Abstract
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease that is caused by an autoimmune response against central nervous system (CNS) structures. Traditionally considered a T-cell-mediated disorder, the contribution of B cells to the pathogenesis of MS has long been debated. Based on recent promising clinical results from CD20-depleting strategies by three therapeutic monoclonal antibodies in clinical phase II and III trials (rituximab, ocrelizumab and ofatumumab), targeting B cells in MS is currently attracting growing interest among basic researchers and clinicians. Many questions about the role of B and plasma cells in MS remain still unanswered, ranging from the role of specific B-cell subsets and functions to the optimal treatment regimen of B-cell depletion and monitoring thereafter. Here, we will assess our current knowledge of the mechanisms implicating B cells in multiple steps of disease pathology and examine current and future therapeutic approaches for the treatment of MS.
Keywords: B lymphocytes, dosage regime, monitoring, multiple sclerosis, ocrelizumab, ofatumumab, rituximab
Introduction
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system (CNS). The complex pathogenesis of MS is still incompletely understood and, while MS has long been considered a ‘classical’ T-cell-mediated autoimmune disorder, intensive research has implicated an involvement of nearly all cell types of the immune system and CNS [Hafler et al. 2005]. A role of the humoral immune system was suggested based on data from histological stainings of MS lesions and the presence of intrathecal immunoglobulin production [O’Brien et al. 2010]. However, T cells have traditionally dominated our view of MS pathophysiology based on data from animal models, mainly experimental autoimmune encephalomyelitis (EAE), that biased pathogenetic concepts towards T helper cells. The contribution of the B cells to MS pathology has been reassessed in recent years due to new findings from basic research and pivotal case reports on the beneficial effect of B-cell-depleting therapies [Monson et al. 2005; Stuve et al. 2005]. The precise mechanisms by which B cells are involved in different stages of MS pathology still remain largely unclear. Several currently approved therapies for MS have at least a partial effect on B cells. Novel therapies addressing B cells either make use of anti-cell surface receptor directed antibodies resulting in cell depletion or aim at B-cell signaling pathways. Three different monoclonal antibodies against CD20-positive B cells (rituximab, ocrelizumab and ofatumumab) have shown overall promising effects in clinical phase II and III trials. CD20 is not expressed in haematopoietic stem cell and plasma cells. B-cell-depleting antibodies have a differential effect on different B-cell subsets and recovery after depletion determines not only treatment efficacy, but also treatment duration and side effects. Therefore, the dosage and mode of application, treatment intervals and monitoring strategies are critical factors that may determine the therapeutic success of B-cell-targeting approaches. Here, we discuss the pathophysiological rationale of targeting B cells and summarize current therapeutic approaches with a special focus on clinical administration regimens and monitoring strategies.
Evidence for B-cell involvement in the pathophysiology of MS
There is growing evidence of an additional involvement of humoral immunity in MS pathogenesis: A seminal study in 1950 first reported the presence of intrathecal immunoglobulin synthesis in MS patients [Kabat et al. 1950]. Oligoclonal bands (OCBs) are distinct protein bands that can be detected in the immunoglobulin region by isoelectrofocusing and immunoblot assay. Their presence in cerebrospinal fluid (CSF) but not in serum shows that synthesis of immunoglobulins has occurred within the CNS. While OCBs are not specific for MS, they are found in nearly 70% of patients with clinically isolated syndrome and nearly 90% of patients with clinically definite MS [Boster et al. 2010; Dobson et al. 2013]. The presence of OCBs has been used as a diagnostic tool in patients with suspected relapsing–remitting MS (RRMS) in the past (McDonald Criteria 2001/2005). While they are not included in the current revised McDonald Criteria 2010 for RRMS due to an increasing value of magnetic resonance imaging (MRI) findings, they are still valid for the diagnosis of primary progressive MS (PPMS) and for differential diagnosis [Polman et al. 2011]. Two studies of OCBs in patients with MS demonstrated that the absence of OCBs was associated with a benign disease course while a high number of OCBs correlated with a worse disease course [Zeman et al. 1996; Villar et al. 2002]. Using microarray approaches to investigate characteristics of MS lesions, another study showed that samples from acute lesions displayed significantly elevated levels of immunoglobulin transcripts compared to chronic silent lesions [Lock et al. 2002]. Various independent histological analysis of CNS lesions from MS patients revealed the presence of B cells, plasma cells and immunoglobulins [Esiri, 1977; Prineas and Wright, 1978]. Prominent immunoglobulin reactivity with depositions of IgG antibodies and C9neo complement is characteristic for myelin destruction in type II plaques according to the classification by Luccinetti and colleagues [Lucchinetti et al. 2000]. Immunoglobulin stainings associated with degenerating myelin and myelin degradation products within macrophages at the active edge of MS plaques argues for an active role in demyelinating lesions. Data from EAE studies support the notion that IgG antibodies facilitate contact between myelin and macrophages leading to myelin phagocytosis [Epstein et al. 1983; Moore and Raine, 1988]. Despite continuous research effort on putative CNS antigens [e.g. myelin basic protein (MBP), myelin oligodendrocyte glycoprotein (MOG) or myelin-associated glycoprotein (MAG)], no single predominant antigen structure for autoantibody responses in MS could be established (see e.g. [Owens et al. 2009] for details on this topic). A possible interpretation of these results is that an individual antibody response can be of differing affinity with different antigenic targets which might change over time by epitope spreading. Recently, it was reported that myelin-specific antibodies produced by autoreactive B cells after activation in the periphery accumulate in antigen-presenting cells in the CNS and significantly enhanced the activation of invading effector T cells [Flach et al. 2016]. Studies of MS pathology have shown the presence of ectopic lymphoid follicles resembling germinal centers containing B cells, T cells and antigen-presenting cells in the meninges of secondary progressive MS [Serafini et al. 2004; Magliozzi et al. 2010] leading to the suggestion that meningeal inflammation perpetuated by lymphoid-like structures are a driven force of cortical neuronal damage especially in later disease phases [Dendrou et al. 2015; Haider et al. 2016]. These follicles also harbor short-lived plasmablasts and plasma cells producing class-switched immunoglobulins that contribute to the compartmentalization of humoral responses in the CNS [Corcione et al. 2004; Michel et al. 2015].
Recent studies applying deep sequencing of IgG heavy chain variable region genes (IgG-VH) in B cells from MS patients revealed the ability of bidirectional B-cell exchange between the CNS and peripheral immune system [Blauth et al. 2015]. Different studies investigated the VH repertoire in the peripheral blood, cervical lymph nodes, meninges, CNS parenchyma and CSF [Von Budingen et al. 2012; Palanichamy et al. 2014a; Stern et al. 2014]. A common observation of all studies were overlapping clonal B-cell populations common to peripheral and CNS compartments. The patterns suggest that B cells are able to travel back and forth across the blood–brain barrier and commonly reenter germinal centers (in the meninges or cervical lymph nodes) to undergo further somatic hypermutations. These findings change our view of lymphocytic surveillance of CNS tissue and underlines that B-cell trafficking is an important topic for future research and therapy strategies.
The three putative biological roles of B cells are antibody production, antigen presentation and production of immunoregulatory cytokines. The later has led to the recognition of different B-cell subtypes producing either proinflammatory or regulatory cytokines (regulatory B cells and B effector cells). Abnormal antibodies and OCBs present in the spinal fluid of MS patients are not significantly reduced following effective B-cell depletion. Study results therefore indicate that peripheral antibodies titers do not impact relapse rate, Expanded Disability Status Scale (EDSS) changes or MRI parameters on a short-term levels. The effects of B-cell-modulating therapies on antibodies in CNS lesions themselves is an unknown topic. This is especially relevant in the light of recent studies showing that CNS-reactive autoantibodies are capable of initiating encephalitogenic immune responses by opsonization of endogenous antigens. These are then recognized by otherwise inactive myeloid antigen-presenting cells [Kinzel et al. 2016]. In the light of newly discovered functional lymphatic vessels lining the dural sinuses [Louveau et al. 2015] and a regular bidirectional trafficking of B cells between brain and periphery (see above), this study points towards novel and relevant B-cell effector pathways. Recent studies also elaborated the importance of antibody-independent functions of B cells in MS. In a recent study, a subtype of memory B cells that produces the proinflammatory cytokine granulocyte macrophage colony-stimulating factor (GM-CSF) was found to be more frequent and active in the blood of MS patients compared with controls [Li et al. 2015b]. These B cells were especially able to switch myeloid cells (and subsequently T cells) to a proinflammatory phenotype. After depletion with rituximab, repopulated B cells showed a reduction in the number of GM-CSF-producing B cells.
