Abstract
Aims
Ibutilide is a rapid-acting antiarrhythmic drug with worldwide use for conversion of recent-onset atrial fibrillation. Vernakalant, approved in the EU in 2010, is likewise used intravenously, with proven efficacy and safety compared with placebo and amiodarone in randomized clinical trials. The aim of our study was to compare the time to conversion and the conversion rate within 90 min in patients with recent-onset atrial fibrillation treated with vernakalant or ibutilide.
Methods and results
A randomized controlled trial registered at clinicaltrials.gov (NCT01447862) was performed in 100 patients with recent-onset atrial fibrillation treated at the emergency department of a tertiary care hospital. Patients received up to two short infusions of vernakalant (n = 49; 3 mg/kg followed by 2 mg/kg if necessary) or ibutilide (n = 51; 1 mg followed by another 1 mg if necessary) according to the manufacturer's instructions. Clinical and laboratory variables, adverse events, conversion rates, and time to conversion were recorded. Time to conversion of AF to sinus rhythm was significantly shorter in the vernakalant group compared with the ibutilide group (median time: 10 vs. 26 min, P = 0.01), and likewise the conversion success within 90 min was significantly higher in the vernakalant group (69 vs. 43%, log-rank P = 0.002). No serious adverse events occurred.
Conclusion
Vernakalant was superior to ibutilide in converting recent-onset atrial fibrillation to sinus rhythm in the emergency department setting.
Keywords: Vernakalant, Ibutilide, Recent-onset atrial fibrillation, Cardioversion, Emergency department
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia and is expected to double till 2060 to 18 million in the EU when compared with 2010.1 It is associated with increased cardiovascular morbidity and mortality2 and devastating stroke. Many studies have investigated the efficacy of different drugs in converting AF to sinus rhythm (SR). There are numerous randomized controlled trials comparing the value of these drugs with placebo, as well as among each other. Efficacy for acute conversion of AF was demonstrated for ibutilide, flecainide, dofetilide, propafenone, and amiodarone, and there was moderate evidence for quinidine.3
Ibutilide, a Class III antiarrhythmic drug that blocks IKr, blocks the rapid component of the cardiac delayed rectifier potassium current and activates a late inward sodium current,4 is approved for acute termination of AF and atrial flutter of recent onset and has been shown to be superior to sotalol5 and equivalent to flecainide6 in this indication in two randomized controlled trials. Recently, the relatively atrial selective antiarrhythmic agent vernakalant has been approved by the European Medicines Agency for the rapid conversion of recent-onset AF to SR in adults. It is recommended for rhythm control by the European Society of Cardiology (ESC) since the 2010 guidelines7 and highlighted in a recent review.8 Vernakalant rapidly terminates AF through blocking of potassium and frequency- and voltage-dependent sodium ion channels in all phases of the atrial action potential.9 In the AVRO trial (a randomized active-controlled study comparing the efficacy and safety of vernakalant to amiodarone in recent-onset AF), vernakalant demonstrated being more effective than amiodarone for acute conversion of recent-onset AF10 as well as in a small single-centre trial from Argentina, intravenous vernakalant was significantly faster in converting AF to SR when compared with oral flecainide.11 Vernakalant and ibutilide have comparable pharmacokinetic properties and are both recommended by the current ESC focused guidelines update for the management of AF in patients with and without moderate, but not severe structural heart disease.7
The aim of our study was to compare the time and the efficacy of conversion to SR within 90 min in patients with recent-onset AF treated with vernakalant or ibutilide intravenously.
Methods
The study protocol was approved by the independent Ethics Committee of the Medical University of Vienna (EK NR: 220/2011). The trial was conducted according to the ICH-GCP guidelines (International Conference on Harmonisation–Good Clinical Practice) as well as the Declaration of Helsinki and registered at Clinicaltrials.gov as NCT01447862 (EudraCT number 2011-000695-34). All subjects gave their written informed consent before inclusion. There was no contribution from the pharmaceutical industry at any stage of the trial.
Study design
One hundred patients with recent-onset AF (symptoms of AF since no longer than 48 h) treated at the emergency department of the Medical University of Vienna have been enrolled in this block-randomized controlled trial.
Prior to randomization we performed several routine baseline and screening examinations, which included medical history, physical exam, standard 12-lead ECG with measurement of QT interval, transthoracic echocardiography, chest X-ray, and routine laboratory testing (including TSH and free T4).
Male and female patients between 18 and 90 years were included in the study. Exclusion criteria were necessity for immediate electrical cardioversion due to haemodynamic instability; heart failure NYHA III/IV; a previously documented left ventricular ejection fraction of ≤35%; history or signs of acute coronary syndrome within the last 30 days; a resting ventricular rate of <80 bpm without pacemaker backup; a QT interval of >440 ms; presence of Wolff–Parkinson–White syndrome; history of Torsade de pointes (TdP) arrhythmia or other polymorphic ventricular tachycardias (VTs); signs of thyrotoxicosis, sick sinus syndrome or atrioventricular block II and III, severe valvular heart disease, clinically meaningful hypertrophic obstructive cardiomyopathy, restrictive cardiomyopathy, or constrictive pericarditis; serious disorders of the hepatic, renal, pulmonary, gastrointestinal, haematological, or central nervous system; serious psychiatric disorders; abnormal serum electrolytes despite adequate therapy; intravenous use of any Class I or III antiarrhythmic drugs within 4 h prior to study drug application; pregnancy; and known hypersensitivity to study medications.
