Abstract
The degradation of benzene, toluene, ethylbenzene and xylene (BTEX) contaminants in groundwater relies largely on anaerobic processes. While the physiology and biochemistry of selected relevant microbes have been intensively studied, research has now started to take the generated knowledge back to the field, in order to trace the populations truly responsible for the anaerobic degradation of BTEX hydrocarbons in situ and to unravel their ecology in contaminated aquifers. Here, recent advances in our knowledge of the identity, diversity and ecology of microbes involved in these important ecosystem services are discussed. At several sites, distinct lineages within the Desulfobulbaceae, the Rhodocyclaceae and the Gram-positive Peptococcaceae have been shown to dominate the degradation of different BTEX hydrocarbons. Especially for the functional guild of anaerobic toluene degraders, specific molecular detection systems have been developed, allowing researchers to trace their diversity and distribution in contaminated aquifers. Their populations appear enriched in hot spots of biodegradation in situ. 13C-labelling experiments have revealed unexpected pathways of carbon sharing and obligate syntrophic interactions to be relevant in degradation. Together with feedback mechanisms between abiotic and biotic habitat components, this promotes an enhanced ecological perspective of the anaerobic degradation of BTEX hydrocarbons, as well as its incorporation into updated concepts for site monitoring and bioremediation.
Keywords: toluene, benzene, groundwater, fumarate-adding enzymes, stable isotope probing, degrader diversity
This review summarises recent advances into the identity, diversity and ecology of anaerobic degraders of monoaromatic hydrocarbons in contaminated aquifers.
INTRODUCTION: BTEX CONTAMINATION OF GROUNDWATER SYSTEMS
Contamination with aromatic hydrocarbons, specifically benzene, toluene, ethylbenzene and xylenes (BTEX), is of major concern to groundwater quality and aquifer ecosystem health. BTEX compounds are prominently placed amongst the US Agency for Toxic Substances and Disease Registry's list of priority pollutants, based on their frequency, toxicity and potential for human exposure (ATSDR 2007). BTEX contamination of the subsurface is often a legacy of petroleum or gasoline processing, transport and storage, of coal gasification, chemical hydrogenation or other industrial activities and accidents (Wilkes and Schwarzbauer 2010).
Since BTEX compounds are relatively soluble and mobile in water and either carcinogenic or neurotoxic to humans, it is relevant to understand and predict their fate in groundwater systems. BTEX contaminations typically spread as plumes, mobilised by advective transport and dispersive mixing downstream of the source (Fig. 1). However, such contaminants are known to be naturally attenuated in aquifers via processes including dilution, dispersion, sorption, abiotic transformation and most important, biodegradation. The latter is considered as the only sustainable component of natural attenuation (Kleinsteuber, Schleinitz and Vogt 2012; Meckenstock et al.2015).
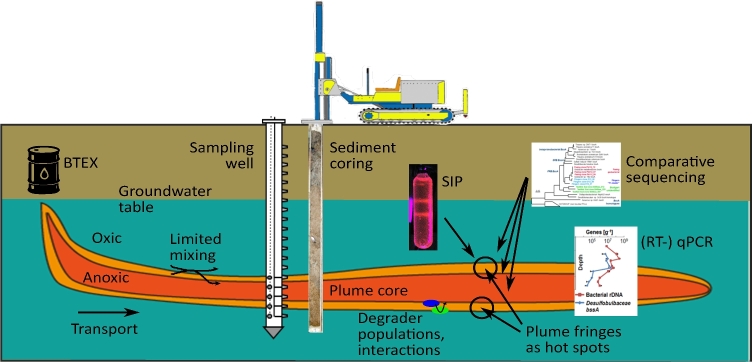
Figure 1.
Conceptual view of longitudinal plume zonation in a BTEX-contaminated aquifer. The plume core is depleted in electron acceptors, while degraders and degradation activities reside especially at the plume fringes. Sampling possibilities for groundwater and sediments are indicated, same as major factors influencing degrader ecology and approaches to investigate them, as discussed in the text.
In contrast to many contaminated soils and surface waters, the saturated subsurface is limited in oxygen replenishment. Hence, increased organic carbon input by contamination results in a rapid depletion of available oxygen. The maximal oxygen concentration of ∼8 mg l−1 (0.5 mM) in pristine groundwater suffices to oxidise a toluene load of ∼5 mg l−1 (∼0.05 mM), much less than what is often found at contaminated sites. Thus, contaminated aquifers are typically reduced and biodegradation depends on anaerobic processes. The classical perspective of such systems involves a reverse longitudinal redox zonation of contaminant plumes, ranging from methanogenic/fermenting conditions in zones of highest contamination to the reduction of energetically more favourable electron acceptors such as sulphate, ferric iron or nitrate along the flow path (Christensen et al.2000). While this perspective has been developed towards a greater importance of redox gradients and biodegradation at the plume fringes (Meckenstock et al.2015), anaerobic degradation and degraders of BTEX compounds for all respective respiratory processes have been reported (Table 1).
Table 1.
Thermodynamics of toluene oxidation under aerobic and anaerobic respiration and in syntrophya.
| Electron acceptor | ΔG0΄ | |
|---|---|---|
| (oxidised/reduced) | Stoichiometry | [kJ (mol C7H8)−1] |
| O2 / H2O | C7H8 + 9 O2 + 3 H2O → 7 HCO3− + 7 H+ | −3790 kJ |
| NO3−/N2 | 5 C7H8 + 36 NO3− + H+ → 35 HCO3− + 18 N2 + 3 H2O | −3555 kJ |
| Fe(OH)3/FeCO3 | C7H8 + 36 Fe(OH)3 + 29 HCO3− + 29 H+ → 36 FeCO3 + 87 H2O | −1497 kJ |
| SO42−/HS− (complete)b | 2 C7H8 + 9 SO42−+ 6 H2O → 14 HCO3− + 9 HS− + 5 H+ | −203 kJ |
| −45 kJ (mol SO42−)−1 | ||
| SO42− HS− (incomplete)b | 2 C7H8 + 3 SO42−+6 H2O → 6 CH3COO− + 2 HCO3− + 3 HS− + 5 H+ | −61 kJ |
| −41 kJ (mol SO42−)−1 | ||
| CO2/CH4 (sum) | 2 C7H8 + 15 H2O → 9 CH4 + 5 HCO3− + 5 H+ | −130 kJ |
| Fermenter | 2 C7H8 + 18 H2O → 6 CH3COO− + 2 HCO3− + 8 H+ + 12 H2 | +166 kJ |
| Hydrogenotroph | 12 H2 + 3 HCO3− + 3 H+ → 3 CH4 + 9 H2O | −203 kJ |
| Acetotroph | 6 CH3COO− + 6 H2O → 6 CH4 + 6 HCO3− | −93 kJ |
ANAEROBES CAPABLE OF OXIDISING BTEX COMPOUNDS
Early research on the degradation of BTEX hydrocarbons was mostly directed towards aerobic degraders and degradation pathways (Zylstra and Gibson 1991; Pérez-Pantoja, González and Pieper 2010). Recent years have seen a substantial increase in the number of isolated anaerobic degraders and enrichment cultures, as well as in physiological and biochemical insights into involved catabolic pathways. As these advances have been intensively reviewed (Weelink, van Eekert and Stams 2010; Widdel, Knittel and Galushko 2010; Fuchs, Boll and Heider 2011; Rabus et al.2016), it is not the intention of this article to expand on this in detail. Instead, it briefly synthesises the fundamentals to then focus on how researchers have taken this knowledge back to the field, in order to identify the microbes truly responsible for anaerobic degradation in situ and to unravel their ecology in contaminated aquifers. Specific attention is given to the study of anaerobic toluene-degrading populations, for which the most significant ecological advances have been achieved.
Cultures and enrichments
Most anaerobic degraders of monoaromatic compounds isolated to date are members of the Betaproteobacteria, Deltaproteobacteria and Clostridia. Under denitrification, a number of Azoarcus, Thauera and ‘Aromatoleum’ spp. and other closely related Rhodocyclaceae are known to degrade toluene and/or ethylbenzene or xylenes (see lists in Weelink, van Eekert and Stams 2010; Widdel, Knittel and Galushko 2010). Within the Alphaproteobacteria, members of the genus Magnetospirillum (Rhodospirillaceae) can also oxidise toluene while respiring nitrate (Shinoda et al.2005). Denitrifying Azoarcus and Dechloromonas strains were actually the first isolates suggested to degrade benzene under anaerobic conditions (Coates et al.2001b; Kasai et al.2006). More recent evidence from denitrifying benzene-degrading enrichments also indicates an involvement of members of the Peptococcaceae (Clostridia) in this process (van der Zaan et al.2012; Luo et al.2014).
Under iron-reducing conditions, several Geobacter spp. (Geobacteraceae) are known to degrade toluene or xylenes (Coates et al.2001a; Kunapuli et al.2010; Prakash et al.2010), as are the betaproteobacterial Georgfuchsia toluolica (Rhodocyclaceae, Weelink et al.2009) and the clostridial Desulfitobacterium aromaticivorans (Peptococcaceae; Kunapuli et al.2010). Geobacter metallireducens has also been proven to degrade benzene under iron reduction (Zhang et al.2012). All of these iron-reducing degraders of monoaromatic hydrocarbons have been isolated from terrestrial samples, which discriminates them from denitrifying degraders where only a few (Kasai et al.2006) are of non-marine origin. Anaerobic benzene degradation has also been shown for the hyperthermophilic, iron-reducing Archaeon Ferroglobus placidus (Holmes et al.2011), yet the relevance of such thermophilic degraders may be limited to very specific habitats.