There is also growing evidence that a subset of regulatory B cells (Bregs) that display an immunoregulatory potential play an important role determining the safety and effectiveness of B-cell targeting therapies [Shen and Fillatreau, 2015]. These Bregs maintain peripheral and central immunological tolerance by secreting immunoregulatory cytokines, especially interleukin (IL)-10 and IL-35 [Fillatreau et al. 2002; Shen et al. 2014]. IL-10 and IL-35 are predominantly provided by distinct sets of regulatory plasma cells, leading to a protective effect in animal models of MS. Clinical experiences showed that Bregs with increased expression of CD38 and CD5 are predominately present after rituximab treatment and these B cells, when activated, express more IL-10 and less pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α, IL-6 and GM-CSF when compared with pretreatment B cells [Pers et al. 2008; Li et al. 2015a]. In line with this, another recent study also showed an elevation of IL10-producing regulatory B cells after therapy with fingolimod [Grutzke et al. 2015]. However, CD20-mediated B-cell depletion is also associated with an activation of monocytes/myeloid cells [Lehmann-Horn et al. 2011]. This underlines that a pan-B-cell depletion might also lead to negative effects as regulatory B cells are depleted as well. Further studies are expected to expand our knowledge on the effect of different B-cell-directed therapies on cytokine-producing B cells in MS. Accumulating evidence suggests that CD20 is also expressed on a subset of T cells (CD3+CD20dim T cells), that is increased in MS patients. While the pathophysiological relevance of CD20-expressing T cells remains to be determined, a depletion of these T cells has been shown under treatment with rituximab thus potentially contributing to the overall clinical effect of CD20-depleting therapies [Palanichamy et al. 2014b].
B cells can per se act as potent antigen-presenting cells and thereby activate T cells. A role of antigen-presenting B cells in MS pathology has been suggested by some studies [Weber et al. 2010; Brimnes et al. 2014], while the relevance of this mechanism is still far from understood. In summary, these studies suggest that B cells play a role in modulating T-cell function driving MS pathology and that antibody binding can directly mediate demyelinating processes within MS lesions.
B cells as therapeutic targets in MS: currently approved therapies
While there is much clinical and experimental data for a role of B cells in the pathogenesis of MS, a direct effect of MS therapies on B cells would provide further support for this hypothesis. Currently approved therapeutic approaches for MS therapy have mostly broad immune-modulatory effects and follow either a general shaping of the immune system towards anti-inflammatory immune responses by multifaceted targets (e.g. interferons, glatiramer acetate) or result in a broad depletion of different immune cells (e.g. mitoxantrone, alemtuzumab). More recently, the field of neuroimmunotherapy is changing as novel therapies aim at a selective modulation of distinct molecular pathways (e.g. natalizumab). Interestingly, nearly all current MS therapies have the potential to affect B cells while the contribution of these biological effects compared with other potential mode of actions are often difficult to quantify. These difficulties and the lack of clinically approved B-cell-specific interventions for MS might have led to an underestimation of B-cell-specific therapeutic effects (see also Krumbholz et al. [2012] and Longbrake and Cross [2016] for more details). Treatment with β-interferons (IFNβ) significantly reduces the number of circulating CD80+ B cells in patients with active disease correlating with clinical amelioration [Genc et al. 1997; Liu et al. 2001]. IFNβ therapy also reduces the expression of CD40, a marker necessary for B-cell differentiation [Liu et al. 2001], as well as the expression of MHC II inhibiting the ability of B cells to stimulate antigen-specific T-cell responses [Jiang et al. 1995]. Glatiramer acetate has been shown to alter the function of antigen-presenting cells (including B cells) in order to shift the phenotype from TH1 to TH2 phenotypes [Neuhaus et al. 2001; Chofflon, 2005] and brain antigen-specific B-cell responses have been proposed to correlate with glatiramer acetate response in MS patients [Rovituso et al. 2015]. Apart from these classical broad immunomodulatory drugs, effects on B cells have also been shown for novel immunotherapeutics. Natalizumab was initially developed to block T cell adherence to the inflamed blood–brain barrier via an inhibition of the molecular interaction between α4β1-integrin (VLA-4) on T lymphocytes and VCAM-1 on endothelial cells. Memory B cells also express high levels VLA-4 and it has been proposed that natalizumab treatment affects directly the migration of B cells into the CNS. However, B cell reduction in the CSF might also be a secondary effect to reduced T cells in the CNS [Alter et al. 2003; Stuve et al. 2006]. Fingolimod acts as a functional antagonist of the sphingosine-1-phosphate (S1P) receptor, necessary for lymphocyte egress from secondary lymphoid structures. As a consequence, naïve and central memory T cells are trapped within the lymphatic tissue. However, a significant drop also of B cells can be observed in the peripheral blood [Kowarik et al. 2011]. Additionally, circulating B cells showed suppressed pro-inflammatory properties with lower levels of CD80, lower TFN levels and higher levels of IL-10 [Miyazaki et al. 2014]. Recently, these findings have been underlined by the finding of a significant increase in the proportion of regulatory B cells upon fingolimod treatment [Grutzke et al. 2015]. Teriflunomide selectively inhibits dihydro-orotate dehydrogenase leading to a reduction in proliferation of activated T and B lymphocytes [Bar-Or et al. 2014b]. Finally, targeting of CD52 by alemtuzumab selectively depletes CD52-bearing immune cells (i.e. T and B lymphocytes and to a lower degree also monocytes, macrophages and eosinophil granulocytes) rapidly after infusion by antibody-dependent and complement-dependent cytolysis. The slow repopulation of these cell populations is believed to rebalance immunological responses mediating long-term beneficial therapeutic effects. B lymphocytes recover early after 3 months showing an overshoot to approximately 150% of baseline levels after 12 months. B-cell reconstitution is associated with a shift towards naïve B cells and a long-term memory B-cell lymphopenia [Thompson et al. 2010; Heidt et al. 2012].
Preclinical therapies targeting B cells in MS
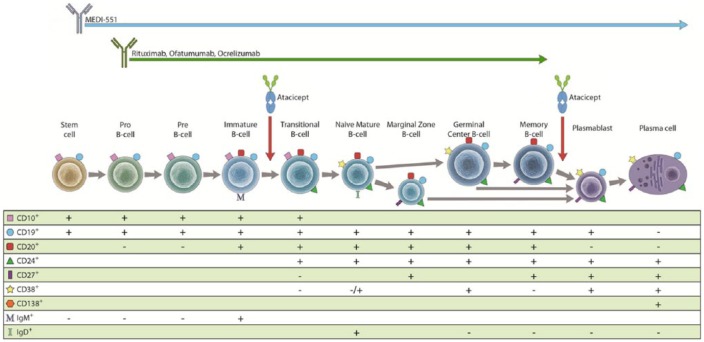
Some of the most compelling lines of evidence for B-cell involvement in MS pathogenesis comes from recent clinical trials that may ultimately lead to the approval of B-cell-specific therapies in the near future. Multiple strategies have been proposed for the modulation of B-cell populations in MS patients (see Figure 1). CD20 is a transmembrane molecule that can be found on naïve and memory B cells, but not on stem cells or fully differentiated plasma cells [Maloney, 2012]. Depletion of CD20-expressing B cells is the common mode of action of rituximab, ocrelizumab and ofatumumab. An overview of currently ongoing clinical trials targeting B cells is provided in Table 1.
Figure 1.
Pathways of B-cell differentiation and characteristic cell surface markers. Targeting of B-cell subtypes by MEDI-551, rituximab, ofatumumab, ocrelizumab and atacicept are shown. See the text for details on the therapeutics.
Table 1.
Currently ongoing or recently terminated clinical trials using B-cell-directed strategies in multiple sclerosis.
