Abstract
Background: Lack of viral load monitoring of ART is known to be associated with slower switch from a failing regimen and thereby higher prevalence of MDR HIV-1. Many countries have continued to use thymidine analogue drugs despite recommendations to use tenofovir in combination with a cytosine analogue and NNRTI as first-line ART. The effect of accumulated thymidine analogue mutations (TAMs) on phenotypic resistance over time has been poorly characterized in the African setting.
Patients and methods: A retrospective analysis of individuals with ongoing viral failure between weeks 48 and 96 in the NORA (Nevirapine OR Abacavir) study was conducted. We analysed 36 genotype pairs from weeks 48 and 96 of first-line ART (14 treated with zidovudine/lamivudine/nevirapine and 22 treated with zidovudine/lamivudine/abacavir). Phenotypic drug resistance was assessed using the Antivirogram assay (v. 2.5.01, Janssen Diagnostics).
Results: At 96 weeks, extensive TAMs (≥3 mutations) were present in 50% and 73% of nevirapine- and abacavir-treated patients, respectively. The mean (SE) number of TAMs accumulating between week 48 and week 96 was 1.50 (0.37) in nevirapine-treated participants and 1.82 (0.26) in abacavir-treated participants. Overall, zidovudine susceptibility of viruses was reduced between week 48 [geometric mean fold change (FC) 1.3] and week 96 (3.4, P = 0.01). There was a small reduction in tenofovir susceptibility (FC 0.7 and 1.0, respectively, P = 0.18).
Conclusions: Ongoing viral failure with zidovudine-containing first-line ART is associated with rapidly increasing drug resistance that could be mitigated with effective viral load monitoring.
Introduction
Combination ART (cART) has led to declining mortality and HIV-1 incidence in high-prevalence settings.1,2 WHO recommends tenofovir/lamivudine/emtricitabine as the NRTI backbone of cART.3 Although countries have scaled up use of tenofovir, thymidine analogues such as stavudine or zidovudine are still in use. Virological failure occurs in 15%–35% of patients treated with thymidine analogue-containing first-line cART within the first year, with the majority harbouring resistance to NNRTI and lamivudine in areas without access to viral load monitoring.4,5 As tenofovir becomes available, increasing numbers of individuals are substituting thymidine analogues for tenofovir without documented viral suppression at switch, carrying risk for acquisition of drug resistance.4,5 There is also evidence pointing to prior undisclosed thymidine analogue-containing ART in patients presenting for first-line tenofovir-based ART in sub-Saharan Africa.6,7 Unsurprisingly, therefore, thymidine analogue mutations (TAMs) have been reported in patients following viral failure of tenofovir-containing first-line regimens,8 contributing to multidrug resistance in these individuals.9 As limitation of emergence and transmission of MDR HIV is a priority, understanding the accumulation and phenotypic impact of TAMs remains important.
Despite a large body of data on HIV-1 resistance mutations in cross-sectional adult treatment studies from Africa,10 there are few longitudinal data on accumulation of drug resistance during prolonged viral failure of first-line cART regimens, and even less information on the phenotypic impact of such accumulated mutations. We sought to address these gaps in current knowledge through a retrospective analysis of individuals with ongoing viral failure between 48 and 96 weeks in the NORA (Nevirapine OR Abacavir) sub-study of the DART (Development of AntiRetroviral Therapy in Africa) trial.
Patients and methods
DART-NORA enrolled 600 previously untreated and asymptomatic Ugandan patients with CD4 counts of <200 cells/mm3, who were randomly assigned to co-formulated zidovudine/lamivudine and either abacavir and nevirapine placebo (n = 300), or abacavir placebo and nevirapine (n = 300). After 24 weeks, participants continued to receive the study drugs open-label and were followed up as part of DART. Of those randomized, 32 died before week 96, and 21 were lost to follow-up. A further 107 participants with CD4 counts ≥300 cells/mm3 at weeks 48 or 72 after ART initiation were randomized to structured treatment interruption (STI) with up to four repeated cycles of 12 weeks on/off therapy and are excluded. In addition, 7 (4 abacavir, 3 nevirapine) participants had switched to lopinavir-based second-line ART by week 96, leaving 433 participants (236 abacavir, 197 nevirapine), for whom 96.7% of week 48 and week 96 HIV-1 RNA measurements were available.