Many sulphate-reducing degraders of BTEX compounds are related to Desulfosarcina, Desulfobacula, Desulfotignum spp. or to other Desulfobacteraceae and have been isolated from marine habitats (see tables in Weelink, van Eekert and Stams 2010; Widdel, Knittel and Galushko 2010). In contrast, the low number of mostly toluene-degrading sulphate reducers of terrestrial origin is phylogenetically more widespread. Here, monoaromatic hydrocarbon-degrading strains related to the deltaproteobacterial Desulforhabdus (Syntrophobacteraceae), Desulfocapsa (Desulfobulbaceae), and the clostridial Desulfotomaculum and Desulfosporosinus spp. (Peptococcaceae) have been isolated (Beller et al.1996; Meckenstock 1999; Liu et al.2004; Morasch et al.2004). The latter were the first non-proteobacterial anaerobic toluene degraders discovered. More recently, further sulfidogenic benzene- or TEX-degrading enrichment cultures dominated by microbes within the Peptococcaceae have been documented in samples from shallow (Kleinsteuber et al.2008; Abu Laban et al.2009) or deep contaminated aquifers (Berlendis et al.2010).
In the absence of all other electron acceptors, methanogenic oxidation of BTEX hydrocarbons occurs via syntrophy between fermenting degraders and methanogenic Archaea (Vogt, Kleinsteuber and Richnow 2011). Here, although defined co-cultures are still lacking, several highly enriched degrader cultures have been phylogenetically dissected. These cultures consistently harboured members of Peptococcaceae, Deltaproteobacteria and different hydrogenotrophic (Methanospirillum, Methanobacterium spp.) or acetotrophic (Methanosaeta spp.) methanogens, irrespective of whether they degraded toluene or benzene (Ficker et al.1999; Ulrich and Edwards 2003; Fowler et al.2012). Detailed functional insights into these enrichment cultures, e.g. as obtained by the use of stable isotope probing (SIP), will be discussed in later sections.
Degradation pathways
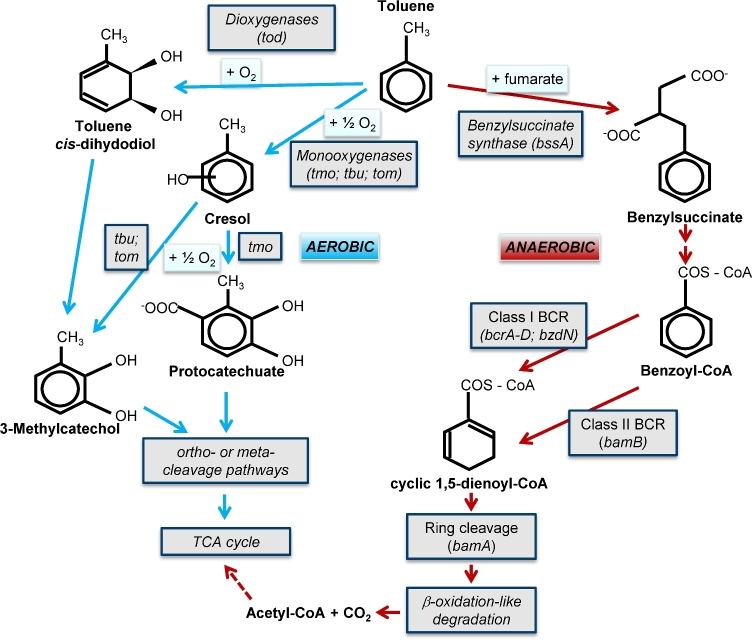
No matter which oxidant is respired, all degradation pathways of aromatic compounds proceed via conserved peripheral and central catabolic routes (Fuchs, Boll and Heider 2011). That is, the aerobic degradation of toluene proceeds via the initial addition of hydroxyl groups by distinct mono- or dioxygenases (Pérez-Pantoja, González and Pieper 2010), relying on molecular oxygen as a co-substrate to destabilise the aromatic ring (Fig. 2). Under anoxic conditions, the initial enzymatic attack of toluene is catalysed by the enzyme benzylsuccinate synthase (Bss), adding the methyl group of toluene to fumarate (so-called fumarate addition) forming benzylsuccinate (Fuchs, Boll and Heider 2011; Rabus et al.2016). This enzymatic step involves a glycyl-radical mechanism, explaining how chemically stable aromatic compounds such as toluene can be activated in the absence of molecular oxygen. Benzylsuccinate is then further activated as a CoA-thioester, which is then transformed, via several intermediates, to benzoyl-CoA. The latter is an important central intermediate in the anaerobic catabolism of aromatic compounds, funnelling metabolites from diverse peripheral degradation pathways into aromatic ring reduction, ring cleavage and finally a modified β-oxidation to acetyl-CoA (Fuchs, Boll and Heider 2011; Rabus et al.2016). The first step of ring reduction can either be conducted by an ATP-dependent class I benzoyl-CoA reductase (BcrA-D) or an ATP-independent class II benzoyl-CoA reductase (BamB-I; Fuchs, Boll and Heider 2011). The former is typically found within facultative anaerobes, while the latter is hosted mostly by strict anaerobes and fermenters (Boll et al.2014). Subsequent ring cleavage by 6-oxocyclohex-1-ene-1-carbonyl-CoA hydrolase (BamA) is followed by β-oxidation-like reactions to acetyl-CoA for assimilation or complete oxidation to CO2 (Fig. 2). The degradation of xylenes and ethylbenzene also often involves fumarate addition as initial activation, though an alternative oxygen-independent hydroxylation has been reported for several denitrifying ethylbenzene degraders (Rabus et al.2016).
Figure 2.
Initial activating reactions, peripheral and central biochemical pathways involved in the aerobic and anaerobic degradation of toluene as representative BTEX compound. Shown aerobic hydroxylation reactions represent the more conserved aerobic activation mechanisms. Important catabolic enzymes (genes) as mentioned in the text are given. Scheme synthesised from Parales et al. (2008) and von Netzer et al. (2016).
The initial enzymatic attack of benzene by anaerobic degraders is still much less understood. It may involve a carboxylation reaction to benzoate (Vogt, Kleinsteuber and Richnow 2011; Meckenstock et al.2016), which is then directly funnelled into central benzoyl-CoA catabolism. A distinct route for benzene catabolism is suggested for the facultatively anaerobic, denitrifying Dechloromonas aromatica (Coates et al. 2001). Instead of genes typically involved in anaerobic aromatics degradation, the genome of this Betaproteobacterium surprisingly contains several oxygenase genes usually found in aerobic aromatics degraders (Salinero et al.2009). This has given rise to the hypothesis of an oxygen-dependent, oxygenase-mediated attack of benzene under nitrate-reducing conditions (Weelink, van Eekert and Stams 2010). The activation mechanism, driven by self-sustained oxygenesis, can be considered analogous to methane oxidation as proposed for the NO-dismutating Candidatus Methylomirabilis oxyfera (Ettwig et al.2010), or to the oxygenase-dependent activation of benzene coupled to chlorite dismutation suggested for D. aromatica (Coates et al.2001b) and Alicycliphilus denitrificans (Weelink et al.2008). However, nitrate is a much more ubiquitous electron acceptor in groundwater than chlorite. In summary, anaerobic degraders of BTEX hydrocarbons have evolved a host of catabolic pathways allowing them to capitalise on hydrocarbons in the environment. However, only few of the involved genes and mechanisms have actually been targeted to trace respective populations and activities in the field.
ANAEROBIC DEGRADERS OF BTEX HYDROCARBONS IN SITU
Over approximately the last decade, researchers have made intensive use of the so-called molecular ecology toolbox to trace anaerobic degraders of monoaromatic hydrocarbons in contaminated aquifers. An unambiguous identification of key players relevant at a distinct site allows elucidating their ecology and potential bottlenecks of biodegradation in situ. There are two major strategies: one involves the detection and phylogenetic affiliation of catabolic biomarkers (genes, transcripts, potentially proteins) in the field, including their quantification. The strength of this approach is that field samples are directly analysed, without prior laboratory handling. A drawback is that prior sequence knowledge of catabolic markers is essential for their detection. The second strategy is based on isotopic labelling of biomarkers (cellular lipids, nucleic acids, proteins, cells) via labelled contaminants, and the subsequent identification of labelled degraders. While this approach, due to the scarcity of biomass in aquifer samples, often requires laboratory incubation (and degrader enrichment to a certain extent), prior knowledge on degrader identity and involved catabolic pathways is not essential. Ideally, both directed (marker-based) and undirected (labelling-assisted) approaches should be combined to functionally and phylogenetically dissect degrader populations at a given site.
Catabolic markers
Anaerobic degraders of BTEX aromatics are a catabolically defined but phylogenetically diverse functional guild, which cannot be comprehensively targeted via ribosomal markers. Thus, some of the conserved key enzymes in BTEX catabolism and their respective genes have been established as catabolic markers for degrader populations in situ. Most prominently, the benzylsuccinate synthase alpha-subunit (bssA) and related fumarate-adding enzyme (FAE) genes are applied for the detection of anaerobic alkylbenzene degraders (von Netzer et al.2016). Initially introduced as a qPCR assay for denitrifying toluene degraders within the Betaproteobacteria (Beller et al.2002), increasing pure culture sequence availability has allowed for the development and continuous improvement of primers and detection assays for degraders active in iron-reducing, sulphate-reducing and methanogenic environments. A comprehensive overview of the assays available is beyond the scope of this review and published elsewhere (von Netzer et al.2016).
