Abstract
Background:
Reinjury rates after anterior cruciate ligament reconstruction (ACLR) are highest among young athletes, who consequently suffer from low rates of return to play. Historically, quantitative measures have been used to determine readiness to return to sport; however, they do not assess modifiable risk factors related to the quality of movement.
Purpose:
To determine the effectiveness of a criteria-based rehabilitation progression and return-to-sport criteria on efficient return to activity and prevention of second injury in young athletes post-ACLR.
Study Design:
Case series; Level of evidence, 4.
Methods:
Between December 2010 and 2013, 42 skeletally immature athletes (mean chronologic age, 12 years; range, 10-15 years) who underwent ACLR using ipsilateral hamstring tendon autograft were prospectively evaluated. All athletes progressed through a criteria-based rehabilitation progression; were assessed at specific time frames for strength, biomechanical, and neuromuscular risk factors predictive of injury; and were provided targeted interventions. The final return to sport phase consisted of quantitative testing as well as a quality of movement assessment of several functional movements with progressive difficulty and sports-specific loading. Clearance for unrestricted activity was determined by achieving satisfactory results on both qualitative and quantitative assessments with consideration for the demands of each sport.
Results:
The mean time for return to unrestricted competitive activity was 12 months. All but 3 (7%) athletes returned to their primary sport. Thirty-five athletes (83%) returned to unrestricted activity. Of the 6 (14%) who sustained a second injury, 3 (50%) were injured in sports they were not cleared for. All ACL reinjuries occurred in a cutting sport. Half of reinjuries occurred within 1 year of surgery, while the remaining occurred between 1 and 2 years. Eighty-three percent of reinjuries involved highly competitive cutting athletes.
Conclusion:
In our cohort, the combination of qualitative and quantitative data served as a good indicator for reducing risk and determining readiness to return to sport.
Keywords: ACL injury, skeletally immature, young athletes, ACL prevention
Anterior cruciate ligament (ACL) injuries continue to be debilitating for young athletes despite extensive research on injury prevention.28,32 With sports specialization on the rise, intrasubstance tears of the ACL in skeletally immature athletes have increased dramatically.4,10,23,36 In addition, return-to-play rates are low and second injury rates are highest among this population.1,22,37 Young athletes are 15 times more likely to reinjure their ACLs within the first year, 6 times more likely within 2 years after returning to sports activity, and only 43% return to their preinjury level.1,5,22,28,30,32,33,38 Reinjury is often career-ending and emotionally devastating.15,32
Advances in ACL reconstruction (ACLR) techniques and fixation methods have significantly expanded surgical options and improved clinical results.4,7,11,29 Therefore, the potential to return to play without second injury may be determined more so by differences in rehabilitation and return-to-sport criteria, which historically have focused on quantitative data such as strength testing, hop tests, and time frames.7,8,17,24,31,39 These assessments do not account for the qualitative components of movement, compensatory movement patterns, or fatigue related to deconditioning that may lead to injury to either limb. For example, an athlete may demonstrate 100% limb symmetry on a single-leg hop test but have significant valgus loading of 1 knee on landing (Figure 1). The athlete depicted in Figure 1 demonstrates poor neuromuscular control on landing, poor proximal hip strength and stability, and therefore, a high risk of reinjury. We believe that a young athlete’s readiness to return to sports should be based not only on their ability to run and jump but also on the quality of their sports-related functional movements.
Figure 1.

A young athlete performing a single-leg hop test with 100% limb symmetry but with poor quality (genu valgum on landing, hip drop, and trunk lean).
There is a wealth of literature on risk factors predictive of ACL injury; however, much of these data focus on the female adolescent athlete.14,15,20,22 Skeletally immature athletes have unique characteristics that may result in increased risk. Growth-related factors such as growth spurts, underdeveloped coordination, high-risk movement patterns, and an inadequate strength base predispose them to injury.11,18,19,40 Refining movement is vital to addressing risk factors associated with poor biomechanics and compensatory movement patterns. We have developed a continuum of care model that incorporates all aspects of care, preparing the athlete from the day they decide to have surgery to the day they step back on the field. It allows for individualized progress and incorporates intervention strategies to address deficits in a timely manner. The model consists of quantitative testing combined with the quality of movement assessment (QMA), which assesses risk factors associated with movement biomechanics. Modifiable risk factors include deficits in strength and flexibility and neuromuscular patterns that place these athletes at risk for ACL injury. Nonmodifiable risk factors include generalized ligamentous laxity (Beighton score >4), recurvatum, structural characteristics such as pathologic anatomic knee valgus (best seen on weightbearing radiographs), narrow notch width, and increased lateral tibial slope.6,34 Clearance is collaborative and involves the physician, physical therapist, athletic trainer, coach, caregiver (typically the parent), and athlete. The purpose of this study was to determine the effectiveness of our continuum of care model including the qualitative and quantitative measures in a population of skeletally immature athletes undergoing ACLR with respect to reinjury and return-to-sport rates.
Methods
Skeletally immature patients (>1 year of growth remaining) who underwent ACLR were evaluated at our institution by 2 senior surgeons. Patients were included if they had an isolated ACLR using either an all-inside, all-epiphyseal reconstruction or an all-inside, partial transphyseal (PTP) reconstruction (crossing the tibial physis but not the femoral physis).13 Exclusion criteria included associated ligamentous reconstruction and articular cartilage injury necessitating articular cartilage restoration. Athletes requiring meniscus repair and or partial meniscectomy were included.
Study Cohort
Between December 2010 and 2013, 42 skeletally immature athletes who underwent ACLR using ipsilateral hamstring tendon autograft were recruited. The cohort consisted of 12 girls and 30 boys, with a mean chronological age of 12 years (range, 10-15 years) at time of surgery. Twenty-five patients underwent an all-inside, all-epiphyseal reconstruction and 17 underwent an all-inside, PTP reconstruction based on skeletal age.13,29 The sport they played at time of injury, their primary sport overall, and the sports they returned to were documented.