Viral loads were measured using Roche Amplicor 1.5. Genotypic resistance testing was by standard population sequencing of pol, with identification of resistance mutations according to the latest IAS-USA list,11 as described previously.12 Phenotypic drug resistance was assessed using the Antivirogram assay (v. 2.5.01, Janssen Diagnostics) using a full-length recombinant HIV vector containing patient-derived protease (PR)–reverse transcriptase (RT) sequences up to codon 400 of RT.13 Nested RT–PCR was used to amplify a 2.2 kb fragment and the resulting PR–RT-coding sequences were then transfected into CD4+ T lymphocytes (MT4), along with the pGEMT3deltaPRT plasmid. Chimeric viruses containing PR- and RT-coding sequences derived from HIV-1 RNA in plasma were generated through homologous recombination in the MT4 cells, and susceptibilities of chimeric viruses to RT inhibitors were determined by an MT4 cell 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide-based cell viability assay.14 Statistical methods included Student’s t-tests and linear regression as appropriate. All P values were two-sided. All analyses were performed using STATA v. 14.0 (StataCorp LP, College Station, TX, USA).
Ethics
Ethics approval both for DART and the NORA sub-study was obtained both in Uganda [Uganda Research Unit on AIDS (UVRI) Science and Ethics Committee] and the UK (Imperial College).
Results
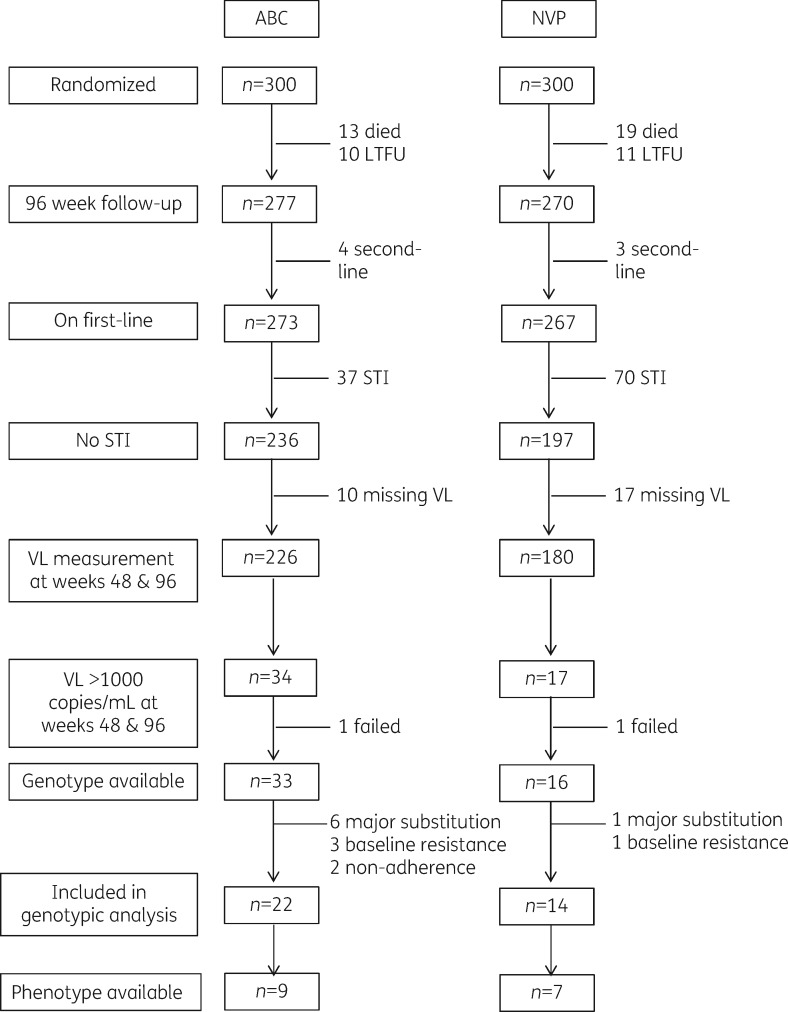
Fifty-one of 433 (12%) participants were identified as having a viral load >1000 copies/mL at both week 48 and week 96 (Figure 1). Genotypic and phenotypic testing was attempted at these timepoints along with a baseline (week 0) sample. Genotyping was successful for 49 pairs of samples. Of these participants, 7 made substitutions to their initial regimen, 4 had detectable resistance mutations prior to starting therapy, and 2 had no major mutations at both weeks 48 and 96, indicating non-adherence; all were excluded from further analyses, leaving 36 individuals with genotype pairs (22 abacavir, 14 nevirapine). Paired phenotypic results from both weeks 48 and 96 were only available in 16 of these 36 participants due to insufficient quantity of sample for phenotyping in the remainder (Figure 1). Twenty-four (67%) of the participants with a genotype were female, with a median (IQR) age at randomization of 37 (30–43) years. The CD4 count at randomization was low, with a median (IQR) value of 44 (17–103) cells/mm3.