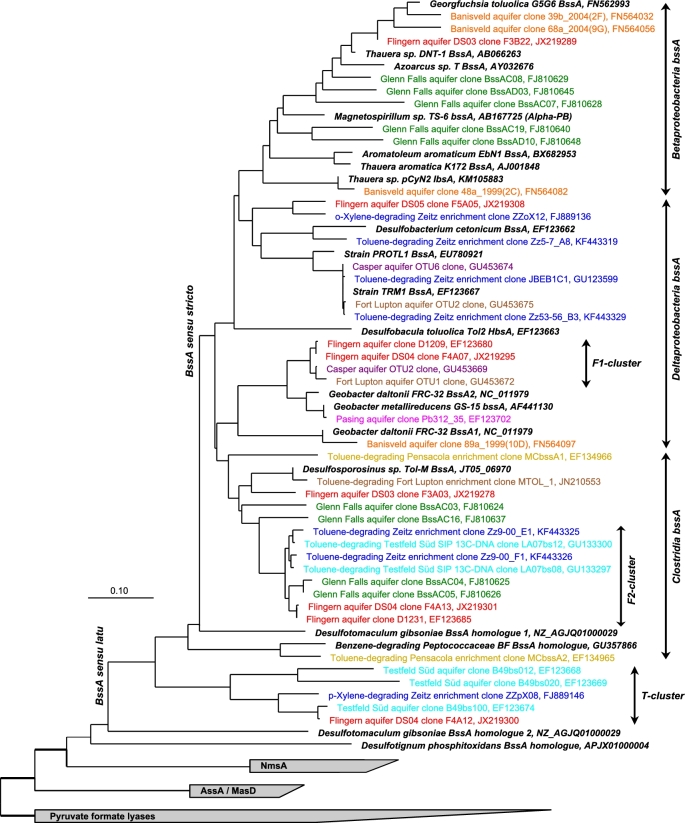
In the field, targeted catabolic gene approaches for anaerobic toluene degradation were first applied for a number of tar oil-contaminated aquifers in Germany (Winderl, Schaefer and Lueders 2007). The authors revealed several unidentified and potentially site-specific degrader lineages, especially at sites with sulphate reduction (Fig. 3). The novel ‘F1-cluster’ bssA genes, more closely related to geobacterial bssA than that of other sulphate reducers, dominated a former gasworks site in Flingern, and was quantitatively enriched at the sulfidogenic lower fringe of the toluene plume (Winderl et al.2008). This suggested a potential of catabolic marker quantification to identify hot spots of biodegradation in situ. However, more deeply branching bssA homologues of unclear affiliation were also recovered, such as the ‘T-cluster’ detected at the Testfeld Süd aquifer near Stuttgart (Winderl, Schaefer and Lueders 2007). The diversity of bssA genes and homologues was next analysed at the hydrocarbon-contaminated Fort Lupton (Colorado) and Casper (Wyoming) aquifers (Callaghan et al.2010). At both sites, specific populations of mostly desulfobulbal bssA sequence types were identified (Fig. 3). A considerable diversity of both betaproteobacterial and more deeply branching bssA genes was found at the coal tar-contaminated South Glens Falls aquifer, New York (Yagi et al.2010). In contrast, rather limited diversities of mostly betaproteobacterial bssA sequence types were found enriched within a landfill-leachate plume at the Banisveld aquifer, the Netherlands, most of them related to Georgfuchsia toluolica (Staats, Braster and Röling 2011). In fact, this iron reducer had originally been isolated from the same site (Weelink et al.2009), thus strengthening the link between studies performed in lab and field.
Figure 3.
Overview of the phylogeny of bssA genes and close homologues for FAEs. Environmental sequences retrieved from different hydrocarbon-contaminated aquifers and enrichment cultures are given in different colours. Major gene lineages as mentioned in the text are named. Outgroup: related pyruvate formate lyase genes. Tree adapted from and reconstructed as in von Netzer et al. (2013, 2016).
A qPCR assay designed to detect bssA genes of sulphate-reducing and syntrophic alkylbenzene degraders was used to monitor degrader abundance and dynamics in two artificially contaminated well galleries at the Vandenberg Air Force Base, California, one of them additionally amended with ethanol (Beller et al.2008). Higher comparative bssA abundance was observed under ethanol amendment, indicating that this could stimulate degrader abundance during bioremediation. Comparative qPCR assays for bssA of betaproteobacterial and deltaproteobacterial degraders were applied to different monitoring wells of a former coal gasification plant in Glassboro, New Jersey, showing that degrader abundance was enriched by several orders of magnitude in central zones of the plume (Oka et al.2011).
Genes for the central enzymes of anaerobic monoaromatic compound degradation have also been employed to monitor degraders in the field. Hence, a substantial diversity of class I benzoyl-CoA reductase (bcrA) genes was first reported for a gasoline-contaminated aquifer in Kumamoto, Japan (Hosoda et al.2005). Distinct gene pools were found in different wells and degrader abundance appeared linked to the level of contamination. Similarly, Fahrenfeld et al. (2014) have reported a quantitative enrichment of class I BCR (bzdN) genes of facultative anaerobes close to the crude oil spill at the Bemidji aquifer, Minnesota. At the Banisveld site, genes for the aromatic ring-cleaving hydrolase (bamA) were monitored parallel to bssA (Staats, Braster and Röling 2011). While the gene pool of bamA was considerably more diverse than that of bssA, it was surprisingly more abundant outside the plume. A comprehensive qualitative assessment of catabolic gene pools for monoaromatics degradation (bcrC, bamB and bamA) has been performed for samples from two aquifers with high (Ruhr area) or low (Gneisenau) benzene contamination in Germany (Kuntze et al.2011). The differentiation between mostly beta- and deltaproteobacterial degraders was largely consistent for both sites.
Apart from these central enzymes, studies tracing peripheral markers of benzene degradation, such as anaerobic benzene carboxylase (AbcDA, Abu Laban et al.2010; Holmes et al.2011), have not been published to date. While such assays should certainly be developed, the detection of central catabolic markers remains an essential tool to characterise benzene degraders in anoxic aquifers. It must be noted, however, that benzoyl-CoA is a central intermediate not only in the anaerobic degradation of BTEX hydrocarbons, but also in the degradation of humics, aromatic amino acids and lignins (Porter and Young 2014). Therefore, the detection of such central markers may not always be strictly linked to populations actually involved in anaerobic aromatic hydrocarbon degradation.
Obviously, all of these gene assays have also been applied to aromatics-degrading laboratory microcosms and enrichments. The detectability of betaproteobacterial bssA was first reported for toluene-degrading aquifer enrichments under denitrification (Beller et al.2002). An unidentified betaproteobacterial bssA lineage was first reported for iron-reducing, toluene-degrading enrichments from the Banisveld aquifer (Botton and Parsons 2007) and later identified to represent the novel isolate G. toluolica (Weelink et al.2009). BssA genes of desulfobulbal affiliation were detected in both toluene- and xylene-degrading sulfidogenic enrichments from the BTEX-contaminated Zeitz aquifer, Germany (Herrmann et al.2009; Jehmlich et al.2010). Deeply branching bssA homologues related to the ‘T-cluster’ FAE genes first detected in the field (Fig. 3) were also reported for the xylene microcosms, again demonstrating vital feedbacks between research performed in lab and field.
The expression of a novel and deeply branching bssA phylotype was first demonstrated by Washer and Edwards (2007) for a methanogenic, toluene-degrading enrichment from the Pensacola aquifer, Florida. In this culture, Desulfotomaculum spp. had been identified as an important component, giving a first hint at the phylogenetic placement of clostridial bssA (Fig. 3). More recently, related bssA lineages of members of the Peptococcaceae have also been reported for a methanogenic, toluene-degrading enrichment from the Fort Lupton aquifer (Fowler et al.2012, 2014) and a number of peptococcal genomes (Poehlein, Daniel and Simeonova 2013; Kuever et al.2014; Abu Laban et al.2015). This substantiates the affiliation of these deeply branching bssA sequence types. Evidence for niche partitioning between distinct bssA gene pools has been elaborated for sulfidogenic toluene-degrading enrichments from the Zeitz aquifer (Kuppardt et al.2014). Here, desulfobulbal bssA dominated enrichments from less contaminated zones, while peptococcal bssA was found in those from highly contaminated inocula. For the comprehensive detection of such peptococcal bssA genes and other more deeply branching FAE genes, optimised primer systems have recently been developed (von Netzer et al.2013).
Amongst the different central catabolic markers, aromatic ring-cleaving bamA hydrolase genes have been detected in a number of sulphate-reducing enrichments from the Zeitz aquifer (Kuntze et al.2008). More recently, the expression of benzene carboxylase transcripts and no less than three distinct pathways for benzoyl-CoA catabolism were proven for nitrate-reducing enrichments from a gasoline-contaminated aquifer in Toronto, Canada (Luo et al.2014). Taken together, the studies summarised here demonstrate how catabolic marker gene detection in lab and field can provide valuable cues for the interpretation of degrader identity and diversity in contaminated systems.
Isotopic labelling of degraders
A second major strategy to trace degraders of BTEX hydrocarbons in contaminated aquifers relies on the isotopic labelling of biomarkers via labelled substrates. This so-called SIP approach can be performed for different cellular biomarkers such as phospholipid fatty acids, nucleic acids, proteins and even entire cells and is widely applied in lab and field. A detailed comparison of the strengths and limitations of the different methodologies and their application to anaerobic hydrocarbon degraders is beyond the scope of this review, but available elsewhere (Vogt et al.2016). Nucleic acid- and protein-based SIP especially have been utilised to characterise anaerobic degraders of BTEX pollutants in aquifer materials, or in related laboratory enrichments. While the results of the first may be more directly linked to processes in the field, the probing of cultures can provide more detailed insights into interactions and carbon sharing within degrader communities (Kleinsteuber, Schleinitz and Vogt 2012; Vogt et al.2016).
For groundwater samples, rRNA-SIP was first applied to identify denitrifying benzene degraders at a gasoline-contaminated aquifer in Kumamoto, Japan (Kasai et al.2006). The identified betaproteobacterial degraders belonged to the genus Azoarcus and could be subsequently isolated in pure culture. Anaerobic benzene degraders were also traced by DNA-SIP at the coal tar-contaminated South Glens Falls aquifer, New York (Liou, DeRito and Madsen 2008). Sediment microcosms incubated under comparative electron acceptor amendments and direct labelling in the field revealed a remarkable diversity of labelled taxa. Most importantly, the betaproteobacterial Pelomonas spp. was consistently labelled. In a follow-up study combining DNA-SIP and metagenomics, denitrifying toluene degraders from the same site were identified as Herminiimonas spp. (Betaproteobacteria), providing a wealth of detail on the catabolic potentials and adaptations of these degraders to the site (Kim et al.2014).