We did not include patients with atrial flutter as vernakalant treatment is not indicated in this patient group due to lack of efficacy.12
Block randomization into two treatment groups with variable block sizes of four to six was performed by an independent epidemiologist using www.randomization.com. To conceal allocation we used sequentially numbered, sealed, opaque envelopes, which were produced before initiation of the study.
If potassium values were not in the high normal range (>4.2 mmol/L), patients received a pre-fabricated electrolyte infusion (Elozell ‘spezial’, 250 mL, Fresenius Kabi, Austria) with 24 mmol of potassium and 6 mmol of magnesium prior to randomization. A total of 43 patients (88%) in the vernakalant group and 38 patients (75%) in the ibutilide group received this treatment.
Additionally, according to local standard of care, most patients received 500 mL of a balanced electrolyte solution (Elo-Mel isoton, Fresenius Kabi Austria) intravenously, in order to increase plasma volume.
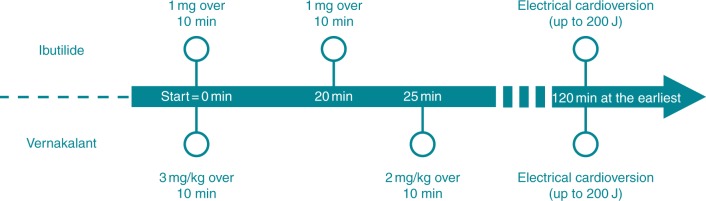
All subjects were randomized to receive either up to two infusions of ibutilide (1 mg each in 100 mL normal saline) intravenously over 10 min with an interruption of 10 min in-between or up to two infusions of vernakalant (3 and 2 mg/kg in 100 mL normal saline) with an interruption of 15 min in-between. The second dose was given only if AF persisted (Figure 1). Subjects weighing <60 kg received 0.01 mg/kg ibutilide according to the manufacturer's instructions, as well as for subjects receiving vernakalant a maximum of 339 mg for the first, and 226 mg for the second infusion was not exceeded.
Figure 1.
Timeline of therapeutic intervention. Time schedule and dose of treatment interventions in the two treatment groups.
We further were interested if vernakalant pre-treatment may also enhances electrical cardioversion efficacy as shown for ibutilide.13 Therefore, according to the study protocol, consented study patients underwent electrical cardioversion if AF persisted 2 h or longer after administration of first study drug dose. We attempted a series of up to three electrical cardioversions (biphasic defibrillation, Philips HeartStart MRx) starting with 100 J and increasing energy up to 150 J and consequently 200 J (Figure 1).
Intravenous administration of ventricular rate controlling drugs (β-blockers, calcium channel blockers, digitalis) was performed in both treatment groups if appropriate. Patients without sufficient anticoagulation received 1 mg/kg of enoxaparin subcutaneously. Monitoring included real-time 12-lead ECG, oxygen saturation, and heart rate. Additionally, arterial blood pressure, QT, and QTc (Fridericia correction) were measured every 20 min for the first 60 min; additional measurements were performed after 120, 240 min, and prior to discharge. To determine the exact time of conversion to SR, patients received continuous ECG monitoring. These traces were centrally stored in a secure database for later analysis.
Treatment had to be discontinued according to the protocol if uncorrected QT interval increased to ≥550 ms or by >25% from baseline, heart rate decreased to <40 bpm, systolic blood pressure increased to >190 mmHg or decreased to <80 mmHg, new bundle-branch block developed or QRS interval increased by ≥50% from baseline, VT, recurrent non-sustained VT (nsVT; defined as three or more ventricular extrasystoles lasting <30 s), a sinus pause of ≥5 s, complete heart block, or intolerable adverse effects occurred. A serious adverse event (SAE) was defined according to the ICH-GCP guidelines as an event resulting in death, a life-threatening condition, hospitalization or prolongation of existing hospitalization, or persistent or significant disability or incapacity. Patients were observed and monitored for at least 6 h after administration of first dosage of study drug.
Outcome measurements
Efficacy
Time to SR measured from start of first study drug administration was the primary endpoint and conversion rate to SR within 90 min was defined as the secondary endpoint. Conversion rate including electrical cardioversion was defined as the tertiary endpoint.
Statistics
Baseline and demographic data were tabulated and informally compared between the groups. We described continuous variables using mean and standard deviation or median and quartiles, as appropriate. Categorized variables were described as absolute count and relative frequency.
Median time to pharmacological conversion was compared using the Mann–Whitney U test. For comparison of cardioversion success, we used survival analysis methods. Cardioversion success until 90 min was analysed using the Kaplan–Meier method with follow-up time starting at initiation of study medication and sustained SR defined as success (15 min at least). As electrical cardioversion time was not protocol directed within the first 90 min, we censored follow-up for this analysis at 90 min. We plotted 95% confidence intervals (95% CI) around the survival probabilities and compared the intervention groups using a log-rank test. As an additional analysis, we repeated this analysis without censoring using the follow-up time until the last success. To assess adverse effects and other categorized outcomes, we calculated absolute risk differences with exact 95% CI and used the Fisher's exact test for hypothesis testing. To assess other ancillary outcomes on a continuous scale, we used the Mann–Whitney U test or t-test, as appropriate. For heart rate, QT interval and blood pressure repeated measurements were available. To allow for the panel structure of these variables, we used multivariable random effects models with each of these measurements as outcome, intervention group, time, and treatment–time interaction as covariates, and patient identifier as the cluster identifier to estimate the difference between the intervention groups based on a Wald test.
