Abstract
Objectives: To investigate the predictors of general practitioner (GP) consultation and antibiotic use in those developing sore throat.
Methods: We conducted a prospective population-based cohort study on 4461 participants in two rounds (2010–11) from 1897 households.
Results: Participants reported 2193 sore throat illnesses, giving a community sore throat incidence of 1.57/ person-year. 13% of sore throat illnesses led to a GP consultation and 56% of these consultations led to antibiotic use. Participants most likely to have sore throats included women and children (e.g. school compared with retirement age); adjusted incidence rate ratio (aIRR) of 1.33 and 1.52, respectively. Participants with sore throat were more likely to consult their GP if they were preschool compared with retirement age [adjusted OR (aOR) 3.22], had more days of sore throat (aOR 1.11), reported more severe pain (aOR 4.24) or reported fever (aOR 3.82). Antibiotics were more often used by chronically ill individuals (aOR 1.78), those reporting severe pain (aOR 4.14), those reporting fever (aOR 2.58) or children with earache (aOR 1.85). Among those who consulted, males and adults who reported feeling anxious were more likely to use antibiotics; aOR 1.87 and 5.36, respectively.
Conclusions: Only 1 in 10 people who have a sore throat see a doctor and more than half of those attending get antibiotics. Further efforts to curb antibiotic use should focus on reducing initial GP consultations through public information promoting safe self-management, targeted at groups identified above as most likely to attend with sore throats.
Introduction
More than one-third of all antibiotics prescribed for respiratory infections are because of sore throat,1 and one in two patients presenting to their general practitioner (GP) with these symptoms receive antibiotics.2,3 There is a national drive in the UK to reduce antibiotic prescribing,4 based on high-level evidence. Meta-analysis of randomized controlled trials of antibiotics for sore throat have shown that they only provide a small reduction in symptom severity and duration (1 day).5 UK observational studies have shown that a very large number of sore throats need to be treated with antibiotics to prevent a single complication of infection.6–10 Survey studies have shown that 1 in 5 patients taking broad-spectrum antibiotics and 1 in 12 taking narrow-spectrum antibiotics suffer side effects such as a rash or gastrointestinal upset,11–15 whilst observational studies using routine data have linked antibiotic use directly to drug resistance at international16,17and local18 levels.
So far, national guidance,4 GP education schemes19–22 and alternative prescribing strategies23 have focused on the GP consultation in an effort to reduce antibiotic use. However, the implementation of consultation-based strategies seems limited since antibiotic use and practice variation are not only high,3 but on the increase.24 There is therefore a continuing need to develop and target strategies that promote patient safe self-management of sore throat in the community and prevent unnecessary consultation. In fact, the last and only population-based study in England to investigate sore throat in the community was conducted in 1974 on 198 women aged 20–44 years over a 28 day observation period.25–27 This small and select sample, with short follow-up, undertaken four decades ago does not provide sufficient information on community burden and risk factors for sore throat, subsequent GP consultation and antibiotic use to inform new public information campaigns. We investigated these issues using a large population-based prospective national household survey including detailed reports of sore throat symptom profiles and GP consultation behaviour over two whole winter seasons in order to inform and target public health campaigns.
Methods
National household survey
One hundred and forty-six general practices volunteered to participate in the study through the primary care research network. GPs sent invitations to a random sample of their register. Around 10% of households invited to participate did so.28 In total, 4461 participants were recruited between the two waves of the survey during the winters of 2009/2010 and 2010/2011. Baseline questionnaires were used to collect basic demographic and medical history information. This included age, gender, postcode, ethnicity, pre-existing medical conditions and number of people living in the same household. Subsequently, participants were asked to complete prospective illness diaries for every day they experienced a ‘sore throat’. Data were collected daily through online surveys to reduce recall bias, with weekly telephone reminders to minimize missing data. Participants were asked about the presence and severity of sore throat and associated symptoms such as feeling feverish, headache, having muscle aches, cough, runny nose, blocked nose and sneezing. During illnesses participants completed a generic health-related quality-of-life measure (EQ5D-3L).29 Participants were also asked if they had sought help from their GP for their problems and whether they used antibiotics. The lead household responder was responsible for the scores entered for any persons in the house younger than 16, entering results by proxy for the very young and supervising more competent children with the process. Further details of the survey methodology have been described elsewhere.30
Variable definition
A sore throat episode was defined as two or more consecutive days of moderate to severe sore throat self-reported by the participant.
An episode was assumed to have ended safely when the participant was free from symptoms for 2 days or more. A new episode was recorded after at least 7 days without symptoms. The relatively short 7 day window between episodes was allowed following sensitivity analyses between 14 and 21 day periods that showed no difference in overall sore throat incidence. If the participant consulted with the GP on multiple occasions during their illness only the first consultation was counted.
Age was categorized as preschool (0–4 years), early school (5–13 years), adolescence and young adult (14–24 years), early adulthood (25–44 years), middle age (45–65 years) and retirement age (>65 years). Ethnicity was defined as white British and other. Postcode was used to define participants’ geographical region in England (North, West Midlands, East Midlands and East of England, London, South-East and South-West), population density (defined as urban or rural) and index of multiple deprivation (categorized into national quintiles).
Sore throat and all associated symptoms (as described above) were reported absent, mild or moderate–severe. The EQ5D-3L consisted of five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression), each with three levels of functioning (no problems, some problems and extreme problems). Both the index score and domain specific values were evaluated.
Weighting
Since the survey was oversampled in South-West England and undersampled in those between 0 and 15 years we weighted analyses to age and regional structure of England to give locally and nationally representative estimates. The final weight also accounted for the method of sampling through households (i.e. participants from a larger household had a greater chance of being sampled compared with those from smaller households).