Sulphate-reducing toluene degraders related to Desulfosporosinus spp. were first identified by DNA-SIP in samples from the tar oil-contaminated Testfeld Süd aquifer (Winderl et al.2010). Via bssA fragments retrieved from 13C-labelled DNA, the authors provided labelling-assisted evidence for the peptococcal affiliation of the previously unidentified F2-cluster bssA (Fig. 3). Similarly, DNA-SIP suggested the F1-cluster bssA genes first detected at the Flingern site to be linked to degraders within the Desulfobulbaceae (Pilloni et al.2011). This was surprising, given the closer affiliation of this lineage with geobacterial bssA than that of other Desulfobulbaceae (Fig. 3) and suggests a possible role of horizontal transfer in local community adaptation to pollution.
Other researchers have used SIP to identify degraders in laboratory cultures previously enriched from contaminated aquifers, often maintained in the lab for a considerable time span. In sulphate-reducing enrichments form the Zeitz aquifer, DNA-SIP identified members of the Desulfobulbaceae as key toluene degraders (Bombach et al.2010), and distinct Peptococcaceae (Pelotomaculum and Cryptoanaerobacter spp.) as primary oxidisers of benzene (Herrmann et al.2010). In a pioneering follow-up study involving protein-SIP, the role of Peptococcaceae was also confirmed via labelled peptides (Taubert et al.2012). This approach led to a conceptual model of organic and inorganic carbon flow between primary degraders and secondary community members, as will be discussed further down. Protein-SIP has also been used to probe the degradation of m-xylene in a sulphate-reducing enrichment from the Zeitz aquifer (Bozinovski et al.2012). Peptides of proteins involved in sulphate reduction, xylene oxidation and C1 metabolism in members of the Desulfobacteraceae were identified as 13C labelled. Finally, the aforementioned methanogenic, toluene-degrading enrichment culture from the Fort Lupton aquifer has recently been dissected using rRNA-SIP (Fowler et al.2014). Here, Desulfosporosinus spp. was revealed as most important assimilator of 13C label.
One general finding of the above SIP studies was the low efficiency of assimilation of 13C from fully labelled BTEX compounds by strictly anaerobic degraders. Typically, this was only ∼50% (Bombach et al.2010; Herrmann et al.2010; Winderl et al.2010; Pilloni et al.2011; Taubert et al.2012), pointing at substantial ratios of assimilated carbon not stemming from the contaminant. This was also directly proven by comparative SIP labelling of the above sulphate-reducing, benzene-degrading enrichment with 13CO2 (Rakoczy et al.2011). This substantial heterotrophic CO2 fixation has been attributed to the fact that strictly anaerobic degraders within the Deltaproteobacteria or Peptococcaceae typically lack the glyoxylate cycle and thus assimilate acetyl-CoA through reductive carboxylation (Winderl et al.2010). Alternatively, evidence for parallel ongoing CO2 fixation by key degraders via the Wood-Ljungdahl pathway has also been provided (Taubert et al.2012). These exceptionally high ratios of inorganic carbon assimilation during heterotrophic contaminant degradation need to be taken into account in the interpretation of labelling results for strictly anaerobic degraders, especially if secondary carbon sharing also occurs.
In summary, this SIP work suggests not only a primary relevance of distinct Beta- and Deltaproteobacteria in BTEX-contaminated aquifers, but also of the Peptococcaceae. This is consistent with labelling results obtained for samples from other terrestrial systems, such as contaminated soils and sediments (Kunapuli, Lueders and Meckenstock 2007; Sun and Cupples 2012; van der Zaan et al.2012; Sun, Sun and Cupples 2014) or oil sands (Abu Laban, Dao and Foght 2015). Besides primary degraders, these studies have also revealed a complex and often overlapping accessory microbiome, including other Deltaproteobacteria, Epsilonproteobacteria, Bacteroidetes and also Archaea. Recent insights into the patterns of carbon and electron sharing in this ‘team play’ of anaerobic aromatics degradation (Kleinsteuber, Schleinitz and Vogt 2012) will be discussed in the following.
ECOLOGICAL CONTROLS OF DEGRADER POPULATIONS AND THEIR ACTIVITY IN SITU
Substrate concentrations
As summarised in the previous section, diverse groups of anaerobic degraders of BTEX aromatics have been identified as relevant in contaminated aquifers. A discussion of their ecology as well as the controls of their activities in situ thus becomes possible, including interactions and feedback mechanisms between degraders and their abiotic as well as biotic environment. Bioavailability is not considered a central limiting factor in the degradation of BTEX compounds due to their good water solubility. In contrast, lower threshold concentrations for anaerobic degradation of monoaromatic hydrocarbons have only rarely been addressed, both in pure culture and in degrader communities. Toluene can be ubiquitous in non-contaminated aquatic systems (in nM background concentrations) due to its formation during the anaerobic degradation of aromatic amino acids (Fischer-Romero, Tindall and Jütner 1996). Threshold concentrations for degradation in sediment microcosms have been reported in the range of ∼1 μM for benzene and ethylbenzene, while they were at least two orders of magnitude lower for toluene and xylenes (Cozzarelli et al.2010). Still, it is unknown whether high-affinity degraders actually exist. Rather, BTEX utilisation at low concentration is believed to occur in cometabolism, e.g. under the simultaneous utilisation of all substrates potentially available to a given degrader. For two anaerobic toluene degraders, it has been shown that a variety of uptake systems or enzymes involved in the catabolism of diverse aromatic and aliphatic substrates (not actually present in the cultures) were upregulated during slow growth under substrate limitation (Trautwein et al.2012; Marozava et al.2014). This can be seen as an ‘alertness’ of the degraders to consume additional substrates, should they transiently become available.
At high BTEX concentrations, toxic effects on aquifer microbes including degraders are possible. As for other hydrocarbons, BTEX compounds tend to accumulate in cellular membranes, increasing membrane fluidity (Heipieper and Martínez 2010). For a number of typical anaerobic degraders, effective concentrations resulting in 50% growth inhibition (EC50) have been reported in the range of ∼0.2 mM for ethylbenzene and xylene, ∼0.5 mM for toluene and 1.5 mM for benzene (Duldhardt et al.2007). Although the solubility of BTEX compounds in complex mixtures often found at contaminated sites is lowered, maximal pollutant concentrations above this range have been reported (Winderl et al.2008; Tischer et al.2013; Kuppardt et al.2014). Thus, toxic effects on in situ degrader populations have to be taken into account, but require further elucidation.
Electron acceptors
The availability of electron acceptors is widely recognised as a critical control for anaerobic degrader activity (Meckenstock et al.2015). The high organic carbon loads in hydrocarbon plumes can be sufficient to deplete all electron acceptors available in the water or sediment matrix. Although methanogenic degradation of monoaromatics is thermodynamically feasible (Table 1) and has been reported for a number of laboratory enrichments (Edwards and Grbić-Galić 1994; Ficker et al.1999; Ulrich and Edwards 2003; Fowler et al.2012), methanogenic degrader ecology has rarely been studied in the field (Tischer et al.2013; Fahrenfeld et al.2014). The comparative contribution of methanogenic degradation to overall biodegradation processes has been questioned (Bekins et al.2001), but methanogenic contaminated aquifers are widespread and certainly await a more fundamental investigation. Especially, possible constraints of groundwater flow on the obligate syntrophic interactions involved remain to be elaborated.
Even if electron acceptors are mostly depleted in the plume core, they are continuously replenished with groundwater flow by advective and dispersive mixing at the plume fringes. This very limited mixing of electron donors and acceptors in porous media has been recognised as one of the key constraints of anaerobic hydrocarbon degradation in situ (Meckenstock et al.2015). If sampled at appropriate resolution, contaminant plumes are indeed characterised by steep vertical counter gradients of electron acceptors and donors at the fringes (van Breukelen and Griffioen 2004; Tuxen, Albrechtsen and Bjerg 2006; Anneser et al.2008; Tischer et al.2013), giving rise to characteristic hot spots, or rather hot zones of biodegradation in situ. Especially at the Flingern site, substantial work has been done to characterise these hot spots, revealing multiple lines of evidence for enhanced biodegradation activity and a quantitative enrichment of specific degrader lineages, respective catabolic genes and metabolites at the plume fringes (Winderl et al.2008; Jobelius et al.2010; Larentis, Hoermann and Lueders 2013; Einsiedl et al.2015). Degrader enrichment also correlated with a decreased overall community diversity in hot spots (Winderl et al.2008), which was in line with the perspective that a high functional organisation of microbial communities can support a most efficient catalysis of specific biogeochemical processes (Marzorati et al.2008).
Degrader diversity
General ecological theory applied to microbial systems suggests that overall community diversity and evenness should be directly related to functional stability upon disturbance (Shade et al.2012). Moreover, the number of functionally redundant species specifically (or potentially) active in a given process can be a measure of system resistance and resilience against disturbance (Konopka 2009). Overall microbial diversity in BTEX plumes has been frequently characterised, and several studies report clear shifts in microbial diversity between plume zones and even increased diversity in highly contaminated samples (Feris et al.2004; Fahy et al.2005; Staats, Braster and Röling 2011; Tischer et al.2013; Fahrenfeld et al.2014). However, highly selected catabolic gene pools and thus a low functional redundancy of anaerobic toluene degraders have also been reported for several sites (Winderl, Schaefer and Lueders 2007; Callaghan et al.2010; Staats, Braster and Röling 2011). For these apparently ‘specialised’ degrader communities, a low functional stability upon habitat disturbance could be implied. Although aquifers are classically considered as steady-state habitats, the impact of hydraulic fluctuations and resulting rearrangements in contaminant distribution and biogeochemical regime are considered a key unknown in aquifer restoration (Qiu 2011). Haack et al. (2004) showed that recharge-connected variations in the groundwater table can be connected to profound rearrangements of overall microbial community structure and to a loss of diversity in specific zones of the BTEX-contaminated Wurtsmith aquifer, Michigan. Although not for a BTEX plume, significant changes of aquifer microbiota driven by fluctuating river water intrusions and related nutrient and electron acceptor fluxes have been reported for the uranium-contaminated Hanford aquifer, Washington (Lin et al.2012). Such couplings between hydraulic and biogeochemical habitat dynamics and the activity of degrader populations should be investigated more extensively in the future. For sites where a larger diversity of bssA genes was found (Yagi et al.2010), it is still unclear whether this indicates functional redundancy amongst degraders, or whether this simply reflects a more complex setting of contamination or redox scenarios at the given site. Indeed, substrate-dependent clustering of bssA gene pools has been reported (Acosta-González, Rosselló-Móra and Marqués 2013; Jarling et al.2015).