A two-sided P-value of <0.05 was considered statistically significant. All analyses were performed using MS Excel 2011 and Stata 11 (StataCorp, College Station, TX, USA) for Mac©.
Sample size calculation
We were planning a study with one control per experimental subject, with a follow-up time of 90 min after initial treatment. Prior data indicated that the median time to conversion in the control treatment was 25 min. If the true median time to conversion in the control and experimental treatments was 25 and 12 min, respectively, we needed to study 30 experimental subjects and 30 control subjects to be able to reject the null hypothesis that the experimental and control survival curves (based on time to conversion) are equal with a power of 80%. The Type I error probability associated with this log-rank test of this null hypothesis is 0.05. To compensate for an expectedly large number of missing data, we increased the study sample to 50 patients per group.
Results
Study population
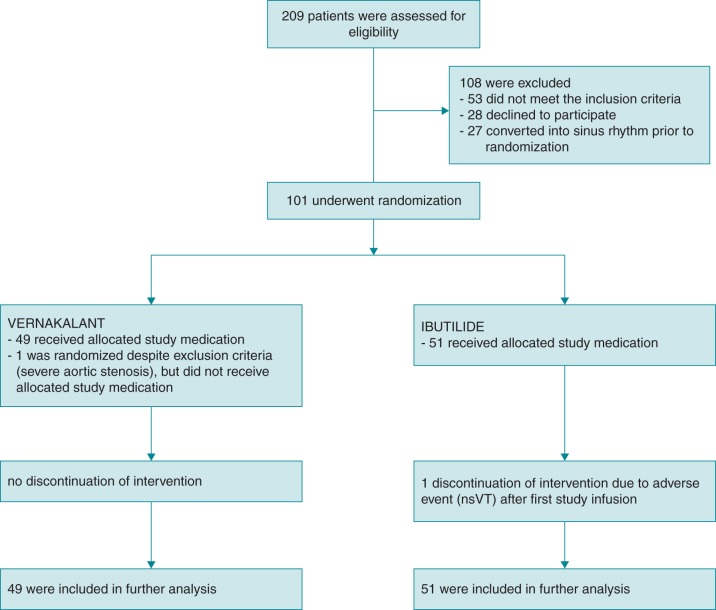
From January 2012 to March 2015, a total of 209 patients were screened for eligibility, of which 101 patients were enrolled in this single-centre randomized controlled trial. A total of 108 patients were excluded, whereof 53 patients did not meet the inclusion criteria, 28 declined to participate, and 27 patients converted to SR during screening phase.
The two treatment groups were well balanced; there were no significant differences regarding demographics and baseline characteristics (Table 1). However, more patients in the ibutilide group had previous ablation (7 vs. 2). This did not affect conversion rate in our study and did not significantly affect further analyses. Interestingly, although AF was recurrent in a large proportion of patients, use of prophylactic oral antiarrhythmic drug therapy was low in both study groups.
Table 1.
Demographics and baseline characteristics
| Treatment group |
Total (n = 100) | P-value | ||
|---|---|---|---|---|
| Vernakalant (n = 49) | Ibutilide (n = 51) | |||
| Baseline characteristics | ||||
| Male, n (%) | 34 (69.4) | 34 (66.7) | 68 | 0.832 |
| Age (year), mean (SD) | 56.2 (14.32) | 56.7 (15.77) | 56.5 (15.00) | 0.873 |
| Weight (kg), mean (SD) | 87.6 (16.57) | 87.0 (15.21) | 87.3 (15.81) | 0.847 |
| No previous episode of AF, n (%) | 21 (42.9) | 15 (29.4) | 36 | 0.212 |
| 1–5 previous episodes of AF, n (%) | 15 (30.6) | 22 (43.1) | 37 | 0.211 |
| 6–10 previous episodes of AF, n (%) | 7 (14.3) | 7 (14.3) | 14 | 1.000 |
| >10 previous episodes of AF, n (%) | 5 (10.2) | 5 (10.2) | 10 | 1.000 |
| History of at least one previous pharmacological cardioversion, n (%) | 12 (24.5) | 21 (41.2) | 33 | 0.091 |
| History of at least one previous electrical cardioversion, n (%) | 15 (30.6) | 17 (33.3) | 32 | 0.832 |
| Paroxysmal AF, n (%) | 8 (16.3) | 6 (11.8) | 14 | 0.573 |
| Persistent AF, n (%) | 20 (40.8) | 30 (58.8) | 50 | 0.109 |
| Median duration of current AF (h), mean (SD) | 10.9 (9.9) | 8.7 (6.2) | 9.7 (8.25) | 0.797 |
| AF duration ≤24 h, n (%) | 43 (87.8) | 50 (98.0) | 93 | 0.057 |
| Medical history, n (%) | ||||
| Hypertension | 30 (61.2) | 36 (70.6) | 66 | 0.400 |
| Diabetes | 5 (10.2) | 6 (11.8) | 11 | 1.000 |
| Dyslipidaemia | 24 (49) | 21 (41.2) | 45 | 0.547 |
| Smoking or previous smoking | 17 (34.7) | 18 (35.3) | 35 | 0.791 |
| Hypothyreosis | 6 (12.2) | 6 (11.8) | 11 | 0.760 |
| Hyperthyreosis | 1 (2.0) | 1 (2.0) | 2 | 1.000 |
| Previous ablation/PVI | 2 (4.1) | 7 (13.7) | 9 | 0.160 |
| Coronary heart disease | 3 (6.1) | 4 (7.8) | 7 | 1.000 |
| Scores, n (%) | ||||
| NYHA I | 42 (85.7) | 49 (96.1) | 91 | 0.105 |
| NYHA II | 6 (12.2) | 2 (3.9) | 8 | 0.160 |
| EHRA ≤2 | 38 (77.6) | 40 (78.4) | 78 | 1.000 |
| EHRA >2 | 11 (22.4) | 11 (21.6) | 22 | 1.000 |
| CHA2DS2VASc (mean, IQR) | 1.7 (1–2) | 1.8 (1–3) | 1.8 (1–2) | 0.665 |