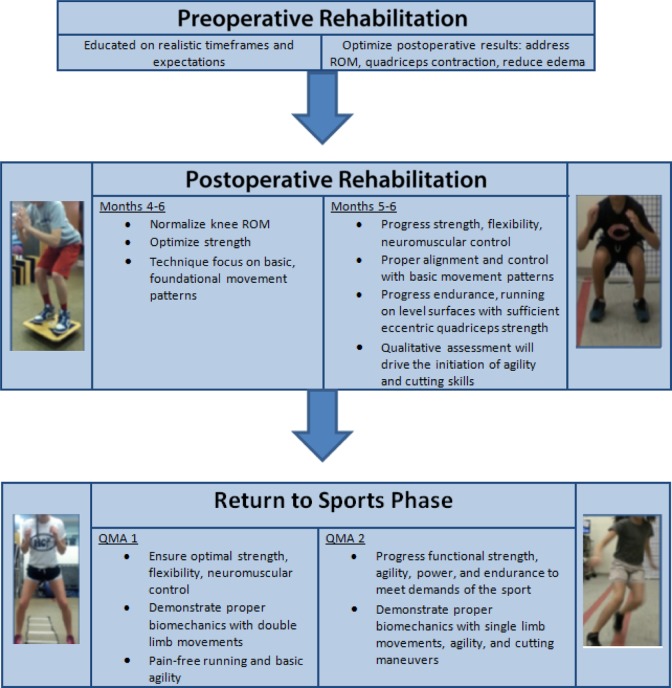
Rehabilitation Phase
Preoperatively, patients were educated on realistic time frames for recovery and criteria for return to play. Parents were advised that return to sport would generally be a minimum of 9 months and more likely 12 months. Postoperatively, athletes were seen on average 2 times per week for the first 12 weeks, after which they underwent a standard ACLR rehabilitation protocol for the first 12 to 16 weeks, and then from months 3 through 6, they transitioned to working with a trainer affiliated with their athletic program 2 to 3 times per week.25 Strength, range of motion (ROM), flexibility, neuromuscular control, and movement patterns were assessed by a physical therapist to minimize complications and encourage timely and safe progression by providing targeted interventions. By 6 months, athletes were expected to have the strength to perform foundational movement patterns such as a squat, single-leg stance, forward step down, and single-leg bridge (Figure 2).
Figure 2.
Criteria-based rehabilitation progression. QMA, quality of movement assessment; ROM, range of motion.
Qualitative and Quantitative Measures
Athletes were cleared for return to sports based on quantitative measures using the limb symmetry index and qualitative measures as well as the ability to meet the demands of their sport. The quantitative assessment included KT-1000 arthrometry (performed prior to or at the first QMA), isokinetic strength testing (performed starting at 5 months and at clearance), and a single-leg hop test (performed starting at 5 months). The KT-1000 examined side-to-side differences for anterior tibial translation and compliance, and isokinetic testing assessed concentric knee extensor and flexor torque values. Standing radiographs and spoiled gradient recalled acquisition magnetic resonance imaging (SPGR MRI) analyses were performed postoperatively at 6 months, 1 year, and annually thereafter until the athletes reached skeletal maturity, but these were not used as criteria for return to sport. All athletes participated in the QMA program starting at approximately 6 to 7 months post-ACLR.
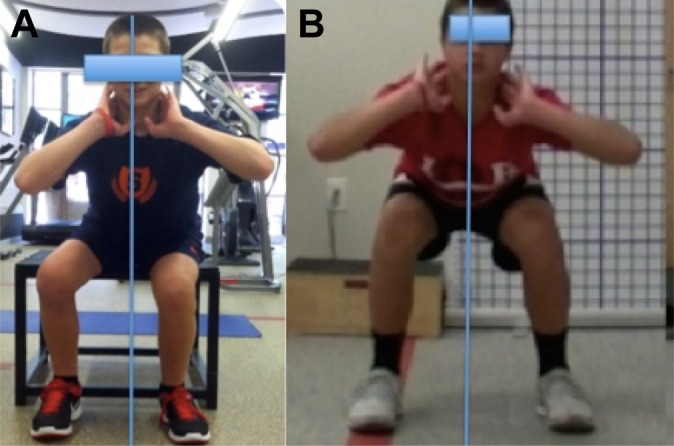
Dartfish Motion Analysis (Dartfish) was used to document a battery of movement patterns that are key to function in sports. The movement patterns progressed by level of difficulty and sports-specific loading pending the individual’s rehabilitative function level. The QMA is a tool for assessing all movements for limitations, asymmetries, and deficits that would demonstrate risk of injury to either limb based on ACL injury prevention principles. Video feedback served as an educational tool to illustrate this to the athlete and parent(s). Targeted exercises were provided to address deficits, and the athlete was progressed to sports-specific drills based on functional milestones. Ideal performance on the QMA requires a balance of mobility, strength, stability, neuromuscular control, and agility. Each movement was assessed for the following criteria: presence or absence of pain, movement strategy, apprehension, dynamic alignment, symmetry, depth, rate of fatigue, and control (Figure 3, A and B). Initiating movement from the hips is a hip strategy.8 Figure 3A depicts a young male performing a squat with a knee strategy as evidenced by his knees shifting over his ankles. Figure 3B demonstrates the appropriate hip strategy performed with a squat, and the athlete is able to center his weight by shifting back through his hips with knees aligned over his ankles.8 Alignment of the trunk, hip, knee, and foot in the sagittal and coronal planes is assessed (Figure 4, A and B). For example, when performing a single-leg squat, if the athlete’s trunk deviates laterally in the frontal plane, valgus loading of the knee may result (Figure 3A). Proper alignment with a single-leg squat would demonstrate the trunk, hip, knee, and foot/ankle aligned with a level pelvis and no valgus loading of the knee (Figure 4B).