Modelling
We used multilevel (patient and general practice level) Poisson regression models to evaluate sociodemographic determinants of sore throat, and logistic regression models to investigate sociodemographic and symptom profiles effects on GP consultation and antibiotic use.
Risk factors found to be significant in univariable analyses were modelled using multilevel multivariable models (at the level of the patient and general practice), in a forward stepwise fashion. We undertook tests for interaction between variables if both variables were independently related to the outcome and had a biologically plausible interdependent relationship to outcome. All statistical analyses were undertaken on Stata SE 13.1.
Missing data
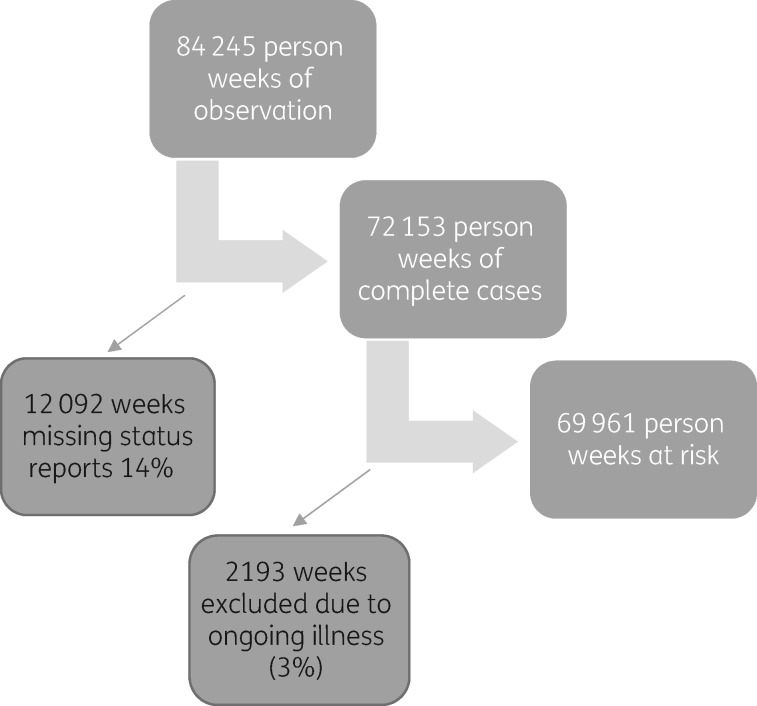
Status reports were missing for 12 092 weeks of the 84 245 weeks of person time, i.e. 14.4% of the data originally available for analysis were missing (see Figure 1). Sensitivity analyses were undertaken to explore the impact of these missing data. In one analysis we assumed that a week missing a status report was a week of no illness, whilst in another analysis we excluded the weeks with missing status reports from analysis (completed weeks analysis). There was minimal difference in the rates produced by either analysis and so we used completed weeks for subsequent analyses.
Figure 1.
Loss to follow-up.
Loss to follow-up was defined by absence of weekly status reports in the last month of study. Less than 3% of the study population was lost to follow-up.
Ethics
This study underwent ethical approval at Oxford A Research Ethics Committee (REC), 10/H0604/56. The study registration number was ISRCTN80214280.
Results
Main findings
The 4461 participants (median age 46 years, IQR 19–62, range 0–99) reported 2193 sore throat illnesses over 489 731 days at risk, giving a community sore throat incidence of 4.29 (95% CI 3.83–4.81)/1000 person-days and 1.57/person-year. In 280 of the 2193 (13%) sore throat illnesses, patients visited their GP, with the remainder self-managing their symptoms. Of those who consulted their GP, 157 (56%) used antibiotics.
Sore throat incidence in the community
Key findings from univariable Poisson analyses (Table 1) include the increased risk of reporting a sore throat in females [incidence rate ratio (IRR) 1.32] and younger people (school age versus retirement age; IRR 1.70). White ethnicity, being a non-smoker, living in a large household (5 versus 2 people), receiving a flu vaccination that season and living in an urban region increased the risk of reporting a sore throat significantly.
Table 1.
Sore throat incidence in the community and multilevel Poisson regression for sore throat risk factors
| Sore throat illnesses | Time at risk (days) | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|---|
| IRR (95% CI) | incidence (episodes/1000 person days) | P | aIRR (95% CI) | P | |||
| 0–4 years | 87 | 22 240 | 1.41 (1.14–1.73) | 3.96 | <0.001 | 1.20 (0.92–1.56) | <0.001 |
| 5–13 years | 283 | 56 819 | 1.70 (1.47–1.97) | 4.78 | 1.52 (1.27–1.82) | ||
| 14–24 years | 169 | 36 121 | 1.54 (1.30–1.82) | 4.3 | 1.54 (1.25–1.89) | ||
| 25–44 years | 533 | 92 143 | 1.88 (1.65–2.14) | 5.28 | 1.79 (1.53–2.09) | ||
| 45–65 years | 787 | 176 474 | 1.47 (1.30–1.65) | 4.13 | 1.36 (1.18–1.57) | ||
| >65 years | 334 | 105 933 | 1 | 2.81 | 1 | ||
| Male | 924 | 240 766 | 0.76 (0.71–0.83) | 3.56 | <0.001 | 0.75 (0.68–0.82) | <0.001 |
| Female | 1269 | 248 965 | 1 | 4.69 | 1 | ||
| North | 214 | 48 046 | 0.96 (0.79–1.17) | 3.83 | 0.07 | – | – |
| West Midlands | 116 | 27 076 | 0.93 (0.74–1.17) | 3.71 | – | ||
| East and East Midlands | 852 | 160 806 | 1.13 (0.98–1.30) | 4.51 | – | ||
| London | 155 | 29 088 | 1.18 (0.95–1.47) | 4.70 | – | ||
| South-East | 223 | 60 849 | 0.88 (0.72–1.09) | 3.51 | – | ||
| South-West | 633 | 163 866 | 1 | 3.99 | – | ||
| Smokers | 88 | 28 809 | 0.60 (0.20–0.73) | 2.61 | <0.001 | – | – |
| Non-smokers | 1737 | 366 203 | 1 | 4.35 | – | – | |
| White ethnicity | 2028 | 422 601 | 1 | 4.36 | 0.04 | – | – |
| Non-white ethnicity | 76 | 15 242 | 0.79 (0.63–0.99) | 3.45 | – | ||
| One person in household | 116 | 27 988 | 1.03 (0.86–1.24) | 3.82 | <0.001 | – | – |
| Two people in household | 898 | 221 787 | 1 | 3.71 | – | ||
| Three people in household | 337 | 73 342 | 1.15 (1.02–1.30) | 4.70 | – | ||
| Four people in household | 596 | 117 010 | 1.30 (1.18–1.44) | 5.32 | – | ||