| Regular oral medication, n (%) | ||||
| β-Blockers | 23 (46.9) | 29 (56.9) | 52 | 0.423 |
| Digitalis glycosides | 2 (4.1) | 1 (2.0) | 3 | 0.614 |
| Amiodarone | 4 (8.2) | 1 (2.0) | 5 | 0.200 |
| Sotalol | 1 (2.0) | 3 (5.9) | 4 | 0.618 |
| ACE inhibitors or ARBs | 26 (53.1) | 29 (56.9) | 55 | 0.841 |
| Thyroid hormone substitution | 6 (12.2) | 6 (11.8) | 12 | 1.000 |
Values are patients, n (%). β-Blockers: oral non-selective and selective β-blockers (excluding sotalol) and α- and β-blocking agents (e.g. carvedilol); digitalis glycosides: digoxin and digitoxin.
AF, atrial fibrillation; ACE, angiotensin-converting enzyme; ARBs, angiotensin II receptor blockers; EHRA, European Heart Rhythm Association; IQR, interquartile range; NYHA, New York Heart Association; PVI, pulmonary vein isolation; SD, standard deviation.
From 101 randomized patients, 100 (49 in the vernakalant group and 51 in the ibutilide group) received at least one dose of study drug and were included in further analyses (Figure 2). One patient in the ibutilide group received one study drug infusion only because of recurrent nsVT. Another patient in this group weighing 48 kg received weight-adjusted reduced dose according to manufacturer's instructions.
Figure 2.
Patient flow diagram. Screening, enrolment, randomization, and inclusion into further analysis. One patient within the ibutilide group did not receive second study drug due to occurrence of recurrent nsVT.
Another patient was erroneously randomized despite the then unknown presence of an exclusion criteria (severe aortic stenosis). Subsequently, no study drug was administered, no further data acquisition or analysis was performed, and the patient was excluded with replacement.
Efficacy
The primary study endpoint ‘time to conversion of AF to SR’ was significantly shorter in the vernakalant group when compared with the ibutilide group [median time: 10 (IQR = 6–17) vs. 26 (IQR = 9–55) minutes, P = 0.01]. Likewise, the secondary endpoint ‘conversion success within 90 min’ was achieved significantly more frequent in the vernakalant group with 34 patients when compared with 22 patients in the ibutilide group (69 vs. 43%, log-rank P = 0.002, Figure 3). Consistently, 29 patients (59%) in the vernakalant group when compared with 14 patients (27%) in the ibutilide group [absolute risk reduction 32% (95% CI 13–50%), P = 0.002] converted into SR prior to administration of second infusion of study drug.
Figure 3.
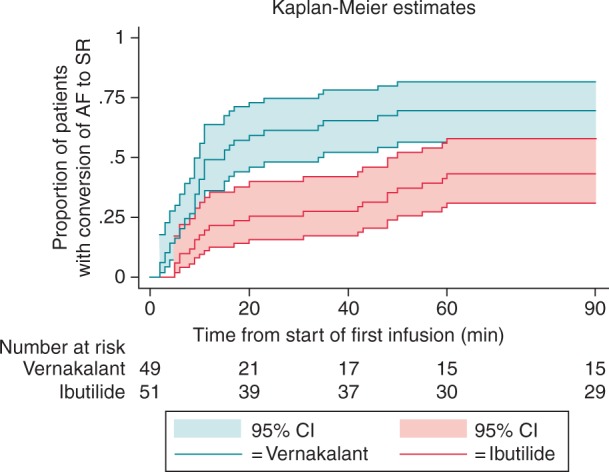
Conversion to sinus rhythm according to study treatment. Time to treatment-induced conversion from AF to SR within 90 min. x-axis: time from start of first infusion (minutes); y-axis: proportion of patients with conversion to sinus rhythm (95% CI).
Late pharmacological conversion (achieving SR 120–240 min after study drug administration) was observed in five patients (two in the vernakalant group and three in the ibutilide group). There was no relapse of AF after successful pharmacological cardioversion in any of the patients in both groups during the observation period of 6 h.
A total of 39 patients (13 in the vernakalant group and 26 in the ibutilide group) did not convert to SR within 2 h or more after start of first study drug administration. All of them consented to undergo electrical cardioversion. Of the 13 patients, 12 (92%) in the vernakalant group underwent successful electrical cardioversion with one shock, whereas only 17 of 26 patients (65%) in the ibutilide group converted to SR after application of one shock. Three patients in the ibutilide group and one patient in the vernakalant group did not convert to SR after a series of three shocks (100J, 150J, 200J). These patients received 300 mg amiodarone intravenously and were successfully electrically cardioverted thereafter.