Figure 3.
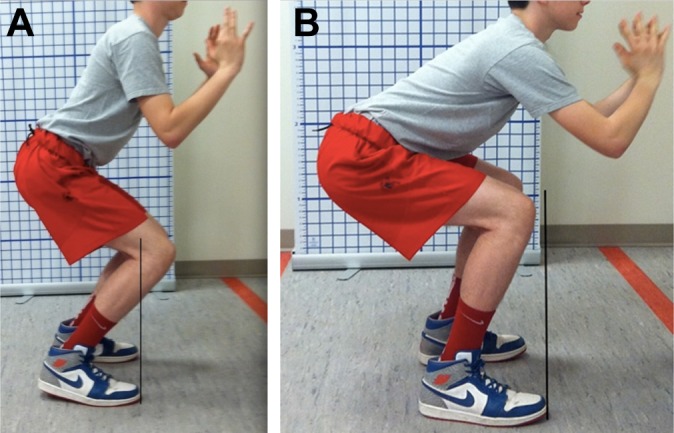
A young athlete performing a squat with (A) a knee strategy and (B) a hip strategy.8
Figure 4.
(A) Poor trunk and lower extremity alignment with lateral trunk deviation in the frontal plane, hip shift, and resultant knee valgus loading of the right lower extremity while performing a single-leg squat. (B) Proper trunk and lower extremity alignment: neutral trunk, level pelvis, and no resultant knee valgus while performing a single-leg squat.
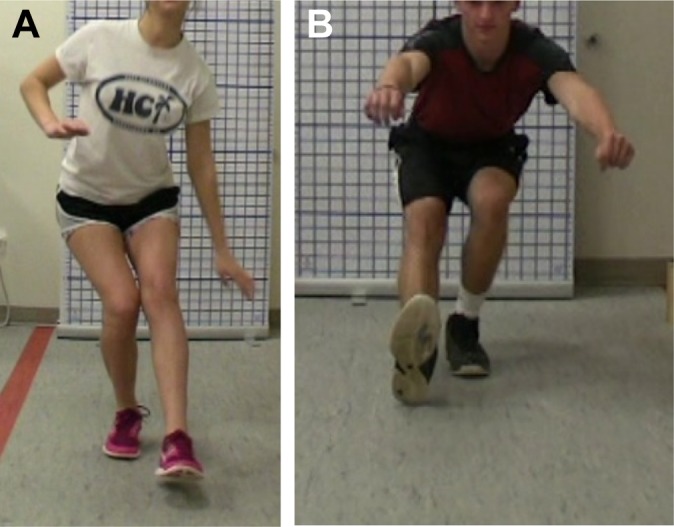
Asymmetry pertains to shifting to 1 lower extremity versus the other and can be observed in double-limb movements (Figure 5). Depth of movement demonstrates appropriate strength, flexibility, and ability to absorb ground-reaction forces (Figure 6, A and B). Apprehension was described as slowed or hesitant movement patterns. Fatigability was noted by the decline in quality of movement with increased repetitions. Athletes were asked about pain with each movement to determine whether pain was altering movement patterns. If an athlete had pain with a movement, the movement was stopped and the athlete was assessed to determine the cause.
Figure 5.
(A) Young athlete demonstrating a shift to the nonoperative left leg while performing a squat at 6 months. (B) The same athlete at 12 months with symmetrical weightbearing during a squat. Note the significant growth in this athlete.
Figure 6.
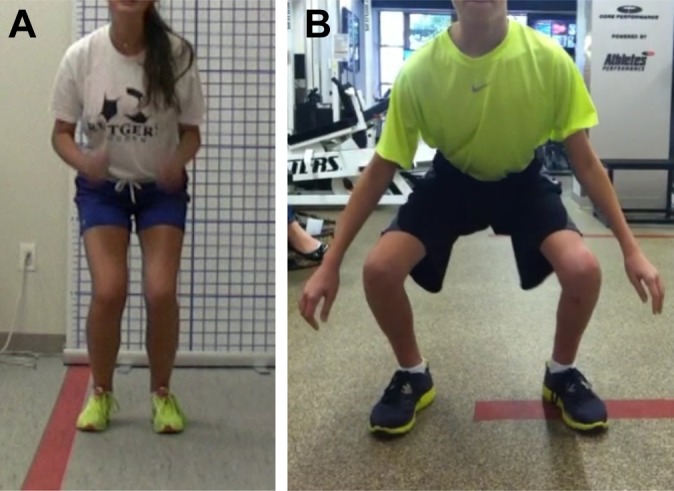
(A) Young athlete performing a jump in place and landing with decreased depth and knee flexion resulting in increased ground-reaction forces and valgus knee loading bilaterally. (B) Trained young athlete shifting through his hips and knees to absorb the jump landing with no valgus loading of either limb.
Basic foundational movements were assessed at the first QMA (squat, single-leg stance, forward step down, single-leg squat, jumps in place, side jumps). Broad jumps and single-leg jumps were assessed pending patient ability and safety with performance (Table 1). Tasks were modified or eliminated if a patient demonstrated significant abnormal movement patterns or pain. KT-1000 and knee flexion/extension isokinetic testing were performed just prior to or at the time of the first QMA. Targeted exercises were provided to address significant abnormal movement patterns. If appropriate pain-free movement patterns and a solid strength base were demonstrated, the patient was cleared to begin a running and plyometric program and progress to single-limb strength training. Athletes were followed up with every 6 to 8 weeks to ensure that deficits were addressed and single-limb movements were performed with optimal form and control (single-leg squat, single-leg hop, hop to opposite leg) (Figure 7A). The ability to demonstrate optimal form when cutting (Figure 7B), changing direction, and decelerating at progressive speeds was assessed pending ability (Figure 7C). If deemed ready, patients were cleared to advance to full speed with all noncontact drills, cutting/agility to 100% speed, and sports-specific drills in a controlled environment to allow quality of movement with increasing speeds. Under direction of a physical therapist or athletic trainer, sport-specific drills were initially performed before advancing to a less supervised environment such as team practice. The QMA was repeated until the athlete was deemed safe to return to sports with proper control and quality of sports-specific movements at 100% speed.