Figure 1.
Flow diagram of participants and samples genotyped and phenotyped. ABC, abacavir; NVP, nevirapine; LTFU, lost to follow-up; VL, viral load.
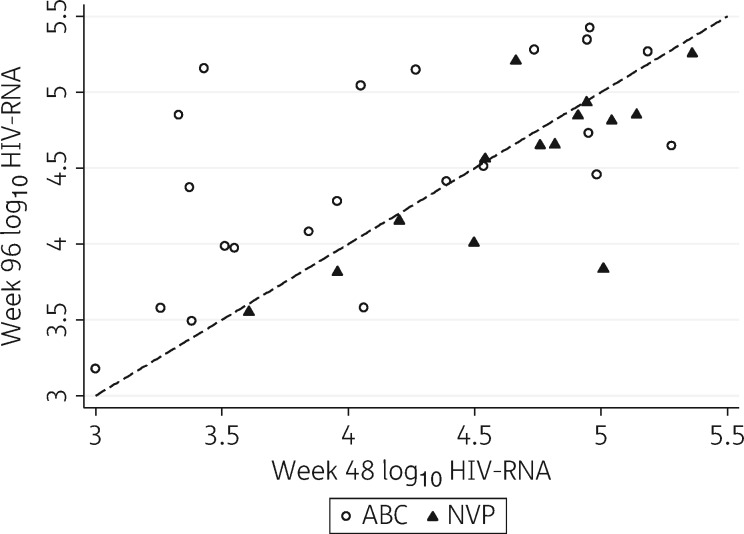
The relationship between an individual’s viral load at weeks 48 and 96 is shown in Figure 2. In participants with paired genotypic data randomized to the nevirapine group (n = 14), mean (SD) viral load was 4.67 (0.49) log10 copies/mL at week 48 and 4.52 (0.55) log10 copies/mL at week 96 [mean decrease of 0.15 log10 copies/mL (95% CI −0.06–0.36)]. In those randomized to abacavir (n = 22), the mean viral load was 4.13 (0.72) log10 copies/mL at week 48 and 4.50 (0.66) log10 copies/mL at week 96 [mean increase of 0.37 log10 copies/mL (95% CI 0.10–0.64)].
Figure 2.
Scatter plot depicting week 48 versus week 96 viral load by treatment group in patients with paired genotypes (n = 36). ABC, abacavir; NVP, nevirapine.
Overall, the prevalence of each TAM increased between weeks 48 and 96 in our sample of 36 patients [mean absolute increase 25% (range 19%–31%)]. TAMs at position 215 were particularly frequent (>70% in each arm) followed by D67N (Table 1). In only one case was there a loss of a specific TAM between timepoints (D67N in the nevirapine arm). At 48 weeks, ≥3 TAMs were present in 14% and 23% of nevirapine- and abacavir-treated patients, respectively, and at 96 weeks the proportions were 50% and 73%, respectively. The mean (SE) number of TAMs accumulating between weeks 48 and 96 was 1.50 (0.37) in nevirapine-treated patients and 1.82 (0.26) in abacavir-treated patients. This rate of accumulation was not related to the absolute number of TAMs at week 48 (P = 0.38), although numbers in each group were small.
Table 1.