Safety
No SAE was observed within the two treatment groups (Table 2).
Table 2.
Summary of adverse events occurring during observational period
| Treatment group |
Risk difference (95% CI) | P-value | ||
|---|---|---|---|---|
| Vernakalant (n = 49) n (%) |
Ibutilide (n = 51) n (%) |
|||
| Any treatment-emergent AEa | 13 (26.5) | 18 (35.3) | 0.09% (−0.09 to 0.27) | 0.392 |
| Common treatment-emergent AEsb | ||||
| PVC | 0 (0.0) | 8 (15.7) | −0.16% (−26 to −05) | 0.004 |
| Non-sustained VT | 0 (0.0) | 7 (13.7) | −0.14% (−23 to −04) | 0.007 |
| Aberrant conduction | 0 (0.0) | 7 (13.7) | −0.14% (−23 to −04) | 0.007 |
| Sneezing | 6 (12.2) | 0 (0.0) | −0.12% (−21 to −03) | 0.011 |
| Atrial flutter | 4 (8.2) | 0 (0.0) | −0.08% (−16 to −00) | 0.054 |
| Paresthesia | 4 (8.2) | 0 (0.0) | −0.08% (−16 to −00) | 0.054 |
| Dysgeusia | 3 (6.1) | 0 (0.0) | −0.06% (−13 to −01) | 0.113 |
| Dry mouth | 1 (2.0) | 2 (3.9) | 0.02% (−05 to 09) | 1.000 |
| Bradycardia | 2 (4.1) | 1 (2.0) | −0.02% (−09 to 05) | 0.613 |
| SVES | 1 (2.0) | 1 (2.0) | −0.00% (−06 to 05) | 1.000 |
| Discontinuations due to AEs | 0 (0.0) | 1 (2.0) | 0.02% (−02 to 06) | 1.000 |
| Any treatment-emergent SAE | 0 (0.0) | 0 (0.0) | — | — |
Values are patients, n (%).
AE, adverse event; PVC, premature ventricular complex; SAE, serious adverse event; SVES, supraventricular extrasystole; VT, ventricular tachycardia.
aTreatment-emergent AEs were defined as any AE that began or worsened following the start of study drug infusion.
bCommon treatment-emergent AEs were those that occurred in ≥2 patients in the study.
There were no cases of TdP, ventricular fibrillation, or polymorphic or sustained VT in either group. Premature ventricular contraction (PVC), nsVT, and aberrant conduction were observed only in the ibutilide group (8 vs. 0, 7 vs. 0, and 7 vs. 0). All but one of these events were asymptomatic and disappeared without therapeutic intervention. However, one patient did not receive the second study drug infusion due to recurrent nsVTs after the first infusion. Further treatment was not mandatory, and the patient underwent successful electrical cardioversion in order to restore SR.
Occurrence of atrial flutter (AFL) after study drug administration was observed in none of the patients in the ibutilide group and in four patients in the vernakalant group (8.2%). Three of them converted successfully to SR within 90 min after study drug administration, and one underwent electrical cardioversion in order to achieve SR.
No atrial flutter events were considered to be serious, and no patient developed 1:1 atrioventricular conduction during the episodes. Minor adverse events like sneezing (12.2%), paraesthesia (8.2%), and dysgeusia (6.1%) occurred in the vernakalant group only. All of these were transient and disappeared without therapeutic intervention.
Overall, in both groups heart rate decreased over time on average. Likewise, systolic and diastolic blood pressure decreased within both groups (Figure 4); however, in patients receiving vernakalant, a slight initial increase in diastolic blood pressure occurred within the first 20–40 min after application of first study drug dose. In both groups, QTcF increased, however, significantly more pronounced in the ibutilide group (Figure 5).
Figure 4.
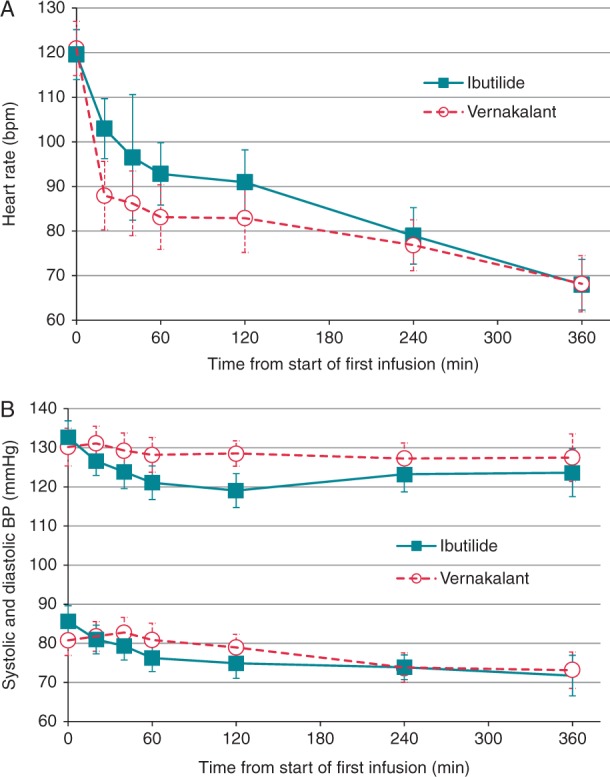
Heart rate (A) and blood pressure (B) over 6 h after administration of first study drug infusion. Absolute values and mean change from baseline within 360 min after administration of first study drug infusion.