| Five people in household | 201 | 41 532 | 1.23 (1.06–1.42) | 5.03 | – | ||
| Six people in household | 45 | 8073 | 1.00 (0.75–1.32) | 4.09 | – | ||
| Received flu vaccination this year | 543 | 110 449 | 1.02 (0.93–1.11) | 4.17 | <0.001 | – | – |
| Did not receive flu vaccine this year | 1608 | 368 198 | 1 | 4.09 | – | ||
| IMD 1 (most deprived) | 59 | 12 889 | 0.99 (0.78–1.25) | 4.38 | 0.11 | – | – |
| IMD 5 (least deprived) | 730 | 144 627 | 1 | 4.43 | – | ||
| Urban | 904 | 183 773 | 1.06 (0.96–1.16) | 4.45 | <0.001 | – | – |
| Rural | 1275 | 270 848 | 1 | 4.20 | – | ||
| Well | 1836 | 374 381 | 1 | 4.30 | 0.6 | – | – |
| Chronic illness | 328 | 71 249 | 0.96 (0.84–1.10) | 4.13 | – | – | |
IMD, index of multiple deprivation (IMD 5 = least deprived quintile).
In the multivariable Poisson analysis only female gender [adjusted IRR (aIRR) 1.33] and being young (aIRR 1.52) increased the risk of reporting a sore throat illness (Table 1). Social deprivation, ethnicity, chronic illness, population density, influenza vaccination and smoking were not related to risks of reporting a sore throat. Interaction testing showed a significant interaction between gender and age with females between the ages of 5 and 44 more likely to report sore throats.
GP consultation behaviour for those with sore throat illnesses
Results from univariable logistic analyses are summarized in Table 2. Sociodemographic factors increasing the risk of a person with sore throat consulting their GP included being young. Disease factors increasing the risk of GP consultation for sore throat included duration of sore throat episode, severity of pain, ear ache, fever and cough. A reduction in health-related quality of life, as measured by increases in certain EQ5D-3L subdomain scores (usual activities, self-care, mobility and anxiety) was associated with increased chance of GP consultation. Multivariable logistic analysis showed that being young [adjusted OR (aOR) 3.22], days of sore throat (aOR 1.11), extreme pain (aOR 4.24) and fever (aOR 3.82) significantly increased the risk of participants consulting their GP for a sore throat. Tests of interaction between age and sore throat symptoms (duration, pain and fever) were not significant.
Table 2.
Risk of GP consultation for sore throat and multilevel logistic regression of risk factors for GP consultation
| Variable | GP visits | Total sore throat episodes | % | Univariable analysis |
Multivariable analysis |
||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | aOR (95% CI) | P | ||||
| Chronically ill | 65 | 442 | 14.71 | 1.81 (1.26–2.60) | 0.001 | — | — |
| Well | 211 | 2425 | 8.70 | 1 | — | ||
| 0–4 years | 37 | 87 | 42.53 | 3.38 (1.92–5.96) | <0.001 | 3.22 (1.81–5.73) | <0.001 |
| 5–13 years | 37 | 283 | 13.07 | 1.26 (0.71–2.23) | 1.25 (0.68–2.27) | ||
| 14–24 years | 27 | 169 | 15.98 | 1.58 (0.82–3.04) | 1.99 (0.90–4.40) | ||
| 25–44 years | 51 | 533 | 9.57 | 0.93 (0.56–1.53) | 0.99 (0.60–1.65) | ||
| 45–65 years | 90 | 787 | 11.44 | 1.04 (0.65–1.66) | 1.04 (0.60–1.77) | ||
| >65 years | 38 | 334 | 11.38 | 1 | 1 | ||
| Female | 169 | 1269 | 13.32 | 1 | 0.3 | 1 | 0.24 |
| Male | 111 | 924 | 12.01 | 0.85 (0.63–1.16) | 0.82 (0.59–1.14) | ||
| Rural | 180 | 1275 | 14.12 | 1 | 0.1 | — | — |
| Urban | 100 | 904 | 11.06 | 0.77 (0.57–1.05) | — | ||
| Non-white | 16 | 76 | 21.05 | 1.76 (0.95–3.25) | 0.07 | — | — |
| White | 252 | 2028 | 12.43 | 1 | — | ||
| IMD 1 (most deprived) | 6 | 59 | 10.17 | 0.71 (0.31–1.61) | 0.63 | — | — |
| IMD 5 (least deprived) | 106 | 730 | 14.52 | 1 | — | ||
| Smokers | 10 | 88 | 11.36 | 1.04 (0.48–2.28) | 0.91 | — | — |
| Non-smokers | 194 | 1737 | 11.17 | 1 | — | ||
| Duration (days) | 271 | 2193 | 12.36 | 1.10 (1.08–1.13) | <0.001 | 1.11 (1.08–1.14) | <0.001 |
| Earache | 101 | 424 | 23.82 | 2.99 (2.19–4.09) | <0.001 | — | — |
| No earache | 179 | 1684 | 10.63 | 1 | — | ||
| Any cough | 236 | 1612 | 14.64 | 2.24 (1.49–3.36) | <0.001 | — | |
| No cough | 44 | 581 | 7.57 | 1 | — | ||
| Fever | 115 | 475 | 24.21 | 4.87 (3.34–7.09) | <0.001 | 3.82 (2.64–5.56) | <0.001 |
| No fever | 94 | 1522 | 6.19 | 1 | 1 | ||
| EQ5D index score | 271 | 2193 | 12.36 | 0.10 (0.05–0.19) | <0.001 | — | — |
| Extreme pain | 24 | 61 | 39.34 | 6.95 (3.23–14.97) | <0.001 | 4.24 (1.81–9.94) | 0.004 |
| Some pain | 47 | 388 | 12.11 | 1.48 (0.94–2.33) | 1.38 (0.83–2.29) | ||
| No pain | 209 | 2449 | 8.53 | 1 | 1 | ||