Respiratory versatility
One mechanism for degrader populations to cope with redox fluctuations would be the utilisation of variable electron acceptors. It is interesting to consider that although all known denitrifying toluene degraders are facultative anaerobes, only two strains have been reported to host both aerobic and anaerobic catabolic pathways (Shinoda et al.2004; Martín-Moldes et al.2015). For a facultative anaerobic degrader population established at the fringe of a BTEX plume, it would be advantageous to utilise either aerobic or anaerobic catabolism depending on oxygen availability. However, since nature does not seem to have evolved many such degraders, other adaptive mechanisms could be in place. Potentially, degrader niches at fluctuating oxic/anoxic redox gradients could be filled by ‘aerobic’ degraders alone. The discovery of the denitrifying methanotroph ‘Methylomirabilis oxyfera’ isolated from a Dutch canal (Ettwig et al.2010) proposes a possible mechanism. Self-sustained oxygenesis via NO dismutation allows this microbe to oxidise methane using aerobic catabolic pathways in redox compartments where molecular oxygen is depleted, but where nitrate or nitrite are available. An analogous monooxygenase-dependent catabolism of alkanes has been suggested for the denitrifying Gammaproteobacterium HdN1 (Zedelius et al.2011) and for the benzene-degrading denitrifying Dechloromonas aromatic (Weelink, van Eekert and Stams 2010). Such physiology could perhaps explain the surprisingly high abundance of toluene monooxygenase genes detected in the anoxic plume core of the BTEX-contaminated Flingern aquifer (Larentis, Hoermann and Lueders 2013).
Furthermore, the discovery of ‘cable bacteria’, i.e. marine Desulfobulbaceae capable of coupling sulphide oxidation to oxygen respiration via filamentous electron transfer over centimetre distances (Pfeffer et al.2012) may have implications for contaminated aquifers. Members of the Desulfobulbaceae have not only been identified as dominating anaerobic degrader populations at a number of BTEX-contaminated sites (Bombach et al.2010; Callaghan et al.2010; Pilloni et al.2011; Sun and Cupples 2012), they have also recently been shown capable of long-distance electron transfer in Flingern aquifer sediments (Müller et al.2016). Besides an indirect role in electron acceptor recycling via electrogenic sulphide oxidation, it remains to be shown whether cable bacteria could also have a direct role in hydrocarbon degradation at plume fringes. This adaptation would allow classical sulphate-reducing degraders to extend their habitat over oxic/anoxic redox gradients, and to maintain anaerobic BTEX catabolism while actually respiring oxygen and thus exploiting much more favourable thermodynamics (Table 1). Apart from these recent discoveries, deltaproteobacterial Geobacter spp. are also known for their versatile use of different anaerobic electron acceptors including metal oxides, nitrate and humics (Coates et al.2001a). This may support their activity under varying redox conditions in situ.
Sharing of labour
Syntrophic interactions and other mechanisms of carbon and electron sharing are recognised as a key feature in anaerobic hydrocarbon degradation, not only in methanogenic systems (Weelink, van Eekert and Stams 2010; Kleinsteuber, Schleinitz and Vogt 2012; Gieg, Fowler and Berdugo-Clavijo 2014). Ulrich and Edwards (2003) reported that several benzene-degrading enrichments could readily switch between sulphate-reducing and methanogenic conditions, suggesting that identical initial degraders were active in both. A role of syntrophy has also been inferred via SIP for several iron-reducing (Kunapuli, Lueders and Meckenstock 2007), sulphate-reducing (Herrmann et al.2010; Taubert et al.2012) and even denitrifying (van der Zaan et al.2012) benzene-degrading enrichments. All were dominated by primary degraders within the Peptococcaceae, and their sharing of hydrogen or electrons with syntrophic partners within the Deltaproteobacteria or Rhodocyclaceae is suggested. The latter culture could also rapidly switch to the reduction of iron or sulphate, suggesting that the same initial benzene degraders could interact with different partners (van der Zaan et al.2012).
It is not yet fully understood whether the primary degraders in such consortia essentially act as fermenters, as incomplete substrate oxidisers or even a mixture of both. The former would be supported by the lack of dissimilatory sulphate reduction genes recently reported for a Desulfosporosinus sp. from a toluene-degrading methanogenic enrichment culture (Abu Laban, Dao and Foght 2015; Abu Laban et al.2015). As shown in Table 1, the incomplete oxidation of toluene to acetate by a sulphate-reducing degrader would also be thermodynamically feasible, especially if calculated per mol of sulphate, much more than the fermentation to acetate and hydrogen. Potentially, incomplete oxidation could be more sustainable in habitats with limited electron acceptor supply such as contaminant plume fringes. However, true syntrophy with sharing of hydrogen (or electrons), acetate and other fermentation products is also supported for some of the above enrichment cultures. Inhibitory effects of both hydrogen and acetate amendment on anaerobic benzene degradation have been shown (Rakoczy et al.2011; van der Zaan et al.2012) and respective protein-SIP work provides evidence for a role of acetate as shared metabolite (Taubert et al.2012; Starke et al.2016). A role of syntrophy in toluene oxidation has also been suggested for systems dominated by Geobacteraceae (Botton et al.2007). Here, Meckenstock (1999) reported that Geobacter can degrade toluene in syntrophy with a fumarate-reducing Wollinella, despite Geobacter's inability to reduce fumarate alone. Potentially, direct interspecies electron transfer (Wang et al.2016) could also play a role in syntrophic BTEX degradation by Geobacteraceae.
Apart from direct syntrophies, indirect interactions and patterns of carbon sharing also seem to be important in the anaerobic degradation of BTEX compounds. Distinct Epsilonproteobacteria related to Sulfurovum and Sulfuricurvum spp. are notoriously detected in respective enrichments and at contaminated sites (Kleinsteuber et al.2008; Herrmann et al.2010; Pilloni et al.2011; Bozinovski et al.2012; Einsiedl et al.2015). Such Epsilonproteobacteria are typically known as chemolithoautotrophic sulphide oxidisers respiring oxygen or nitrate. While their physiology in anaerobic hydrocarbon-degrading consortia is not yet fully understood, recent evidence suggests potentials roles as acetate scavengers and in polysulfide cycling (Keller et al.2015; Starke et al.2016). Taken together, syntrophic interactions and other mechanisms of carbon and electron sharing seem to be an almost universal feature of microbial consortia active in anaerobic aromatics degradation.
BIOREMEDIATION AND AQUIFER RESTORATION
As reviewed above, considerable advances of our understanding of the diversity and ecology of anaerobic degraders of BTEX hydrocarbons in aquifers have been accomplished. It remains to be discussed how this can actually benefit the management and restoration of contaminated sites. Such benefit should be apparent on two levels: that of monitored natural attenuation and that of bioremediation. The monitoring of natural attenuation involving tools of molecular environmental microbiology is still far from routine. Wilson, Thornton and Mackay (2004) suggested that monitoring should focus on the localisation of key degradation activities in critical zones of a contaminated aquifer. Spatially resolved sampling is thus a crucial issue which may not always be readily accomplished. In today's routine monitoring schemes, depth-resolved sampling of aquifers is done at metre rather than at centimetre scales, if at all. Spatial heterogeneity is thus not assessed at relevant resolution, and hot spots of biodegradation may be missed (Meckenstock et al.2015).
Still it is clear that catabolic gene detection offers an unparalleled means to localise and quantify degraders at contaminated sites. The à priori identification of dominating degrader populations, e.g. by SIP, can be an important prerequisite (Vogt et al.2016). A considerable number of field studies have included quantitative marker gene-based monitoring of the spatial distribution or temporal development of anaerobic degraders (Hosoda et al.2005; Beller et al.2008; Winderl et al.2008; Oka et al.2011; Staats, Braster and Röling 2011; Fahrenfeld et al.2014). A direct correlation between bssA gene abundance and anaerobic toluene degradation rates has been reported for aquifer microcosms (Kazy, Monier and Alvarez 2010). However, it must be remembered that quantitative gene detection allows assessing established potentials, and not actually ongoing degradation activities. Therefore, the detection and quantification of respective catabolic gene transcripts is of special interest for the assessment and prediction of biodegradation rates (von Netzer et al.2016). The quantitative monitoring of bssA transcript-to-gene ratios has indeed been shown to reflect anaerobic toluene degradation activities in an indoor model aquifer (Brow et al.2013). However, the incorporation of more advanced metagenomic, transcriptomic or even metabolomic approaches are still far from routine in contaminated site monitoring (Callaghan 2013; Tan et al.2015).
The identification, localisation and quantification of intrinsic degraders can also guide the development of site-specific restoration strategies. Molecular monitoring results can assist targeted amendments of electron acceptors or nutrients, or help to evaluate degrader amendments in bioaugmentation (Kasai et al.2007). Any enhanced site management scheme based on direct knowledge on intrinsic degraders will be an important step towards more integrated concepts in bioremediation. Optimally, such schemes will be based on multiple lines of evidence for active degradation processes, including biogeochemical monitoring, contaminant stable isotopes, molecular markers and metabolites (Callaghan 2013; Meckenstock et al.2015; von Netzer et al.2016).
CONCLUSIONS AND PERSPECTIVES
This review summarises recent advances into the diversity and ecology of anaerobic degraders of BTEX hydrocarbons in contaminated aquifers. A number of novel or previously unrecognised degrader lineages within the Desulfobulbaceae, the Rhodocyclaceae and the Gram-positive Peptococcaceae, which are still only poorly represented within our culture collections, have been identified to dominate the degradation of monoaromatic pollutants at a range of different sites. However, it has become clear that in most settings, complex microbial interactions or even obligate syntrophic sharing of labour will be involved in anaerobic hydrocarbon degradation in situ. Thus, not only a key-player perspective, but also a microbiome perspective becomes very relevant to understand pollutant degradation processes in complex natural systems. A more systematic application of latest ‘meta-omics’ approaches directly at contaminated sites will facilitate further insights into new ecophysiologies and the microbial interaction networks involved, and how they can potentially be employed in a population-based site restoration.