Figure 5.
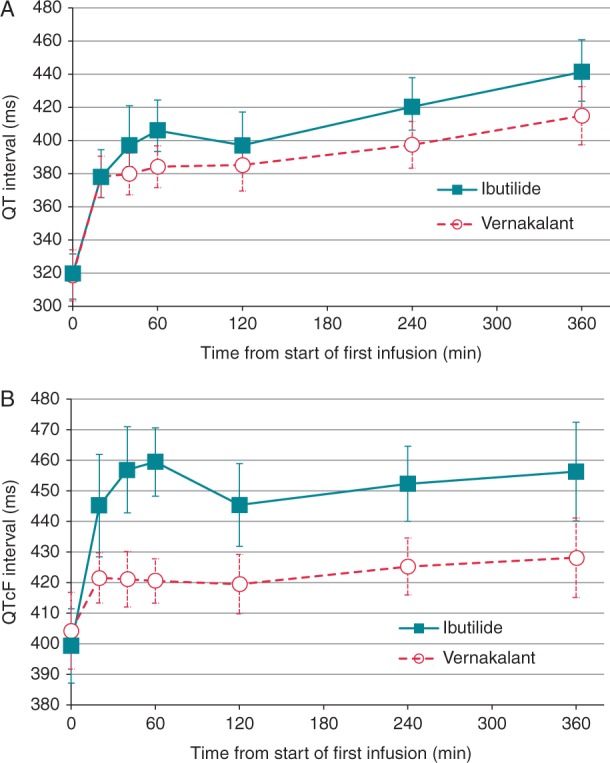
Mean QT interval (A) and QTcF interval (B) over 6 h after administration of first study drug infusion. Absolute values and mean change from baseline within 360 min after administration of first study drug infusion. (B) Mean QT interval corrected for heart rate using Fridericia's formula.
Discussion
This is, to our knowledge, the first study that compared the efficacy and safety of vernakalant and ibutilide, two anti-arrhythmic drugs with similar pharmacokinetic properties and clinical indications according to the current ESC focused guidelines update for the management of AF.7
The study has demonstrated that vernakalant is significantly faster and more effective than ibutilide for achieving SR in recent-onset AF in the emergency department setting. The rapid time to conversion in vernakalant-treated patients is consistent with Phase III studies of intravenous vernakalant14,15 including the AVRO trial10 and a recent single-centre study, which compared intravenous vernakalant to oral flecainide.11 The efficacy of vernakalant in converting recent-onset AF was even higher in our study than previously reported in several trials. The majority of those studies observed conversion rates of ∼50%, with an increased conversion success of ∼60% in one post hoc analysis.16 In one recent Swedish cohort study including 251 patients, a conversion rate of 70% was reported, which is comparable to our findings.17 Just two small single-centre studies from Argentina, each with 17 patients receiving vernakalant, reported even higher conversion rates of 76.4% within 30 min11 and 93% within 2 h.18 The high conversion rate in our study might be related with the low number of patients with greater co-morbidities (e.g. structural heart disease), the rather young patient age and the relatively short duration of AF prior to study inclusion.
The conversion rate in the ibutilide group was in the same range as in previous trials that compared the efficacy of ibutilide with placebo or other antiarrhythmic agents. Conversion rates of 35–51% have been observed in those studies, with the highest efficacy in patients with atrial flutter5,19 and in patients with AF lasting <48 h.6,20 Likewise, the time to conversion is comparable to our results (mean conversion time of 27–33 min).19
The electrophysiological actions of the mixed ion channel blocker vernakalant are largely characterized by frequency-dependent alterations of peak sodium channel currents including effective refractory period prolongation due to induction of post-repolarization refractoriness.21 Additionally, vernakalant acts by blockade of the ultra-rapid delayed potassium atrial rectifier current (IKur), with consequent prolongation of the atrial effective refractory period.22 These effects may explain the prominent anti-AF efficacy and consequently the superiority when compared with ibutilide, which acts by blocking the rapid component of the cardiac delayed rectifier potassium and by activation of a late inward sodium current.4
The benefits of an early cardioversion and a comprehensive rhythm control therapy23 include a reduction in the risk of recurrent AF episodes,24 less need for electrical cardioversion,20 prevention of prolonged and expensive hospital admissions, and might improve overall outcome, a question addressed in the on-going EAST (Early Treatment of Atrial Fibrillation for Stroke Prevention Trial) study.25 Indeed, significantly less patients in the vernakalant group needed electrical cardioversion (13 when compared with 26 patients in the ibutilide group), and duration of emergency department stay could have been significantly shortened, especially in the vernakalant group, if an observational period of at least 6 h would not have been required by our study protocol. Accordingly, the cumulative time to conversion to SR, summarizing pharmacological and electrical cardioversion, was shorter in the vernakalant group when compared with the ibutilide group [median time: 15 (IQR = 8–140) vs. 124 (IQR = 20–241)].