Prevalence of TAMs and M184V in patients with paired genotypes (n = 36) at weeks 48 and 96, by treatment arm
| ABC (n = 22) |
NVP (n = 14) |
|||||||
|---|---|---|---|---|---|---|---|---|
| week 48 |
week 96 |
week 48 |
week 96 |
|||||
| n | % | n | % | n | % | n | % | |
| Mutation | ||||||||
| 184V | 22 | 100 | 22 | 100 | 12 | 86 | 13 | 93 |
| 41L | 2 | 9 | 8 | 36 | 1 | 7 | 4 | 29 |
| 67N | 9 | 41 | 15 | 68 | 5 | 36 | 6 | 43 |
| 70R | 9 | 41 | 14 | 64 | 2 | 14 | 4 | 29 |
| 210W | 0 | 0 | 7 | 32 | 1 | 7 | 5 | 36 |
| 215F | 3 | 14 | 7 | 32 | 0 | 0 | 5 | 36 |
| 215Y | 3 | 14 | 9 | 41 | 2 | 14 | 5 | 36 |
| 219QE | 4 | 18 | 11 | 50 | 1 | 7 | 4 | 29 |
| No. of TAMs | ||||||||
| 0 | 9 | 41 | 3 | 14 | 8 | 57 | 3 | 21 |
| 1 | 2 | 9 | 1 | 5 | 2 | 14 | 1 | 7 |
| 2 | 6 | 27 | 2 | 9 | 2 | 14 | 3 | 21 |
| 3 | 4 | 18 | 4 | 18 | 2 | 14 | 2 | 14 |
| ≥4 | 1 | 5 | 12 | 55 | 0 | 0 | 5 | 36 |
ABC, abacavir; NVP, nevirapine.
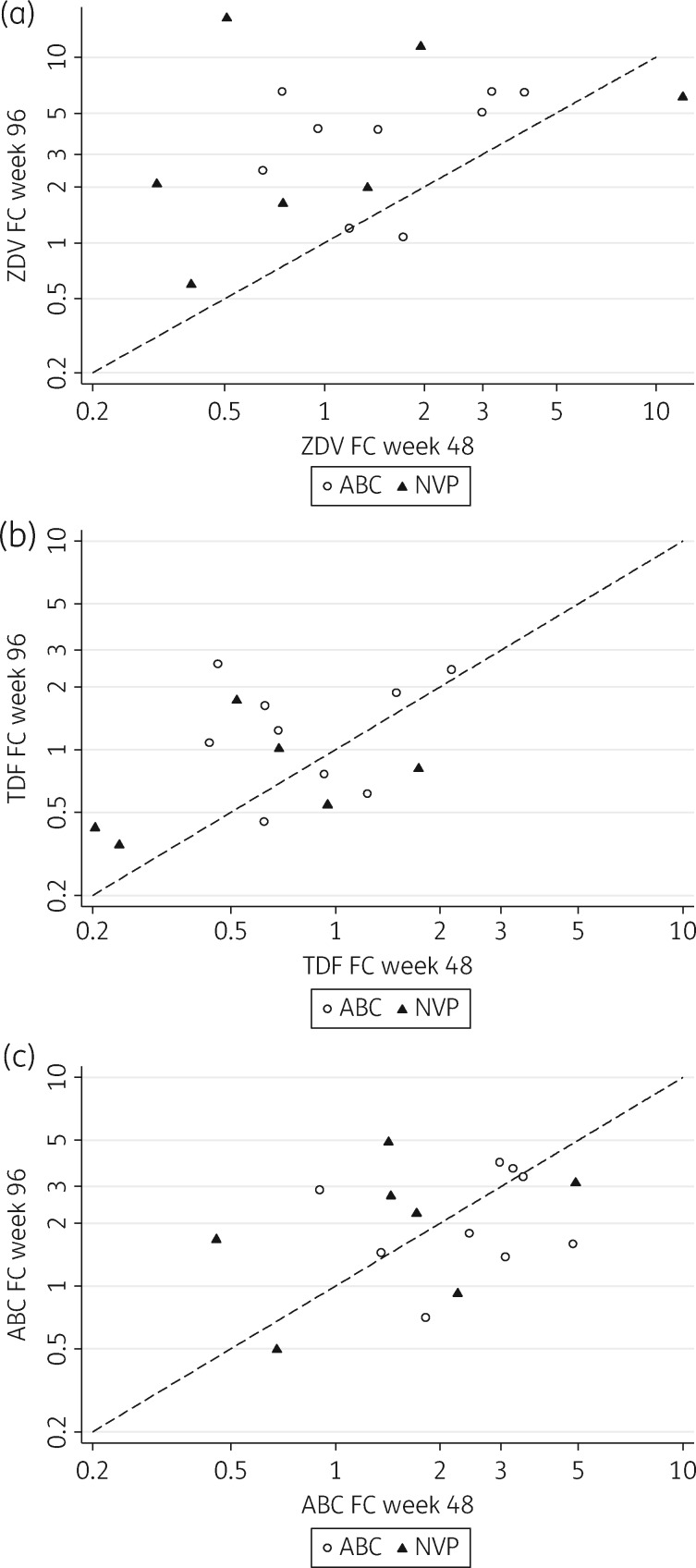
Combining both treatment arms, the zidovudine susceptibility of viruses was reduced between week 48 [geometric mean fold change (FC) 1.3] and week 96 (geometric mean FC 3.4, P = 0.01) (Figure 3a). Similarly, there was a small, non-significant reduction in tenofovir disoproxil fumarate susceptibility over time (FC 0.7 and 1.0, respectively, P = 0.18) (Figure 3b) with most patients remaining fully susceptible. However, there were no observed changes in susceptibility to abacavir (FC 1.9 and 1.9, respectively, P = 0.96) between these timepoints (Figure 3c).