| Extreme loss of usual activity | 28 | 84 | 33.33 | 5.44 (2.92–10.12) | <0.001 | — | — |
| Some loss of usual activity | 34 | 222 | 15.32 | 1.97 (1.13–3.41) | |||
| No loss of usual activity | 218 | 2592 | 8.41 | 1 | — | ||
| Extreme inability to self-care | 9 | 16 | 56.25 | 12.77 (3.32–49.08) | <0.001 | — | — |
| Some inability to self-care | 11 | 40 | 27.50 | 3.77 (1.41–10.10) | |||
| Normal self-care | 260 | 2842 | 9.15 | 1 | — | ||
| Extreme loss of mobility | 20 | 50 | 40.00 | 6.95 (4.09–11.80) | <0.001 | — | — |
| Some loss of mobility | 18 | 83 | 21.69 | 2.89 (1.39–6.01) | |||
| Normal mobility | 242 | 2765 | 8.75 | 1 | — | ||
| Severe anxiety | 4 | 11 | 36.36 | 5.67 (1.24–25.98) | 0.001 | — | — |
| Some anxiety | 22 | 111 | 19.82 | 2.45 (1.32–4.55) | |||
| No anxiety | 254 | 2776 | 9.15 | 1 | — | ||
IMD, index of multiple deprivation (IMD 5 = least deprived quintile).
Antibiotic prescribing for sore throat
Initial multivariable multilevel logistic analyses of antibiotic use in relation to all sore throat episodes showed that chronic illness was the only patient factor related to antibiotic use (aOR 1.78). Sore throat symptom features related to antibiotic use included earache (aOR 1.85), fever (aOR 2.58) and extreme pain (aOR 4.14) (see Table 3 for all results). Further testing only showed an interaction between age and earache: children were more likely to receive antibiotics if they reported a sore throat as well as earache compared with adults.
Table 3.
Risk of antibiotic use amongst those with sore throat and multilevel logistic regression risk factors for antibiotic use
| Variable | Antibiotics taken | Total sore throat episodes | % | Univariable analysis |
Multivariable analysis |
||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | aOR (95% CI) | P | ||||
| Chronically ill | 56 | 328 | 17.07 | 1.99 (1.37–2.88) | <0.001 | 1.78 (1.24–2.62) | 0.004 |
| Well | 167 | 1836 | 9.10 | 1 | 1 | ||
| 0–4 years | 23 | 87 | 26.44 | 2.61 (1.21–5.63) | 0.1 | 2.23 (0.99–5.01) | 0.23 |
| 5–13 years | 25 | 283 | 8.83 | 1.08 (0.54–2.14) | 1.01 (0.47–2.19) | ||
| 14–24 years | 19 | 169 | 11.24 | 1.36 (0.62–2.97) | 1.61 (0.74–1.49) | ||
| 25–45 years | 46 | 533 | 8.63 | 1.08 (0.60–1.95) | 1.08 (0.58–2.03) | ||
| 46–65 years | 84 | 787 | 10.67 | 1.27 (0.75–2.17) | 1.22 (0.70–2.11) | ||
| >65 years | 29 | 334 | 8.68 | 1 | 1 | ||
| Female | 132 | 1269 | 10.40 | 1 | 0.94 | 1 | 0.82 |
| Male | 94 | 924 | 10.17 | 0.99 (0.70–1.39) | 1.04 (0.73–1.49) | ||
| Rural | 137 | 1275 | 10.75 | 1 | 0.60 | — | |
| Urban | 89 | 904 | 9.85 | 0.91 (0.63–1.31) | — | ||
| Non-white | 14 | 76 | 18.42 | 1.75 (0.81–3.78) | 0.15 | — | |
| White | 206 | 2028 | 10.16 | 1 | — | ||
| IMD 1 (most deprived) | 4 | 59 | 6.78 | 0.69 (0.24–1.98) | 0.85 | — | |
| IMD 5 (least deprived) | 73 | 730 | 10.00 | 1 | — | ||
| Smokers | 11 | 88 | 12.50 | 1.21 (0.54–2.74) | 0.64 | — | |
| Non-smokers | 215 | 2105 | 10.21 | 1 | — | ||
| Duration (days) | 2193 | 2193 | 100.00 | 1.09 (1.06–1.11) | <0.001 | 1.08 (1.06–1.10) | |
| Earache | 80 | 424 | 18.87 | 2.36 (1.68–3.29) | <0.001 | 1.85 (1.25–2.75) | 0.002 |
| No earache | 146 | 1684 | 8.67 | 1 | 1 | ||
| Any cough | 191 | 1612 | 11.85 | 1.90 (1.18–3.03) | 0.008 | — | |
| No cough | 35 | 572 | 6.12 | 1 | — | ||
| Fever | 83 | 367 | 22.62 | 3.02 (2.19–4.17) | <0.001 | 2.58 (1.74–3.82) | <0.001 |
| No fever | 143 | 1826 | 7.83 | 1 | 1 | ||
| EQ5D index score | 2193 | 2193 | 100.00 | 0.13 (0.06–0.28) | <0.001 | — | |
| Extreme pain | 21 | 52 | 40.38 | 5.88 (2.81–12.30) | <0.001 | 4.14 (1.74–3.81) | 0.002 |
| Some pain | 37 | 331 | 11.18 | 1.33 (0.82–2.15) | 1.18 (0.74–1.89) | ||
| No pain | 168 | 1810 | 9.28 | 1 | 1 | ||
| Extreme loss of usual activity | 20 | 76 | 26.32 | 3.34 (1.77–6.30) | <0.001 | — | |
| Some loss of usual activity | 27 | 191 | 14.14 | 1.62 (0.94–2.78) | — | ||
| No loss of usual activity | 179 | 1926 | 9.29 | 1 | — | ||
| Extreme inability to self-care | 9 | 12 | 75.00 | 13.93 (3.20–60.66) | <0.0004 | — | |
| Some inability to self-care | 10 | 32 | 31.25 | 3.44 (1.18–10.02) | — | ||
| Normal self-care | 207 | 2149 | 9.63 | 1 | — | ||
| Extreme loss of mobility | 16 | 45 | 35.56 | 5.20 (2.89–9.38) | <0.001 | — | |
| Some loss of mobility | 12 | 73 | 16.44 | 1.92 (0.93–3.96) | — | ||
| Normal mobility | 198 | 2075 | 9.54 | 1 | — | ||
| Severe anxiety | 4 | 9 | 44.44 | 6.15 (1.31–28.96) | 0.004 | — | |