The perspective of the porous medium as microbial habitat, with all its special features and limitations, is now also recognised as a key control of degrader populations and their activity. Hydraulic and geochemical regimes define windows of opportunity in which degradation can take place. However, hot spots and also hot moments of biodegradation activity are currently still only rarely considered in site monitoring and reactive transport modelling schemes. Even though BTEX compounds are typically considered as legacy contaminants and many sites have been contaminated for decades or centuries, the detailed understanding of the microbes and processes controlling their degradation which is now available make them ideal model systems to test how general ecological theories such as disturbance ecology or redundancy concepts apply to contaminated systems. This will not only help to develop stronger predictions of possible feedbacks of climate change and changing land use on groundwater quality, it will also be valuable to safeguard groundwater resources against contaminants of emerging concern. Thus, the anaerobic degradation of BTEX hydrocarbons will continue to serve as a prime model system to generate a generic ecological and mechanistic understanding of contaminant degradation processes in groundwater.
Acknowledgments
The author wishes to thank the European Research Council (ERC; Grant 616644), the Deutsche Forschungsgemeinschaft (DFG; Grants LU 1188/4-1 and -2), as well as the Helmholtz Society for financial support during the writing of this review and of own research covered. The author is also grateful to Lauren Bradford (Helmholtz Zentrum München) for English language editing.
Conflict of interest. None declared.
REFERENCES
- Abu Laban N, Dao A, Foght J. DNA stable-isotope probing of oil sands tailings pond enrichment cultures reveals different key players for toluene degradation under methanogenic and sulfidogenic conditions. FEMS Microbiol Ecol 2015;91:fiv039. [DOI] [PubMed] [Google Scholar]
- Abu Laban N, Selesi D, Jobelius C et al. . Anaerobic benzene degradation by Gram-positive sulfate-reducing bacteria. FEMS Microbiol Ecol 2009;68:300–11 [DOI] [PubMed] [Google Scholar]
- Abu Laban N, Selesi D, Rattei T et al. . Identification of enzymes involved in anaerobic benzene degradation by a strictly anaerobic iron-reducing enrichment culture. Environ Microbiol 2010;12:2783–96 [DOI] [PubMed] [Google Scholar]
- Abu Laban N, Tan B, Dao A et al. . Draft genome sequence of uncultivated Desulfosporosinus sp. strain Tol-M, obtained by stable isotope probing Using [13C6]-toluene. Genome Announc 2015;3:e01422–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-González A, Rosselló-Móra R, Marqués S. Diversity of benzylsuccinate synthase-like (bssA) genes in hydrocarbon-polluted marine sediments suggests substrate-dependent clustering. Appl Environ Microb 2013;79:3667–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anneser B, Einsiedl F, Meckenstock RU et al. . High-resolution monitoring of biogeochemical gradients in a tar oil-contaminated aquifer. Appl Geochem 2008;23:1715–30 [Google Scholar]
- ATSDR Agency for Toxic Substances and Disease Registry (ATSDR). CERCLA Priority List of Hazardous Substances Atlanta, GA:U.S. Department of Health and Human Services, Public Health Service, 2007 [Google Scholar]
- Bekins BA, Cozzarelli IM, Godsy EM et al. . Progression of natural attenuation processes at a crude oil spill site: II. Controls on spatial distribution of microbial populations. J Contam Hydrol 2001;53:387–406 [DOI] [PubMed] [Google Scholar]
- Beller HR, Kane SR, Legler TC et al. . A real-time polymerase chain reaction method for monitoring anaerobic, hydrocarbon-degrading bacteria based on a catabolic gene. Environ Sci Technol 2002;36:3977–84 [DOI] [PubMed] [Google Scholar]
- Beller HR, Kane SR, Legler TC et al. . Comparative assessments of benzene, toluene, and xylene natural attenuation by quantitative polymerase chain reaction analysis of a catabolic gene, signature metabolites, and compound-specific isotope analysis. Environ Sci Technol 2008;42:6065–72 [DOI] [PubMed] [Google Scholar]
- Beller HR, Spormann AM, Sharma PK et al. . Isolation and characterization of a novel toluene-degrading, sulfate-reducing bacterium. Appl Environ Microb 1996;62:1188–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlendis S, Lascourreges J-F, Schraauwers B et al. . Anaerobic biodegradation of BTEX by original bacterial communities from an underground gas storage aquifer. Environ Sci Technol 2010;44:3621–8 [DOI] [PubMed] [Google Scholar]
- Boll M, Löffler C, Morris BEL et al. . Anaerobic degradation of homocyclic aromatic compounds via arylcarboxyl-coenzyme A esters: organisms, strategies and key enzymes. Environ Microbiol 2014;16:612–27 [DOI] [PubMed] [Google Scholar]
- Bombach P, Chatzinotas A, Neu TR et al. . Enrichment and characterization of a sulfate-reducing toluene-degrading microbial consortium by combining in situ microcosms and stable isotope probing techniques. FEMS Microbiol Ecol 2010;71:237–46 [DOI] [PubMed] [Google Scholar]
- Botton S, Parsons J. Degradation of BTX by dissimilatory iron-reducing cultures. Biodegradation 2007;18:371–81 [DOI] [PubMed] [Google Scholar]
- Botton S, van Harmelen M, Braster M et al. . Dominance of Geobacteraceae in BTX-degrading enrichments from an iron-reducing aquifer. FEMS Microbiol Ecol 2007;62:118–30 [DOI] [PubMed] [Google Scholar]
- Bozinovski D, Herrmann S, Richnow H-H et al. . Functional analysis of an anaerobic m-xylene-degrading enrichment culture using protein-based stable isotope probing. FEMS Microbiol Ecol 2012;81:134–44 [DOI] [PubMed] [Google Scholar]
- Brow CN, O'Brien Johnson R, Johnson RL et al. . Assessment of anaerobic toluene biodegradation activity by bssA transcript/gene ratios. Appl Environ Microb 2013;79:5338–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan AV. Metabolomic investigations of anaerobic hydrocarbon-impacted environments. Curr Opin Biotechnol 2013;24:506–15 [DOI] [PubMed] [Google Scholar]
- Callaghan AV, Davidova IA, Savage-Ashlock K et al. . Diversity of benzyl- and alkylsuccinate synthase genes in hydrocarbon-impacted environments and enrichment cultures. Environ Sci Technol 2010;44:7287–94 [DOI] [PubMed] [Google Scholar]
- Christensen TH, Bjerg PL, Banwart SA et al. . Characterization of redox conditions in groundwater contaminant plumes. J Contam Hydrol 2000;45:165–241 [Google Scholar]
- Coates JD, Bhupathiraju VK, Achenbach LA et al. . Geobacter hydrogenophilus, Geobacter chapellei and Geobacter grbiciae, three new, strictly anaerobic, dissimilatory Fe(III)-reducers. Int J Syst Evol Micr 2001a;51:581–8 [DOI] [PubMed] [Google Scholar]
- Coates JD, Chakraborty R, Lack JG et al. . Anaerobic benzene oxidation coupled to nitrate reduction in pure culture by two strains of Dechloromonas. Nature 2001b;411:1039–43 [DOI] [PubMed] [Google Scholar]
- Cozzarelli IM, Bekins BA, Eganhouse RP et al. . In situ measurements of volatile aromatic hydrocarbon biodegradation rates in groundwater. J Contam Hydrol 2010;111:48–64 [DOI] [PubMed] [Google Scholar]
- Duldhardt I, Nijenhuis I, Schauer F et al. . Anaerobically grown Thauera aromatica, Desulfococcus multivorans, Geobacter sulfurreducens are more sensitive towards organic solvents than aerobic bacteria. Appl Microbiol Biot 2007;77:705–11 [DOI] [PubMed] [Google Scholar]
- Edwards EA, Grbić-Galić D. Anaerobic degradation of toluene and o-xylene by a methanogenic consortium. Appl Environ Microb 1994;60:313–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einsiedl F, Pilloni G, Ruth-Anneser B et al. . Spatial distributions of sulphur species and sulphate-reducing bacteria provide insights into sulphur redox cycling and biodegradation hot-spots in a hydrocarbon-contaminated aquifer. Geochim Cosmochim Acta 2015;156:207–21 [Google Scholar]
- Ettwig KF, Butler MK, Le Paslier D et al. . Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 2010;464:543–8 [DOI] [PubMed] [Google Scholar]
- Fahrenfeld N, Cozzarelli I, Bailey Z et al. . Insights into biodegradation through depth-resolved microbial community functional and structural profiling of a crude-oil contaminant plume. Microbial Ecol 2014;68:453–62 [DOI] [PubMed] [Google Scholar]
- Fahy A, Lethbridge G, Earle R et al. . Effects of long-term benzene pollution on bacterial diversity and community structure in groundwater. Environ Microbiol 2005;7:1192–9 [DOI] [PubMed] [Google Scholar]
- Feris KP, Hristova K, Gebreyesus B et al. . A shallow BTEX and MTBE contaminated aquifer supports a diverse microbial community. Microbial Ecol 2004;48:589–600 [DOI] [PubMed] [Google Scholar]
- Ficker M, Krastel K, Orlicky S et al. . Molecular characterization of a toluene-degrading methanogenic consortium. Appl Environ Microb 1999;65:5576–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Romero C, Tindall BJ, Jütner F. Tolumonas auensis gen. nov., sp. nov., a toluene-producing bacterium from anoxic sediments of a freshwater lake. Int J Syst Bacteriol 1996;46:183–8 [DOI] [PubMed] [Google Scholar]
- Fowler SJ, Dong X, Sensen CW et al. . Methanogenic toluene metabolism: community structure and intermediates. Environ Microbiol 2012;14:754–64 [DOI] [PubMed] [Google Scholar]
- Fowler SJ, Gutierrez-Zamora M-L, Manefield M et al. . Identification of toluene degraders in a methanogenic enrichment culture. FEMS Microbiol Ecol 2014;89:625–36 [DOI] [PubMed] [Google Scholar]