Of interest, vernakalant was at least as effective as ibutilide in enhancing electrical cardioversion. Of 13 patients, 12 (92%) in the vernakalant group were successfully electrically cardioverted with one shock of 100 J, when compared with only 17 of 26 patients (65%) in the ibutilide group. This finding is in line with a recently published study from Germany in which vernakalant was more effective than amiodarone in facilitating electrical cardioversion in patients with either immediate AF recurrence or failed electrical cardioversion after antiarrhythmic-naïve electrical cardioversion.26
Both study drugs were well tolerated in our study, and most observed adverse events were in the range of previous trials. Dysgeusia, sneezing, and paraesthesia were comparable to the summarized findings from the Phase II and Phase III trials of intravenous vernakalant vs. placebo (dysgeusia 20.4%, sneezing 15%, paraesthesia 8.8%)27 and the AVRO trial (dysgeusia 6.9%, sneezing 3.4%).10 Likewise, atrial flutter occurred with the same incidence as in the AVRO trial (8.2 vs. 8.6%).
Non-sustained VTs occurred in 14% (n = 7) of the patients in the ibutilide group. This incidence is higher as reported in previous studies with ibutilide, where nsVTs were observed in 4.9–8.3% of treated patients.19,28 In line with previous reports, QT and QTc prolongation was particularly pronounced in the ibutilide group.19
Of interest, no patient experienced relevant arterial hypotension, and blood pressure did not differ between treatment groups, although hypotension is a potential dangerous adverse event developing after vernakalant administration in ∼6% of patients.15 This might be at least partly attributable to our local standard of care intravenous fluid therapy (on average 0.75 L electrolyte infusion) in patients with AF and the fact that just a minority of our study patients showed impaired left ventricular function, a known risk factor for developing haemodynamic instability.
Our study has several limitations. First, we did not include a placebo group due to our concerns of leaving symptomatic patients untreated. Moreover, placebo controlled trials already exist for both drugs.14,19 Second, study treatment was not blinded, so we cannot exclude some type of bias. However, our predefined study endpoints were not prone to subjective assessment. Third, we performed a single-centre trial and cannot rule out that local standard of care affected study outcomes. Indeed, the administration of an electrolyte infusion to raise potassium values to high normal values during the screening phase might be linked with the absence of TdP arrhythmia or other polymorphic VT in our study patients. Forth, AF duration prior the study inclusion was short, and most of our study patients had good ejection fraction and were relatively young.
Conclusion
Vernakalant was superior to ibutilide in converting recent-onset AF to SR in the emergency department setting.
Funding
This project was supported by a grant from the Jubilaeumsfonds of the Austrian National Bank (#14891 to A.O.S). Funding to pay the Open Access publication charges for this article was provided by Medical University of Vienna.
Conflict of interest: none declared.
Acknowledgements
The authors would like to thank the emergency department staff for their support during trial conduct.
References
- 1. Krijthe BP, Kunst A, Benjamin EJ, Lip GYH, Franco OH, Hofman A et al. . Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 2013;34:2746–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pedersen OD, Søndergaard P, Nielsen T, Nielsen SJ, Nielsen ES, Falstie-Jensen N et al. , DIAMOND Study Group Investigators. Atrial fibrillation, ischaemic heart disease, and the risk of death in patients with heart failure. Eur Heart J 2006;27:2866–70. [DOI] [PubMed] [Google Scholar]
- 3. McNamara RL, Tamariz LJ, Segal JB, Bass EB. Management of atrial fibrillation: review of the evidence for the role of pharmacologic therapy, electrical cardioversion, and echocardiography. Ann Intern Med 2003;139:1018–33. [DOI] [PubMed] [Google Scholar]
- 4. Foster RH, Wilde MI, Markham A. Ibutilide: a review of its pharmacological properties and clinical potential in the acute management of atrial flutter and fibrillation. Drugs 1997;54:312–30. [DOI] [PubMed] [Google Scholar]
- 5. Vos MA, Golitsyn SR, Stangl K, Ruda MY, Van Wijk LV, Harry JD et al. . Superiority of ibutilide (a new class III agent) over DL-sotalol in converting atrial flutter and atrial fibrillation. The Ibutilide/Sotalol Comparator Study Group. Heart 1998;79:568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reisinger J, Gatterer E, Lang W, Vanicek T, Eisserer G, Bachleitner T et al. . Flecainide versus ibutilide for immediate cardioversion of atrial fibrillation of recent onset. Eur Heart J 2004;25:1318–24. [DOI] [PubMed] [Google Scholar]
- 7. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH et al. . 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Europace 2012;14:1385–413. [DOI] [PubMed] [Google Scholar]
- 8. Savelieva I, Graydon R, Camm AJ. Pharmacological cardioversion of atrial fibrillation with vernakalant: evidence in support of the ESC Guidelines. Europace 2014;16:162–73. [DOI] [PubMed] [Google Scholar]
