bartoletti R, Bjerklund Johansen TE. et al. Guidelines on Urological Infections.2015. http://uroweb.org/wp-content/uploads/19-Urological-infections_LR2.pdf.
- 13. Gupta K, Hooton TM, Naber KG. et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52: e103–20. [DOI] [PubMed] [Google Scholar]
- 14. Ikatan Ahli Urologi Indonesia (IAUI). Penatalaksanaan Infeksi Saluran Kemih dan Genitalia Pria; 2015http://www.iaui.or.id/ast/file/GL_ISK_2015.pdf.
- 15. Hadi U, Qiptiyah M, Paraton H.. Problem of antibiotic use and antimicrobial resistance in Indonesia: are we really making progress? Indones J Trop Infect Dis 2013; 4: 5–8. [Google Scholar]
- 16. Laupland KB, Ross T, Pitout JDD. et al. Investigation of sources of potential bias in laboratory surveillance for anti-microbial resistance. Clin Invest Med 2007; 30: E159–66. 1 [DOI] [PubMed] [Google Scholar]
- 17. Rempel OR, Laupland KB.. Surveillance for antimicrobial resistant organisms: potential sources and magnitude of bias. Epidemiol Infect 2009; 137: 1665–73. [DOI] [PubMed] [Google Scholar]
- 18. Schultsz C, Lan NPH, Van Dung N. et al. Network building and knowledge exchange with telemicrobiology. Lancet Glob Health 2014; 2: e78. [DOI] [PubMed] [Google Scholar]
- 19. Centers for Disease Control and Prevention (CDC). CDC/NHSN Surveillance Definition of Healthcare-Associated Infection and Criteria for Specific Types of Infections in the Acute Care Setting 2012. http://www.cdc.gov/nhsn/PDFs/pscManual/17pscNosInfDef_current.pdf.
- 20. Vandepitte J, Verhaegen J, Engbaeck K. et al. Basic Laboratory Procedures in Clinical Biology, 2nd edn Geneva: WHO, 2003. [Google Scholar]
- 21. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests—Eleventh Edition: Approved Standard M02-A11. CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- 22. Lu P-L, Liu Y-C, Toh H-S. et al. Epidemiology and antimicrobial susceptibility profiles of Gram-negative bacteria causing urinary tract infections in the Asia-Pacific region: 2009-2010 results from the Study for Monitoring Antimicrobial Resistance Trends (SMART). Int J Antimicrob Agents 2012; 40 Suppl: S37–43. [DOI] [PubMed] [Google Scholar]
- 23. Murugan K, Savitha T, Vasanthi S.. Retrospective study of antibiotic resistance among uropathogens from rural teaching hospital, Tamilnadu, India. Asian Pac J Trop Dis 2012; 2: 375–80. [Google Scholar]
- 24. Huttner A, Verhaegh EM, Harbarth S. et al. Nitrofurantoin revisited: a systematic review and meta-analysis of controlled trials. J Antimicrob Chemother 2015; 70: 2456–64. [DOI] [PubMed] [Google Scholar]
- 25. Sabharwal ER, Sharma R.. Fosfomycin: an alternative therapy for the treatment of UTI amidst escalating antimicrobial resistance. J Clin Diagn Res 2015; 9: DC06–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sastry S, Doi Y.. Fosfomycin: resurgence of an old companion. J Infect Chemother 2016; 22: 273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Radyowijati A, Haak H.. Improving antibiotic use in low-income countries: an overview of evidence on determinants. Soc Sci Med 2003; 57: 733–44. [DOI] [PubMed] [Google Scholar]
- 28. Van Leth F, den Heijer CDJ, Beerepoot MAJ. et al. Rapid assessment of antimicrobial resistance prevalence using a lot quality assurance sampling approach. Future Microbiol 2017; in press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.