| Some anxiety | 18 | 100 | 18.00 | 2.48 (1.26–4.88) | — | ||
| No anxiety | 204 | 2084 | 9.79 | 1 | — | ||
IMD, index of multiple deprivation (IMD 5 = least deprived quintile).
Subsequent analyses of antibiotic use were specifically restricted to the sore throat episodes which resulted in GP consultation. Results from multivariable multilevel logistic analyses are summarized in Table 4 and showed that being male (aOR 1.87) and self-reporting greater anxiety (aOR 5.36) significantly increased the risk of receiving antibiotics following a visit to the GP for a sore throat. Interaction testing between age and anxiety showed that adults who reported anxiety were more likely to receive antibiotics compared with children.
Table 4.
Risk of antibiotic use amongst those who consult their GP for sore throat and multilevel multivariable logistic regression for risk factors for antibiotic use
| Variable | Antibiotic taken | GP consultations | % | Multivariable analysis |
|
|---|---|---|---|---|---|
| aOR (95% CI) | P | ||||
| 0–4 years | 16 | 37 | 43.243 | 0.50 (0.16–1.53) | 0.51 |
| 5–13 years | 18 | 37 | 48.649 | 0.65 (0.21–2.07) | |
| 14–24 years | 16 | 27 | 59.259 | 1.09 (0.32–3.70) | |
| 25–44 years | 31 | 51 | 60.784 | 1.22 (0.39–3.81) | |
| 45–65 years | 54 | 90 | 60 | 0.98 (0.42–2.30) | |
| >65 years | 22 | 38 | 57.895 | 1 | |
| Female | 88 | 169 | 52.071 | 1 | 0.05 |
| Male | 69 | 111 | 62.162 | 1.87 (1.00–3.48) | |
| Anxiety | 20 | 26 | 76.923 | 5.36 (1.44–19.91) | 0.01 |
| No anxiety | 137 | 254 | 53.937 | 1 | |
Discussion
This study demonstrates a high incidence of sore throat in the community (1.57 episodes/person-year) with the majority of sore throat illnesses (87.2%) safely managed without GP consultation or prescription. Younger age not only increased the risk of reporting a sore throat (school compared with retirement age: aIRR 1.52) but also increased the chance of consulting a GP once a sore throat was reported (school age compared with retirement age: aOR 4.05). Whilst women were more likely to report a sore throat (aIRR 1.33) they were just as likely to consult their GP as men once a sore throat had been reported. Participants were more likely to consult their GP for a sore throat if their illness was associated with more days of sore throat (aOR 1.11), extreme pain (aOR 4.24) or fever (aOR 3.82). Despite some targeting of antibiotic use in sore throat illnesses to those with chronic illness (aOR 1.78), extreme pain (aOR 4.14) and fever (aOR 2.58) more than half (57%) of those attending still received antibiotics.
This is the largest population-based survey of sore throat to date, weighted to represent the national population. The prospective nature of data collection, through daily health diaries and weekly telephone calls, allowed us to reduce the recall bias inherent in retrospective interview studies. This survey method also allowed us to reduce our missing data (14% missing weekly status reports). Sensitivity analyses of different ways of accounting for our missing data showed no change in our conclusions. In contrast to electronic healthcare record studies, we were able to accurately assess the role of associated symptoms and health-related quality of life in the management of sore throat episodes, by using detailed disease profile questions. In addition, we were able to directly measure antibiotic use through patient self-report, rather than indirectly through prescription rates. This study has three limitations. Firstly, very young children, residents of North England and those of lowest socioeconomic status were under-represented in the study population. Therefore, the survey was weighted to allow the incidence to be more representative of local and national populations. Secondly, 1 year of the study was conducted in a pandemic influenza outbreak year when there was considerable media coverage, which may have increased symptom vigilance and affected consultation behaviour. Lastly, we did not have data on GP prescription rates. Therefore, the rate of delayed antibiotic prescribing cannot be ascertained. We have only reported whether antibiotics were taken or not. We have therefore not been able to evaluate the effectiveness of the delayed antibiotic prescribing strategy. Even if a delayed prescribing strategy was used frequently, the fact that more than half of sore throat consultations ended in antibiotic use shows further strategies are needed.