TABLE 1.
Return-to-Sport Progression Based on Quality of Movement Assessment (QMA) and Quantitative Assessment Results
| Assessment | Qualitative Assessment | Quantitative Assessment | Sport-Specific Progression |
|---|---|---|---|
| 1 |
Squat Single-leg stance 8-inch forward step
Single-leg squat Jump in place |
|
|
| 2 |
|
|
|
| 3 |
Run forward and back peddle Shuttle run Sprint stop and go on command Cutting 90° Side shuffle 90° |
|
|
aProgression beyond QMA 1 is highly individualized and requires a customized approach based on patient progression, sport specificity, and physical therapist discretion.
bIf deemed safe to perform.
cIf deficits persists from assessment 2.
Figure 7.

(A) Hop to opposite leg performed by young athlete with proper alignment on his left nonoperative lower extremity but with valgus knee loading when performed to his right lower extremity. (B) Young athlete cutting at 90° with proper trunk and lower extremity alignment followed by a young female athlete with valgus loading. (C) Trained male athlete with proper trunk, hip, knee, and foot/ankle alignment followed by young, untrained female athlete stopping on command with hip internal rotation and knee valgus.
Results
Return to Sport Time Frame: Impact on Second Injury
The mean time for return to unrestricted competitive activity was 12 ± 2.0 months postoperatively. Nineteen athletes were returned prior to 12 months (10.5 ± 1.1 months), of which 21% (n = 4) incurred a second injury. The QMA indicated that no athlete was able to perform a 2-legged squat without some degree of compensation at 6 months, and none were ready to return to sport before 9 months secondary to compensatory movement patterns. All but 3 athletes demonstrated risk factors with single-limb movements such as single-leg hops at the 9-month mark.
Thirty-nine (93%) athletes returned to their primary sports with the exception of 3 (7%): 1 reinjured secondary to noncompliance at 3 months, 1 switched from football to hockey, and the other was advised to avoid soccer secondary to a connective tissue disorder (Table 2). Thirty-five athletes (83%) returned to play at an average of 12 months without reinjury. KT and isokinetic testing was conducted between 5 to 6 months postoperatively. The mean KT-1000 side-to-side difference was 1.1 ± 0.6 mm, and isokinetic testing showed a mean deficit of 8.3% ± 6.8% in extension torque and 11.3% ± 8.2% in flexion torque at a speed of 180 deg/s on clearance. The single-leg hop test scored 93% compared with the uninjured side. The International Knee Documentation Committee (IKDC) score was 93.1 ± 7.2, the mean Lysholm score was 97.6 ± 4.5, and the mean Hospital for Special Surgery Modified Marx Activity Rating Scale score was 23.2 ± 8.3. With the exception of the 1 patient who was reinjured in the playground 3 months postoperatively, all patients (including the 3 remaining subjects who were subsequently reinjured) had negative Lachman and pivot-shift tests.
TABLE 2.
Results by Sex and Sport Categorization Levela
| Age at Injury, y | Sex | Initial ACL Surgery | Injury Activity | Cleared to Return to Activity | Primary Sport | Sports Cleared For | Mechanism of Reinjury | Reinjury | Sport Categorization Levelb |
|---|---|---|---|---|---|---|---|---|---|
| 10 | Female | AE | Softball | Yes | Softball | Softball | 1A | ||
| 12 | Female | AE | Soccer | Yes | Soccer | Soccer | 1A | ||
| 12 | Female | PTP | Soccer | Yes | Soccer | Soccer | 1A | ||
| 13 | Female | AE | Soccer | Yes | Soccer | Soccer | 1A | ||
| 13 | Female | PTP | Lacrosse | Not lacrosse | 1A | ||||
| 14 | Female | PTP | Lacrosse | Yes | Lacrosse | Rowing | Lacrosse game | Ipsilateral ACL | 1A |
| 15 | Female | PTP | Lacrosse | Yes | Lacrosse | Lacrosse | Lacrosse game | Contralateral ACL | 1A |
| 15 | Female | PTP | Lacrosse | Yes | Hockey/lacrosse | Both | 1A | ||
| 10 | Female | AE | Soccer | Yes | Tennis | Tennis | 2 | ||
| 11 | Female | AE | Soccer | Yes | Softball | Softball | 2 | ||
| 12 | Female | AE | Soccer | Yes | Soccer | Soccer-all | 2 | ||
| 10 | Female | AE | Basketball | Yes | All | All | 3 | ||
| 11 | Male | AE | Baseball | Yes | Baseball | Baseball | 1A | ||
| 11 | Male | AE | Lacrosse | Yes | Football | Football | 1A | ||
| 11 | Male | AE | Lacrosse | Yes | Lacrosse | Lacrosse | 1A | ||
| 11 | Male | AE | Lacrosse | Yes | Lacrosse | Lacrosse | 1A | ||
| 11 | Male | AE | Football | Yes | Football | Hockey-all | |||
| 12 | Male | AE | Soccer | Yes | Soccer | Soccer-all | 1A | ||
| 12 | Male | AE | Lacrosse | Yes | Football | Football | 1A | ||
| 12 | Male | AE | Lacrosse | Yes | Lacrosse | Lacrosse | 1A | ||
| 13 | Male | PTP | Lacrosse | Yes | Lacrosse | Lacrosse | Lacrosse game | Ipsilateral ACL | 1A |
| 13 | Male | PTP | Soccer | Yes | Lacrosse | Lacrosse | 1A | ||
| 13 | Male | PTP | Lacrosse | Yes | Lacrosse | Lacrosse | 1A | ||
| 14 | Male | PTP | Soccer | Yes | Soccer | Soccer | 1A | ||
| 14 | Male | PTP | Soccer | Yes | Soccer | Soccer | 1A | ||