- 9. Fedida D, Orth PMR, Chen JYC, Lin S, Plouvier B, Jung G et al. . The mechanism of atrial antiarrhythmic action of RSD1235. J Cardiovasc Electrophysiol 2005;16:1227–38. [DOI] [PubMed] [Google Scholar]
- 10. Camm AJ, Capucci A, Hohnloser SH, Torp-Pedersen C, Van Gelder IC, Mangal B et al. . A randomized active-controlled study comparing the efficacy and safety of vernakalant to amiodarone in recent-onset atrial fibrillation. J Am Coll Cardiol 2011;57:313–21. [DOI] [PubMed] [Google Scholar]
- 11. Conde D, Costabel JP, Caro M, Ferro A, Lambardi F, Corrales Barboza A et al. . Flecainide versus vernakalant for conversion of recent-onset atrial fibrillation. Int J Cardiol 2013;168:2423–5. [DOI] [PubMed] [Google Scholar]
- 12. Camm AJ, Toft E, Torp-Pedersen C, Vijayaraman P, Juul-Moller S, Ip J et al. . Efficacy and safety of vernakalant in patients with atrial flutter: a randomized, double-blind, placebo-controlled trial. Europace 2012;14:804–9. [DOI] [PubMed] [Google Scholar]
- 13. Oral H, Souza JJ, Michaud GF, Knight BP, Goyal R, Strickberger SA et al. . Facilitating transthoracic cardioversion of atrial fibrillation with ibutilide pretreatment. N Engl J Med 1999;340:1849–54. [DOI] [PubMed] [Google Scholar]
- 14. Kowey PR, Dorian P, Mitchell LB, Pratt CM, Roy D, Schwartz PJ et al. , Atrial Arrhythmia Conversion Trial Investigators. Vernakalant hydrochloride for the rapid conversion of atrial fibrillation after cardiac surgery: a randomized, double-blind, placebo-controlled trial. Circ Arrhythm Electrophysiol 2009;2:652–9. [DOI] [PubMed] [Google Scholar]
- 15. Roy D, Pratt CM, Torp-Pedersen C, Wyse DG, Toft E, Juul-Moller S et al. , Atrial Arrhythmia Conversion Trial Investigators. Vernakalant hydrochloride for rapid conversion of atrial fibrillation: a phase 3, randomized, placebo-controlled trial. Circulation 2008;117:1518–25. [DOI] [PubMed] [Google Scholar]
- 16. Stiell IG, Dickinson G, Butterfield NN, Clement CM, Perry JJ, Vaillancourt C et al. . Vernakalant hydrochloride: a novel atrial-selective agent for the cardioversion of recent-onset atrial fibrillation in the emergency department. Acad Emerg Med 2010;17:1175–82. [DOI] [PubMed] [Google Scholar]
- 17. Juul-Moller S. Vernakalant in recently developed atrial fibrillation: how to translate pharmacological trials into clinical practice. Eur J Cardiovasc Med 2013;2:226–33. [Google Scholar]
- 18. Conde D, Costabel JP, Aragon M, Lambardi F, Klein A, Corrales Barbosa A et al. . Propafenone versus vernakalant for conversion of recent-onset atrial fibrillation. Cardiovasc Ther 2013;31:377–80. [DOI] [PubMed] [Google Scholar]
- 19. Stambler BS, Wood MA, Ellenbogen KA, Perry KT, Wakefield LK, Vanderlugt JT. Efficacy and safety of repeated intravenous doses of ibutilide for rapid conversion of atrial flutter or fibrillation. Ibutilide Repeat Dose Study Investigators. Circulation 1996;94:1613–21. [DOI] [PubMed] [Google Scholar]
- 20. Domanovits H, Schillinger M, Thoennissen J, Nikfardjam M, Janata K, Brunner M et al. . Termination of recent-onset atrial fibrillation/flutter in the emergency department: a sequential approach with intravenous ibutilide and external electrical cardioversion. Resuscitation 2000;45:181–7. [DOI] [PubMed] [Google Scholar]
- 21. Burashnikov A, Pourrier M, Gibson JK, Lynch JJ, Antzelevitch C. Rate-dependent effects of vernakalant in the isolated non-remodeled canine left atria are primarily due to block of the sodium channel: comparison with ranolazine and DL-sotalol. Circ Arrhythm Electrophysiol 2012;5:400–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Savelieva I, Camm J. Anti-arrhythmic drug therapy for atrial fibrillation: current anti-arrhythmic drugs, investigational agents, and innovative approaches. Europace 2008;10:647–65. [DOI] [PubMed] [Google Scholar]
- 23. Cosio FG, Aliot E, Botto GL, Heidbuchel H, Geller CJ, Kirchhof P et al. . Delayed rhythm control of atrial fibrillation may be a cause of failure to prevent recurrences: reasons for change to active antiarrhythmic treatment at the time of the first detected episode. Europace 2008;10:21–7. [DOI] [PubMed] [Google Scholar]
- 24. Dittrich HC, Erickson JS, Schneiderman T, Blacky AR, Savides T, Nicod PH. Echocardiographic and clinical predictors for outcome of elective cardioversion of atrial fibrillation. Am J Cardiol 1989;63:193–7. [DOI] [PubMed] [Google Scholar]
- 25. Aliot E, Brandes A, Eckardt L, Elvan A, Gulizia M, Heidbuchel H et al. . The EAST study: redefining the role of rhythmcontrol therapy in atrial fibrillation: EAST, the Early treatment of Atrial fibrillation for Stroke prevention Trial. Eur Heart J 2015;36:255–6. [DOI] [PubMed] [Google Scholar]
- 26. Müssigbrodt A, John S, Kosiuk J, Richter S, Hindricks G, Bollmann A. Vernakalant-facilitated electrical cardioversion: comparison of intravenous vernakalant and amiodarone for drug-enhanced electrical cardioversion of atrial fibrillation after failed electrical cardioversion. Europace 2016;18:51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tian D, Frishman WH. Vernakalant: a new drug to treat patients with acute onset atrial fibrillation. Cardiol Rev 2011;19:41–4. [DOI] [PubMed] [Google Scholar]
- 28. Kowey PR, Vanderlugt JT, Luderer JR. Safety and risk/benefit analysis of ibutilide for acute conversion of atrial fibrillation/flutter. Am J Cardiol 1996;78:46–52. [DOI] [PubMed] [Google Scholar]