- Fuchs G, Boll M, Heider J. Microbial degradation of aromatic compounds - from one strategy to four. Nat Rev Microbiol 2011;9:803–16 [DOI] [PubMed] [Google Scholar]
- Gieg LM, Fowler SJ, Berdugo-Clavijo C. Syntrophic biodegradation of hydrocarbon contaminants. Curr Opin Biotechnol 2014;27:21–9 [DOI] [PubMed] [Google Scholar]
- Haack SK, Fogarty LR, West TG et al. . Spatial and temporal changes in microbial community structure associated with recharge-influenced chemical gradients in a contaminated aquifer. Environ Microbiol 2004;6:438–48 [DOI] [PubMed] [Google Scholar]
- Heipieper HJ, Martínez PM. Toxicity of hydrocarbons to microorganisms. Timmis KN. Handbook of Hydrocarbon and Lipid Microbiology Berlin, Heidelberg:Springer, 2010, 1563–73 [Google Scholar]
- Herrmann S, Kleinsteuber S, Chatzinotas A et al. . Functional characterization of an anaerobic benzene-degrading enrichment culture by DNA stable isotope probing. Environ Microbiol 2010;12:401–11 [DOI] [PubMed] [Google Scholar]
- Herrmann S, Vogt C, Fischer A et al. . Characterization of anaerobic xylene biodegradation by two-dimensional isotope fractionation analysis. Environ Microbiol Rep 2009;1:535–44 [DOI] [PubMed] [Google Scholar]
- Holmes DE, Risso C, Smith JA et al. . Anaerobic oxidation of benzene by the hyperthermophilic archaeon Ferroglobus placidus. Appl Environ Microb 2011;77:5926–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda A, Kasai Y, Hamamura N et al. . Development of a PCR method for the detection and quantification of benzoyl-CoA reductase genes and its application to monitored natural attenuation. Biodegradation 2005;16:591–601 [DOI] [PubMed] [Google Scholar]
- Jarling R, Kühner S, Basílio Janke E et al. . Versatile transformations of hydrocarbons in anaerobic bacteria: substrate ranges and regio- and stereochemistry of activation reactions. Front Microbiol 2015;6 10.3389/fmicb.2015.00880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehmlich N, Kleinsteuber S, Vogt C et al. . Phylogenetic and proteomic analysis of an anaerobic toluene-degrading community. J Appl Microbiol 2010;109:1937–45 [DOI] [PubMed] [Google Scholar]
- Jobelius C, Ruth B, Griebler C et al. . Metabolites indicate hot spots of biodegradation and biogeochemical gradients in a high-resolution monitoring well. Environ Sci Technol 2010;45:474–81 [DOI] [PubMed] [Google Scholar]
- Kasai Y, Kodama Y, Takahata Y et al. . Degradative capacities and bioaugmentation potential of an anaerobic benzene-degrading bacterium strain DN11. Environ Sci Technol 2007;41:6222–7 [DOI] [PubMed] [Google Scholar]
- Kasai Y, Takahata Y, Manefield M et al. . RNA-based stable isotope probing and isolation of anaerobic benzene-degrading bacteria from gasoline-contaminated groundwater. Appl Environ Microb 2006;72:3586–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazy S, Monier A, Alvarez P. Assessing the correlation between anaerobic toluene degradation activity and bssA concentrations in hydrocarbon-contaminated aquifer material. Biodegradation 2010;21:793–800 [DOI] [PubMed] [Google Scholar]
- Keller AH, Schleinitz KM, Starke R et al. . Metagenome-based metabolic reconstruction reveals the ecophysiological function of Epsilonproteobacteria in a hydrocarbon-contaminated sulfidic aquifer. Front Microbiol 2015;6:1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-J, Park S-J, Jung M-Y et al. . An uncultivated nitrate-reducing member of the genus Herminiimonas degrades toluene. Appl Environ Microb 2014;80:3233–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinsteuber S, Schleinitz K, Vogt C. Key players and team play: anaerobic microbial communities in hydrocarbon-contaminated aquifers. Appl Microbiol Biot 2012;94:851–73 [DOI] [PubMed] [Google Scholar]
- Kleinsteuber S, Schleinitz KM, Breitfeld J et al. . Molecular characterization of bacterial communities mineralizing benzene under sulfate-reducing conditions. FEMS Microbiol Ecol 2008;66:143–57 [DOI] [PubMed] [Google Scholar]
- Konopka A. What is microbial community ecology?. ISME J 2009;3:1223–30 [DOI] [PubMed] [Google Scholar]
- Kuever J, Visser M, Loeffler C et al. . Genome analysis of Desulfotomaculum gibsoniae strain Groll(T) a highly versatile Gram-positive sulfate-reducing bacterium. Stand Genomic Sci 2014;9:821–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunapuli U, Jahn MK, Lueders T et al. . Desulfitobacterium aromaticivorans sp. nov. and Geobacter toluenoxydans sp. nov., iron-reducing bacteria capable of anaerobic degradation of monoaromatic hydrocarbons. Int J Syst Evol Micr 2010;60:686–95 [DOI] [PubMed] [Google Scholar]
- Kunapuli U, Lueders T, Meckenstock RU. The use of stable isotope probing to identify key iron-reducing microorganisms involved in anaerobic benzene degradation. ISME J 2007;1:643–53 [DOI] [PubMed] [Google Scholar]
- Kuntze K, Shinoda Y, Moutakki H et al. . 6-Oxocyclohex-1-ene-1-carbonyl-coenzyme A hydrolases from obligately anaerobic bacteria: characterization and identification of its gene as a functional marker for aromatic compounds degrading anaerobes. Environ Microbiol 2008;10:1547–56 [DOI] [PubMed] [Google Scholar]
- Kuntze K, Vogt C, Richnow H-H et al. . Combined application of PCR-based functional assays for the detection of aromatic-compound-degrading anaerobes. Appl Environ Microb 2011;77:5056–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppardt A, Kleinsteuber S, Vogt C et al. . Phylogenetic and functional diversity within toluene-degrading, sulphate-reducing consortia enriched from a contaminated aquifer. Microbial Ecol 2014;68:222–34 [DOI] [PubMed] [Google Scholar]
- Larentis M, Hoermann K, Lueders T. Fine-scale degrader community profiling over an aerobic/anaerobic redox gradient in a toluene-contaminated aquifer. Environ Microbiol Rep 2013;5:225–34 [DOI] [PubMed] [Google Scholar]
- Lin X, McKinley J, Resch CT et al. . Spatial and temporal dynamics of the microbial community in the Hanford unconfined aquifer. ISME J 2012;6:1665–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou JSC, DeRito CM, Madsen EL. Field-based and laboratory stable isotope probing surveys of the identities of both aerobic and anaerobic benzene-metabolizing microorganisms in freshwater sediment. Environ Microbiol 2008;10:1964–77 [DOI] [PubMed] [Google Scholar]
- Liu A, Garcia-Dominguez E, Rhine ED et al. . A novel arsenate respiring isolate that can utilize aromatic substrates. FEMS Microbiol Ecol 2004;48:323–32 [DOI] [PubMed] [Google Scholar]
- Luo F, Gitiafroz R, Devine CE et al. . Metatranscriptome of an anaerobic benzene-degrading, nitrate-reducing enrichment culture reveals involvement of carboxylation in benzene ring activation. Appl Environ Microb 2014;80:4095–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marozava S, Röling WFM, Seifert J et al. . Physiology of Geobacter metallireducens under excess and limitation of electron donors. Part II. Mimicking environmental conditions during cultivation in retentostats. Syst Appl Microbiol 2014;37:287–95 [DOI] [PubMed] [Google Scholar]
- Martín-Moldes Z, Zamarro MT, del Cerro C et al. . Whole-genome analysis of Azoarcus sp. strain CIB provides genetic insights to its different lifestyles and predicts novel metabolic features. Syst Appl Microbiol 2015;38:462–71 [DOI] [PubMed] [Google Scholar]
- Marzorati M, Wittebolle L, Boon N et al. . How to get more out of molecular fingerprints: practical tools for microbial ecology. Environ Microbiol 2008;10:1571–81 [DOI] [PubMed] [Google Scholar]
- Meckenstock RU. Fermentative toluene degradation in anaerobic defined syntrophic cocultures. FEMS Microbiol Lett 1999;177:67–73 [DOI] [PubMed] [Google Scholar]
- Meckenstock RU, Boll M, Mouttaki H et al. . Anaerobic degradation of benzene and polycyclic aromatic hydrocarbons. J Mol Microb Biotech 2016;26:92–118 [DOI] [PubMed] [Google Scholar]
- Meckenstock RU, Elsner M, Griebler C et al. . Biodegradation: updating the concepts of control for microbial cleanup in contaminated aquifers. Environ Sci Technol 2015;49:7073–81 [DOI] [PubMed] [Google Scholar]
- Morasch B, Schink B, Tebbe CC et al. . Degradation of o-xylene and m-xylene by a novel sulfate-reducer belonging to the genus Desulfotomaculum. Arch Microbiol 2004;181:407–17 [DOI] [PubMed] [Google Scholar]
- Müller H, Bosch J, Griebler C et al. . Long-distance electron transfer by cable bacteria in aquifer sediments. ISME J 2016 10.1038/ismej.2015.1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka AR, Phelps CD, Zhu X et al. . Dual biomarkers of anaerobic hydrocarbon degradation in historically contaminated groundwater. Environ Sci Technol 2011;45:3407–14 [DOI] [PubMed] [Google Scholar]
- Parales RE, Parales JV, Pelletier DA et al. . Diversity of microbial toluene degradation pathways. Laskin AI, Sariaslani S, Gadd GM. Advances in Applied Microbiology Vol. 64San Diego:Elsevier Academic Press, 2008, 1–73 [DOI] [PubMed] [Google Scholar]
- Pérez-Pantoja D, González B, Pieper DH. Aerobic degradation of aromatic hydrocarbons. Timmis K. Handbook of Hydrocarbon and Lipid Microbiology Berlin, Heidelberg:Springer, 2010, 799–837 [Google Scholar]
- Pfeffer C, Larsen S, Song J et al. . Filamentous bacteria transport electrons over centimetre distances. Nature 2012;491:218–21 [DOI] [PubMed] [Google Scholar]