Figure 3.
Drug susceptibility of patient-derived virus isolates to abacavir, zidovudine and tenofovir disoproxil fumarate (modelled as FCs relative to a reference isolate) by treatment group in patients with paired phenotypes (n = 16). ABC, abacavir; NVP, nevirapine; TDF, tenofovir disoproxil fumarate; ZDV, zidovudine.
Lamivudine resistance (M184V) was present in all patients with viral failure at 96 weeks except one participant on the nevirapine arm (Table 1), and had emerged by week 48 in all but one patient on the nevirapine arm. Other NRTI-associated mutations were present only in participants on the abacavir arm, and had emerged by week 48: one individual acquired the multinucleoside resistance 69 insertion, one acquired K65R and two acquired Y115F (Table S1, available as Supplementary data at JAC Online). The abacavir-selected mutation L74V mutation was not observed in any participant (Table S1).
The connection domain mutation N348I conferring resistance to zidovudine15,16 was acquired in 4/14 (28.6%) of nevirapine-treated patients and in 6/22 (27.3%) of abacavir-treated patients.
Major NNRTI mutations occurred in 12/14 participants treated with nevirapine by week 48. In those who were already resistant by week 48, only one participant acquired a new mutation by week 96 (V106A at week 48, K101E + G190A at week 96), and both participants without NNRTI mutations at week 48 went on to develop G190A by week 96.
Discussion
NNRTI-based cART appears to be associated with rapid accumulation of TAMs in those with virological failure (1.50 TAMs per year for zidovudine/lamivudine/nevirapine-treated patients) in this resource-limited setting where routine viral load monitoring is not performed. We observed that abacavir/zidovudine/lamivudine also resulted in rapid TAM accumulation (1.82 TAMs per year), although WHO guidelines do not currently recommend triple nucleoside-based cART for first-line cART. Our estimate of TAM accumulation for NNRTI-based ART is higher than reported previously in South African adults17 (0.84 per year), and in Zambian children18 (0.5 per year). Both those studies involved significant stavudine use, as opposed to zidovudine. Stavudine is known to select not only for TAM mutational pathways, but also selects for K65R and this could partly explain the discrepancy with our data.
Over half of all patients with viral failure by week 48 in this study had ≥3 TAMs in total by week 96. Accumulated TAMs were associated with reduced phenotypic drug susceptibility amongst a smaller sample of patients, with zidovudine susceptibility significantly falling between weeks 48 and 96, and a small non-significant decrease observed for tenofovir.
We know from previous studies that boosted PI-based first-line ART is protective against the development of NRTI mutations, in comparison with NNRTI-based first-line therapy.19–21 This is important to bear in mind as first-line treatment strategies in sub-Saharan Africa may need to be changed in the coming years due to the rising prevalence of transmitted NNRTI drug resistance.22 It will be of interest to know whether the use of integrase inhibitors is also protective against NRTI resistance in such settings.
Limitations
This study was limited by a relatively small number of available phenotype pairs and the conclusions are largely drawn from genotypic information. Furthermore the data are derived from nevirapine-treated patients and efavirenz is now the most widely used NNRTI in resource-limited settings.23
Despite its associated toxicity, zidovudine has emerged as an important component of cART regimens in resource-limited settings; moreover, dose reduction reduces incidence of moderate to severe anaemia.24 WHO recommends the use of zidovudine in second-line regimens following failure of first-line regimens containing tenofovir. This presents a possible problem where patients have accumulated TAMs prior to tenofovir replacing zidovudine during first-line cART, though two large studies have not shown a detrimental effect of TAMs on boosted PI-based second-line therapy.25,26
Transmission of NRTI drug resistance compromises subsequent response to first-line therapies,27,28 and therefore limitation of accumulated TAMs should be a public health priority as cART is scaled up. It is also appropriate to limit multidrug resistance as this may allow for recycling of drugs in the future as the epidemic matures.