The only other prospective population-based study in England was undertaken in Lambeth in 1974 on 198 women, aged 20–44, who were asked to keep a prospective health diary for 28 days each. During the observation period 90 sore throat episodes were reported [an annual sore throat incidence of 5.9 (95% CI 4.7–7.3) sore throat episodes per person-year] with 33 subsequent GP consultations [a consultation rate of 37% (95% CI 25%–51%)]. However, since this was a small study in a select population it is difficult to draw meaningful comparisons. Our results add to the growing body of evidence from general population studies of respiratory infections28,31 that the majority of sore throats are managed safely in the community. The rate of antibiotic prescribing for sore throat found in our study is comparable to previous primary care studies.2,3 However, these studies retrospectively defined their sore throat population using GP diagnostic codes which are known to be inconsistently used for this condition32 and indirectly approximated antibiotic usage through prescription rates. The present study was able to prospectively define its population based on symptoms experienced and to directly measure antibiotic use through patient self-report.
We found that GP consultation for sore throat was predicted by young age. Studies of all respiratory infections also confirm that young age is a major driver for primary care use in the UK33 with qualitative studies showing that parents’ decision to bring their children to the GP is influenced by perceived threat, disease severity, the perceived benefits of consulting, and an expectation of assessment, information, advice or treatment.33–37 Our study also found that severity of throat pain, duration of sore throat and presence of fever were related to GP consultation. Explanations for these results can be offered from qualitative research into people with acute sore throat38 and respiratory tract infections31 showing that most commonly seek help from their GP for pain relief, perceived symptom severity and non-resolution of symptoms. Amongst those who see their GP for sore throat, being male and self-reporting anxiety were the only factors related to an increased chance of taking antibiotics. However, it should be noted that whilst women were more likely to report a sore throat, gender had no overall relationship between sore throat and antibiotic use (see Table 3). Having controlled for pain and duration, increased anxiety was still an important predictor for receiving antibiotics in adults. Whilst patient anxiety has not previously been studied as a driver for high antibiotic prescribing, GPs have been shown to prescribe antibiotics under perceived patient pressure.39,40 Further qualitative research could be undertaken to explore the mechanism through which patient anxiety leads GPs to prescribe antibiotics for sore throat more frequently. Our study shows no relationship between sore throat severity, fever, cough, severity of pain, reduced health-related quality of life on antibiotic use for those who consulted. Whilst these factors affected participants’ decisions to consult, they did not appear to affect subsequent antibiotic prescription in this cohort. Qualitative studies investigating high antibiotic use have focused on GP behaviour and have shown a complex interplay of factors including perceived clinical need,40,41 perceived patient/parent pressure for antibiotics,39 clinical uncertainty42–44 and the desire to maintain a good relationship with the patient (or parent).42,43,45
Interventions to reduce antibiotic prescribing such as GP outreach programmes,46 and more recently web-based GP education programmes,47 have shown some benefit but have not been widely implemented in the UK. In fact, antibiotic prescribing in primary care increased by 6.2% from 2011 to 2014,24 and even though there was a 5.4% reduction from 2015 to 2016,48 there were more antibiotic prescriptions in 2016 compared with 2011. In addition, review of the literature shows that a multifaceted approach that includes patients and the public has the greatest impact on antibiotic prescription.49,50 Therefore, there is an urgent need to design additional strategies targeted at risk groups within the general population to help them better understand and self-manage sore throat, minimizing unnecessary GP consultations and subsequent antibiotic prescribing. Whilst the general poster and leaflet campaign in England encouraging the public not to take antibiotics for common colds had little effect on antibiotic prescriptions,51 studies of targeted patient education have shown reductions in antibiotic use.52–54 Public information particularly targeted at groups that our study found to be at risk of sore throat or most likely to consult, such as women and children, could aim to promote safe self-management and reduce anxiety associated with sore throat. This could emphasize that moderate to severe pain, prolonged duration and fever are frequent self-limiting features of sore throat. There is also a need to moderate the messages by highlighting ‘red-flag’ symptoms (e.g. trismus, difficulty breathing, neck swelling, torticollis), so the threshold for help-seeking behaviour may align more accurately with patients who would benefit from antibiotics in sore throat infections.55–57 Messages which highlight the fact that antibiotics provide minimal additional benefit over and above simple over-the-counter medications and often result in unwanted side effects may be more effective than those focusing on the less immediate problem of resistance.58 Tackling the problem of over-prescribing of antibiotics in primary care requires development of joined-up strategies targeting the general public, those who present with sore throat and their doctors.
Funding
This project was funded by the Medical Research Council and the Wellcome Trust.
Funding bodies had no role in: study design; collection, analysis and interpretation of data; writing of the report; and decision to submit the article for publication. Researchers were independent from funders.
Transparency declarations
None to declare.
Author contributions
N. M. and A. H. are guarantors. E. F. and A. H. acquired the data. N. M., A. S., E. F., O. D., H. E. R. E., L. M., S. C. S. and P. L. analysed and interpreted the data. All authors revised the manuscript and agreed to be accountable.