- Pilloni G, von Netzer F, Engel M et al. . Electron acceptor-dependent identification of key anaerobic toluene degraders at a tar-oil-contaminated aquifer by Pyro-SIP. FEMS Microbiol Ecol 2011;78:165–75 [DOI] [PubMed] [Google Scholar]
- Poehlein A, Daniel R, Simeonova DD. Draft genome sequence of Desulfotignum phosphitoxidans DSM 13687 strain FiPS-3. Genome Announc 2013;1 10.1128/genomeA.00227-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter AW, Young LY. Benzoyl-CoA, a universal biomarker for anaerobic degradation of aromatic compounds. Sariaslani S, Gadd GM. Advances in Applied Microbiology Vol. 88San Diego:Elsevier Academic Press, 2014, 167–203 [DOI] [PubMed] [Google Scholar]
- Prakash O, Gihring TM, Dalton DD et al. . Geobacter daltonii sp. nov., an Fe(III)- and uranium(VI)-reducing bacterium isolated from a shallow subsurface exposed to mixed heavy metal and hydrocarbon contamination. Int J Syst Evol Micr 2010;60:546–53 [DOI] [PubMed] [Google Scholar]
- Qiu J. China to spend billions cleaning up groundwater. Science 2011;334:745. [DOI] [PubMed] [Google Scholar]
- Rabus R, Boll M, Heider J et al. . Anaerobic microbial degradation of hydrocarbons: from enzymatic reactions to the environment. J Mol Microb Biot 2016;26:5–28 [DOI] [PubMed] [Google Scholar]
- Rakoczy J, Schleinitz KM, Müller N et al. . Effects of hydrogen and acetate on benzene mineralisation under sulphate-reducing conditions. FEMS Microbiol Ecol 2011;77:238–47 [DOI] [PubMed] [Google Scholar]
- Salinero K, Keller K, Feil W et al. . Metabolic analysis of the soil microbe Dechloromonas aromatica str. RCB: indications of a surprisingly complex life-style and cryptic anaerobic pathways for aromatic degradation. BMC Genomics 2009;10:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shade A, Peter H, Allison SD et al. . Fundamentals of microbial community resistance and resilience. Front Microbiol 2012;3:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda Y, Akagi J, Uchihashi Y et al. . Anaerobic degradation of aromatic compounds by Magnetospirillum strains: Isolation and degradation genes. Biosci Biotechnol Biochem 2005;69:1483–91 [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Sakai Y, Uenishi H et al. . Aerobic and anaerobic toluene degradation by a newly isolated denitrifying bacterium, Thauera sp. strain DNT-1. Appl Environ Microb 2004;70:1385–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staats M, Braster M, Röling WFM. Molecular diversity and distribution of aromatic hydrocarbon-degrading anaerobes across a landfill leachate plume. Environ Microbiol 2011;13:1216–27 [DOI] [PubMed] [Google Scholar]
- Starke R, Keller A, Jehmlich N et al. . Pulsed 13C2-acetate protein-SIP unveils Epsilonproteobacteria as dominant acetate utilizers in a sulfate-reducing microbial community mineralizing benzene. Microbial Ecol 2016;71:901–11 [DOI] [PubMed] [Google Scholar]
- Sun W, Cupples AM. Diversity of five anaerobic toluene-degrading microbial communities investigated using stable isotope probing. Appl Environ Microb 2012;78:972–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Sun X, Cupples AM. Identification of Desulfosporosinus as toluene-assimilating microorganisms from a methanogenic consortium. Int Biodeter Biodegr 2014;88:13–9 [Google Scholar]
- Tan B, Ng CM, Nshimyimana JP et al. . Next-generation sequencing (NGS) for assessment of microbial water quality: current progress, challenges, and future opportunities. Front Microbiol 2015;6 10.3389/fmicb.2015.01027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubert M, Vogt C, Wubet T et al. . Protein-SIP enables time-resolved analysis of the carbon flux in a sulfate-reducing, benzene-degrading microbial consortium. ISME J 2012;6:2291–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischer K, Kleinsteuber S, Schleinitz KM et al. . Microbial communities along biogeochemical gradients in a hydrocarbon-contaminated aquifer. Environ Microbiol 2013;15:2603–15 [DOI] [PubMed] [Google Scholar]
- Trautwein K, Lahme S, Wöhlbrand L et al. . Physiological and proteomic adaptation of “Aromatoleum aromaticum” EbN1 to low growth rates in benzoate-limited, anoxic chemostats. J Bacteriol 2012;194:2165–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuxen N, Albrechtsen H-J, Bjerg PL. Identification of a reactive degradation zone at a landfill leachate plume fringe using high resolution sampling and incubation techniques. J Contam Hydrol 2006;85:179–94 [DOI] [PubMed] [Google Scholar]
- Ulrich AC, Edwards EA. Physiological and molecular characterization of anaerobic benzene-degrading mixed cultures. Environ Microbiol 2003;5:92–102 [DOI] [PubMed] [Google Scholar]
- van Breukelen BM, Griffioen J. Biogeochemical processes at the fringe of a landfill leachate pollution plume: potential for dissolved organic carbon, Fe(II), Mn(II), NH4, and CH4 oxidation. J Contam Hydrol 2004;73:181–205 [DOI] [PubMed] [Google Scholar]
- van der Zaan BM, Saia FT, Stams AJM et al. . Anaerobic benzene degradation under denitrifying conditions: Peptococcaceae as dominant benzene degraders and evidence for a syntrophic process. Environ Microbiol 2012;14:1171–81 [DOI] [PubMed] [Google Scholar]
- Vogt C, Kleinsteuber S, Richnow H-H. Anaerobic benzene degradation by bacteria. Microb Biotechnol 2011;4:710–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt C, Lueders T, Richnow HH et al. . Stable isotope probing approaches to study anaerobic hydrocarbon degradation and degraders. J Mol Microb Biot 2016;26:195–210 [DOI] [PubMed] [Google Scholar]
- von Netzer F, Kuntze K, Vogt C et al. . Functional gene markers for fumarate-adding and dearomatizing key enzymes in anaerobic aromatic hydrocarbon degradation in terrestrial environments. J Mol Microb Biot 2016;26:180–94 [DOI] [PubMed] [Google Scholar]
- von Netzer F, Pilloni G, Kleindienst S et al. . Enhanced gene detection assays for fumarate-adding enzymes allow uncovering anaerobic hydrocarbon degraders in terrestrial and marine systems. Appl Environ Microb 2013;79:543–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L-Y, Nevin K, Woodard TL et al. . Expanding the diet for DIET: electron donors supporting direct interspecies electron transfer (DIET) in defined co-cultures. Front Microbiol 2016;7:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washer CE, Edwards EA. Identification and expression of benzylsuccinate synthase genes in a toluene-degrading methanogenic consortium. Appl Environ Microb 2007;73:1367–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weelink SAB, Doesburg Wv, Saia FT et al. . A strictly anaerobic betaproteobacterium Georgfuchsia toluolica gen. nov., sp. nov. degrades aromatic compounds with Fe(III), Mn(IV) or nitrate as an electron acceptor. FEMS Microbiol Ecol 2009;70:575–85 [DOI] [PubMed] [Google Scholar]
- Weelink SAB, Tan NCG, ten Broeke H et al. . Isolation and characterization of Alicycliphilus denitrificans strain BC, which grows on benzene with chlorate as the electron acceptor. Appl Environ Microb 2008;74:6672–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weelink SAB, van Eekert MHA, Stams AJM. Degradation of BTEX by anaerobic bacteria: physiology and application. Rev Environ Sci BioTechnol 2010;9:359–85 [Google Scholar]
- Widdel F, Knittel K, Galushko A. Anaerobic hydrocarbon-degrading microorganisms: an overview. Timmis KN. Handbook of Hydrocarbon and Lipid Microbiology Berlin, Heidelberg:Springer, 2010, 1997–2021 [Google Scholar]
- Wilkes H, Schwarzbauer J. Hydrocarbons: an introduction to structure, physico-chemical properties and natural occurrence. Timmis K. Handbook of Hydrocarbon and Lipid Microbiology Berlin, Heidelberg:Springer, 2010, 1–48 [Google Scholar]
- Wilson RD, Thornton SF, Mackay DM. Challenges in monitoring the natural attenuation of spatially variable plumes. Biodegradation 2004;15:359–69 [DOI] [PubMed] [Google Scholar]
- Winderl C, Anneser B, Griebler C et al. . Depth-resolved quantification of anaerobic toluene degraders and aquifer microbial community patterns in distinct redox zones of a tar oil contaminant plume. Appl Environ Microb 2008;74:792–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winderl C, Penning H, von Netzer F et al. . DNA-SIP identifies sulfate-reducing Clostridia as important toluene degraders in tar-oil-contaminated aquifer sediment. ISME J 2010;4:1314–25 [DOI] [PubMed] [Google Scholar]
- Winderl C, Schaefer S, Lueders T. Detection of anaerobic toluene and hydrocarbon degraders in contaminated aquifers using benzylsuccinate synthase (bssA) genes as a functional marker. Environ Microbiol 2007;9:1035–46 [DOI] [PubMed] [Google Scholar]
- Yagi JM, Suflita JM, Gieg LM et al. . Subsurface cycling of nitrogen and anaerobic aromatic hydrocarbon biodegradation revealed by nucleic acid and metabolic biomarkers. Appl Environ Microb 2010;76:3124–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zedelius J, Rabus R, Grundmann O et al. . Alkane degradation under anoxic conditions by a nitrate-reducing bacterium with possible involvement of the electron acceptor in substrate activation. Environ Microbiol Rep 2011;3:125–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Bain TS, Nevin KP et al. . Anaerobic benzene oxidation by Geobacter species. Appl Environ Microb 2012;78:8304–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylstra GJ, Gibson DT. Aromatic hydrocarbon degradation: a molecular approach. Genet Eng 1991;13:183–203 [DOI] [PubMed] [Google Scholar]