Effective viral load monitoring has been identified as a priority area29 and could avoid prolonged viral failure with accumulation of TAMs.30 In such individuals with virological failure, adherence counselling with possible switch to second-line therapy should be undertaken, thus avoiding ongoing first-line failure and accumulation of drug resistance.8
Supplementary Material
Acknowledgements
We thank all the patients and staff from all the centres participating in the DART trial.
DART Virology Group: P. Kaleebu (Co-Chair), D. Pillay (Co-Chair), P. Awio, M. Chirara, D. Dunn, D. M. Gibb, C. Gilks, R. Goodall, A. Kapaata, M. Katuramur, F. Lyagoba, B. Magambo, K. Mataruka, L. Mugarura, T. Musunga, M. Nabankkema, J. Nkalubo, P. Nkurunziza, C. Parry, V. Robertson, M. Spyer, D. Mulima, D. E. Williams, I. Nankya, S. Nassimbwa, E. Ndashimye, E. Nabulime, M. Phiri, K. Mutasa and S. Mukasa.
MRC/UVRI Uganda Research Unit on AIDS, Entebbe, Uganda: H. Grosskurth, P. Munderi, G. Kabuye, D. Nsibambi, R. Kasirye, E. Zalwango, M. Nakazibwe, B. Kikaire, G. Nassuna, R. Massa, K. Fadhiru, M. Namyalo, A. Zalwango, L. Generous, P. Khauka, N. Rutikarayo, W. Nakahima, A. Mugisha, J. Todd, J. Levin, S. Muyingo, A. Ruberantwari, P. Kaleebu, D. Yirrell, N. Ndembi, F. Lyagoba, P. Hughes, M. Aber, A. Medina Lara, S. Foster, J. Amurwon and B. Nyanzi Wakholi.
Joint Clinical Research Centre, Kampala, Uganda: P. Mugyenyi, C. Kityo, F. Ssali, D. Tumukunde, T. Otim, J. Kabanda, H. Musana, J. Akao, H. Kyomugisha, A. Byamukama, J. Sabiiti, J. Komugyena, P. Wavamunno, S. Mukiibi, A. Drasiku, R. Byaruhanga, O. Labeja, P. Katundu, S. Tugume, P. Awio, A. Namazzi, G. T. Bakeinyaga, H. Katabira, D. Abaine, J Tukamushaba, W. Anywar, W. Ojiambo, E. Angweng, S. Murungi, W. Haguma, S. Atwiine and J. Kigozi.
University of Zimbabwe, Harare, Zimbabwe: A. Latif, J. Hakim, V. Robertson, A. Reid, E. Chidziva, R. Bulaya-Tembo, G. Musoro, F. Taziwa, C. Chimbetete, L. Chakonza, A. Mawora, C. Muvirimi, G. Tinago, P. Svovanapasis, M. Simango, O. Chirema, J. Machingura, S. Mutsai, M. Phiri, T. Bafana, M. Chirara, L. Muchabaiwa and M. Muzambi.
Infectious Diseases Institute (formerly the Academic Alliance) Makerere University, Mulago, Uganda: E. Katabira, A. Ronald, A. Kambungu, F. Lutwama, A. Nanfuka, J. Walusimbi, E. Nabankema, R. Nalumenya, T. Namuli, R. Kulume, I. Namata, L. Nyachwo, A. Florence, A. Kusiima, E. Lubwama, R. Nairuba, F. Oketta, E. Buluma, R. Waita, H. Ojiambo, F. Sadik, J. Wanyama and P. Nabongo.
The AIDS Support Organization (TASO), Uganda: R. Ochai and D. Muhweezi.
Imperial College, London, UK: C. Gilks, K. Boocock, C. Puddephatt, D. Winogron and J. Bohannon.
MRC Clinical Trials Unit at UCL, London, UK: J. Darbyshire, D. M. Gibb, A. Burke, D. Bray, A. Babiker, A. S. Walker, H. Wilkes, M. Rauchenberger, S. Sheehan, L. Peto, K. Taylor, M. Spyer, A. Ferrier, B. Naidoo, D. Dunn and R. Goodall.
Independent DART Trial Monitors: R. Nanfuka and C. Mufuka-Kapuya.