| 15 | Male | PTP | Lacrosse | Yes | Lacrosse | Lacrosse | Lacrosse game | Contralateral ACL | 1A |
| 16 | Male | PTP | Soccer | Yes | Soccer | Soccer | 1A | ||
| 17 | PTP | Lacrosse | Lacrosse | Yes | Lacrosse | Lacrosse | Lacrosse practice | Ipsilateral medial meniscus injury | 1A |
| 10 | Male | AE | Ski racing | Yes | Ski race | All | 1B | ||
| 10 | Male | AE | Ski racing | Yes | All | All | |||
| 13 | Male | AE | Ski racing | Yes | Ski race | Ski race | 1B | ||
| 10 | Male | AE | Soccer | Yes | All | All | 2 | ||
| 11 | Male | AE | Soccer | Not cleared | Soccer | Not soccer | Playground 3 months | Ipsilateral ACL | 2 |
| 12 | Male | AE | Soccer | Yes | Soccer | Soccer | 2 | ||
| 12 | Male | AE | Soccer | Yes | Soccer | Soccer | |||
| 13 | Male | AE | Skiing | Yes | All | All | 2 | ||
| 13 | Male | AE | Soccer | Not for cutting | Soccer–All | No cutting | 2 | ||
| 14 | Male | PTP | Skateboard | Yes | All | All | 2 | ||
| 14 | Male | AE | Soccer | No | All | All | 2 | ||
| 14 | Male | PTP | Football | Yes | Football | Football | 2 | ||
| 14 | Male | PTP | Basketball | Not for basketball | Soccer | Soccer | Basketball | Ipsilateral ACL | 2 |
| 15 | Male | PTP | Running | Yes | All | All | 3 |
aACL, anterior cruciate ligament; AE, all-epiphyseal ACL reconstruction with hamstring autograft; PTP, partial transphyseal ACL reconstruction with hamstring autograft.
bAs delineated by Maron et al.27
Of the 7 who suffered a second injury, 6 (14%) injured their ACL (4 ipsilateral, 2 contralateral) and 1 injured his meniscus. Four (67%) of the patients who reinjured their ACL had been cleared to return to unrestricted activity, while 3 (50%) returned against medical advice. One athlete injured his ipsilateral knee secondary to an incompletely healed meniscus repair. All of the ACL reinjuries were noncontact injuries. Fifty percent (n = 3) were injured within the first 12 months of surgery and 50% (n = 3) between 12 and 24 months (Table 3). Of the 4 athletes who were cleared to return to sport and were reinjured, 3 (75%) had bilateral knee joint hyperextension with recurvatum greater than 10°. None of the athletes had a significant growth disturbance (>1.5-cm change) on follow-up imaging (standing radiographs and SPGR MRI analysis).
TABLE 3.
Summary of Athletes Who Sustained a Second ACL Injurya
| Sex | Limb Injured | Surgery | Age at Second Injury, y | Cleared for Activity | Month Cleared for Activity | Time Frame of Second Injury (mo postoperative) | Mechanism of Injury | Competitive Level | Risk Factors Present |
|---|---|---|---|---|---|---|---|---|---|
| Male | Ipsilateral | AE | 12 | No | Not cleared | 3 | Noncontact playground | 2 | 3 months postoperative |
| Male | Contralateral | PTP | 16 | Yes | 11 | 12 | Noncontact lacrosse game | 1A | Significant hyperflexion ROM of bilateral knees |
| Male | Ipsilateral | PTP | 15 | Advised against playing basketball | 9 (for soccer) | 12 | Noncontact basketball game | 2 | Jump-landing deficits |
| Male | Ipsilateral | PTP | 14 | Yes | 12 | 15-16 | Noncontact lacrosse game | 1A | Significant hyperflexion ROM of bilateral knees |
| Male | Ipsilateral medial meniscus tear | PTP | 18 | Yes | 9 | 10 | Noncontact lacrosse practice | 1A | Hyperextension ROM of bilateral knees |
| Female | Contralateral | PTP | 15 | All except lacrosse | 10 | 11.5 | Noncontact lacrosse game | 1A | None observed |
| Female | Ipsilateral | PTP | 15 | Yes | 14 | 25 | Noncontact lacrosse game | 1A | Coordination deficits—reached maximum potential |
aACL, anterior cruciate ligament; AE, all-epiphyseal ACL reconstruction with hamstring autograft; PTP, partial transphyseal ACL reconstruction with hamstring autograft; ROM, range of motion.
Level of Competition and Injury Rates
The most common sports played by our patients were soccer (35.7%, n = 15) and lacrosse (28.5%, n = 12). Males accounted for 71% (n = 30) of the cohort and 71% (n = 5) of reinjuries. A second injury was sustained by 5 of the 30 males (16.7%) and 2 of the 12 females (16.7%). Ipsilateral injuries were sustained by 4 of the 5 males and 1 of the 2 females. The mean age at the time of second injury was 15.5 years for females and 15.0 years for males. Because competition level is believed to play a role in injury, athletes were categorized based on level of competition.26 Level 1 athletes were highly competitive and participated in travel leagues. This level was further divided into cutting sports (level 1A) and noncutting sports such as ski-racing (level 1B). Level 2 were multisport athletes and level 3 were those who participated in physical education or recreational sports. Level 1 athletes made up 64% (n = 27) of the cohort, of which 3 were ski-racers (level 1B); 31% (n = 13) were level 2 and 5% level 3. Eighty-three percent of those reinjured were from the competitive level 1 group; 100% were cutting athletes, and 50% (n = 3) were injured within the first 12 months of surgery. Neither ski-racers nor level 3 athletes were reinjured.