All authors, external and internal, had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
The lead author affirms that this manuscript is an honest, accurate and transparent account of the study being reported, that no important aspects of the study have been omitted and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
References
- 1.Petersen I, Hayward AC. On behalf of the SACAR Surveillance Subgroup. Antibacterial prescribing in primary care. J Antimicrob Chemother 2007; 60: i43–7. [DOI] [PubMed] [Google Scholar]
- 2.Ashworth M, Cox K, Latinovic R. et al. Why has antibiotic prescribing for respiratory illness declined in primary care? A longitudinal study using the General Practice Research Database. J Pub Health 2004; 26: 268–74. [DOI] [PubMed] [Google Scholar]
- 3.Hawker JI, Smith S, Smith GE. et al. Trends in antibiotic prescribing in primary care for clinical syndromes subject to national recommendations to reduce antibiotic resistance, UK 1995-2011: analysis of a large database of primary care consultations. J Antimicrob Chemother 2014; 69: 3423–30. [DOI] [PubMed] [Google Scholar]
- 4.Centre for Clinical Practice at NICE (UK). Respiratory Tract Infections - Antibiotic Prescribing: Prescribing of Antibiotics for Self-Limiting Respiratory Tract Infections in Adults and Children in Primary Care. London: National Institute for Health and Clinical Excellence (UK; ), 2008. [PubMed] [Google Scholar]
- 5.Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane Database Syst Rev 2013; issue 11: CD000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen I, Johnson AM, Islam A. et al. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ 2007; 335: 982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Little P, Gould C, Williamson I. et al. Reattendance and complications in a randomised trial of prescribing strategies for sore throat: the medicalising effect of prescribing antibiotics. BMJ 1997; 315: 350–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zwart S, Sachs AP, Ruijs GJ. et al. Penicillin for acute sore throat: randomised double blind trial of seven days versus three days treatment or placebo in adults. BMJ 2000; 320: 150–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper RJ, Hoffman JR, Bartlett JG. et al. Principles of appropriate antibiotic use for acute pharyngitis in adults: background. Ann Intern Med 2001; 134: 509–17. [DOI] [PubMed] [Google Scholar]
- 10.Del Mar CB, Glasziou PP, Spinks AB. Antibiotics for sore throat. Cochrane Database Syst Rev 2006; issue 4: CD000023.. [DOI] [PubMed] [Google Scholar]
- 11.Lode H. Safety and tolerability of commonly prescribed oral antibiotics for the treatment of respiratory tract infections. Am J Med 2010; 123 Suppl: S26–38. [DOI] [PubMed] [Google Scholar]
- 12.Arason VA, Kristinsson KG, Sigurdsson JA. et al. Do antimicrobials increase the carriage rate of penicillin resistant pneumococci in children? Cross sectional prevalence study. BMJ 1996; 313: 387–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shehab N, Patel PR, Srinivasan A. et al. Emergency department visits for antibiotic‐associated adverse events. Clin Infect Dis 2008; 47: 735–43. [DOI] [PubMed] [Google Scholar]
- 14.Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med 2002; 346: 334–9. [DOI] [PubMed] [Google Scholar]
- 15.Kuehn J, Ismael Z, Long PF. et al. Reported rates of diarrhea following oral penicillin therapy in pediatric clinical trials. J Pediatr Pharmacol Ther 2015; 20: 90–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Editorial. Antimicrobial resistance: a global threat. Essential Drugs Monitor 2000.
- 17.Thapa B. Antimicrobial resistance: a global threat. Int J Infection Microbiol 2012; 1: 41–2. [Google Scholar]
- 18.Ashiru-Oredope D, Hopkins S; on behalf of the English Surveillance Programme for Antimicrobial Utilization and Resistance Oversight Group. Antimicrobial stewardship: English Surveillance Programme for Antimicrobial Utilization and Resistance (ESPAUR). J Antimicrob Chemother 2013; 68: 2421–3. [DOI] [PubMed] [Google Scholar]
- 19.Grimshaw JMJ, Eccles MPM, Walker AEA. et al. Changing physicians’ behavior: what works and thoughts on getting more things to work. J Contin Educ Health Prof 2002; 22: 237–43. [DOI] [PubMed] [Google Scholar]
- 20.Grimshaw JM, Russell IT. Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. Lancet 1993; 342: 1317–22. [DOI] [PubMed] [Google Scholar]
- 21.Wensing M, van der Weijden T, Grol R. Implementing guidelines and innovations in general practice: which interventions are effective? Br J Gen Pract 1998; 48: 991–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Couper MR. Strategies for the rational use of antimicrobials. Clin Infect Dis 1997; 24 Suppl 1: S154–6. [DOI] [PubMed] [Google Scholar]
- 23.Arroll B, Kenealy T, Kerse N. Do delayed prescriptions reduce antibiotic use in respiratory tract infections? A systematic review. Br J Gen Pract 2003; 53: 871–7. [PMC free article] [PubMed] [Google Scholar]
- 24.PHE. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) 2010 to 2014 2015. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/477962/ESPAUR_Report_2015.pdf.