| Drug | Mode of action | Phase | ClinicalTrials.gov identifier | Study rationale | Patients | Control group | Primary endpoint | Duration | Status |
|---|---|---|---|---|---|---|---|---|---|
| Rituximab | Depletion of CD20 B cells | 2 | NCT00097188 | Safety and efficacy of 2 × 1 g intravenous rituximab on patients with RRMS | 104 | Placebo | Total count of gadolinium-enhancing lesions in MRI | 2 years | Hauser et al. [2008] |
| 2/3 | NCT00087529 | Efficacy of intravenous repeating doses of rituximab on patients with PPMS | 439 | Placebo | Time to confirmed disease progression (increase in EDSS) | 4 years | Hawker et al. [2009] | ||
| 1/2 | NCT01212094 | Safety and efficacy of combined systemic and intrathecal treatment in patients with SPMS | 44 | Placebo | Brain atrophy progression | 2 years | Active, not recruiting | ||
| 1 | NCT02253264 | Safety and efficacy of intrathecal treatment in patients with progressive MS showing meningeal inflammation | 12 | None | Number of serious adverse events | 1 year | Recruiting | ||
| 2 | NCT02545959 | Efficacy of intrathecal treatment on CSF markers in patients with progressive MS | 12 | Methyl-prednisolone | Change in osteopontin level in CSF | 1 year | Recruiting | ||
| 2 | NCT01719159 | Safety and efficacy of intrathecal treatment in patients with progressive MS | 30 | None | Number of participants with adverse events | 1 year | Recruiting | ||
| Ocrelizumab | Depletion of CD20 B cells | 2 | NCT00676715 | Dose finding study to evaluate the efficacy and safety of 2 dose regimens of ocrelizumab in patients with RRMS | 220 | Placebo, IFN-β 1a i.m. | Total count of gadolinium-enhancing lesions in MRI | 24 weeks | Kappos et al. [2011] |
| 3 | NCT01412333 | Efficacy and safety of ocrelizumab in patients with RRMS | 835 | IFN-β 1a s.c. | Annualized relapse rate by 2 years | 96 weeks | Completed, results not published yet | ||
| 3 | NCT01247324 | Efficacy and safety of ocrelizumab in patients with RRMS | 821 | IFN-β 1a s.c. | Annualized relapse rate by 2 years | 96 weeks | Completed, results not published yet | ||
| 3 | NCT01194570 | Efficacy and safety of ocrelizumab in patients with PPMS | 732 | Placebo | Time to onset of sustained disability progression | Up to 5.5 years | Completed, results not published yet | ||
| Ofatumumab | Depletion of CD20 B cells | 2 | NCT00640328 | Safety of escalating doses of intravenous ofatumumab in patients with RRMS | 38 | Placebo | Safety | 24 weeks | Sorensen et al. [2014] |
| 2 | NCT01457924 | Safety and efficacy of subcutaneous doses of ofatumumab in patients with RRMS | 232 | Placebo | Cumulative number of new Gd-enhanced lesions in cMRI (up to week 12) | 24 weeks | Completed, results not published yet | ||
| MEDI-551 | Depletion of CD19 B cells | 1 | NCT01585766 | Safety and tolerability of i.v. and s.c. MEDI-551 in patients with RRMS | 56 | None | Safety and tolerability | 170 days | Ongoing, not recruiting |
| VAY736 | Blockade of BAFF-R | 2 | NCT02038049 | Safety, tolerability and efficacy of VAY736 | Unknown | None | Cumulative number of new Gd-enhanced lesions | 16 weeks | Finished, awaiting results. |
Literature search was performed on www.clinicaltrials.gov in September 2015. All published, finished (but unpublished) and continuing studies are listed.
CSF, cerebrospinal fluid; EDSS, Expanded Disability Status Scale; Gd, gadolinium; MS, multiple sclerosis; PPMS, primary progressive multiple sclerosis; RRMS, relapsing–remitting multiple sclerosis; SPMS, secondary progressive multiple sclerosis.
Rituximab
Rituximab is a chimeric monoclonal antibody leading to depletion of CD20+ B cells. This established B-cell targeting therapy has been approved for the treatment of different lymphomas, rheumatoid arthritis (RA) and antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis in Europe and has been shown to be effective in different additional autoimmune disorders such as systemic lupus erythematodes or Sjögren’s syndrome [Tokunaga et al. 2007; Gurcan et al. 2009]. In MS, four clinical trials have so far been completed [Barun and Bar-Or, 2012]. Two smaller open-label phase I/II trials showed good tolerability and a significant reduction in gadolinium (Gd)-enhanced lesions [Bar-Or et al. 2008; Naismith et al. 2010]. In a randomized, placebo-controlled phase II trial in 104 patients with RRMS (HERMES trial), patients treated with rituximab showed a 91% reduction of Gd-enhanced lesions and a reduction of relapse rates by 49% after 48 weeks [Hauser et al. 2008]. In contrast, a phase II/III trial in 439 patients with PPMS (OLYMPUS trial) failed to show a reduction in confirmed disease progression after 96 weeks [Hawker et al. 2009]. However, when looking at subgroups of patients younger than 50 years or with Gd-enhanced lesions at baseline, there was a significant effect. Despite promising effects in the HERMES trial and subgroups in the OLYMPUS trial, no further phase III trials necessary for regulatory approval were launched for rituximab. Instead, clinical research focused on newer-generation anti-CD20 mAbs ocrelizumab and ofatumumab. While these fully humanized antibodies might prove superior over a chimeric antibody especially concerning safety, tolerability and biological properties, it has been suggested that licensing issues have had an additional influence on this decision [Buttmann, 2010; Gasperi et al. 2016; Milo, 2016].
Rituximab treatment has shown not only to affect peripheral, but also centrally localized B cells. Animal studies have shown that anti-CD20 treatment depleted B cells within the CNS of mice with disease systems [Weber et al. 2010]. Investigations of rituximab-treated patients have shown a depletion in the CSF and in the perivascular spaces as revealed by histological stainings [Martin Mdel et al. 2009]. These findings are especially relevant in light of the particular relevance of B cells in meningeal compartments and B-cell trafficking routes in and out of the CNS (see above). However, direct evidence from a therapeutic effect on MS lesions has so far not been reported. Based on the observation of germinal center-like structures in the meninges of progressive MS patients, intrathecal administration of low doses of rituximab has been investigated, demonstrating a sustained depletion of peripheral B cells, but only a moderate decline of CSF B cells [Svenningsson et al. 2015]. Further smaller trials are ongoing focusing on the intrathecal administration or the combined systemic and intrathecal use of rituximab [ClinicalTrials.gov identifier: NCT01212094] (Table 1). Of note, serious adverse events have been reported in patients treated with rituximab in different disorders, including cases of progressive multifocal leukoencephalopathy (PML), a rare viral infection of the CNS caused by JC virus. While no PML cases have been reported in MS patients receiving rituximab, the number of individuals with MS treated with rituximab is relatively small.
Ocrelizumab
Ocrelizumab is an anti-CD20 antibody causing B-cell depletion by complement-dependent lysis and antibody-dependent cytotoxicity [Gasperi et al. 2016]. In contrast to rituximab, a chimeric antibody, ocrelizumab is a humanized IgG1 antibody with decreased immunogenicity. Ocrelizumab binds to a different but overlapping epitope than rituximab. Compared with rituximab in vitro, ocrelizumab demonstrated increased antibody-dependent cellular cytotoxicity and reduced complement-dependent cytotoxicity, which might additionally lead to reduced immunogenicity and increased tolerability [Oflazoglu and Audoly, 2010]. Development of ocrelizumab in RA and systemic lupus erythematosus (SLE) were terminated since phase III trials did not demonstrate additional benefit of ocrelizumab over existing therapies [Mysler et al. 2013; Emery et al. 2014]. Serious infects were more frequent in both RA and SLE trials, however, ocrelizumab was used as a combination therapy together with methotrexate or glucocorticoids and mycophenolate mofetil.
For MS, ocrelizumab has been used as a monotherapy. A phase II randomized, placebo-controlled trial in patients with RRMS showed a significant reduction of annualized relapse rates (ARRs) and Gd-enhanced lesions in the ocrelizumab groups compared to both placebo and IFNb-1a treatment [Kappos et al. 2011]. Due to one death due to acute-onset thrombotic microangiopathy in the higher dose arm (2000 mg i.v.), following phase III trials focused on the lower-dose regimen (600 mg i.v.). Results of two phase III trials (OPERA I and II [ClinicalTrials.gov identifiers: NCT01247324 and NCT01412333]) in RRMS patients and one phase III trial (ORATORIA [ClinicalTrials.gov identifier: NCT01194570]) in PPMS patients were recently reported at the ECTRIMS congress 2015 [Sorensen and Blinkenberg, 2016]. In the OPERA I and II trials, 600 mg ocrelizumab i.v. administered every 24 weeks to 1656 patients led to a significant reduction of ARRs (~47%) compared with IFNβ-1a treatment after 96 weeks. Ocrelizumab also reduced the risk of confirmed disability progression by 40% after 12 and 24 weeks of treatment. Moreover, a drastic effect was observed for reduction of Gd-enhanced T1 lesions by 94–95%. Most common adverse events associated with ocrelizumab were infusion-related reactions in 34% versus 10%. Most infusion-related reactions were mild-to-moderate in severity and decreased with number of treatment cycles. In the OPERAI/II trials, serious adverse events were comparable with the IFNβ-1a group. Higher rates of upper respiratory tract and nasopharyngitis infections were observed (approximately 15% versus 10%) but especially no opportunistic infections were reported. However, PML has been reported in patients treated with anti-CD20 antibodies for other indications. In contrast to experiences from alemtuzumab (depleting B and T lymphocytes), no cases of secondary autoimmunity were reported so far.
In parallel, the efficacy of ocrelizumab was tested in a placebo-controlled phase III trial (ORATORIO) in patients with PPMS. A total of 488 patients received 600 mg ocrelizumab every 24 weeks compared with placebo (244 patients) and primary endpoint was confirmed disability progression after 12 weeks of treatment. Secondary endpoints included confirmed disability progression after 24 weeks of treatment, timed walked test, T2-weighted lesion volume and loss of total brain volume. Ocrelizumab treatment resulted in a 24% lower risk for confirmed disability progression after 12 weeks and a 25% lower risk after 24 weeks. At 120 weeks, patients had a 29% reduction in walking time. T2-weighted lesion volumes were 3.4% lower than at baseline and brain volume loss was 17.5% lower than in patients who received placebo. Ocrelizumab was therefore the first treatment ever to show clinical efficacy in patients with PPMS. However, the occurrence of side effects in the ORATORIO trial has raised questions about its safety profile. Specifically, the frequency of malignancies was higher in patients treated with ocrelizumab compared with placebo (11 versus 2). If basal cell carcinomas were excluded (as they are sometimes considered not to be malignancies), the rate was still 8 to 1. This is considerably higher than in pooled OPERAI/II data (2 versus 4 malignancies) for yet unknown reasons. When looking at the type of malignancy, breast cancer occurred altogether in 6 ocrelizumab patients versus 0 in placebo groups. It is so far unclear, whether there is indeed a link between ocrelizumab and an increased risk for malignancies which would also not have been suspected from experiences with rituximab.