Trial Steering Committee: I. Weller (Chair), A. Babiker (Trial Statistician), S. Bahendeka, M. Bassett, A. Chogo Wapakhabulo, J. Darbyshire, B. Gazzard, C. Gilks, H. Grosskurth, J. Hakim, A. Latif, C. Mapuchere, O. Mugurungi, P. Mugyenyi. Observers: C. Burke, M. Distel, S. Jones, E. Loeliger, P. Naidoo, C. Newland, G. Pearce, S. Rahim, J. Rooney, M. Smith, W. Snowden and J.-M. Steens.
Data and Safety Monitoring Committee: A. Breckenridge (Chair), A. McLaren (Chair, deceased), C. Hill, J. Matenga, A. Pozniak and D. Serwadda.
Endpoint Review Committee: T. Peto (Chair), A. Palfreeman, M. Borok and E. Katabira.
Funding
DART is funded by the UK Medical Research Council, the UK Department for International Development (DFID), and the Rockefeller Foundation. GlaxoSmithKline/ViiV Healthcare and Boehringer-Ingelheim donated first-line drugs for NORA. Additional support for viral load and resistance assays in NORA was provided by GlaxoSmithKline/ViiV Healthcare. This work was supported by a Wellcome Trust Fellowship to R. K. G. (WT108082AIA), and the UK Medical Research Council.
Transparency declarations
T. P. is an employee of Janssen. All other authors: none to declare.
Supplementary data
Table S1 is available as Supplementary data at JAC Online.
References
- 1. Bor J, Herbst AJ, Newell ML.. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science 2013; 339: 961–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tanser F, Barnighausen T, Grapsa E. et al. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science 2013; 339: 966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO. March 2014 Supplement to the 2013 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection http://www.who.int/hiv/pub/guidelines/arv2013/arvs2013upplement_march2014/en/. [PubMed]
- 4. Gupta RK, Hill A, Sawyer AW. et al. Virological monitoring and resistance to first-line highly active antiretroviral therapy in adults infected with HIV-1 treated under WHO guidelines: a systematic review and meta-analysis. Lancet Infect Dis 2009; 9: 409–17. [DOI] [PubMed] [Google Scholar]
- 5. Hoffmann CJ, Charalambous S, Sim J. et al. Viremia, resuppression, and time to resistance in human immunodeficiency virus (HIV) subtype C during first-line antiretroviral therapy in South Africa. Clin Infect Dis 2009; 49: 1928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brooks K, Diero L, DeLong A. et al. Treatment failure and drug resistance in HIV-positive patients on tenofovir-based first-line antiretroviral therapy in western Kenya. J Int AIDS Soc 2016; 19: 20798.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaleebu P, Kirungi W, Watera C. et al. Virological response and antiretroviral drug resistance emerging during antiretroviral therapy at three treatment centers in Uganda. PLoS One 2015; 10: e0145536.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. TenoRes Study G. Global epidemiology of drug resistance after failure of WHO recommended first-line regimens for adult HIV-1 infection: a multicentre retrospective cohort study. Lancet Infect Dis 2016; 16: 565–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gregson J, Kaleebu P, Marconi VC. et al. Occult HIV-1 drug resistance to thymidine analogues following failure of first-line tenofovir combined with a cytosine analogue and nevirapine or efavirenz in sub Saharan Africa: a retrospective multi-centre cohort study. Lancet Infect Dis 2016; doi: 10.1016/S1473-3099(16)30469-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hosseinipour MC, Gupta RK, Van Zyl G. et al. Emergence of HIV drug resistance during first- and second-line antiretroviral therapy in resource-limited settings. J Infect Dis 2013; 207 Suppl 2: S49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wensing AM, Calvez V, Gunthard HF. et al. Update of the drug resistance mutations in HIV-1. Topics Antiviral Med 2015; 23: 132–41. [PMC free article] [PubMed] [Google Scholar]
- 12. Ndembi N, Goodall RL, Dunn DT. et al. Viral rebound and emergence of drug resistance in the absence of viral load testing: a randomized comparison between zidovudine-lamivudine plus nevirapine and zidovudine-lamivudine plus abacavir. J Infect Dis 2009; 201: 106–13. [DOI] [PubMed] [Google Scholar]