Discussion
Skeletally immature athletes are a challenging population because of high reinjury rates and low return to preinjury levels after ACLR.12,24,30,35,36,39 It is imperative to reduce modifiable risk factors and implement thorough return-to-sport criteria to ensure readiness and reduce reinjury rates. Research is lacking with regard to sport readiness criteria for this population as well as a thorough rehabilitation progression that follows an athlete through all phases of movement and ensures no compensatory movement patterns with sports-specific demands. Time frames are frequently used but do not account for the determination of risk factors and do not consider the impact of growth spurts on coordination and movement patterns. Quantitative assessments may provide valid and reliable measures of strength and power but lack information about alignment. The model proposed in this study is unique in that it uses a continuum of care and incorporates the QMA that consists of individualized benchmarks to guide athletes back to their respective sports in a timely and safe manner. To our knowledge, this is the first guideline that combines both qualitative and quantitative criteria to enable safe return to play in skeletally immature athletes after ACLR. The study allowed us to determine unique risk factors within this cohort, to develop areas of improvement to the program, and develop new questions for further research.
Impact of Rapid Developmental Change on Movement Patterns
The impact of rapid developmental change and early specialization is an underappreciated and underreported phenomenon in this challenging group of athletes. Physiological changes result in increased height, body mass, and bony lever arms, resulting in altered center of mass affecting alignment and quality of movement.2,16,40 This correlates with the need for increased time spent during the rehabilitation process to address the impact on coordination, core strength, and body alignment while allowing the athlete to adjust to their new body frame. For example, several athletes returned from summer break with significant growth changes and maturation affecting the quality of movements they had mastered prior to the break. Longer lever arms and altered centers of gravity required retraining of the skill sets as well as additional work on flexibility and strengthening. In addition, regular follow-up may be critical to assess movement and strength during skeletal maturation. Female athletes aged 12 to 13 years who are high risk at 14 to 16 years of age16,22 should be reassessed during maturation to ensure optimal neuromuscular patterns. The 2 females in the current study who sustained second injuries both fell in the at-risk age range of 14 to 16 years.
Sex-Specific Differences
Because of the fact that 71% of the patients in this cohort were male, statements or conclusions regarding sex-specific differences are not possible. We did observe that male and female second injury rates were equal at 16.7%, and that ipsilateral second injuries accounted for 4 of the 5 males and 1 of the 2 females.
Reduced Risk of Second Injury
The risk of sustaining a second ACL injury within 24 months of returning to activity has been reported at 29.5% in those aged 10 to 25 years of age, with 20.5% sustaining contralateral injury and 9% ipsilateral injury.3,15 The reinjury rate in our cohort was 14%, of which 4.5% (n = 2) sustained contralateral injury and 9.5% (n = 4) sustained ipsilateral injury. The reinjury rate would be 9%, excluding the 2 athletes who returned against medical advice. We believe our outcomes are a direct result of a close collaboration among all members of a multidisciplinary team working on combining both qualitative and quantitative measures to determine readiness to meet the demand of the sport. We believe contralateral injury occurs when compensatory movement patterns are present to offload the surgical limb and/or when risk factors are present within that limb. Fatigue will affect quality of movement.3 It is imperative to ensure that patients not only have appropriate strength, range of motion, and neuromuscular control with movements but also the appropriate endurance for their support to allow for reproducible safe movement patterns.
Return to Preinjury Level
Research reports that 43% of young athletes return to preinjury levels of play within 2 years.27 In this cohort, 91% (n = 39) returned to their primary sport with the exception of 3 athletes: 1 was noncompliant, 1 switched from football to hockey, while the other was advised not to participate in soccer secondary to a nonmodifiable risk factor (a connective tissue disorder). Communication was critical to ensure understanding of the importance of quality of movement and reduction of risk factors prior to returning to play. Video feedback served as a useful tool in this regard. Only 2 athletes returned to high-risk sports against our advice, and both incurred ACL injuries.
Quantitative and Qualitative Testing
Historically, return-to-sport criteria after ACLR focused on quantitative data such as muscle testing and hop tests.7,17 The battery of tests commonly used for assessing muscle strength (leg extension and leg press) and various hop tests (vertical jump and hop for distance) may not be sensitive enough to identify side-to-side differences, and therefore, new criteria may be required.7,41,42 This article reports on an attempt to minimize subsequent risk of reinjury using a continuum of care model incorporating regular physical therapy check-ups and a QMA combined with quantitative data.
A wealth of data has been published supporting successful ACL injury prevention programs by addressing biomechanical risk factors.9,18–21,26,39 ACL injury prevention principles served as the foundation for this model, and targeted exercises were provided to address specific deficits at each follow-up appointment. The risk of injury to either limb is substantially greater in those with a previous injury.20,35 Asymmetrical loading of limbs during jump landings after ACLR has been noted and identified as a risk factor for both the contralateral and ipsilateral limb.19,20,35 All athletes in the current study displayed asymmetrical loading at 6 months with a squat and compensatory movement patterns with single-limb movements before 9 months. We believe that because of the high rate of poor-quality functional movements seen at 6 and 9 months postoperatively, pediatric athletes should not be cleared to return to sports based on time frame alone.
Impact of Competitive Level
Our results demonstrated that the level of competition may impact secondary ACL injury rates in this cohort. All athletes who suffered reinjury were playing a cutting or jumping sport and participated at highly competitive levels. Sixty percent of reinjuries occurred within the first 12 months of surgery. This may indicate that return to a high level of play may require advanced return-to-sport criteria. There were no reinjuries in the group that returned to physical education and/or recreational leagues.
Risk Factors
As a result of an increasing number of ACL injuries occurring at younger ages, it is important to address modifiable risk factors during the rehabilitation process to prevent reinjury. According to our study, the most common patients in our cohort were males between the ages of 10 and 15 years who played cutting and jumping sports, specifically soccer and lacrosse. Furthermore, all reinjuries occurred during noncontact, cutting sports, which indicates that proper alignment and neuromuscular control are vital. Those playing at competitive levels are most at risk, given 65% of primary ACL injuries occurred in highly competitive leagues and 83% of reinjuries were within that group, suggesting that this subgroup of skeletally immature athletes needs special attention. Finally, with respect to timing, it appears that young athletes are at most risk when returning within 12 months of surgery.