Of note, different clinical trials in patients with PPMS have been shown to vary concerning the proportion of patients with Gd-enhanced lesions at baseline ranging from 14.1% in the PROMiSe trial (glatiramer acetate) to 24.5% in the OLYMPUS trial (rituximab) and 27.5% in the ORATORIA trial raising concerns about a putative bias towards more inflammatory active patients. While in the ORATORIA trial, the number of patients with Gd-enhanced lesions was quite comparable in the placebo versus ocrelizumab arms (24.7% versus 27.5%), the actual number of Gd-enhanced lesions was twice as high in the active arm as in placebo (0.6 versus 1.2). Nevertheless, a subgroup analysis presented at the ACTRIMS congress (02/2016) showed that ocrelizumab showed no significant differences in efficacy in patients with and without T1 Gd-enhanced lesions at baseline. However, as the study was not powered to show statistical significance between subgroups, no definite conclusions can be drawn as to which patients might benefit most from ocrelizumab treatment.
Ofatumumab
Ofatumumab is a fully humanized anti-CD20 antibody binding to a different epitope than rituximab (and ocrelizumab) resulting in a pronounced complement-mediated cytotoxicity in vitro [Klotz and Wiendl, 2013]. Ofatumumab has a higher binding affinity to CD20 compared with rituximab and it binds to an additional antigenic determinant. It is currently approved for the treatment of chronic lymphatic leukemia. However, whether this differences translate into distinct clinical differences in patients with MS is currently unknown [Zhang, 2009]. In a placebo-controlled phase I/II trial in 38 patients with RRMS treated with two intravenous administrations of 100, 300 or 700 mg ofatumumab, a significant reduction of Gd-enhanced lesions and new and enlarged T2 lesions was observed after 24 weeks in all doses [Sorensen et al. 2014]. Treatment was not associated with any unexpected safety concerns and was generally well tolerated. A subsequent phase II placebo-controlled in 232 patients (MIRROR trial) assessed the efficacy of subcutaneously administrated ofatumumab (3, 30 and 60 mg every 4 weeks) over 6 months [ClinicalTrials.gov identifier NCT01457924]. The cumulative number of new T1 Gd-enhanced lesions was reduced by 65%. A dose-dependent CD19 B-cell depletion, reconstitution rate and effect on MRI activity was seen across regimens [Bar-Or et al. 2014a]. Phase III trials have been announced but no further details are available yet.
MEDI-551
In MS and neuromyelitis optica (NMO), there are substantial data from clinical trials that B-cell depletion with anti-CD20 antibodies is relatively safe and effective. CD20 is detected on pre-B cells, immature B cells, naïve B cells and mature B cells. However, both plasmablasts and plasma cells are spared by these therapeutic approaches. Therefore, the depletion of CD19-expressing cells might offer potential advantages with regard to efficacy by additionally depleting antibody-producing plasmablasts, but potentially with a higher risk of infections. Mature plasma cells are not directly affected by anti-CD19 treatment [Halliley et al. 2015]. The most advanced compound in clinical trials targeting CD19 is MEDI-551, a humanized monoclonal antibody. MEDI-551 is currently been tested in a phase I trial and no results have been released so far [ClinicalTrials.gov identifier: NCT01585766].
Atacicept and VAY736
Atacicept is a human recombinant fusion protein with an Ig Fc domain that combines the binding portions of receptors for both BLyS/BAFF (B-Lymphocyte Stimulator or B-Cell activating Factor of the TNF Family) and APRIL (A Proliferation-Inducing Ligand). BAFF and APRIL are two TNF family ligands regulating B lymphocyte and plasma-cell survival, antibody isotype switching, antibody responses and T-cell costimulation via binding to different receptors (see e.g. Schneider [2005] for a detailed review on this topic). Atacicept binds soluble BAFF and APRIL molecules thereby preferentially impairing mature B cells and plasma cells with less impact on progenitor cells memory cells [Edwards and Cambridge, 2006]. Treatment with this blocking antibody causes the arrest of splenic B-cell development at the immature transitional T1 stage [Boster et al. 2010] and suppresses the production of autoreactive antibodies in mouse models of RA and SLE. However, a phase II trial in patients with MS who received weekly subcutaneous doses of atacicept (25, 75 or 150 mg, ATAMS study) was terminated due to an increase of clinical disease activity [Kappos et al. 2014]. ARRs more than doubled in all atacicept groups compared to placebo (ARR placebo: 0.38; ARR atacicept: 0.79–0.98). Of note, MRI findings did not reflect these differences as mean numbers of Gd-enhanced T1 lesions per scan were similar in all groups. The differences between atacicept and anti-CD20 drugs points towards a complex role of B cells in the pathophysiology of MS. An additional expression for BAFF and APRIL has for example been described not only on B cells but also on T cells. One other possible explanation might have to do with the specific B-cell subsets preferentially targeted by atacicept. It has been hypothesized that atacicept might preferentially intervene with regulatory B-cell subtypes possessing high expression levels of APRIL [Stuve et al. 2014].
Another current approach is VAY736, a human monoclonal antibody targeting BAFF-receptor, that is currently been tested in a phase II trial [ClinicalTrials.gov identifier: NCT02038049]. Results are awaited for 2016.
Dosing of B-cell-depleting therapies: experiences from rituximab
Experiences from B-cell-depleting therapies have underlined the importance of an optimal treatment regimen to balance clinical safety and efficacy. Phase III trials for ocrelizumab used slightly different regimens: for OPERA I/II, 600 mg i.v. were applied every 6 months (first cycle: twice 300 mg i.v. 2 weeks apart, then single dose 600 mg). For ORATORIA, a continued splitting of the dosage in 300 mg i.v., 2 weeks apart, was chosen for every 6-month cycle. It is however unclear, whether this treatment regimen is optimal and whether individual patients’ responses should be taken into account. Available studies from closely related rituximab in MS and other indications (e.g. NMOSD) might help to give further insights.
Rituximab is currently approved for the treatment of CD20-positive non-Hodgkin lymphomas, CD20-positive chronic lymphocytic leukemia (CLL), RA and ANCA-associated systemic vasculitis; however its off-label use extends to various other autoimmune disorders. In MS it is used more often than expected for an unapproved drug. The Swedish register of neurological diseases allocates 14% of MS therapies in adults to rituximab (August 2015, see http://www.neuroreg.se) [Salzer et al. 2016]. Alongside the variety of indications, there are many different dosing and application strategies. In MS rituximab is commonly administered intravenously either at a dose of 1000 mg on days 1 and 15, or 375 mg/m2 in 4-weekly doses every 6–12 months [Kim et al. 2013; Melzer and Meuth, 2014]. Concomitant steroids, antihistaminic and antipyretic drugs are used to reduce infusion-associated adverse reactions. Apart from the usage as monotherapy, rituximab was also tested as add-on therapy (4-weekly doses of 375 mg/m2, added on to IFNs or glatiramer acetate) in patients with RRMS demonstrating an additional reduction of Gd-enhanced brain lesions [Naismith et al. 2010]. The RIVITALISE trial [ClinicalTrials.gov identifier: NCT01212094] evaluated the efficacy of intrathecally administered rituximab compared with placebo in SPMS following the hypothesis of compartmentalized inflammation in the progressive phase of MS. 2% of serum concentrations were measured in the CSF representing a 20-fold increase in bioavailability of serum values achievable without intrathecal dosing [Komori et al. 2016]. However, the trial was prematurely terminated due to not meeting efficacy endpoints during the interim analysis. In contrast to commonly applied regimens, the same study combined only 200 mg i.v. rituximab on day 1 and day 15 with intrathecally administered rituximab. The authors demonstrated that higher rituximab concentrations paradoxically decreased the rituximab concentration on the surface of B cells, due to facilitated internalization of CD20/rituximab complexes in vitro [Komori et al. 2016]. Therefore, the 200 mg dose was considered sufficient to saturate CD20 leading to long-lasting depletion of B cells from peripheral circulation [Komori et al. 2016]. However, greater surface binding does not mean more-efficient depletion and the study did not investigate B-cell depletion in vivo for the different rituximab dosages to allow definite conclusions. Nevertheless, the duration of depletion is dose-dependent as shown in a study by Greenberg and colleagues investigating B-cell repopulation dynamics in NMO and MS patients. In patients given 100 mg twice the CD19 positive B-cell population was greater than 2% in a mean of 99 days (range 43–172), for the 1000 mg dose in 184 days (range 52–288) [Greenberg et al. 2012]. Higher dosages might be able to penetrate B-cell-containing secondary lymphoid tissues in higher magnitude and therefore may be critical to delay reconstitution.