- 13. Kellam P, Larder BA.. Recombinant virus assay: a rapid, phenotypic assay for assessment of drug susceptibility of human immunodeficiency virus type 1 isolates. Antimicrob Agents Chemother 1994; 38: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hertogs K, de Bethune MP, Miller V. et al. A rapid method for simultaneous detection of phenotypic resistance to inhibitors of protease and reverse transcriptase in recombinant human immunodeficiency virus type 1 isolates from patients treated with antiretroviral drugs. Antimicrob Agents Chemother 1998; 42: 269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCormick AL, Parry CM, Crombe A. et al. Impact of the N348I mutation in HIV-1 reverse transcriptase on nonnucleoside reverse transcriptase inhibitor resistance in non-subtype B HIV-1. Antimicrob Agents Chemother 2011; 55: 1806–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yap SH, Sheen CW, Fahey J. et al. N348I in the connection domain of HIV-1 reverse transcriptase confers zidovudine and nevirapine resistance. PLoS Med 2007; 4: e335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sigaloff KC, Ramatsebe T, Viana R. et al. Accumulation of HIV drug resistance mutations in patients failing first-line antiretroviral treatment in South Africa. AIDS Res Hum Retroviruses 2012; 28: 171–5. [DOI] [PubMed] [Google Scholar]
- 18. Gupta RK, Ford D, Mulenga V. et al. Drug resistance in human immunodeficiency virus type-1 infected Zambian children using adult fixed dose combination stavudine, lamivudine, and nevirapine. Pediatr Infect Dis J 2010; 29: e57–62. [DOI] [PubMed] [Google Scholar]
- 19. Hill A, McBride A, Sawyer AW. et al. Resistance at virological failure using boosted protease inhibitors versus nonnucleoside reverse transcriptase inhibitors as first-line antiretroviral therapy–implications for sustained efficacy of art in resource-limited settings. J Infect Dis 2013; 207 Suppl 2: S78–84. [DOI] [PubMed] [Google Scholar]
- 20. Gupta R, Hill A, Sawyer AW. et al. Emergence of drug resistance in HIV type 1-infected patients after receipt of first-line highly active antiretroviral therapy: a systematic review of clinical trials. Clin Infect Dis 2008; 47: 712–22. [DOI] [PubMed] [Google Scholar]
- 21. Scherrer AU, Boni J, Yerly S. et al. Long-lasting protection of activity of nucleoside reverse transcriptase inhibitors and protease inhibitors (PIs) by boosted PI containing regimens. PLoS One 2012; 7:e50307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gupta RK, Jordan MR, Sultan BJ. et al. Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: a global collaborative study and meta-regression analysis. Lancet 2012; 380: 1250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection 2013 http://www.who.int/hiv/pub/guidelines/arv2013/download/en/index.html.
- 24. Rougemont M, Nchotu Ngang P, Stoll B. et al. Safety of zidovudine dose reduction in treatment-naive HIV infected patients. A randomized controlled study (MiniZID). HIV Med 2016; 17: 206–15. [DOI] [PubMed] [Google Scholar]
- 25. Paton NI, Kityo C, Hoppe A. et al. Assessment of second-line antiretroviral regimens for HIV therapy in Africa. N Engl J Med 2014; 371: 234–47. [DOI] [PubMed] [Google Scholar]
- 26. Sigaloff KC, Hamers RL, Wallis CL. et al. Second-line antiretroviral treatment successfully resuppresses drug-resistant HIV-1 after first-line failure: prospective cohort in sub-Saharan Africa. J Infect Dis 2012; 205: 1739–44. [DOI] [PubMed] [Google Scholar]
- 27. Hamers RL, Schuurman R, Sigaloff KC. et al. Effect of pretreatment HIV-1 drug resistance on immunological, virological, and drug-resistance outcomes of first-line antiretroviral treatment in sub-Saharan Africa: a multicentre cohort study. Lancet Infect Dis 2012; 12: 307–17. [DOI] [PubMed] [Google Scholar]
- 28. Wittkop L, Günthard HF, de Wolf F. et al. Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord-CHAIN joint project): a European multicohort study. Lancet Infect Dis 2011; 11: 363–71. [DOI] [PubMed] [Google Scholar]
- 29. Ford N, Roberts T, Calmy A.. Viral load monitoring in resource-limited settings: a medical and public health priority. AIDS 2012; 26: 1719–20. [DOI] [PubMed] [Google Scholar]
- 30. Sigaloff KC, Hamers RL, Wallis CL. et al. Unnecessary antiretroviral treatment switches and accumulation of HIV resistance mutations; two arguments for viral load monitoring in Africa. J Acquir Immune Defic Syndr 2011; 58: 23–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.