Limitations
There are several limitations to our study, the first of which is that our study was not a randomized controlled trial so it did not examine cause and effect. Rather, it correlated good results on qualitative and quantitative assessments to reduced reinjury risk and readiness to return to sport. Additionally, the patient population included in the study was selective in terms of sports they played and lacked diversity in socioeconomic status. Similarly, it is important to note that implementing a QMA program for all patient populations may be unreasonable due to time and financial demands. Furthermore, we acknowledge that the surgeons who performed the surgeries were highly specialized pediatric sports medicine surgeons, to whom all patients may not have access. Finally, we did not assess nonmodifiable risk factors and their influence on reinjury.
Conclusion
A criteria-based progression for return to sport that considered both quantitative and qualitative data demonstrated excellent subjective and objective clinical outcomes in skeletally immature athletes. When quantitative data are combined with a qualitative assessment to address modifiable risk factors, sports medicine providers can better advise adolescent athletes when to return to sport. Such a program has the potential to decrease the risk of reinjury in these young athletes. Continued prospective analysis of large cohorts of skeletally immature athletes with respect to modifiable and nonmodifiable risk factors and the further development of multifactorial criteria for return to sport is required to further refine the recommendations made in this study.
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.
Ethical approval for this study was obtained from the Hospital for Special Surgery Institutional Review Board (Study #2015-357).
References
- 1. Ardern CL, Webster KE, Taylor NF, Feller JA. Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. Br J Sports Med. 2011;45:596–606. [DOI] [PubMed] [Google Scholar]
- 2. Carter CW, Micheli LJ. Training the child athlete: physical fitness, health and injury. Br J Sports Med. 2011;45:880–885. [DOI] [PubMed] [Google Scholar]
- 3. Chappell JD, Herman DC, Knight BS, Kirkendall DT, Garrett WE, Yu B. Effect of fatigue on knee kinetics and kinematics in stop-jump tasks. Am J Sports Med. 2005;33:1022–1029. [DOI] [PubMed] [Google Scholar]
- 4. Cordasco FA, Mayer SW, Green DW. All-inside, all-epiphyseal anterior cruciate ligament reconstruction in skeletally immature athletes: return to sport, incidence of second surgery, and 2-year clinical outcomes. Am J Sports Med. 2017;45:856–863. [DOI] [PubMed] [Google Scholar]
- 5. Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22:632–644. [DOI] [PubMed] [Google Scholar]
- 6. Dare DM, Fabricant PD, McCarthy MM, et al. Increased lateral tibial slope is a risk factor for pediatric anterior cruciate ligament injury: an MRI-based case-control study of 152 patients. Am J Sports Med. 2015;43:1632–1639. [DOI] [PubMed] [Google Scholar]
- 7. Delay BS, Smolinksi RJ, Wind WM, Bowman DS. Current practices and opinions in ACL reconstruction and rehabilitation: results of a survey of the American Orthopaedic Society for Sports Medicine. Am J Knee Surg. 2001;14:85–91. [PubMed] [Google Scholar]
- 8. DeMille P, Nguyen J, Brown A, Do H, Selvaggio E, Chiaia T. Quality of movement for athletes six months post ACL reconstruction. Orthop J Sports Med. 2016;4(7 suppl):2325967116S00202. [Google Scholar]
- 9. DiStefano LJ, Blackburn TJ, Marshall SW, Guskiewicz KM, Garrett WE, Padua DA. Effects of an age-specific anterior cruciate ligament injury prevention program on lower extremity biomechanics in children. Am J Sports Med. 2011;39:949–957. [DOI] [PubMed] [Google Scholar]
- 10. Dodwell ER, Lamont LE, Green DW, Pan TJ, Marx RG, Lyman S. 20 years of pediatric anterior cruciate ligament reconstruction in New York State. Am J Sports Med. 2014;42:675–680. [DOI] [PubMed] [Google Scholar]
- 11. Fabricant PD, Jones KJ, Delos D, et al. Reconstruction of the anterior cruciate ligament in the skeletally immature athlete: a review of current concepts: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95:e28. [DOI] [PubMed] [Google Scholar]
- 12. Ford KR, Myer GD, Hewett TE. Valgus knee motion during landing in high school female and male basketball players. Med Sci Sports Exerc. 2003;35:1745–1750. [DOI] [PubMed] [Google Scholar]
- 13. Ford KR, Myer GD, Smith RL, Vianello RM, Seiwert SL, Hewett TE. A comparison of dynamic coronal plane excursion between matched male and female athletes when performing single leg landings. Clin Biomech (Bristol, Avon). 2006;21:33–40. [DOI] [PubMed] [Google Scholar]
- 14. Ford KR, Shapiro R, Myer GD, Van Den Bogert AJ, Hewett TE. Longitudinal sex differences during landing in knee abduction in young athletes. Med Science Sports Exerc. 2010;42:1923–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Freedman KB, Glasgow MT, Glasgow SG, Bernstein J. Anterior cruciate ligament injury and reconstruction among university students. Clin Orthop Relat Res. 1998;356:208–212. [DOI] [PubMed] [Google Scholar]
- 16. Hass CJ, Schick EA, Tillman MD, Chow JW, Brunt D, Cauraugh JH. Knee biomechanics during landings: comparison of pre- and postpubescent females. Med Sci Sports Exerc. 2005;37:100–107. [DOI] [PubMed] [Google Scholar]
- 17. Henja W, Rosenberg A, Buturusis D, Krieger A. The prevention of sports injuries in high school students through strength training. Natl Strength Cond Assoc J. 1982;4:28–31. [Google Scholar]