In RA several trials and cohort studies suggested comparable efficacy of high-dose (1000 mg twice) and low-dose (500 mg twice or 1000 mg once) rituximab treatment. A meta-analysis of four randomized trials showed non-inferiority of the low dose for the most composite measures of disease activity and patient-reported outcomes. However, some trends were found for long-term clinical outcomes and early withdrawal due to lack of efficacy favoring high-dose rituximab [Bredemeier et al. 2014]. In immune thrombocytopenia a multicenter retrospective study compared rituximab 1000 mg on days 1 and 15 with the standard regimen of 4-weekly doses of 375 mg/m2 including 107 patients. A total of 22/61 patients (36%) treated with the standard regimen and 23/46 (50%) with the 1000 mg twice dosing reached the definition for treatment response without being significantly different [Mahevas et al. 2013]. So far there have been no randomized controlled trials in MS comparing efficacy and safety of the different dosing strategies. To the current knowledge both commonly administered dosing regimens of rituximab seem to be feasible to induce long-term B-cell depletion in MS. Higher doses of rituximab could be associated with more prolonged B-cell depletion. However, the individual variety in magnitude and duration of B-cell depletion demonstrates the importance of a close monitoring in B-cell-targeted therapies.
Monitoring of rituximab therapy
Retreatment
Rituximab is an expensive treatment with unclear long-term safety of repeated infusions. Monitoring strategies guiding when and how to retreat patients might help to minimize unnecessary exposure to the drug and therefore to optimize the individual efficacy and safety and also allow for significant cost savings. In RA patients are regularly monitored for disease activity using different clinical composite measures and rituximab treatment is adjusted to achieve predefined goals [Emery et al. 2011]. However, in MS the clinical and radiological signs of disease activity do not present gradually and are no clearly defined tools to continuously monitor disease activity or treatment effects. Of note, outcome in RA are distinct from MS and rituximab is often discontinued unless there is evidence of continued activity and is often used in conjunction with other medications.
Therefore, initial treatment strategies in NMO or MS followed uncontrolled fixed intervals. The every-6-month rituximab dosing strategy was based on data suggesting that most patients show B-cell depletion for approximately 6 months [Roll et al. 2006; Greenberg et al. 2012]. However, there are high interindividual variations of B-cell repopulation as stated above and therefore these uncontrolled dosing regimens might not be adequate to control disease activity in many patients. In a cohort study of NMO and MS patients, 5 of 29 (17%) patients demonstrated B-cell repopulation before 6 months after the last rituximab infusion [Greenberg et al. 2012]. Interestingly, patients with early repopulation did not always show the same repopulation dynamic in repeated infusion courses emphasizing the necessity of individual monitoring strategies. Greenberg and colleagues suggested monitoring of the B-cell population by analyzing CD19 positive cell counts as it has been established for RA before [Dass et al. 2008]. In contrast to previously defined limits of B-cell depletion with CD19 counts below 0.01*109/l, this study set a CD19 percentage of ⩾2% of all peripheral blood mononuclear cells (PBMCs) as cut-off for retreatment, which was based on the observation of a significant rise of CD19 counts when reaching this limit [Pellkofer et al. 2011]. A subsequent study by Kim and colleagues found that CD19 counts as appropriate monitoring tool for rituximab therapy. In 30 NMO patients 65% of relapses or 60% respectively occurred in patients with CD19 counts <0.5% or <0.01 × 109/l of PBMCs [Kim et al. 2013]. In RA the return of disease activity correlated with higher numbers of memory B cells [Leandro et al. 2006] and in NMO their depletion was associated with clinical response to rituximab [Roll et al. 2008]. Therefore, Kim and colleagues suggested evaluation of the reemerging CD27+ memory B-cell frequency to decide on retreatment. PBMCs were obtained every 6 weeks throughout the first year, every 8 weeks throughout the second year and every 10 weeks thereafter to evaluate lymphocytes subsets. Patients were retreated with one additional infusion of rituximab whenever the frequency of CD27+ memory B cells exceeded 0.05% of PBMCs in the first 2 years or 0.1% thereafter. Control disease activity was maintained in almost all patients with longer retreatment intervals after 2 years than during the initial 2-year study (about 8 versus 5 months, respectively) and the cumulative dose of rituximab was much lower when compared with a calculated fixed treatment interval of every 6 months. Of note, the reemergence of CD27+ memory B cells was not necessarily proportional to the reconstitution of CD19+ B cells and might exceed the limits even with low CD19 counts [Kim et al. 2011, 2013]. In a phase II trial of ocrelizumab in RRMS long-lasting memory B-cell repopulation coincided with sustained suppression of MS disease activity indicating that memory B-cell counts might also be a tool to guide retreatment strategies in MS for B-cell-targeted therapies. Even after multiple rituximab cycles memory B-cell repopulation follows comparable patterns supporting its use in long-term monitoring [Roll et al. 2015]. However, with analyzing the CD27+ memory B-cell pool relapses can still be observed in NMO patients during periods where memory B cells were lower than the therapeutic target as has been shown in a follow-up study of 100 rituximab-treated NMO patients [Kim et al. 2015] demanding new and more developed monitoring strategies in NMO, MS and other indications. Moreover, there is no randomized clinical trial comparing the different surveillance and fixed-dose strategies to draw definite conclusions for rituximab use.
Based on the expression of IgD, IgM and CD27 the memory B-cell pool can be further subdivided into CD27+IgM+IgD+, IgM-only, IgD-only, CD27+IgM−IgD− (CD27+, class-switched), and CD27− IgM−IgD− memory B cells (CD27−, class-switched) [Wu et al. 2011]. CD27− memory B cells represent 1–4% of all peripheral B cells corresponding to approximately 25% of IgG+ blood B cells and questioning CD27 as exclusive memory B-cell marker [Fecteau et al. 2006]. In RA both the number of CD27+IgD– or CD27–IgD– class-switched memory B cells has been shown to correlate with response to rituximab therapy [Moller et al. 2009; Tony et al. 2015]. Other tools such as markers for late-stage B-cell lineage plasmablasts and fragment c gamma receptor 3A (FCGR3A) gene polymorphisms have been suggested to predict efficacy of B-cell-targeted therapies and might help to identify eligible patients [Owczarczyk et al. 2011; Kim et al. 2015].
In MS rituximab was shown to deplete B cells from the CSF [Cross et al. 2006] and might also be able to diminish B cells in the brain tissue [Martin Mdel et al. 2009] opening new opportunities for monitoring. The evaluation of B-cell-related biomarkers such as IL-6, BAFF (B-cell activating factor), APRIL (A proliferation-inducing ligand) and soluble CD21 or repopulation dynamics of different B cell subsets in the CSF might be more suitable to predict treatment response and the need for retreatment due to the close proximity to the inflammatory responses in MS. In accordance, rituximab has been shown to normalize IL-6 and GM-CSF levels secreted by B cells isolated from RRMS patients in vitro [Barr et al. 2012; Li et al. 2015]. With the upcoming variety of B-cell-targeted therapies for MS new monitoring strategies are urgently needed to improve patient selection and guidance and therefore therapeutic efficacy and safety.
Precautions/adverse events
Before rituximab initiation all patients should be screened for HBV, HCV and HIV infection. All vaccines following the respective guidelines should be completed at least 4–6 weeks prior to therapy start. After rituximab administration CBC with differential and platelets should be done in weekly to monthly intervals with higher frequencies in patients with cytopenias. Frequent adverse events are infusion-associated reactions within 24 h after the first infusion and mild to moderate infections (nasopharyngitis, upper respiratory tract infections, urinary tract infections and sinusitis). Infrequent, but severe adverse events are toxic epidermal necrolysis (Lyell syndrome), Stevens–Johnson syndrome and PML. In RA the PML risk is estimated at 1 per 25,000 treated individuals [Clifford et al. 2011]. In addition, hypogammaglobinemia has been reported in rituximab-treated patient cohorts. In a 5-year follow up of 41 NMO patients 53%, 29%, and 10% had low IgM, IgG, and IgA levels, respectively [Kim et al. 2015]. However, infection rates were not increased with low IgM or IgG levels. In contrast, in a cohort of lymphoma patients 6.6% patients needed treatment with intravenous immunoglobulins for recurrent infections [Casulo et al. 2013]. Therefore, monitoring of serum immunoglobulin concentrations might be needed in patients receiving rituximab, along with other therapies for oncologic indications.