- 25.Morrell DC, Wale CJ. Symptoms perceived and recorded by patients. J R Coll Gen Pract 1976; 26: 398–403. [PMC free article] [PubMed] [Google Scholar]
- 26.Banks MHM, Beresford SAS, Morrell DCD. et al. Factors influencing demand for primary medical care in women aged 20-44 years: a preliminary report. Int J Epidemiol 1975; 4: 189–95. [DOI] [PubMed] [Google Scholar]
- 27.Morrell DC, Avery AJ, Watkins CJ. Management of minor illness. Br Med J 1980; 280: 769–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayward AC, Fragaszy EB, Bermingham A. et al. Comparative community burden and severity of seasonal and pandemic influenza: results of the Flu Watch cohort study. Lancet Respir Med 2014; 2: 445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.EuroQol Group. EuroQol - a new facility for the measurement of health-related quality of life. Health Policy 1990; 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 30.Fragaszy EB, Quinlivan M, Breuer J. et al. Population-Level Susceptibility, Severity and Spread of Pandemic Influenza: Design of, and Initial Results from, a Pre-Pandemic and Hibernating Pandemic Phase Study Using Cross-Sectional Data from the Health Survey for England (HSE). Southampton: NIHR Journals Library, 2015; 1–24. [PubMed] [Google Scholar]
- 31.McNulty CA, Nichols T, French DP. et al. Expectations for consultations and antibiotics for respiratory tract infection in primary care: the RTI clinical iceberg. Br J Gen Pract 2013; 63: 429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marshall TT, Mohammed MAM, Lim HTH. Understanding variation for clinical governance: an illustration using the diagnosis and treatment of sore throat. Br J Gen Pract 2002; 52: 277–83. [PMC free article] [PubMed] [Google Scholar]
- 33.Wyke S, Hewison J, Russell IT. Respiratory illness in children: what makes parents decide to consult? Br J Gen Pract 1990; 40: 226–9. [PMC free article] [PubMed] [Google Scholar]
- 34.Neill SJ. Acute childhood illness at home: the parents’ perspective. J Adv Nurs 2000; 31: 821–32. [DOI] [PubMed] [Google Scholar]
- 35.Kai J. Parents’ difficulties and information needs in coping with acute illness in preschool children: a qualitative study. BMJ 1996; 313: 987–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kai J. What worries parents when their preschool children are acutely ill, and why: a qualitative study. BMJ 1996; 313: 983–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cabral C, Lucas PJ, Ingram J. et al. “It’s safer to … …” parent consulting and clinician antibiotic prescribing decisions for children with respiratory tract infections: an analysis across four qualitative studies. Soc Sci Med 2015; 136–137: 156–64. [DOI] [PubMed] [Google Scholar]
- 38.van Driel ML, De Sutter A, Deveugele M. et al. Are sore throat patients who hope for antibiotics actually asking for pain relief? Ann Fam Med 2006; 4: 494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mangione-Smith R, McGlynn EA, Elliott MN. et al. The relationship between perceived parental expectations and pediatrician antimicrobial prescribing behavior. Pediatrics 1999; 103: 711–8. [DOI] [PubMed] [Google Scholar]
- 40.Little P, Dorward M, Warner G. et al. Importance of patient pressure and perceived pressure and perceived medical need for investigations, referral, and prescribing in primary care: nested observational study. BMJ 2004; 328: 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischer DD, Stewart ALA, Bloch DAD. et al. Capturing the patient’s view of change as a clinical outcome measure. JAMA 1999; 282: 1157–62. [DOI] [PubMed] [Google Scholar]
- 42.Tonkin-Crine S, Yardley L, Little P. Antibiotic prescribing for acute respiratory tract infections in primary care: a systematic review and meta-ethnography. J Antimicrob Chemother 2011; 66: 2215–23. [DOI] [PubMed] [Google Scholar]
- 43.Tonkin-Crine S, Yardley L, Coenen S. et al. GPs’ views in five European countries of interventions to promote prudent antibiotic use. Br J Gen Pract 2011; 61: 252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whaley LE, Businger AC, Dempsey PP. et al. Visit complexity, diagnostic uncertainty, and antibiotic prescribing for acute cough in primary care: a retrospective study. BMC Fam Pract 2013; 14: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petursson P. GPs’ reasons for “non-pharmacological” prescribing of antibiotics. A phenomenological study. Scand J Prim Health Care 2005; 23: 120–5. [DOI] [PubMed] [Google Scholar]
- 46.Davey P, Brown E, Charani E. et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2013; issue 4: CD003543.. [DOI] [PubMed] [Google Scholar]
- 47.Little P, Stuart B, Francis N. et al. Effects of internet-based training on antibiotic prescribing rates for acute respiratory-tract infections: a multinational, cluster, randomised, factorial, controlled trial. Lancet 2013; 382: 1175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.NHS England. Success in NHS Push to Reduce Avoidable Antibiotic Prescribing https://www.england.nhs.uk/2016/03/antibiotic-prescribing/.
- 49.Arnold SR, Straus SE. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst Rev 2005; issue 4: CD003539.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Velden AW, Pijpers EJ, Kuyvenhoven MM. et al. Effectiveness of physician-targeted interventions to improve antibiotic use for respiratory tract infections. Br J Gen Pract 2012; 62: e801–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McNulty CAM, Nichols T, Boyle PJ. et al. The English antibiotic awareness campaigns: did they change the public’s knowledge of and attitudes to antibiotic use? J Antimicrob Chemother 2010; 65: 1526–33. [DOI] [PubMed] [Google Scholar]
- 52.Trepka MJ, Belongia EA, Chyou PH. et al. The effect of a community intervention trial on parental knowledge and awareness of antibiotic resistance and appropriate antibiotic use in children. Pediatrics 2001; 107: E6. [DOI] [PubMed] [Google Scholar]
- 53.Samore MH, Bateman K, Alder SC. et al. Clinical decision support and appropriateness of antimicrobial prescribing: a randomized trial. JAMA 2005; 294: 2305–14. [DOI] [PubMed] [Google Scholar]
- 54.Taylor JA, Kwan-Gett TSC, McMahon EM., Jr. Effectiveness of a parental educational intervention in reducing antibiotic use in children. Pediatr Infect Dis J 2005; 24: 489–93. [DOI] [PubMed] [Google Scholar]
- 55.Kataria G, Saxena A, Bhagat S. et al. Deep neck space infections: a study of 76 cases. Iran J Otorhinolaryngol 2015; 27: 293–9. [PMC free article] [PubMed] [Google Scholar]
- 56.Kilty SJ, Gaboury I. Clinical predictors of peritonsillar abscess in adults. J Otolaryngol Head Neck Surg 2008; 37: 165–8. [PubMed] [Google Scholar]
- 57.Shoemaker M, Lampe RM, Weir MR. Peritonsillitis: abscess or cellulitis? Pediatr Infect Dis 1986; 5: 435–9. [PubMed] [Google Scholar]
- 58.Wellcome Trust. Exploring the Consumer Perspective on Antimicrobial Resistance http://wellcomelibrary.org/item/b24978000#?c=0&m=0&s=0&cv=0.