- 18. Hewett TE, Ford KR, Hoogenbaum BJ, Myer GD. Understanding and preventing ACL injuries: current biomechanical and epidemiologic considerations. Am J Sports Med. 2007;35:235–241. [PMC free article] [PubMed] [Google Scholar]
- 19. Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes: a prospective study. Am J Sports Med. 1999;27:699–706. [DOI] [PubMed] [Google Scholar]
- 20. Hewett TE, Myer GD, Ford KR, Paterno MV, Quatman CE. The 2012 ABJS Nicolas Andry Award: the sequence of prevention: a systematic approach to prevent anterior cruciate ligament injury. Clin Orthop Relat Res. 2012;470:2930–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hewett TE, Paterno MV, Myer GD. Strategies for enhancing proprioception and neuromuscular control of the knee. Clin Orthop Relat Res. 2002;402:76–94. [DOI] [PubMed] [Google Scholar]
- 22. Kaeding CC, Aros B, Pedroza A, et al. Allograft versus autograft anterior cruciate reconstruction: predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kocher MS, Micheli LJ, Gerbino P, Hresko MT. Tibial eminence fractures in children: prevalence of meniscal entrapment. Am J Sports Med. 2003;31:404–407. [DOI] [PubMed] [Google Scholar]
- 24. Kvist J. Rehabilitation following anterior cruciate ligament injury: current recommendations for sports participation. Sports Med. 2004;34:269–280. [DOI] [PubMed] [Google Scholar]
- 25. Malempati C, Jurjans J, Noehren B, Ireland M, Johnson D. Current rehabilitation concepts for anterior cruciate ligament surgery in athletes. Orthopedics. 2015;38:689–696. [DOI] [PubMed] [Google Scholar]
- 26. Mandelbaum BR, Silvers HJ, Watanabe DS, et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am J Sports Med. 2005;33:1003–1010. [DOI] [PubMed] [Google Scholar]
- 27. Maron BJ, Isner JM, Mckenna WJ. 26th Bethesda conference: recommendations for determining eligibility for competition in athletes with cardiovascular abnormalities. Task Force 3: hypertrophic cardiomyopathy, myocarditis and other myopericardial diseases and mitral valve prolapse. J Am Coll Cardiol. 1994;24:880–885. [DOI] [PubMed] [Google Scholar]
- 28. MARS Group, Wright RW, Huston LJ, et al. Descriptive epidemiology of the Multicenter ACL Revision Study (MARS) cohort. Am J Sports Med. 2010;38:1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McCarthy MM, Graziano J, Green DW, Cordasco FA. All-epiphyseal, all-inside anterior cruciate ligament reconstruction technique for skeletally immature patients. Arthroscopy Tech. 2012;1:231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McCullough KA, Phelps KD, Spindler KP, et al. Return to high school-and-college-level football after anterior cruciate ligament reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) cohort study. Am J Sports Med. 2012;40:2523–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Myer GD, Paterno MV, Ford KR, Quatman CE, Hewett TE. Rehabilitation after anterior cruciate ligament reconstruction: criteria-based progression through the return-to-sport phase. J Orthop Sports Phys Ther. 2006;36:385–402. [DOI] [PubMed] [Google Scholar]
- 32. Nwachukwu BU, Schairer WW, Bernstein JL, Dodwell ER, Marx RG, Allen AA. Cost-effectiveness analyses in orthopaedic sports medicine: a systematic review. Am J Sports Med. 2015;43:1530–1537. [DOI] [PubMed] [Google Scholar]
- 33. Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of second ACL injury after primary ACL reconstruction and return to sport. Am J Sports Med. 2014;42:1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Price MJ, Tuca M, Cordasco FA, Green DW. Nonmodifiable risk factors for anterior cruciate ligament injury. Curr Opin Pediatri. 2017;29:55–64. [DOI] [PubMed] [Google Scholar]
- 35. Salmon L, Russell V, Musgrove T, Pinczewski L, Refshauge K. Incidence and risk factors for graft rupture and contralateral rupture after anterior cruciate ligament reconstruction. Arthroscopy. 2005;21:948–957. [DOI] [PubMed] [Google Scholar]
- 36. Shea KG, Pfeiffer R, Wang JH, Curtin M, Apel PJ. Anterior cruciate ligament injury in pediatric and adolescent soccer players: an analysis of insurance data. J Pediatr Orthop. 2004;24:623–628. [DOI] [PubMed] [Google Scholar]
- 37. Shelbourne KD, Gray T, Haro M. Incidence of subsequent injury to either knee within 5 years after anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med. 2009;37:246–251. [DOI] [PubMed] [Google Scholar]
- 38. Swartz EE, Decoster LC, Russell PJ, Croce RV. Effects of developmental stage and sex on lower extremity kinematics and vertical ground reaction forces during landing. J Athl Train. 2005;40:9–14. [PMC free article] [PubMed] [Google Scholar]
- 39. Thomeé R, Kaplan Y, Kvist J, et al. Muscle strength and hop performance criteria prior to return to sports after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19:1798–1805. [DOI] [PubMed] [Google Scholar]
- 40. van Mechelen W, Hlobil H, Kemper HC. Incidence, severity, aetiology and prevention of sports injuries: a review of concepts. Sports Med. 1992;14:82–99. [DOI] [PubMed] [Google Scholar]
- 41. Wilk KE, Macrina LC, Cain EL, Dugas JR, Andrews JR. Recent advances in the rehabilitation of anterior cruciate ligament injuries. J Orthop Sports Phys Ther. 2012;42:153–171. [DOI] [PubMed] [Google Scholar]
- 42. Zazulak BT, Hewett TE, Reeves NP, Goldberg B, Cholewicki J. The effects of core proprioception on knee injury: a prospective biomechanical-epidemiological study. Am J Sports Med. 2007;35:368–373. [DOI] [PubMed] [Google Scholar]