Conclusion
Recent clinical trial results showed that antibody-mediated depletion of B cells using anti-CD20 antibody, significantly reduced new CNS inflammation and relapses in MS patients and progression in those with gradually worsening disease. Marketing authorization for ocrelizumab as a novel treatment option for MS is expected to be pursued in the near future opening up new questions for both researchers and clinicians: Which pathways and specific B-cell subtypes exactly drive disease pathology? How are other parts of the immune system affected by antigen presentation and release of inflammatory mediators by B cells in the periphery and the CNS compartment? What is the optimal dosing strategy and what monitoring parameters do best reflect treatment success and indicate the need for retreatment? Growing clinical experience will contribute to an assessment of adverse events providing information where to place B-cell-depleting therapies in the growing armamentarium of MS treatments.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SB has received honoraria for lecturing, travel expenses for attending meetings and financial research support from Bayer Schering AG, Biogen Idec, Merck Serono, Novartis and TEVA. TR has received travel expenses and financial research support from Genzyme and has received honoraria for lecturing from Genzyme, Biogen and Teva. OG has received honoraria for lecturing and travel expenses for attending meetings from Roche and Bristol-Myers-Squibb. HW has received honoraria for lecturing, travel expenses for attending meetings from Bayer Health Care, Biogen Idec/Elan Corporation, Lilly, Lundbeck Merck Serono, Novartis, Sanofi Aventis, and TEVA Neuroscience; has served/serves as a consultant for Biogen Idec, Merck Serono, Novartis Pharma Sanofi-Aventis; and receives research support from Bayer Schering Pharma, Biogen Idec/Elan Corporation, Merck Serono, Novartis, Novo Nordisk and Sanofi-Aventis. SGM has received honoraria for lecturing and travel expenses for attending meetings and has received financial research support from Bayer, Bayer Schering, Biogen Idec, Genzyme, Merck Serono, MSD, Novartis, Novo Nordisk, Sanofi-Aventis and Teva.
Contributor Information
Stefan Bittner, Department of Neurology, University of Mainz, Mainz, Germany.
Tobias Ruck, Department of Neurology, University of Münster, Münster, Germany.
Heinz Wiendl, Department of Neurology, University of Münster, Münster, Germany.
Oliver M. Grauer, Department of Neurology, University of Münster, Münster, Germany
Sven G. Meuth, Department of Neurology, University of Münster, Münster, Germany
References
- Alter A., Duddy M., Hebert S., Biernacki K., Prat A., Antel J., et al. (2003) Determinants of human B cell migration across brain endothelial cells. J Immunol 170: 4497–4505. [DOI] [PubMed] [Google Scholar]
- Bar-Or A., Calabresi P., Arnold D., Markowitz C., Shafer S., Kasper L., et al. (2008) Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol 63: 395–400. [DOI] [PubMed] [Google Scholar]
- Bar-Or A., Grove R., Austin A., Tolson J., Vanmeter S., Lewis E., et al. (2014a) The MIRROR study: a randomized, double-blind, placebo-controlled, parallel-group, dose-ranging study to investigate the safety and MRI efficacfy of subcutaneous ofatumumab in subjects with relapsing-remitting multiple sclerosis (RRMS). Neurology 82(Suppl. 10): I7–1.007. [Google Scholar]
- Bar-Or A., Pachner A., Menguy-Vacheron F., Kaplan J., Wiendl H. (2014b) Teriflunomide and its mechanism of action in multiple sclerosis. Drugs 74: 659–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr T., Shen P., Brown S., Lampropoulou V., Roch T., Lawrie S., et al. (2012) B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med 209: 1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barun B., Bar-Or A. (2012) Treatment of multiple sclerosis with anti-CD20 antibodies. Clin Immunol 142: 31–37. [DOI] [PubMed] [Google Scholar]
- Blauth K., Owens G., Bennett J. (2015) The ins and outs of B cells in multiple sclerosis. Front Immunol 6: 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boster A., Ankeny D., Racke M. (2010) The potential role of B cell-targeted therapies in multiple sclerosis. Drugs 70: 2343–2356. [DOI] [PubMed] [Google Scholar]
- Bredemeier M., De Oliveira F., Rocha C. (2014) Low- versus high-dose rituximab for rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res 66: 228–235. [DOI] [PubMed] [Google Scholar]
- Brimnes M., Hansen B., Nielsen L., Dziegiel M., Nielsen C. (2014) Uptake and presentation of myelin basic protein by normal human B cells. PloS one 9: e113388. doi: 10.1371/journal.pone.0113388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttmann M. (2010) Treating multiple sclerosis with monoclonal antibodies: a 2010 update. Expert Rev Neurother 10: 791–809. [DOI] [PubMed] [Google Scholar]
- Casulo C., Maragulia J., Zelenetz A. (2013) Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin Lymphoma Myeloma Leuk 13: 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chofflon M. (2005) Mechanisms of action for treatments in multiple sclerosis: does a heterogeneous disease demand a multi-targeted therapeutic approach? BioDrugs 19: 299–308. [DOI] [PubMed] [Google Scholar]
- Clifford D., Ances B., Costello C., Rosen-Schmidt S., Andersson M., Parks D., et al. (2011) Rituximab-associated progressive multifocal leukoencephalopathy in rheumatoid arthritis. Arch Neurol 68: 1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcione A., Casazza S., Ferretti E., Giunti D., Zappia E., Pistorio A., et al. (2004) Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc Natl Acad Sci U S A 101: 11064–11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A., Stark J., Lauber J., Ramsbottom M., Lyons J. (2006) Rituximab reduces B cells and T cells in cerebrospinal fluid of multiple sclerosis patients. J Neuroimmunol 180: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dass S., Rawstron A., Vital E., Henshaw K., Mcgonagle D., Emery P. (2008) Highly sensitive B cell analysis predicts response to rituximab therapy in rheumatoid arthritis. Arthritis Rheum 58: 2993–2999. [DOI] [PubMed] [Google Scholar]
- Dendrou C., Fugger L., Friese M. (2015) Immunopathology of multiple sclerosis. Nat Rev Immunol 15: 545–558. [DOI] [PubMed] [Google Scholar]
- Dobson R., Ramagopalan S., Davis A., Giovannoni G. (2013) Cerebrospinal fluid oligoclonal bands in multiple sclerosis and clinically isolated syndromes: a meta-analysis of prevalence, prognosis and effect of latitude. J Neurol Neurosurg Psychiatry 84: 909–914. [DOI] [PubMed] [Google Scholar]
- Edwards J., Cambridge G. (2006) B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunol 6: 394–403. [DOI] [PubMed] [Google Scholar]
- Emery P., Mease P., Rubbert-Roth A., Curtis J., Muller-Ladner U., Gaylis N., et al. (2011) Retreatment with rituximab based on a treatment-to-target approach provides better disease control than treatment as needed in patients with rheumatoid arthritis: a retrospective pooled analysis. Rheumatology 50: 2223–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery P., Rigby W., Tak P., Dorner T., Olech E., Martin C., et al. (2014) Safety with ocrelizumab in rheumatoid arthritis: results from the ocrelizumab phase III program. PloS one 9: e87379. doi: 10.1371/journal.pone.0087379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L., Prineas J., Raine C. (1983) Attachment of myelin to coated pits on macrophages in experimental allergic encephalomyelitis. J Neurol Sci 61: 341–348. [DOI] [PubMed] [Google Scholar]
- Esiri M. (1977) Immunoglobulin-containing cells in multiple-sclerosis plaques. Lancet 2: 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau J., Cote G., Neron S. (2006) A new memory CD27-IgG+ B cell population in peripheral blood expressing VH genes with low frequency of somatic mutation. J Immunol 177: 3728–3736. [DOI] [PubMed] [Google Scholar]
- Fillatreau S., Sweenie C., Mcgeachy M., Gray D., Anderton S. (2002) B cells regulate autoimmunity by provision of IL-10. Nat Immunol 3: 944–950. [DOI] [PubMed] [Google Scholar]
- Flach A., Litke T., Strauss J., Haberl M., Gomez C., Reindl M., et al. (2016) Autoantibody-boosted T-cell reactivation in the target organ triggers manifestation of autoimmune CNS disease. Proc Natl Acad Sci U S A 113: 3323–3328 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasperi C., Stuve O., Hemmer B. (2016) B cell-directed therapies in multiple sclerosis. Neurodegener Dis Manag 6: 37–47. [DOI] [PubMed] [Google Scholar]
- Genc K., Dona D., Reder A. (1997) Increased CD80(+) B cells in active multiple sclerosis and reversal by interferon beta-1b therapy. J Clin Invest 99: 2664–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg B., Graves D., Remington G., Hardeman P., Mann M., Karandikar N., et al. (2012) Rituximab dosing and monitoring strategies in neuromyelitis optica patients: creating strategies for therapeutic success. Mult Scler 18: 1022–1026. [DOI] [PubMed] [Google Scholar]
- Grutzke B., Hucke S., Gross C., Herold M., Posevitz-Fejfar A., Wildemann B., et al. (2015) Fingolimod treatment promotes regulatory phenotype and function of B cells. Ann Clin Transl Neurol 2: 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurcan H., Keskin D., Stern J., Nitzberg M., Shekhani H., Ahmed A. (2009) A review of the current use of rituximab in autoimmune diseases. Int Immunopharmacol 9: 10–25. [DOI] [PubMed] [Google Scholar]
- Hafler D., Slavik J., Anderson D., O’connor K., De Jager P., Baecher-Allan C. (2005) Multiple sclerosis. Immunol Rev 204: 208–231. [DOI] [PubMed] [Google Scholar]
- Haider L., Zrzavy T., Hametner S., Hoftberger R., Bagnato F., Grabner G., et al. (2016) The topograpy of demyelination and neurodegeneration in the multiple sclerosis brain. Brain 139: 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliley J., Tipton C., Liesveld J., Rosenberg A., Darce J., Gregoretti I., et al. (2015) Long-lived plasma cells are contained within the CD19(-)Cd38(hi)Cd138(+) subset in human bone marrow. Immunity 43: 132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser S., Waubant E., Arnold D., Vollmer T., Antel J., Fox R., et al. (2008) B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 358: 676–688. [DOI] [PubMed] [Google Scholar]
- Hawker K., O’Connor P., Freedman M., Calabresi P., Antel J., Simon J., et al. (2009) Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol 66: 460–471. [DOI] [PubMed] [Google Scholar]
- Heidt S., Hester J., Shankar S., Friend P., Wood K. (2012) B cell repopulation after alemtuzumab induction-transient increase in transitional B cells and long-term dominance of naive B cells. Am J Transplant 12: 1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Milo R., Swoveland P., Johnson K., Panitch H., Dhib-Jalbut S. (1995) Interferon beta-1b reduces interferon gamma-induced antigen-presenting capacity of human glial and B cells. J Neuroimmunol 61: 17–25. [DOI] [PubMed] [Google Scholar]
- Kabat E., Freedman D., Murray J., Knaub V. (1950) A study of the crystalline albumin, gamma globulin and total protein in the cerebrospinal fluid of 100 cases of multiple sclerosis and in other diseases. Am J Med Sci 219: 55–64. [DOI] [PubMed] [Google Scholar]
- Kappos L., Hartung H., Freedman M., Boyko A., Radu E., Mikol D., et al. (2014) Atacicept in multiple sclerosis (ATAMS): a randomised, placebo-controlled, double-blind, phase II trial. Lancet Neurol 13: 353–363. [DOI] [PubMed] [Google Scholar]
- Kappos L., Li D., Calabresi P., O’Connor P., Bar-Or A., Barkhof F., et al. (2011) Ocrelizumab in relapsing-remitting multiple sclerosis: a phase II, randomised, placebo-controlled, multicentre trial. Lancet 378: 1779–1787. [DOI] [PubMed] [Google Scholar]
- Kim S., Huh S., Lee S., Joung A., Kim H. (2013) A 5-year follow-up of rituximab treatment in patients with neuromyelitis optica spectrum disorder. JAMA Neurol 70: 1110–1117. [DOI] [PubMed] [Google Scholar]
- Kim S., Jeong I., Hyun J., Joung A., Jo H., Hwang S., et al. (2015) Treatment outcomes with rituximab in 100 patients with neuromyelitis optica: influence of FCGR3A polymorphisms on the therapeutic response to rituximab. JAMA Neurol 72: 989–995. [DOI] [PubMed] [Google Scholar]
- Kim S., Kim W., Li X., Jung I., Kim H. (2011) Repeated treatment with rituximab based on the assessment of peripheral circulating memory B cells in patients with relapsing neuromyelitis optica over 2 years. Arch Neurol 68: 1412–1420. [DOI] [PubMed] [Google Scholar]
- Kinzel S., Lehmann-Horn K., Torke S., Hausler D., Winkler A., Stadelmann C., et al. (2016) Myelin-reactive antibodies initiate T cell-mediated CNS autoimmune disease by opsonization of endogenous antigen. Acta Neuropathol 132: 43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz L., Wiendl H. (2013) Monoclonal antibodies in neuroinflammatory diseases. Expert Opin Biol Ther 13: 831–846. [DOI] [PubMed] [Google Scholar]
- Komori M., Lin Y., Cortese I., Blake A., Ohayon J., Cherup J., et al. (2016) Insufficient disease inhibition by intrathecal rituximab in progressive multiple sclerosis. Ann Clin Transl Neurol 3: 166–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowarik M., Pellkofer H., Cepok S., Korn T., Kumpfel T., Buck D., et al. (2011) Differential effects of fingolimod (FTY720) on immune cells in the CSF and blood of patients with MS. Neurology 76: 1214–1221. [DOI] [PubMed] [Google Scholar]
- Krumbholz M., Derfuss T., Hohlfeld R., Meinl E. (2012) B cells and antibodies in multiple sclerosis pathogenesis and therapy. Nat Rev Neurol 8: 613–623. [DOI] [PubMed] [Google Scholar]
- Leandro M., Cambridge G., Ehrenstein M., Edwards J. (2006) Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum 54: 613–620. [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn K., Schleich E., Hertzenberg D., Hapfelmeier A., Kumpfel T., von Bubnoff N., et al. (2011) Anti-CD20 B-cell depletion enhances monocyte reactivity in neuroimmunological disorders. J Neuroinflammation 8: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Rezk A., Healy L., Muirhead G., Prat A., Gommerman J., et al. (2015a) Cytokine-defined B cell responses as therapeutic targets in multiple sclerosis. Front Immunol 6: 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Rezk A., Miyazaki Y., Hilgenberg E., Touil H., Shen P., et al. (2015b) Proinflammatory GM-CSF-producing B cells in multiple sclerosis and B cell depletion therapy. Sci Transl Med 7: 310ra166. doi: 10.1126/scitranslmed.aab4176. [DOI] [PubMed] [Google Scholar]
- Liu Z., Pelfrey C., Cotleur A., Lee J., Rudick R. (2001) Immunomodulatory effects of interferon beta-1a in multiple sclerosis. J Neuroimmunol 112: 153–162. [DOI] [PubMed] [Google Scholar]
- Lock C., Hermans G., Pedotti R., Brendolan A., Schadt E., Garren H., et al. (2002) Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med 8: 500–508. [DOI] [PubMed] [Google Scholar]
- Longbrake E., Cross A. (2016) Effect of multiple sclerosis disease-modifying therapies on B cells and humoral immunity. JAMA Neurol 73: 219–225. [DOI] [PubMed] [Google Scholar]
- Louveau A., Smirnov I., Keyes T., Eccles J., Rouhani S., Peske J., et al. (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchinetti C., Bruck W., Parisi J., Scheithauer B., Rodriguez M., Lassmann H. (2000) Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 47: 707–717. [DOI] [PubMed] [Google Scholar]
- Magliozzi R., Howell O., Reeves C., Roncaroli F., Nicholas R., Serafini B., et al. (2010) A gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol 68: 477–493. [DOI] [PubMed] [Google Scholar]
- Mahevas M., Ebbo M., Audia S., Bonnotte B., Schleinitz N., Durand J., et al. (2013) Efficacy and safety of rituximab given at 1,000 mg on days 1 and 15 compared to the standard regimen to treat adult immune thrombocytopenia. Am J Hematol 88: 858–861. [DOI] [PubMed] [Google Scholar]
- Maloney D. (2012) Anti-CD20 antibody therapy for B-cell lymphomas. N Engl J Med 366: 2008–2016. [DOI] [PubMed] [Google Scholar]
- Martin Mdel P., Cravens P., Winger R., Kieseier B., Cepok S., Eagar T., et al. (2009) Depletion of B lymphocytes from cerebral perivascular spaces by rituximab. Arch Neurol 66: 1016–1020. [DOI] [PubMed] [Google Scholar]
- Melzer N., Meuth S. (2014) Disease-modifying therapy in multiple sclerosis and chronic inflammatory demyelinating polyradiculoneuropathy: common and divergent current and future strategies. Clin Exp Immunol 175: 359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel L., Touil H., Pikor N., Gommerman J., Prat A., Bar-Or A. (2015) B Cells in the multiple sclerosis central nervous system: trafficking and contribution to CNS-compartmentalized inflammation. Front Immunol 6: 636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milo R. (2016) Therapeutic strategies targeting B-cells in multiple sclerosis. Autoimmun Rev 15: 714–718. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y., Niino M., Fukazawa T., Takahashi E., Nonaka T., Amino I., et al. (2014) Suppressed pro-inflammatory properties of circulating B cells in patients with multiple sclerosis treated with fingolimod, based on altered proportions of B-cell subpopulations. Clin Immunol 151: 127–135. [DOI] [PubMed] [Google Scholar]
- Moller B., Aeberli D., Eggli S., Fuhrer M., Vajtai I., Vogelin E., et al. (2009) Class-switched B cells display response to therapeutic B-cell depletion in rheumatoid arthritis. Arthritis Res Ther 11: R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson N., Cravens P., Frohman E., Hawker K., Racke M. (2005) Effect of rituximab on the peripheral blood and cerebrospinal fluid B cells in patients with primary progressive multiple sclerosis. Arch Neurol 62: 258–264. [DOI] [PubMed] [Google Scholar]
- Moore G., Raine C. (1988) Immunogold localization and analysis of IgG during immune-mediated demyelination. Lab Invest 59: 641–648. [PubMed] [Google Scholar]
- Mysler E., Spindler A., Guzman R., Bijl M., Jayne D., Furie R., et al. (2013) Efficacy and safety of ocrelizumab in active proliferative lupus nephritis: results from a randomized, double-blind, phase III study. Arthritis Rheum 65: 2368–2379. [DOI] [PubMed] [Google Scholar]
- Naismith R., Piccio L., Lyons J., Lauber J., Tutlam N., Parks B., et al. (2010) Rituximab add-on therapy for breakthrough relapsing multiple sclerosis: a 52-week phase II trial. Neurology 74: 1860–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus O., Farina C., Wekerle H., Hohlfeld R. (2001) Mechanisms of action of glatiramer acetate in multiple sclerosis. Neurology 56: 702–708. [DOI] [PubMed] [Google Scholar]
- O’Brien K., Gran B., Rostami A. (2010) T-cell based immunotherapy in experimental autoimmune encephalomyelitis and multiple sclerosis. Immunotherapy 2: 99–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oflazoglu E., Audoly L. (2010) Evolution of anti-CD20 monoclonal antibody therapeutics in oncology. MAbs 2: 14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owczarczyk K., Lal P., Abbas A., Wolslegel K., Holweg C., Dummer W., et al. (2011) A plasmablast biomarker for nonresponse to antibody therapy to CD20 in rheumatoid arthritis. Sci Transl Med 3: 101ra192. doi: 10.1126/scitranslmed.3002432. [DOI] [PubMed] [Google Scholar]
- Owens G., Bennett J., Lassmann H., O’Connor K., Ritchie A., Shearer A., et al. (2009) Antibodies produced by clonally expanded plasma cells in multiple sclerosis cerebrospinal fluid. Ann Neurol 65: 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanichamy A., Apeltsin L., Kuo T., Sirota M., Wang S., Pitts S., et al. (2014a) Immunoglobulin class-switched B cells form an active immune axis between CNS and periphery in multiple sclerosis. Sci Transl Med 6: 248ra106. doi: 10.1126/scitranslmed.3008930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanichamy A., Jahn S., Nickles D., Derstine M., Abounasr A., Hauser S., et al. (2014b) Rituximab efficiently depletes increased CD20-expressing T cells in multiple sclerosis patients. J Immunol 193: 580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellkofer H., Krumbholz M., Berthele A., Hemmer B., Gerdes L., Havla J., et al. (2011) Long-term follow-up of patients with neuromyelitis optica after repeated therapy with rituximab. Neurology 76: 1310–1315. [DOI] [PubMed] [Google Scholar]
- Pers J., Daridon C., Bendaoud B., Devauchelle V., Berthou C., Saraux A., et al. (2008) B-cell depletion and repopulation in autoimmune diseases. Clin Rev Allergy Immunol 34: 50–55. [DOI] [PubMed] [Google Scholar]
- Polman C., Reingold S., Banwell B., Clanet M., Cohen J., Filippi M., et al. (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prineas J., Wright R. (1978) Macrophages, lymphocytes, and plasma cells in the perivascular compartment in chronic multiple sclerosis. Lab Invest 38: 409–421. [PubMed] [Google Scholar]
- Roll P., Dorner T., Tony H. (2008) Anti-CD20 therapy in patients with rheumatoid arthritis: predictors of response and B cell subset regeneration after repeated treatment. Arthritis Rheum 58: 1566–1575. [DOI] [PubMed] [Google Scholar]
- Roll P., Mahmood Z., Muhammad K., Feuchtenberger M., Dorner T., Tony H. (2015) Long-term repopulation of peripheral B-cell subsets after single and repeated rituximab infusions in patients with rheumatoid arthritis. Clin Exp Rheumatol 33: 347–353. [PubMed] [Google Scholar]
- Roll P., Palanichamy A., Kneitz C., Dorner T., Tony H. (2006) Regeneration of B cell subsets after transient B cell depletion using anti-CD20 antibodies in rheumatoid arthritis. Arthritis Rheum 54: 2377–2386. [DOI] [PubMed] [Google Scholar]
- Rovituso D., Duffy C., Schroeter M., Kaiser C., Kleinschnitz C., Bayas A., et al. (2015) The brain antigen-specific B cell response correlates with glatiramer acetate responsiveness in relapsing-remitting multiple sclerosis patients. Sci Rep 5: 14265. doi: 10.1038/srep14265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer J., Lycke J., Wickstrom R., Naver H., Piehl F., Svenningsson A. (2016) Rituximab in paediatric onset multiple sclerosis: a case series. Journal of Neurology 263: 322–326. [DOI] [PubMed] [Google Scholar]
- Schneider P. (2005) The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol 17: 282–289. [DOI] [PubMed] [Google Scholar]
- Serafini B., Rosicarelli B., Magliozzi R., Stigliano E., Aloisi F. (2004) Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol 14: 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen P., Fillatreau S. (2015) Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol 15: 441–451. [DOI] [PubMed] [Google Scholar]
- Shen P., Roch T., Lampropoulou V., O’Connor R., Stervbo U., Hilgenberg E., et al. (2014) IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 507: 366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen P., Blinkenberg M. (2016) The potential role for ocrelizumab in the treatment of multiple sclerosis: current evidence and future prospects. Ther Adv Neurol Disord 9: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen P., Lisby S., Grove R., Derosier F., Shackelford S., Havrdova E., et al. (2014) Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: a phase II study. Neurology 82: 573–581. [DOI] [PubMed] [Google Scholar]
- Stern J., Yaari G., Vander Heiden J., Church G., Donahue W., Hintzen R., et al. (2014) B cells populating the multiple sclerosis brain mature in the draining cervical lymph nodes. Sci Transl Med 6: 248ra107. doi: 10.1126/scitranslmed.3008879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuve O., Cepok S., Elias B., Saleh A., Hartung H., Hemmer B., et al. (2005) Clinical stabilization and effective B-lymphocyte depletion in the cerebrospinal fluid and peripheral blood of a patient with fulminant relapsing-remitting multiple sclerosis. Arch Neurol 62: 1620–1623. [DOI] [PubMed] [Google Scholar]
- Stuve O., Marra C., Jerome K., Cook L., Cravens P., Cepok S., et al. (2006) Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol 59: 743–747. [DOI] [PubMed] [Google Scholar]
- Stuve O., Warnke C., Deason K., Stangel M., Kieseier B., Hartung H., et al. (2014) CD19 as a molecular target in CNS autoimmunity. Acta Neuropathol 128: 177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson A., Bergman J., Dring A., Vagberg M., Birgander R., Lindqvist T., et al. (2015) Rapid depletion of B lymphocytes by ultra-low-dose rituximab delivered intrathecally. Neurol Neuroimmunol Neuroinflamm 2: e79. doi: 10.1212/NXI.0000000000000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S., Jones J., Cox A., Compston D., Coles A. (2010) B-cell reconstitution and BAFF after alemtuzumab (Campath-1H) treatment of multiple sclerosis. J Clin Immunol 30: 99–105. [DOI] [PubMed] [Google Scholar]
- Tokunaga M., Saito K., Kawabata D., Imura Y., Fujii T., Nakayamada S., et al. (2007) Efficacy of rituximab (anti-CD20) for refractory systemic lupus erythematosus involving the central nervous system. Ann Rheum Dis 66: 470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tony H., Roll P., Mei H., Blumner E., Straka A., Gnuegge L., et al. (2015) Combination of B cell biomarkers as independent predictors of response in patients with rheumatoid arthritis treated with rituximab. Clin Exp Rheumatol 33: 887–894. [PubMed] [Google Scholar]
- Villar L., Masjuan J., Gonzalez-Porque P., Plaza J., Sadaba M., Roldan E., et al. (2002) Intrathecal IgM synthesis in neurologic diseases: relationship with disability in MS. Neurology 58: 824–826. [DOI] [PubMed] [Google Scholar]
- Von Budingen H., Kuo T., Sirota M., Van Belle C., Apeltsin L., Glanville J., et al. (2012) B cell exchange across the blood-brain barrier in multiple sclerosis. J Clin Invest 122: 4533–4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M., Prod’homme T., Patarroyo J., Molnarfi N., Karnezis T., Lehmann-Horn K., et al. (2010) B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity. Ann Neurol 68: 369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Kipling D., Dunn-Walters D. (2011) The relationship between CD27 negative and positive B cell populations in human peripheral blood. Front Immunol 2: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman A., Kidd D., Mclean B., Kelly M., Francis D., Miller D., et al. (1996) A study of oligoclonal band negative multiple sclerosis. J Neurol Neurosurg Psychiatry 60: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B. (2009) Ofatumumab. MAbs 1: 326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]